Abstract
To investigate the importance of tyrosine recognition by the AP-1B clathrin adaptor subunit μ1B for basolateral sorting of integral membrane proteins in polarized epithelial cells, we have produced and characterized a mutant form of μ1B. The mutant (M-μ1B) contains alanine substitutions of each of the four conserved residues, which in the AP-2 adaptor subunit μ2 are critical for interacting with tyrosine-based endocytosis signals. We show M-μ1B is defective for tyrosine binding in vitro, but is nevertheless incorporated into AP-1 complexes in transfected cells. Using LLC-PK1 cells expressing either wild type or M-μ1B, we find that there is inefficient basolateral expression of membrane proteins whose basolateral targeting signals share critical tyrosines with signals for endocytosis. In contrast, membrane proteins whose basolateral targeting signals are distinct from their endocytosis signals (transferrin and low-density lipoprotein receptors) accumulate at the basolateral domain normally, although in a manner that is strictly dependent on μ1B or M-μ1B expression. Our results suggest that μ1B interacts with different classes of basolateral targeting signals in distinct ways.
INTRODUCTION
The plasma membrane of epithelial cells is physically separated by the tight junction into two distinct domains: the apical and the basolateral membranes. These two membrane domains have distinct lipid and protein compositions, which is thought to be important for the polarity and function of epithelial cells (Mellman, 1996; Aroeti et al., 1998; Mostov et al., 2000). To maintain the “polar” distribution of newly synthesized membrane proteins, as well as those endocytosed from the cell surface, proteins must be transported to the proper plasma membrane domain from the trans-Golgi network (TGN) or from the endosomal compartments, respectively.
Polarized targeting of basolateral plasma membrane proteins is largely dependent on distinct sorting signals present in their cytoplasmic domains (Mellman, 1996; Aroeti et al., 1998; Mostov et al., 2000). Some of these basolateral sorting signals show a sequence similarity with tyrosine-based or dileucine-based endocytosis signals, which are well known as clathrin-coated pit targeting signals (Matter and Mellman, 1994). Because these coated pit targeting signals directly interact with adaptor protein (AP) complexes of clathrin coats (Ohno et al., 1995; Boll et al., 1996; Dell'Angelica et al., 1997; Rapoport et al., 1998; Rodionov and Bakke, 1998; Hofmann et al., 1999), it had been hypothesized early on that an AP or AP-like complex may play a similar role in basolateral sorting in epithelial cells (Hunziker et al., 1991).
AP complexes comprise a family of heterotetrameric protein complexes (AP-1 through AP-4) consisting of two large (α, γ, δ or ε, and β), one medium (μ), and one small (ς) subunit (Hirst and Robinson, 1998; Bonifacino and Dell'Angelica, 1999). Recently, we cloned a novel medium subunit, μ1B, which is expressed only in epithelial cells (Ohno et al., 1999). μ1B can assemble in combinatorial manner with three subunits of AP-1A (γ, β1, and ς1) to generate an AP-1B complex (Folsch et al., 1999). Importantly, AP-1B plays an essential role in basolateral targeting of a variety of membrane proteins such as the transferrin receptor (TfR) and the low-density lipoprotein receptor (LDLR) (Folsch et al., 1999, 2001). AP-1A cannot substitute for AP-1B in basolateral sorting, consistent with the fact that only AP-1B, and not AP-1A, complexes interact physically with basolateral targeting signals (Folsch et al., 2001). Because the only apparent difference between these complexes is identity of their μ subunits, it is reasonable to suspect that the μ1B subunit itself is responsible for recognizing basolateral targeting signals.
Indeed, it is well known that all μ subunits at least in vitro interact directly with sorting signals that contain critical tyrosine residues, where those signals conform to the consensus sequence YXXØ (where Y is tyrosine; X is any amino acid; and Ø is a bulky, hydrophobic residue) (Ohno et al., 1995, 1999; Boll et al., 1996; Dell'Angelica et al., 1997; Aguilar et al., 2001). However, μ subunits interact with distinct subsets of tyrosine-based signals with different affinities, a feature that is likely to reflect their ability to select different cargo proteins during transport (Ohno et al., 1996, 1998). An interesting feature of basolateral targeting signals is that they tend to be highly heterogeneous, with many not conforming to the YXXØ motif. Even in these instances, however, transport to the basolateral surface is completely dependent on AP-1B (Folsch et al., 1999). Conceivably, these different classes of signals interact with μ1B in distinct ways.
Thus far, the only μ chain whose structure has been at least partially solved is the μ2 subunit of the AP-2 adaptor complex (Owen and Evans, 1998). By analyzing the position of a peptide containing YXXØ-type signal bound to μ2, several residues in μ2 were identified that seemed to be responsible for signal binding. Because these residues are also conserved in the sequence of μ1B, we asked whether they were also important for the binding of basolateral signals. Indeed, they were but only in the case of signals that depended on critical tyrosine residues that were also required for endocytosis.
MATERIALS AND METHODS
Antibodies
A rabbit polyclonal antibody specific for a μ1B C-terminal peptide was described previously (Folsch et al., 1999). A rabbit antiserum recognizing β4 was raised against a glutathione S-transferase-β4 fragment (corresponding amino acid residues 452–806 of human β4). An anti-human asialoglycoprotein receptor (AGPR) subunit H1 antiserum was a gift from Dr. Martin Spiess (University of Basel, Basel, Switzerland). The following antibodies were obtained from the American Type Culture Collection (Manassas, VA): 7G7.B6, a monoclonal antibody (mAb) recognizing Tac, the interleukin-2 receptor α subunit; L5.1, a mAb specific for the human TfR; and a mAb specific for the human LDLR, C7. A mouse anti-γ-adaptin mAb, 100/3, was purchased from Sigma-Aldrich (St. Louis, MO). The following were purchased from Molecular Probes (Eugene, OR): Alexa Fluor 488-conjugated anti-mouse and anti-rabbit IgG antibodies; Alexa Fluor 546-conjugated anti-mouse and anti-rabbit IgG antibodies; and an Alexa Fluor 488 phalloidin. Anti-mouse and anti-rabbit IgG, 125I-labeled whole antibody, were purchased from Amersham Biosciences (Piscataway, NJ).
Plasmids
GAL4ad-μ1B, GAL4bd-EITYWFL, and GAL4bd-RSLYRRL were described previously (Ohno et al., 1999). A mutant human μ1B cDNA (M-μ1B), in which four amino acids (Phe172, Asp174, Trp408, and Arg410) were replaced with alanine, was produced by polymerase chain reaction-based site-directed mutagenesis, and subcloned into pcDNA3 (Invitrogen, Carlsbad, CA) for transfection, or into pACT2 (CLONTECH, Palo Alto, CA) for two-hybrid analyses. The expression vector for the human AGPR subunit H1 and its tyrosine-to-alanine mutant, AGPR-H1(5A), were a gift from Dr. Martin Spiess. Tac-lysosomal-associated membrane protein-1 (Lamp-1) was made by polymerase chain reaction-based recombination and subcloned into pcDNA3 as described previously (Humphrey et al., 1993) and has the sequence of the luminal and transmembrane domains of Tac and the cytoplasmic domain of Lamp-1. In Tac-Lamp1.YA, tyrosine in the cytoplasmic domain of Lamp-1 was substituted with alanine. cDNA encoding the human TfR (a gift from Dr. Juan S. Bonifacino, National Institutes of Health, Bethesda, MD) was subcloned into pcDNA3. Expression constructs for LDLR were described previously (Matter et al., 1992).
Yeast Two-Hybrid Analysis
The yeast strain HF7c (CLONTECH) was maintained on YEPD (rich) medium. Transformation and two-hybrid analyses were performed as described in the instructions for the MATCHMAKER two-hybrid system (CLONTECH). In brief, GAL4-binding domain (bd) and GAL4-activation domain (ad) constructs were cotransformed into HF7c. Half of the transformants were cultured on dropout media lacking leucine and tryptophan (indicated as +His) as a control of transformation, and half were plated on media lacking leucine, tryptophan, and histidine (denoted as −His). Transformants growing on the −His plate were judged positive for protein–protein interactions.
Cell Culture and Transfection
LLC-PK1 porcine kidney cells were cultured in Dulbecco's modified Eagle's medium (Sigma-Aldrich) supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin (Sigma-Aldrich) (regular medium). LLC-PK1 cells stably transfected with human μ1B (LLC-PK1::μ1B) (Folsch et al., 1999) were grown in regular medium supplemented with 1.8 mg/ml geneticin (Invitrogen). To obtain LLC-PK1 cells stably expressing M-μ1B, cells were transfected using the calcium phosphate precipitation, and the positive clones were selected and maintained in regular medium supplemented with 1.8 mg/ml geneticin.
Immunoprecipitation, Gel Filtration, and Immunoblotting
LLC-PK1 transfectants were split in six-well plates 1 d before the experiment. The cells (on ice) were washed twice with ice-cold phosphate-buffered saline (PBS), and then buffer A (1% Triton X-100 [wt/vol], 0.3 M NaCl, 50 mM Tris-HCl [pH 7.4], 0.1% bovine serum albumin [wt/vol], and protease inhibitors [240 μg/ml pBASF, 2 μg/ml aprotinin, 157 μg/ml benzamidine, 10 μg/ml leupeptin, 10 μg/ml chymostatin, and 10 μg/ml pepstatin A]) was added. The cells were recovered using a cell scraper and passed four times through a 21-gauge needle. Lysis was judged complete after a 30-min incubation on ice. The lysates were clarified by centrifugation for 15 min at 13,000 rpm in an Eppendorf centrifuge at 4°C. The resulting supernatants were used for immunoprecipitation with the 100/3 anti-γ-adaptin antibody prebound to protein G-Sepharose (Amersham Biosciences) at 4°C. As a negative control, the 7G7 anti-Tac mAb was used in parallel. Immunoprecipitates were washed twice with buffer A, once with buffer A without Triton X-100, and eluted in SDS-PAGE sample buffer. The samples were subjected to SDS-PAGE, transferred onto Hybond-ECL membranes (Amersham Biosciences), immunoblotted with the anti-μ1B antibody or the anti-γ-adaptin antibody, and detected using the enhanced chemiluminescence system (Amersham Biosciences).
For gel filtration analysis, 400 μl/well of buffer A without bovine serum albumin was used for lysis, and 200 μl of lysis supernatant was subsequently applied to a Superose 6 gel filtration column equilibrated with buffer B (0.5 mM EDTA, 1% Triton X-100, 0.3 M NaCl, 50 mM Tris-HCl [pH 7.4]). Fractions (0.5 ml) were collected and precipitated by adding trichloroacetic acid to a final concentration of 10% (wt/vol). Samples were resolved on SDS-PAGE and subjected to Western blot analysis by using anti-μ1B, anti-γ-adaptin, anti-α-adaptin, anti-ς3, and anti-β4 antibodies.
Immunofluorescence
LLC-PK1 cells stably expressing μ1B or M-μ1B were plated on polycarbonate membrane filters at a density of 5.6 × 104 cells/6.5-mm filter (Transwell units, 0.4-μm pore size; Corning-Costar, Corning, NY) and cultured for 4 d with daily changes of medium. Cells were transfected with the indicated expression plasmids by using GenePORTER2 (Gene Therapy Systems, San Diego, CA). After 2 d of incubation, the cells were washed twice with PBS, and the indicated antibodies were added to both the apical and the basolateral sides. After an incubation of 30 min at 4°C, cultures were washed twice with PBS and fixed in 3% paraformaldehyde/PBS for 15 min at room temperature. Subsequently, the filters were washed twice with PBS and incubated with the secondary antibodies Alexa Fluor 488 anti-mouse IgG for the apical side and Alexa Fluor 546 anti-mouse IgG for the basolateral side, respectively, for 1 h. When parental LLC-PK1 cells were stained, cells were plated at a density of 1.7 × 105 cells/12-mm filter (0.4-μm pore size), washed twice in PBS, incubated with the primary antibodies for 30 min at 4°C, washed twice in PBS, fixed in 3% paraformaldehyde/PBS, and incubated with Alexa Fluor 546 anti-mouse IgG for 1 h. This is because the parental cells usually fail to make a continuous monolayer. The filters were then cut off and washed four times with PBS over a period of 30 min.
For staining with Alexa Fluor 488 phalloidin, cells were cultured for 6 d with daily changes of medium, fixed in 3% paraformaldehyde, permeabilized with 0.1% Triton X-100, washed two times with PBS, and incubated for 30 min. Samples were analyzed using an LSM 510 laser scanning confocal microscope (Carl Zeiss, Thornwood, NY).
Binding of Radioiodinated Antibodies
Parental LLC-PK1 cells or cells stably expressing μ1B or M-μ1B were plated (at a density of 1.7 × 105 cells/12-mm filter) and transfected as described for immunofluorescence experiments. After 2 d of incubation, the cells were washed twice with ice-cold PBS, and the indicated antibodies were added from either the apical or basolateral side. After an incubation of 30 min at 4°C, cultures were washed twice with PBS and fixed in 3% paraformaldehyde/PBS for 15 min at room temperature. Subsequently, the filters were washed twice with PBS and incubated with secondary antibodies (anti-mouse or anti-rabbit IgG, 125I-labeled whole antibody), added to both sides of the filter membrane, for 1 h at room temperature. Finally, the filters were washed twice with PBS, cut off, and cell-associated radioactivity was measured with a gamma counter. Nonspecific binding was determined by measuring binding to cultures incubated with the secondary antibodies alone, which were subtracted from the cell-associated radioactivity determined as described above. All given values represent the mean of three independent experiments performed in duplicate. Mean values of the three experiments for the sum of the apically and basolaterally associated radioactivity in parental, μ1B-, and M-μ1B–expressing LLC-PK1 cells were 481, 443, and 524 cpm for AGPR-H1 transfection; 296, 264, and 222 cpm for Tac-Lamp1 transfection; 133, 147, and 125 cpm for LDLR; and 143, 118, and 113 cpm for TfR transfection, respectively.
RESULTS
Production of a Mutant μ1B Deficient at Binding Tyrosine-based Motifs
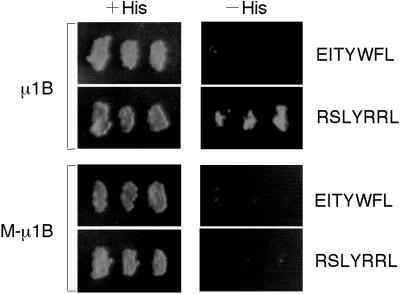
To investigate the importance of μ1B-recognition of tyrosine-based sorting signals in basolateral sorting, we generated a mutant form of μ1B in which those residues possibly involved in tyrosine recognition were altered. The residues selected for mutagenesis were those identified from the μ2 crystal structure as being involved in binding YXXØ signals, four of which were precisely conserved in the μ1B sequence (Owen and Evans, 1998; Bonifacino and Dell'Angelica, 1999). Phe172, Asp174, Trp408, and Arg410 were each replaced with alanines to produce the M-μ1B mutant. Initially, we examined the ability of this protein to bind tyrosine motifs in a yeast two-hybrid assay. We picked two YXXØ sequences from combinatorial library clones according to the previous study (Ohno et al., 1999); YWFL as a negative control and YRRL as a positive control, respectively, for μ1B binding. As expected, M-μ1B failed to interact with a test YXXØ motif, YRRL, which interacted with μ1B (Figure 1). Thus, altering the four conserved residues required for tyrosine interactions in μ2 greatly reduced the ability of μ1B to interact with YXXØ motifs.
Figure 1.
M-μ1B does not interact with tyrosine-based sorting signals. HF7c yeast cells were cotransformed with a plasmid encoding GAL4ad fused to human μ1B or a mutant μ1B (M-μ1B) and a plasmid encoding GAL4bd fused to EITYWFL or RSLYRRL. Cotransformants were tested for their ability to grow in the presence (+His) or absence (−His) of histidine. Growth on the −His plate indicates that the products of GAL4bd and GAL4ad constructs can interact.
Mutant μ1B (M-μ1B) Is Incorporated into AP-1B Complexes
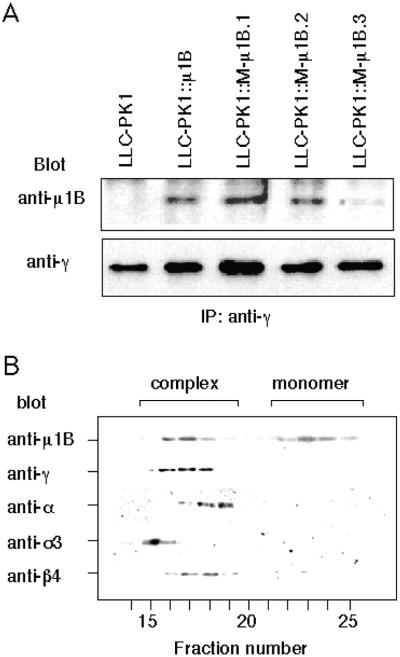
We next asked whether the mutations introduced into M-μ1B affected the incorporation of M-μ1B into AP-1B complexes. For this purpose, we established LLC-PK1 cell lines stably expressing M-μ1B and determined whether an anti-γ adaptin antibody could coprecipitate M-μ1B from these cells. As previously shown, wild-type μ1B coprecipitates with γ-adaptin, a large-chain adaptin of AP-1 (Figure 2A) (Folsch et al., 1999, 2001). In the present study, M-μ1B was detected in anti-γ-adaptin precipitates from lysates of three stable cell lines expressing M-μ1B (Figure 2). This suggests that M-μ1B is incorporated into AP-1B complexes.
Figure 2.
M-μ1B is specifically incorporated into AP-1B complexes. (A) AP-1 complexes were immunoprecipitated with an anti-γ-adaptin antibody 100/3 from lysates of LLC-PK1 cells stably expressing μ1B (LLC-PK1::μ1B) or a mutant μ1B (LLC-PK1::M-μ1B). Immunoprecipitants were subjected to SDS-PAGE, transferred onto Hybond-ECL membranes, immunoblotted with the anti-μ1B antibody or the anti-γ-adaptin antibody, and detected using the enhanced chemiluminescence system as described in MATERIALS AND METHODS. (B) Cytosol from LLC-PK1 cells stably expressing M-μ1B (LLC-PK1::μ1B) was fractionated by gel filtration chromatography on a Superose 6 column, and fractions (0.5 ml) were collected and analyzed by SDS-PAGE and Western blotting by using antibodies to various AP subunits as described in MATERIALS AND METHODS.
We also verified that M-μ1B was specifically assembled into AP-1 but not into the other AP complexes (i.e., AP-2, AP-3, or AP-4). Cytosol from LLC-PK1 cells stably expressing M-μ1B was fractionated by gel filtration chromatography on a Superose 6 column, and fractions were collected and subjected to Western blot analysis by using anti-AP subunit antibodies. As shown in Figure 2B, and consistent with previous observations for μ1B stably expressed in LLC-PK1 (Folsch et al., 1999), M-μ1B was eluted in two peaks. One peak coeluted with AP-1, as indicated by the presence of γ-adaptin in the same fractions. The second peak likely represented unassembled monomeric M-μ1B, as previously reported for μ1B (Folsch et al., 1999). Figure 2B also showed that the subunits of other AP complexes tested had different elution profiles from M-μ1B. Our elution profiles are consistent with studies demonstrating that each AP complex exhibits somewhat different apparent molecular weights (Dell'Angelica et al., 1997, 1999). In combination, these data suggest that M-μ1B assembles into an AP-1 complex, with some not incorporating and existing as a monomer, in our LLC-PK1 cells. Also, M-μ1B does not seem to incorporate into the other AP complexes, a finding in agreement with previous studies of μ1B. Comparison of the expression levels by immunoblotting of the serial dilution of the lysates showed a similar amount of μ1B expression for LLC-PK1::μ1B and LLC-PK1::M-μ1B.1 cells (our unpublished data). The results presented in this study were obtained using LLC-PK1::M-μ1B.1, but similar results were observed using LLC-PK1::M-μ1B.2 cells (our unpublished data).
Recognition of Tyrosine by μ1B Is Required for YXXØ-Motif–dependent Basolateral Sorting In Vivo
We have demonstrated that μ1B is required for the basolateral sorting of membrane proteins containing basolateral targeting signals, such as TfR and LDLR (Folsch et al., 1999, 2001). Because M-μ1B was incorporated into AP-1B complexes (Figure 2), we asked whether it could support the proper targeting of basolateral membrane proteins, as does μ1B. We first examined the steady-state localization on the plasma membrane of AGPR-H1 transiently expressed in filter-grown LLC-PK1 cells stably expressing μ1B or M-μ1B. AGPR-H1 is a basolateral membrane protein that cycles between the plasma membrane and endosomes in hepatocytes and transfected Madin-Darby canine kidney (MDCK) cells. A tyrosine-based sorting motif YQDL is essential for both efficient internalization and polarized expression of AGPR-H1 (Fuhrer et al., 1991; Geffen et al., 1993).
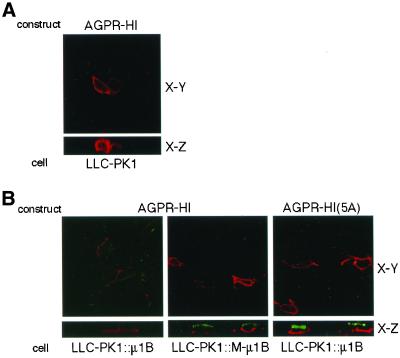
An analysis of the transfected AGPR-H1 localization by using immunofluorescence confocal microscopy is presented in Figure 3. As expected, AGPR-H1 was localized predominantly on the basolateral plasma membrane in LLC-PK1::μ1B cells (Figure 3B). However, it was detected on the apical and basolateral plasma membranes in LLC-PK1::M-μ1B cells, much as it was when expressed in the μ1B-negative parental LLC-PK1 cells (Figure 3, A and B). We also determined the distribution of AGPR-H1(5A), in which the tyrosine in the YQDL motif was substituted with alanine (Geffen et al., 1993). Herein, AGPR-H1(5A) distributed on both apical and basolateral plasma membranes even in LLC-PK1::μ1B cells (Figure 3B). These results were confirmed by a quantitative antibody binding assay (see below).
Figure 3.
Basolateral sorting of AGPR-H1 depends on the tyrosine-binding ability of μ1B. (A) LLC-PK1 cells grown on the Transwell filters were transiently transfected with the AGPR-H1 expression plasmid and incubated with an anti-H1 antiserum. Subsequently, cells were fixed and stained with the Alexa Fluor 546 anti-rabbit IgG as described in MATERIALS AND METHODS. (B) LLC-PK1 cells stably expressing μ1B (LLC-PK1::μ1B) or a mutant μ1B (LLC-PK1::M-μ1B) grown on the Transwell filters were transiently transfected with AGPR-H1 or AGPR-H1(5A) expression plasmids, and incubated with an anti-H1 antiserum. Subsequently, cells were fixed and stained with the secondary antibodies, the Alexa Fluor 488 anti-rabbit IgG from the apical side, and Alexa Fluor 546 anti-rabbit IgG from the basolateral side as described in MATERIALS AND METHODS. Samples were analyzed using an LSM 510 laser scanning confocal microscope (Carl Zeiss, Thornwood, NY), and representative X-Y and X-Z sections are shown.
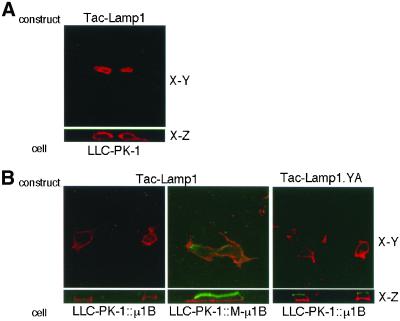
We next tested another tyrosine-based basolateral sorting signal, YQTI, from Lamp-1. Lamp-1 is a lysosomal membrane protein, and the YQTI sequence in its cytoplasmic tail has been reported to be required for direct lysosomal sorting, endocytosis as well as basolateral targeting (Hunziker et al., 1991; Harter and Mellman, 1992; Honing and Hunziker, 1995). We used a chimeric protein Tac-Lamp1, in which the luminal and transmembrane domains of Tac, the α subunit of interleukin-2 receptor, is appended with the Lamp-1 cytoplasmic tail containing the YQTI motif. Similar results were obtained as described above for AGPR-H1. As shown in Figure 4B, Tac-Lamp1 was primarily localized on the basolateral plasma membrane when expressed in LLC-PK1::μ1B cells. In contrast, it was expressed both apically and basolaterally in LLC-PK1::M-μ1B cells as well as in parental LLC-PK1 cells (Figure 4, A and B). Tac-Lamp1.YA, bearing a tyrosine-to-alanine substitution in YQTI motif, was similarly expressed on both apical and basolateral plasma membranes in LLC-PK1::μ1B cells, as expected (Figure 4B).
Figure 4.
Basolateral sorting of Tac-Lamp1 depends on the tyrosine-binding ability of μ1B. (A) LLC-PK1 cells grown on the Transwell filters were transiently transfected with the Tac-Lamp1 or Tac-Lamp1.YA expression plasmid, and incubated with an anti-Tac mAb 7G7.B6. Subsequently, cells were fixed and stained with the Alexa Fluor 546 anti-mouse IgG as described in MATERIALS AND METHODS. (B) LLC-PK1 cells stably expressing μ1B (LLC-PK1::μ1B) or a mutant μ1B (LLC-PK1::M-μ1B) grown on the Transwell filters were transiently transfected with the Tac-Lamp1 expression plasmid and incubated with an anti-Tac mAb 7G7.B6. Subsequently, cells were fixed and stained with the secondary antibodies Alexa Fluor 488 anti-mouse IgG from the apical side and Alexa Fluor 546 anti-mouse IgG from the basolateral side as described in MATERIALS AND METHODS. Samples were analyzed using an LSM 510 laser scanning confocal microscope (Carl Zeiss), and representative X-Y and X-Z sections are shown.
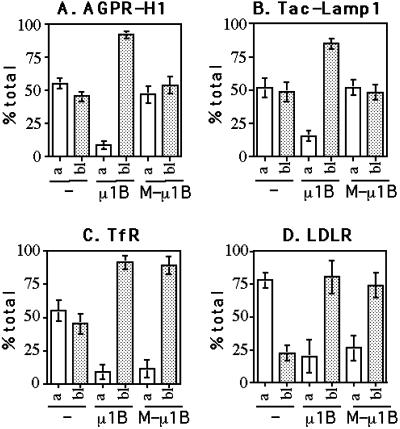
Finally, we determined the steady-state distribution of AGPR-H1 and Tac-Lamp1 quantitatively (Figure 7, A and B). As expected from the qualitative immunofluorescence results, both AGPR-H1 and Tac-Lamp1 were predominantly (80–90%) expressed at the basolateral surface of LLC-PK1::μ1B cells but were randomly expressed on both the apical and basolateral plasma membranes in parental LLC-PK1 as well as in LLC-PK1::M-μ1B cells.
Figure 7.
Quantitative determination of the steady-state distribution on the plasma membrane of AGPR-H1, Tac-Lamp1, TfR, and LDLR. Parental LLC-PK1 cells, LLC-PK1 cells stably expressing μ1B (LLC-PK1::μ1B), or a mutant μ1B (LLC-PK1::M-μ1B) grown in the Transwell filters were transiently transfected with AGPR-H1 (A), Tac-Lamp1 (B), TfR (C), or LDLR (D) expression plasmid and incubated with the corresponding primary antibodies. Subsequently, cells were fixed and incubated with 125I-labeled anti-mouse or anti-rabbit IgG, and the cell-associated radioactivity was measured as described in MATERIALS AND METHODS. Values are given as the percentage of total cell surface radioactivity and represent mean ± SD of three independent experiments performed in duplicate.
Taken together, these results suggest that the tyrosine-based basolateral sorting signals of AGPR-H1 and Lamp-1 require interactions with the presumptive tyrosine-binding pocket of μ1B for proper basolateral targeting in vivo.
Tyrosine Binding by μ1B Is Not Required for Basolateral Targeting of TfR and LDLR
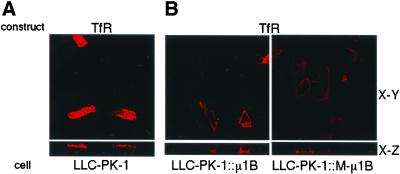
We next studied the steady-state plasma membrane distribution of TfR transiently expressed in parental LLC-PK1 cells and LLC-PK1 cells containing μ1B or M-μ1B (Figure 5). As shown previously (Folsch et al., 1999), the localization of TfR at the basolateral surface of LLC-PK1 cells was dependent on μ1B expression. Interestingly, and in contrast to results obtained for AGPR-H1 and Lamp-1, TfR was also found at the basolateral surface of cells expressing M-μ1B. Although the basolateral targeting signal of TfR has not been precisely defined, it is clear that the signal is distinct from the tyrosine-containing motif (YTRF) that is required for TfR endocytosis in clathrin-coated pits (Dargemont et al., 1993; Odorizzi and Trowbridge, 1997).
Figure 5.
Basolateral sorting of TfR does not depend on the tyrosine-binding ability of μ1B. (A) LLC-PK1 cells grown on the Transwell filters were transiently transfected with the TfR expression plasmid and incubated with an anti-TfR mAb L5.1. Subsequently, cells were fixed and stained with the Alexa Fluor 546 anti-mouse IgG as described in MATERIALS AND METHODS. (B) LLC-PK1 cells stably expressing μ1B (LLC-PK1::μ1B) or a mutant μ1B (LLC-PK1::M-μ1B) grown in the Transwell filters were transiently transfected with the TfR expression plasmid, and incubated with an anti-TfR mAb L5.1. Subsequently, cells were fixed and stained with the secondary antibodies Alexa Fluor 488 anti-mouse IgG from the apical side and Alexa Fluor 546 anti-mouse IgG from the basolateral side as described in MATERIALS AND METHODS. Samples were analyzed using an LSM 510 laser scanning confocal microscope (Carl Zeiss), and representative X-Y and X-Z sections are shown.
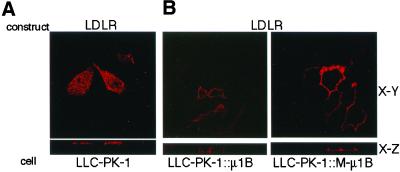
We next determined whether basolateral localization of LDLR was dependent on the tyrosine-recognition ability of μ1B. The LDLR cytoplasmic domain contains two basolateral targeting signals, both of which depend on critical tyrosines but only one of these (tyrosine 18) is also required for endocytosis. The second signal (involving tyrosine-35) is the dominant of the two and interacts with μ1B (Folsch et al., 2001). As shown in Figure 6, and like TfR, LDLR was targeted to the basolateral plasma membrane of LLC-PK1 cells expressing either μ1B or M-μ1B, although it is predominantly expressed on the apical plasma membrane in parental LLC-PK1 cells.
Figure 6.
Basolateral sorting of LDLR does not depend on the tyrosine-binding ability of μ1B. (A) LLC-PK1 cells grown on the Transwell filters were transiently transfected with the LDLR expression plasmid and incubated with an anti-LDLR mAb C7. Subsequently, cells were fixed and stained with the Alexa Fluor 546 anti-mouse IgG as described in MATERIALS AND METHODS. (B) LLC-PK1 cells stably expressing μ1B (LLC-PK1::μ1B) or a mutant μ1B (LLC-PK1::M-μ1B) grown in the Transwell filters were transiently transfected with the LDLR expression plasmid and incubated with an anti-LDLR mAb C7. Subsequently, cells were fixed and stained with the secondary antibodies Alexa Fluor 488 anti-mouse IgG from the apical side and Alexa Fluor 546 anti-mouse IgG from the basolateral side as described in MATERIALS AND METHODS. Samples were analyzed using an LSM 510 laser scanning confocal microscope (Carl Zeiss), and representative X-Y and X-Z sections are shown.
The polarized distribution of both TfR and LDLR was then determined by a quantitative antibody binding assay (Figure 7, C and D). As found previously in parental LLC-PK1 cells (Folsch et al., 1999), TfR was randomly distributed on the apical and basolateral plasma membranes, whereas LDLR was predominantly (75%) expressed at the apical plasma membrane; the latter finding is consistent with the notion that LDLR possesses a recessive apical determinant (Matter et al., 1992; Matter and Mellman, 1994). As expected from the immunofluorescence data (Figures 5 and 6), both TfR (∼90%) and LDLR (80%) were predominantly expressed on the basolateral plasma membrane in LLC-PK1::M-μ1B cells as well as LLC-PK1::μ1B cells.
Taken together, these results suggest that the basolateral targeting signals of TfR and LDLR do not require the tyrosine-motif binding ability of μ1B for their proper targeting to the basolateral plasma membrane. This feature is consistent with the fact that, unlike AGPR-H1 and Lamp-1, neither depends exclusively on a tyrosine-containing endocytosis-type signal for polarity.
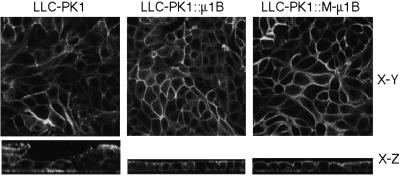
Monolayer Formation of LLC-PK1 Cells Is Supported in the Presence of M-μ1B as Well as μ1B
LLC-PK1 cells, unlike MDCK cells, do not always produce perfect monolayers typical of epithelial cells in culture, but occasionally pile up instead (Folsch et al., 1999). Expression of μ1B in LLC-PK1 cells corrects this phenotype resulting in monolayer-type growth (Folsch et al., 1999). Herein, we took advantage of this morphological difference in LLC-PK1 cells in the presence or absence of μ1B to measure the function of M-μ1B in monolayer formation. When LLC-PK1 cells expressing M-μ1B were grown on filter membranes, they grew in monolayers similar to cells expressing μ1B (Figure 8). This finding suggests that the molecule(s) required for growth of LLC-PK1 cells in a monolayer depend on the presence of μ1B or M-μ1B for function, but do not seem to require the tyrosine-binding ability by μ1B.
Figure 8.
M-μ1B supports the growth of LLC-PK1 cells in monolayer. Parental LLC-PK1 cells, LLC-PK1 cells stably expressing μ1B (LLC-PK1::μ1B), or a mutant μ1B (LLC-PK1::M-μ1B) were grown on the Transwell filers, fixed, permeabilized, and stained with Alexa Fluor 488 phalloidin as described in MATERIALS AND METHODS. Samples were analyzed using an LSM 510 laser scanning confocal microscope (Carl Zeiss), and representative X-Y and X-Z sections are shown.
DISCUSSION
Although it is clear that expression of μ1B plays a critical role in ensuring the polarized targeting of a wide array of basolateral plasma proteins in epithelial cells, little is understood about how this one AP-1B subunit interacts with the diverse set of basolateral sorting signals it seems to decode. Some basolateral signals depend on tyrosine residues that are also critical for AP-2–dependent clathrin-mediated endocytosis (e.g., Lamp-1 and AGPR-H1), some depend on tyrosines that are not required for endocytosis (LDLR), and some do not involve tyrosine residues at all (TfR). We characterized the importance of tyrosine recognition by replacing in four residues conserved among μ family members thought to be important for tyrosine binding (Owen and Evans, 1998). A similar strategy has successfully been applied to study the importance of tyrosine recognition by μ2 in endocytosis (Nesterov et al., 1999). Although the mutant μ1B (M-μ1B) was incorporated into functional AP-1B complexes, it lost the ability to decode tyrosine-dependent basolateral signals, or at least those that share tyrosines important for endocytosis such as AGPR-H1 and Lamp-1.
In contrast, basolateral expression of TfR and LDLR was not obviously affected by the removal of residues required for tyrosine binding μ1B. It has been reported that the basolateral sorting of TfR is mainly determined by the GDNS sequence downstream of the YTRF endocytosis/coated pit localization signal (Dargemont et al., 1993; Odorizzi and Trowbridge, 1997). Although the precise features of the TfR basolateral targeting signal have yet to be characterized, it is clear that the tyrosine required for endocytosis is not involved. Thus, it was interesting to learn that four residues in μ1B that are required for the coordination of tyrosine-containing determinants were not required for the basolateral targeting of TfR.
LDLR was an even more interesting case. This receptor's cytoplasmic tail contains two independent basolateral targeting determinants, both of which are tyrosine-dependent for activity (Matter et al., 1992, 1994). The membrane proximal signal overlaps with, but is distinct from, the NPVY endocytosis signal. The distal signal's critical tyrosine, on the other hand, does not direct endocytosis. Basolateral expression of LDLR was not affected by the μ1B mutations, suggesting that at least one of the LDLR basolateral signals does not bind to the tyrosine-binding pocket of μ1B. This may not be surprising, because the sequence surrounding the tyrosine of either signal does not conform to the canonical YXXØ sequence that is recognized by μ chains, including μ1B (Ohno et al., 1995, 1999; Boll et al., 1996; Dell'Angelica et al., 1997; Aguilar et al., 2001). Moreover, recent work has demonstrated that it is the distal basolateral targeting signal in LDLR that serves primarily to control basolateral targeting of this receptor (Koivisto et al., 2001).
Based on the present study, together with previous reports (Roush et al., 1998; Folsch et al., 1999), basolateral sorting signals so far identified can be divided into at least the following three classes. First, there are signals such as the dileucine-based determinant found in the IgG receptor FcRII-B2 (Hunziker and Fumey, 1994; Matter et al., 1994), which can mediate basolateral targeting in the absence of μ1B. Second, YXXØ-type basolateral signals such as those in AGPR-H1 (Fuhrer et al., 1991; Geffen et al., 1993) and Lamp-1 (Hunziker et al., 1991; Honing and Hunziker, 1995), which require the interaction of a critical tyrosine residue with μ1B for their sorting function. This same tyrosine is also required for rapid endocytosis of these membrane proteins via the AP-2 adapter complex. Finally, signals such as those in TfR and LDLR, which clearly require the presence of μ1B (and by extension the AP-1B complex), but not the ability of μ1B to bind tyrosine via μ1B residues required for interacting with tyrosines involved in endocytosis.
Although it is clear that basolateral proteins such as LDLR and TfR interact directly and selectively with the AP-1B adaptor complex, the actual interacting subunit has not been identified. A priori, μ1B is the most likely candidate. It is clear that its homolog μ2 directly binds the internalization motifs in endocytic receptors. Moreover, the single substitution of μ1B for μ1A in the AP-1 complex switches the affinity of the complex from those proteins involved in TGN/endosome transport in all cells to proteins that are transported to the basolateral surface of epithelial cells. Only 47 (of ∼270) amino acids differ between the carboxyl-terminal domains of μ1A and μ1B. The μ1 carboxyl-terminal domain is thought to protrude from the trunk of the AP-1 complex and to be important for interactions with sorting signals (Owen and Evans, 1998; Bonifacino and Dell'Angelica, 1999). These carboxyl-terminal μ1B residues may, therefore, participate in providing the binding surface for the signals from TfR and LDLR. Alternatively, these signals may bind to a region of μ1B that overlaps where the YXXØ-type signal binds, but bind in a different register, or perhaps interacting with different residues in this region. Some flexibility in the mode of interaction of internalization signals with μ2 has recently been observed (Owen et al., 2001).
Another explanation, although we think it less likely, is that the signals could interact with AP-1B subunits other than μ1B. The presence of AP-1A cannot support the basolateral sorting of TfR or LDLR (Roush et al., 1998; Folsch et al., 1999). Because AP-1A and AP-1B are believed to share the subunits other than μ1A and μ1B (Folsch et al., 1999), it is difficult to imagine that these common subunits cause the difference in sorting phenotype. Nevertheless, it might be possible that the difference between μ1A and μ1B could cause the conformational change(s) of the other subunits to generate the binding surface for the basolateral sorting signals from TfR and LDLR. Thirty-six residues differ between μ1A and μ1B in their amino-terminal domains, the region thought to be involved in mediating interactions with other adaptor subunits; conceivably, alterations in such interactions may lead to alterations in substrate specificity. Final understanding of how μ1B can accommodate such seemingly different signals for such a common, fundamental function as polarized targeting in epithelia will require direct structural information on the μ1B and adaptors in general.
Finally, it should be pointed out that the precise site of action of AP-1B in polarized sorting remains to be determined. Other kidney epithelial cells, such as MDCK cells, have been shown to sort apical from basolateral proteins upon their emergence from the Golgi complex, before their first appearance at the plasma membrane. Hepatocytes, which are μ1B negative, sort by an indirect route whereby both apical and basolateral proteins are transported from the Golgi to the basolateral surface from which they are internalized and then sorted from each other in endosomes. Because in MDCK cells the signals that mediate biosynthetic and endocytic basolateral sorting are similar (Matter et al., 1994; Odorizzi and Trowbridge, 1997), it is conceivable that μ1B acts on both pathways. Indeed, there is ample evidence that AP-1 adaptors can be found at the TGN and in endosomes (Futter et al., 1998; Folsch et al., 2001). It is also possible that expression of μ1B confers upon the TGN the ability to mediate apical vs. basolateral sorting. Thus, it is possible that LLC-PK1 cells sort indirectly (like hepatocytes), whereas μ1B-expressing LLC-PK1 cells sort directly (like MDCK cells). The fact that a tyrosine mutant of AGPR-H1 was found apically argues against indirect sorting in μ1B-expressing LLC-PK1 cells. For such a mutant to reach the apical surface by the indirect route, transcytosis from the basolateral domain would be required. Yet, transcytosis might be rendered less efficient because the same tyrosine residue required for basolateral targeting is also required for rapid endocytosis.
ACKNOWLEDGMENTS
We thank Drs. Juan S. Bonifacino (National Institutes of Health) and Martin Spiess (University of Basel) for generously providing the reagents. This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to H.O.), and also, in part, by the Uehara Memorial Foundation (to H.O.). I.M. and H.K. are supported by the Ludwig Institute for Cancer Research and by a grant from the National Institutes of Health (GM-29765). C.M. is supported by a National Research Council Associateship.
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E01–10–0096. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E01–10–0096.
REFERENCES
- Aguilar RC, Boehm M, Gorshkova I, Crouch RJ, Tomita K, Saito T, Ohno H, Bonifacino JS. Signal-binding specificity of the {micro}4 subunit of the adaptor protein complex AP-4. J Biol Chem. 2001;276:13145–13152. doi: 10.1074/jbc.M010591200. [DOI] [PubMed] [Google Scholar]
- Aroeti B, Okhrimenko H, Reich V, Orzech E. Polarized trafficking of plasma membrane proteins: emerging roles for coats, SNAREs, GTPases and their link to the cytoskeleton. Biochim Biophys Acta. 1998;1376:57–90. doi: 10.1016/s0304-4157(98)00005-7. [DOI] [PubMed] [Google Scholar]
- Boll W, Ohno H, Songyang Z, Rapoport I, Cantley LC, Bonifacino JS, Kirchhausen T. Sequence requirements for the recognition of tyrosine-based endocytic signals by clathrin AP-2 complexes. EMBO J. 1996;15:5789–5795. [PMC free article] [PubMed] [Google Scholar]
- Bonifacino JS, Dell'Angelica EC. Molecular bases for the recognition of tyrosine-based sorting signals. J Cell Biol. 1999;145:923–926. doi: 10.1083/jcb.145.5.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dargemont C, Le Bivic A, Rothenberger S, Iacopetta B, Kuhn LC. The internalization signal and the phosphorylation site of transferrin receptor are distinct from the main basolateral sorting information. EMBO J. 1993;12:1713–1721. doi: 10.1002/j.1460-2075.1993.tb05816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Angelica EC, Mullins C, Bonifacino JS. AP-4, a novel protein complex related to clathrin adaptors. J Biol Chem. 1999;274:7278–7285. doi: 10.1074/jbc.274.11.7278. [DOI] [PubMed] [Google Scholar]
- Dell'Angelica EC, Ohno H, Ooi CE, Rabinovich E, Roche KW, Bonifacino JS. AP-3: an adaptor-like protein complex with ubiquitous expression. EMBO J. 1997;16:917–928. doi: 10.1093/emboj/16.5.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folsch H, Ohno H, Bonifacino JS, Mellman I. A novel clathrin adaptor complex mediates basolateral targeting in polarized epithelial cells [see comments] Cell. 1999;99:189–198. doi: 10.1016/s0092-8674(00)81650-5. [DOI] [PubMed] [Google Scholar]
- Folsch H, Pypaert M, Schu P, Mellman II. Distribution, and Function of AP-1 clathrin adaptor complexes in polarized epithelial cells. J Cell Biol. 2001;152:595–606. doi: 10.1083/jcb.152.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrer C, Geffen I, Spiess M. Endocytosis of the ASGP receptor H1 is reduced by mutation of tyrosine-5 but still occurs via coated pits. J Cell Biol. 1991;114:423–431. doi: 10.1083/jcb.114.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futter CE, Gibson A, Allchin EH, Maxwell S, Ruddock LJ, Odorizzi G, Domingo D, Trowbridge IS, Hopkins CR. In polarized MDCK cells basolateral vesicles arise from clathrin-gamma-adaptin-coated domains on endosomal tubules. J Cell Biol. 1998;141:611–623. doi: 10.1083/jcb.141.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geffen I, Fuhrer C, Leitinger B, Weiss M, Huggel K, Griffiths G, Spiess M. Related signals for endocytosis and basolateral sorting of the asialoglycoprotein receptor. J Biol Chem. 1993;268:20772–20777. [PubMed] [Google Scholar]
- Harter C, Mellman I. Transport of the lysosomal membrane glycoprotein lgp120 (lgp-A) to lysosomes does not require appearance on the plasma membrane. J Cell Biol. 1992;117:311–325. doi: 10.1083/jcb.117.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst J, Robinson MS. Clathrin and adaptors. Biochim Biophys Acta. 1998;1404:173–193. doi: 10.1016/s0167-4889(98)00056-1. [DOI] [PubMed] [Google Scholar]
- Hofmann MW, Honing S, Rodionov D, Dobberstein B, von Figura K, Bakke O. The leucine-based sorting motifs in the cytoplasmic domain of the invariant chain are recognized by the clathrin adaptors AP1 and AP2 and their medium chains. J Biol Chem. 1999;274:36153–36158. doi: 10.1074/jbc.274.51.36153. [DOI] [PubMed] [Google Scholar]
- Honing S, Hunziker W. Cytoplasmic determinants involved in direct lysosomal sorting, endocytosis, and basolateral targeting of rat lgp120 (lamp-I) in MDCK cells. J Cell Biol. 1995;128:321–332. doi: 10.1083/jcb.128.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey JS, Peters PJ, Yuan LC, Bonifacino JS. Localization of TGN38 to the trans-Golgi network: involvement of a cytoplasmic tyrosine-containing sequence. J Cell Biol. 1993;120:1123–1135. doi: 10.1083/jcb.120.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker W, Fumey C. A di-leucine motif mediates endocytosis and basolateral sorting of macrophage IgG Fc receptors in MDCK cells. EMBO J. 1994;13:2963–2967. doi: 10.1002/j.1460-2075.1994.tb06594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker W, Harter C, Matter K, Mellman I. Basolateral sorting in MDCK cells requires a distinct cytoplasmic domain determinant. Cell. 1991;66:907–920. doi: 10.1016/0092-8674(91)90437-4. [DOI] [PubMed] [Google Scholar]
- Koivisto UM, Hubbard AL, Mellman I. A novel cellular phenotype for familial hypercholesterolemia due to a defect in polarized targeting of LDL receptor. Cell. 2001;105:575–585. doi: 10.1016/s0092-8674(01)00371-3. [DOI] [PubMed] [Google Scholar]
- Matter K, Hunziker W, Mellman I. Basolateral sorting of LDL receptor in MDCK cells: the cytoplasmic domain contains two tyrosine-dependent targeting determinants. Cell. 1992;71:741–753. doi: 10.1016/0092-8674(92)90551-m. [DOI] [PubMed] [Google Scholar]
- Matter K, Mellman I. Mechanisms of cell polarity: sorting and transport in epithelial cells. Curr Opin Cell Biol. 1994;6:545–554. doi: 10.1016/0955-0674(94)90075-2. [DOI] [PubMed] [Google Scholar]
- Matter K, Yamamoto EM, Mellman I. Structural requirements and sequence motifs for polarized sorting and endocytosis of LDL and Fc receptors in MDCK cell. J Cell Biol. 1994;126:991–1004. doi: 10.1083/jcb.126.4.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I. Endocytosis and molecular sorting. Annu Rev Cell Dev Biol. 1996;12:575–625. doi: 10.1146/annurev.cellbio.12.1.575. [DOI] [PubMed] [Google Scholar]
- Mostov KE, Verges M, Altschuler Y. Membrane traffic in polarized epithelial cells. Curr Opin Cell Biol. 2000;12:483–490. doi: 10.1016/s0955-0674(00)00120-4. [DOI] [PubMed] [Google Scholar]
- Nesterov A, Carter RE, Sorkina T, Gill GN, Sorkin A. Inhibition of the receptor-binding function of clathrin adaptor protein AP-2 by dominant-negative mutant mu2 subunit and its effects on endocytosis. EMBO J. 1999;18:2489–2499. doi: 10.1093/emboj/18.9.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odorizzi G, Trowbridge IS. Structural requirements for basolateral sorting of the human transferrin receptor in the biosynthetic and endocytic pathways of Madin-Darby canine kidney cells. J Cell Biol. 1997;137:1255–1264. doi: 10.1083/jcb.137.6.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno H, Aguilar RC, Yeh D, Taura D, Saito T, Bonifacino JS. The medium subunits of adaptor complexes recognize distinct but overlapping sets of tyrosine-based sorting signals. J Biol Chem. 1998;273:25915–25921. doi: 10.1074/jbc.273.40.25915. [DOI] [PubMed] [Google Scholar]
- Ohno H, Fournier MC, Poy G, Bonifacino JS. Structural determinants of interaction of tyrosine-based sorting signals with the adaptor medium chains. J Biol Chem. 1996;271:29009–29015. doi: 10.1074/jbc.271.46.29009. [DOI] [PubMed] [Google Scholar]
- Ohno H, Stewart J, Fournier MC, Bosshart H, Rhee I, Miyatake S, Saito T, Gallusser A, Kirchhausen T, Bonifacino JS. Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science. 1995;269:1872–1875. doi: 10.1126/science.7569928. [DOI] [PubMed] [Google Scholar]
- Ohno H, Tomemori T, Nakatsu F, Okazaki Y, Aguilar RC, Foelsch H, Mellman I, Saito T, Shirasawa T, Bonifacino JS. Mu1B, a novel adaptor medium chain expressed in polarized epithelial cells. FEBS Lett. 1999;449:215–220. doi: 10.1016/s0014-5793(99)00432-9. [DOI] [PubMed] [Google Scholar]
- Owen DJ, Evans PR. A structural explanation for the recognition of tyrosine-based endocytotic signals. Science. 1998;282:1327–1332. doi: 10.1126/science.282.5392.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen DJ, Setiadi H, Evans PR, McEver RP, Green SA. A third specificity-determining site in mu 2 adaptin for sequences upstream of Yxx phi sorting motifs. Traffic. 2001;2:105–110. doi: 10.1034/j.1600-0854.2001.020205.x. [DOI] [PubMed] [Google Scholar]
- Rapoport I, Chen YC, Cupers P, Shoelson SE, Kirchhausen T. Dileucine-based sorting signals bind to the beta chain of AP-1 at a site distinct and regulated differently from the tyrosine-based motif-binding site. EMBO J. 1998;17:2148–2155. doi: 10.1093/emboj/17.8.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodionov DG, Bakke O. Medium chains of adaptor complexes AP-1 and AP-2 recognize leucine-based sorting signals from the invariant chain. J Biol Chem. 1998;273:6005–6008. doi: 10.1074/jbc.273.11.6005. [DOI] [PubMed] [Google Scholar]
- Roush DL, Gottardi CJ, Naim HY, Roth MG, Caplan MJ. Tyrosine-based membrane protein sorting signals are differentially interpreted by polarized Madin-Darby canine kidney and LLC-PK1 epithelial cells. J Biol Chem. 1998;273:26862–26869. doi: 10.1074/jbc.273.41.26862. [DOI] [PubMed] [Google Scholar]