Abstract
Few studies have explored the impact of blue light-emitting diode (BL) irradiation combined with different storage temperatures on antioxidant defense and cell wall metabolic activities related to the quality deterioration of postharvest strawberries. This study investigates the effects of BL exposure as a non-chemical preservation strategy to improve the postharvest quality of strawberries stored at 22 °C and 8 °C. Over a 10-day storage period, BL irradiation significantly reduced respiratory and ethylene production rates, while preserving fruit firmness and increasing the contents of soluble sugar and total phenol at both temperatures. Additionally, increases of the enzymatic activities of antioxidant defense metabolism (e.g. superoxide dismutase (SOD) and peroxidase (POD)) accompanied with decreased levels of reactive oxygen species (ROS) (e.g. superoxide anion (O2−), and hydrogen peroxide (H2O2)) and the content of secondary metabolites (e.g. ascorbic acid (AsA)) accompanied with enhanced activities of ascorbate peroxidase (APX) and glutathione reductase (GR) were found in BL irradiated postharvest strawberries at the later stages of storage. Meanwhile, BL irradiation also mitigated the activity of cell wall-degrading enzymes, thereby reducing the degradation rates of protopectin (PP), cellulose (CEL), and hemicellulose (HCEL), while maintaining the structural integrity of cell walls. According to fuzzy mathematics analysis, the membership function values for BL irradiated postharvest strawberries at different storage temperatures ranked as follows: BL + 8 °C (0.60) > BL + 22 °C (0.52) > 8 °C (0.46) > 22 °C (0.36). These findings suggest that BL irradiation not only extends the shelf life of strawberries by modulating antioxidant defense and cell wall metabolic activities but also maintains their commercial quality, particularly under low-temperature storage conditions. Therefore, BL irradiation holds significant promise for minimizing quality deterioration in postharvest strawberries during cold storage.
Keywords: Strawberry, Blue light, Postharvest quality, Antioxidant capacity, Ascorbic acid, Cell wall metabolism
Highlights
-
•
BL irradiation unevenly improved the fruit quality and self life of postharvest strawberries during room and low temperature storage period.
-
•
Combined effect of BL irradiation with low temperature storage was more effective, special for later storage.
-
•
In the later storage of 6 d to 10 d, BL irradiation in combination with low temperature storage significantly suppressed respiration, increased soluble sugar, actived SOD and APX, decreased PPO, PG and PME, resulting in a greater firmness and stable cellular structure.
1. Introduction
Consumers appreciate fresh fruits for their ideal flavor, appealing taste, rich nutrition, and health-promoting benefits (Zhang, Li, et al., 2022). However, before fruits reach consumers' tables, postharvest losses throughout the fruit supply chain can exceed 30 % (Chen et al., 2021). Extensive research has identified physiological aging, mechanical injury and fungal infection as major factors contributing to postharvest losses (Zhang, Zhang, et al., 2022). Thus, synthetic or naturally extracted chemicals, known for their simplicity and cost-effectiveness, have been widely used for many years to control the postharvest quality and extend the shelf life of fresh fruits (Freche et al., 2022). Given the increasing concern for environmental protection and food safety, there is an urgent need to develop environmentally sustainable and consumer-friendly preservation techniques that can effectively minimize postharvest losses and extend shelf life of fresh produce.
Temperature serves as a vital environmental regulator of physiological and biochemical metabolism in vivo, and it is equally pivotal in determining the postharvest storage conditions of fresh fruits. Considerable studies have showed that low-temperature, a common physical preservation technique, reduce postharvest losses and extend shelf life by suppressing metabolic activities of fruits during transport and storage. As a result, the recommended storage temperatures for soft-textured fruits or those at red maturity stages range between 0-10 °C (Chai et al., 2024; Hassan et al., 2021; Liu et al., 2019; Park et al., 2024; Tao et al., 2024). However, concerns such as chilling injury, starchy taste and increased energy consumption highlight the need for careful management of low-temperature, despite their benefits in preserving flavor and extending shelf life. Moreover, fruits stored in low-temperature environments continue to produce ethylene and reactive oxygen species (ROS), which can slowly promote over-ripening, damages cell membrane, and lead to rotting and odorous decay (Hassan et al., 2021). Therefore, developing an integrated low-temperature storage technology is crucial to further improve the storage quality and extend the shelf life of postharvest fruits.
With the rapid advancement of light-emitting diode (LED) technology, its application in irradiation has become a safe, effective, low-cost, and environmentally friendly approach for the postharvest preservation of fruits and vegetables (Song et al., 2020; Zhou et al., 2022; Lauria et al., 2023;). Consequently, blue LED light (BL) was used to pretreated mature green tomatoes (Solanum lycopersicum L.) for seven days at room-temperature, resulting in a yellowish color and a high level of firmness (Dhakal & Baek, 2014). This finding indicated that BL illumination can be an effective approach to extend shelf life by delaying softening and ripening of the produce. Then, the technology of BL irradiation was innovatively used for postharvest preservation of sweet cherries (Prunus avium L.) (Kokalj et al., 2019), pitayas (Hylocereus undatus Haw. Britton & Rose) (Wu et al., 2020), peppers (Capsicum annuum L.) (Liu et al., 2022), and citrus fruits (Du et al., 2023), demonstrating its versatility across various fruits. In addition, research has progressively explored the mechanisms by which BL irradiation delays fruit senescence and enhances shelf life, notably through suppressing ethylene biosynthesis, starch degradation and cell wall metabolism (Sun et al., 2024; Xu et al., 2024). Recent studies have further demonstrated that BL irradiation effectively suppresses the development of sour rot in citrus (Citrus reticulata Blanco) and the growth of pathogens such as Salmonella and Escherichia coli in pineapples (Ananas comosus L. Merr.) without compromising the storage quality of postharvest fruits (Du et al., 2023; Ghate et al., 2017). Therefore, the shelf life and storage quality of fruits can be increased by applying BL irradiation in both pre-harvest and storage conditions. However, despite its benefits as a pollution-free and green preservation method, BL irradiation has yet to gain substantial attention for its synergistic effects with low-temperature storage on antioxidant defense and cell wall metabolism (CWM) during the storage of postharvest fruits.
Strawberry (Fragaria × ananassa Duch. ‘Benihoppe’), a member of the Rosaceae family and the Fragaria genus, is one of the high economic and nutritional value fruit crops cultivated worldwide. After harvest, fresh strawberries are prone to mechanical damage, microbial decay, and moisture loss. Meanwhile, their strong respiration and perishable nature further contribute to a reduced short shelf life and reduced quality (Thamyres et al., 2024). Thus, various postharvest preservation techniques, such as freezing and pressure applications (Guo et al., 2024), packaging with biodegradable edible film (Ratna et al., 2023), and the use of chlorine dioxide gas slow-release film (Liu, Wang, et al., 2023), have been developed to extend postharvest shelf life of strawberries. Recent advances have also explored the efficacy of BL treatment in extending the storage shelf life of strawberries by enhancing their antioxidant capacities related to the Indole-3-acetic acid (IAA) or ascorbic acid (AsA)-related antioxidant capacity (Mesa et al., 2024; Sun et al., 2024; Xu et al., 2014), and in preventing gray mold reproduction of Botrytis cinerea (Suthaparan et al., 2024). Additionally, BL in combination with salicylic acid (SA) treatment was another effective application to extend the shelf-life of postharvest strawberry by increasing activities of enzymatic antioxidants superoxide dismutase (SOD) and ascorbate peroxidase (APX) (Zhang, Li, et al., 2022). However, it is very impossible to further enhance postharvest quality of strawberry stored in BL irradiation combined with low-temperature storage. Therefore, this study aims to investigate the combination effects of continuous BL irradiation with room- and low-temperature storage on the postharvest quality and shelf life of strawberry by focusing on antioxidant defense and cell wall metabolic activities, providing postharvest preservation method for strawberry as well as other berries.
2. Materials and methods
2.1. Fruit materials and treatments
The maturity of the strawberry is classified according to the level of redness of their surface. Strawberries exhibiting 70 % to 80 % surface redness were selected in this study. Strawberries were harvested from a sunlight greenhouse in Xushui County, Baoding City, Hebei Province, China (39°01′05″N, 115°51′24″E). The fruits were immediately transported to the laboratory in a cooler box. Only healthy fruits, uniform in size and shape and free from pests or mechanical damage, were retained for the experiments. Four groups of fruits, each consisting of 100 individual fruits, were placed in a growth chamber under four different conditions: either in complete darkness or under a 446 nm BL (35 μmol m−2 s−1), with the temperature set at either 22 °C or 8 °C. Fruits were positioned 20 cm beneath the BL source. The chamber was maintained at 80 % relative humidity with continuous BL exposure for 10 days, the dark treatment was set as CK. Sampling was conducted every two days, with five strawberries randomly selected per replicate from each treatment. Images of the fruits were captured immediately, firmness, respiratory rate and ethylene production were measured, and the flesh samples were frozen in liquid nitrogen before storage at −80 °C.
2.2. Determination of firmness, total phenol and soluble sugar content
Measurements were repeated three times, using three fruits each time, to ensure biological reproducibility. The fruit firmness was measured using a GY-4 hardness tester, was made to evaluate the firmness of strawberries fruit and three equidistant positions were selected at the equator of strawberries and their average value was recorded in Newtons (N). The content of total phenol was quantified using specific assay kits (G0117F, Great Biosciences, Suzhou, China), with the results expressed as mg g−1FW. Approximately 200 mg of frozen strawberry fruit was ground with 10 mL of distilled water, and the samples were centrifuged at 12,000 × r min−1 for 10 min at 4 °C. The supernatants were then diluted to 50 mL with distilled water, and 1 mL of extract was combined with 1 mL of 2 g L−1 anthrone in 706 g L−1 H2SO4. The mixture was incubated at 100 °C for 15 min and cooled to room-temperature in a water bath. The total soluble sugar content was measured by anthrone colorimetry and expressed as a percentage (%).
2.3. Determination of respiratory rate and ethylene production
The respiratory rate was assessed with a multichannel dynamic respiration analyzer (YZQ—201B, Yi Zong Qi Technology, Co., LTD, China), and the results were expressed as μmol O2 kg−1 s−1. For ethylene production assessments, 16 fruits were randomly selected from each group, placed into four glass containers, and stored at room-temperature (22 ± 1 °C) for six hours. Gas was then collected via a drainage method, and ethylene production was quantified using a gas chromatograph (GC-2014; Shimadzu, Kyoto, Japan) and expressed as C2H4 μL kg−1 s−1.
2.4. Determination of reactive oxygen species
The generation rate of superoxide anion (O2−) and the content of hydrogen peroxide (H2O2) were measured using standardized methods. The malondialdehyde (MDA) content was then quantified using a commercial assay kit (G0109F, Great Biosciences, Suzhou, China). The concentrations of H2O2 and MDA were expressed as μmol g−1 and nmol g−1, respectively, whereas the O2− generation rate was expressed as nmol min−1 g−1FW.
2.5. Measurement of antioxidant enzyme activities
Approximately 0.5 g of strawberry powder was extracted with 1 mL of extraction solution (Great Biosciences, Suzhou, China) and subsequently homogenized. The activities of SOD, peroxidase (POD), catalase (CAT), polyphenol oxidase (PPO), and phenylalanine ammonia-lyase (PAL) were measured using antioxidant enzyme detection kits (G0101F, G0107F, G0303F, G0113F and G0114F; Great Biosciences, Suzhou, China). Each assay was conducted in four biological replicates. Enzymatic activities of SOD, POD, and PPO were measured in U g−1. CAT activity was expressed as μmol min−1 g−1, and PAL activity was expressed as OD290 h−1 g−1FW.
2.6. Determination of ascorbic acid-glutathione cycle
AsA content was measured using the method described by (Kampfenkel et al., 1995) and expressed as μg g−1. Reduced glutathione (GSH) content was measured using the method established by (Brehe & Burch, 1976) and expressed as μmol g−1. For determination of APX and glutathione reductase (GR), The activities of APX and GR were measured using APX and GR assay kits (G0203F and G0209F; Great Biosciences, Suzhou, China). Measurements were expressed as μmol min−1 g−1.
2.7. Determination of CWM activity
2.7.1. Determination of protopectin (PP)
Referring to a previously published method (Ning et al., 2022) with slight modification, In the CK and BL treatments, 0.5 g of strawberry powder was weighed and mixed with 1.5 mL of 80 % ethanol. After reacting in a water bath at 85 °C for 10 min, the mixture was centrifuged, discarding the supernatant and retaining the precipitate. Then, 1 mL of distilled water was added, and the mixture was reacted in a water bath at 50 °C for 30 min, followed by centrifugation for 10 min at 8000 ×g. The supernatant was used to measure the soluble pectin (SP) content. To determine the PP content, the precipitate was mixed with 1 mL of extraction solution (G0703F, Great Biosciences, Suzhou, China) and reacted in a water bath at 95 °C for 60 min, followed by centrifugation to obtain the solution for analysis. To this solution, 70 μL was added along with 420 μL of concentrated sulfuric acid, and the mixture was reacted in a water bath at 85 °C for 15 min. After that, 14 μL of reagent one was added. The reaction was kept in the dark at 25 °C for 30 min, and the absorbance was measured at a wavelength of 530 nm, Each assay was performed in three biological replicates and the results were expressed as mg g−1.
2.7.2. Determination of SP content
The SP content was measured using a pectin assay kit (G0704F, Great Biosciences, Suzhou, China). A sample of 0.5 g of strawberry powder was prepared similarly to that for PP, repeated three times to remove other substances. In an EP tube, 70 μL of sample and 420 μL of concentrated sulfuric acid were added, followed by a 15-min reaction in a water bath at 85 °C. Afterward, 14 μL of reagent one was added. The mixture was kept in the dark at 25 °C for 30 min, and the absorbance was measured at a wavelength of 530 nm and the results were expressed as mg g−1.
2.7.3. Determination of cellulose (CEL) and hemicellulose (HCEL) content
CEL and HCEL content were determined using cell wall analysis kits (G0715F, G0716F; Great Biosciences, Suzhou, China). Each assay was performed in three biological replicates and the results were expressed as mg g−1.
2.7.4. Determination of polygalacturonase (PG) activity
Following previous methods, the measurement was conducted using a PG enzyme kit (G0701F, Great Biosciences, Suzhou, China) with slight modifications. A sample of 0.5 g of strawberry powder was ground with ethanol, centrifuged, and the supernatant was discarded. 80 % ethanol was added to the precipitate, and after another centrifugation, 1 mL of extraction solution was added to the precipitate, mixed, and centrifuged again to obtain the solution for analysis. To this solution, 20 μL was added along with 130 μL of reagent one, and incubated in a water bath at 40 °C for 30 min, followed by the addition of 130 μL of reagent three; in the control tube, reagent two was replaced with reagent three. The mixture was heated in a boiling water bath for 5 min, and the absorbance was measured at a wavelength of 530 nm. The results were expressed as mg h−1 g−1.
2.7.5. Determination of pectin methylesterase (PME) activity
The measurement was conducted using a PME assay kit (G0707D, Great Biosciences, Suzhou, China). A sample of 0.5 g of strawberry powder was mixed with 1.5 mL of extraction solution, ground, and repeated three times to remove other substances. After centrifugation, the solution for analysis was obtained. To this solution, 1 mL of the sample, 25 μL of reagent two, and 4 mL of reagent one were added, and reagent three was used to adjust the color to pink. The mixture was incubated at 37 °C for 60 min, with reagent four added as needed to maintain the pink color. The absorbance was measured at a wavelength of 540 nm. The results were expressed as μmol min−1 g−1.
2.7.6. Determination of β-glucosidase (β-Glu) and cellulase (Cx) activities
β-Glu and Cx activity detection kit (G0522F, G0533F; Great Biosciences, Suzhou, China) was used to measure the samples, and the results were expressed as nmol min−1 g−1, and μg h−1 g−1.
2.8. Membership function value (MFV)
The trend of postharvest storage tolerance in strawberries treated with BL and temperature was evaluated using MFV. MFV is calculated based on the measured values of each indicator under the combined treatment of BL and temperature. Then, the MFV1 or MFV2 for each indicator was calculated according to the following formula.
In the formula, Xi represents the measured value of a specific indicator, while Xmin and Xmax denote the minimum and maximum values among all indicators, respectively. MFV2 is used for indicators that are negatively correlated with storage tolerance, meaning that lower values indicate higher storage tolerance (e.g., O2−, H2O2 and MDA).
2.9. Statistical analysis
All data are presented as mean ± standard error (SE). Statistical analyses and significance testing were performed using SPSS 25.0 software, employing a three-way ANOVA. A significance threshold of p < 0.05 was set for all tests. Graphs were generated using Origin 2021 software.
3. Results and discussion
3.1. Effect of BL irradiation on the firmness, total phenol content, and soluble sugar content of postharvest strawberry at different storage temperatures
Fruit firmness is a key indicator for assessing storage quality and shelf life of postharvest fruits (Sun et al., 2024; Wu et al., 2020). Therefore, researchers have suggested equipping storage chamber, refrigerators, or coolers with LED illumination to enhance the firmness or delay the ripening process of fruits (Pérez et al., 2018). In this study, BL irradiation increased the firmness of postharvest strawberries during the room- and low-temperature storage period (Fig. 1A). This finding aligns with previous studies on strawberry (Xu et al., 2014), pitaya (Wu et al., 2020), kiwifruit (Xu et al., 2024), and citrus (Du et al., 2023). For example, the shelf life of postharvest kiwifruit was prolonged to about 30 d because BL treatment effectively maintained postharvest firmness and delayed its softening (Xu et al., 2024). Meanwhile, regardless of the postharvest storage temperature, the contents of total phenol and soluble sugars of BL-treated strawberries are also significantly higher than those of darkness (P < 0.05) during the later storage period (Fig. 1B and C), which reaches its peak on day six of storage, with increase of 59.5 % and 33.3 % at 22 °C, and 28.4 % and 22.8 % at 8 °C, respectively. On the one hand, this enhancement of soluble sugars is likely linked to the upregulation expression of SUUS, HXK, MrSPS and MrINV genes (Shi et al., 2016; Tao et al., 2024), which stabilize the level of soluble sugar in berry fruit. On the other hand, previous researches have also demonstrated that BL irradiation promotes the accumulation of secondary metabolites (e.g. phenolics, flavonoids), which plays a critical role in the scavenging of ROS in pepper fruits, cherry tomato and citrus fruit, and enhances their nutritional value and antioxidant capacity (Liu et al., 2022; MarMaría et al., 2020; Sun et al., 2024). Thus, BL irradiation as a valuable preservation technology plays an important role in maintaining postharvest quality and extending shelf life, especially for unstorable berries including strawberry.
Fig. 1.
Effects of BL irradiation on firmness (A), total phenol content (B) and soluble sugar content (C) of postharvest strawberries at different storage temperatures over a 10-day period. Treatments include darkness and BL irradiation at two temperatures: 22 °C and 8 °C. Each point represents the mean value, with error bars indicating the standard error of the mean. Statistical significance between different treatments at each time point is denoted by different letters, with a significance level set at p < 0.05. The arrangement of letters from top to bottom indicates the statistical ranking from highest to lowest value at each storage time. The notation “ns” indicates non-significant.
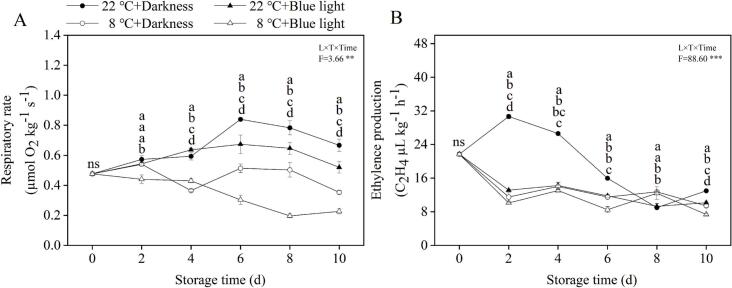
3.2. Effect of BL irradiation on respiratory rate and ethylene production of postharvest strawberry at different storage temperatures
Although strawberry as a non-climacteric fruit, the respiratory rate and ethylene production were still measured to investigate the effect of BL irradiation on extension of postharvest quality of strawberries at different storage temperatures. In our study, the strawberries subjected to BL irradiation exhibited significantly lower respiratory rate in later low-temperature storage, with decrease of 63.9 % - 74.9 % compared with darkness (Fig. 2A). Therefore, BL irradiation further prolonged the storage quality of strawberries by inhibiting the respiration rate. However, banana exhibit higher respiration rate even at lower temperature (Zhu et al., 2018), which can accelerate the decay of postharvest fruit during the short storage period. Therefore, the proper temperature management should be also considered in application of BL treatment to extend the postharvest quality and shelf-life of fruits.
Fig. 2.
Effects of BL irradiation on respiratory rate (A) and ethylence production (B) of postharvest strawberries at different storage temperatures over a 10-day period. Treatments include darkness and BL irradiation at two temperatures: 22 °C and 8 °C. Each point represents the mean value, with error bars indicating the standard error of the mean. Statistical significance between different treatments at each time point is denoted by different letters, with a significance level set at P < 0.05. The arrangement of letters from top to bottom indicates the statistical ranking from highest to lowest value at each storage time. The notation “ns” indicates non-significant.
Low-temperature usually suppresses ethylene production during the postharvest storage period (Liu et al., 2019; Qin et al., 2024). In our study, both BL-irradiated and untreated fruit showed a gradual decline in ethylene production with prolonged storage (Fig. 2B). Notably, BL-irradiated fruit exhibited a more reduction in ethylene production in early stages of storage, with decreases of 26.1 % - 57.2 % at 22 °C and 7.1 % - 26.2 % at 8 °C compared to the dark control (Fig. 2B). The inhibition of BL irradiation on ethylene production in pears (Pyrus bretschneideri Rehder) (Liu, Zhang, et al., 2023) and apples (Malus pumila Mill.) (Wang et al., 2023) has also been found by suppressing the activity of enzymes (e.g. ACS, ACO) related to ethylene biosynthesis. In contrast, some studies have shown that BL irradiation can significantly increase ethylene production and accelerate fruit ripening in peaches (Prunus persica L. Batsch) and bananas by upregulating expression of genes related to ethylene biosynthesis (Gong et al., 2015; Huang et al., 2018). The delaying effect of BL on self life and ripening of postharvest fruit through inhibition of ethylene may vary among different species and phototechnology. Thus, the intensity and duration of BL irradiation should be carefully adapted to the postharvest fruit type to decrease ethylene production and maintain better postharvest quality during different storage temperatures.
3.3. Effect of BL irradiation on MDA, O2−, and H2O2 contents in postharvest strawberry at different storage temperatures
The oxidative damage is often indicated by an increase in MDA content, which is a critical marker to judge the degree of membrane lipid peroxidation (MLP) (Jiang et al., 2020). In other words, higher MDA levels are associated with more severe cellular membrane damage. Our research showed that the contents of MDA and O2− in the control and BL-treated strawberries increased gradually during the room- and low-temperature storage period (Fig. 3A and B), and there was a slightly decrease in H2O2 content in the BL-treated strawberries than in the control strawberries (Fig. 3C). Furthermore, the MDA content in strawberries exposed to BL compared to the dark-treated control from day two to six of storage, decreased by 5.4 %, 7.1 %, 7.3 % at 22 °C, and 29.4 %, 2.7 %, 10.4 % at 8 °C, respectively. Meanwhile, the O2− production rate from day two to six of storage, in BL-treated strawberries was reduced by 46.3 %, 16.9 %, 2 % at 22 °C, and 42 %, 22.2 %, 1.9 % at 8 °C, respectively. However, with prolonged storage time, no significant difference between the BL-irradiated and dark-treated groups was observed in the O2− and H2O2 content. These findings are mostly consistent with results observed in cherry tomato (Sun et al., 2024) and pitaya (Wu et al., 2020), because the 1,1-Diphenyl-2-picrylhydrazyl (DPPH) radical scavenging capacity after BL irradiation was significantly higher than that in control samples in later stages of storage (Wu et al., 2020; Zhang et al., 2024). These data suggested that the overall antioxidant system was significant enhanced to decrease ROS production and prevent oxidative damage in postharvest fruits and vegetables.
Fig. 3.
Effects of BL irradiation on MDA content (A), O2− production rate (B) and H2O2 content (C) of postharvest strawberries at different storage temperatures over a 10-day period. Treatments include darkness and BL irradiation at two temperatures: 22 °C and 8 °C. Each point represents the mean value, with error bars indicating the standard error of the mean. Statistical significance between different treatments at each time point is denoted by different letters, with a significance level set at p < 0.05. The arrangement of letters from top to bottom indicates the statistical ranking from highest to lowest value at each storage time. The notation “ns” indicates non-significant.
3.4. Effect of BL on AsA, GSH, APX, and GR contents in postharvest strawberry at different storage temperatures
AsA is a beneficial natural antioxidant, and strawberries are rich in this compound (Xu et al., 2014; Zhang, et al., 2022). In this study, BL irradiation gradually increased the AsA content under room- and low-temperature storage conditions (Fig. 4A), which the maximum content of AsA accompanied with 89.3 μg g−1 at 22 °C and 81.6 μg g−1 at 8 °C present on the day six of storage, respectively. Previous studies have shown that BL treatment can significantly increase AsA levels in various fruits and vegetables, including fresh-cut tomatoes (Kong et al., 2021), vegetable sprouts (Kang et al., 2020), and blueberries (Vaccinium spp.) (Li et al., 2024), thereby enhancing their ROS scavenging capabilities and maintaining the structural integrity of cells. Additionally, as increase of storage duration, the GSH content in BL-irradiated strawberries remained significantly higher than that in the dark control (P < 0.05). On day six of storage, the GSH contents in BL-treated strawberries were 18.6 % and 7.2 % higher at 22 °C and 8 °C (Fig. 4B), respectively, compared to the dark control. Therefore, we speculate that BL irradiation regulates the AsA-GSH cycle, leading to the production of metabolites that help maintain membrane stability and intracellular osmotic balance of postharvest strawberries. Furthermore, the significant increases of APX activity and GR activity of postharvest strawberries were also found in the later storage of low-temperature and room-temperature (Fig. 4C and D). However, BL irradiation does not affect the activity of APX in tomato accompanied with increase of AsA content, suggesting that the increase in AsA content may result from the upregulation expression of PguAPX2, PguGR1 related the AsA biosynthesis rather than from enhanced activity of ROS-scavenging antioxidant enzymes (Kazufumi et al., 2020). Therefore, further studies are needed to understand the molecular mechanism underlying the enhancement of AsA metabolic pathways by BL irradiation, particularly under the low-temperature storage conditions.
Fig. 4.
Effects of BL irradiation on AsA content (A), GSH content (B), APX activity (C) and GR reductase activity (D) of postharvest strawberries at different storage temperatures over a 10-day period. Treatments include darkness and BL irradiation at two temperatures: 22 °C and 8 °C. Each point represents the mean value, with error bars indicating the standard error of the mean. Statistical significance between different treatments at each time point is denoted by different letters, with a significance level set at p < 0.05. The arrangement of letters from top to bottom indicates the statistical ranking from highest to lowest value at each storage time. The notation “ns” indicates non-significant.
3.5. Effect of BL irradiation on the antioxidant enzymes of postharvest strawberry at different storage temperatures
Oxidative stress remains a significant concern for maintaining the postharvest quality of fruits (Piechowiak et al., 2024; Suthaparan et al., 2024), because the disruption in the balance between ROS generation and clearance usually lead to elevated ROS levels, which increasing to lipid peroxidation, reducing membrane integrity, and decreasing antioxidant enzyme activities. Thus, the ROS antioxidant metabolism is triggered to mitigate oxidative stress through converting excessive O2− into H2O2 by SOD, which is then decomposed into H2O and O2 by CAT and POD (Xia et al., 2023). In this study, the activities of SOD and POD in strawberry increased rapidly during the initial eight days of storage (Fig. 5A and B). On day eight of storage, the activities of SOD and POD peaked in strawberries irradiated with BL, showing enhancements of 37 % and 75 % at 22 °C, and 69.8 % and 41 % at 8 °C, respectively, compared to the dark control. The enhanced activities of SOD and POD can combat ROS accumulation in strawberry fruit during room- and low-temperature storage. These results are similar to those for strawberries, suggesting that BL irradiation plays a major role in extending the shelf life of postharvest fruits by increasing enzymatic metabolic activity (Xu et al., 2014). The CAT activity in BL-treated strawberries was significantly higher than that in the dark-treated control under the initial six days of both room- and low-temperature storage conditions (Fig. 5C), but BL irradiation decreased the PPO activity during the same storage period (Fig. 5D). Meanwhile, the significant increase of PAL activity was found in BL-treated strawberries during later storage of room- and low-temperature period (Fig. 5E), demonstrating that BL could stimulate downstream production of secondary metabolites (e.g. phenolic) and strengthen cell walls to prevent oxidative damage in the later stages. It has also been reported that BL improved the activities of PAL in cherry tomato, sweet cherries, and Shine Muscat grape (Huo et al., 2024; Kokalj et al., 2019; Sun et al., 2024). Furthermore, the blue-green light irradiation after harvest significantly up-regulated the accumulation of total phenol and total flavone during the postharvest Chinese cabbage, which further delay the senescence of the leaves and extend the shelf life (Zhang et al., 2024). Therefore, BL treatment can effectively reduced ROS levels and oxidative damage by enhancing the overall enzymatic and non-enzymatic antioxidant defense system to maintain the quality of postharvest strawberries and extend their shelf life.
Fig. 5.
Effects of BL irradiation on activity of SOD (A), POD (B), CAT (C), PPO (D) and PAL (E) of postharvest strawberries at different storage temperatures over a 10-day period. Treatments include darkness and BL irradiation at two temperatures: 22 °C and 8 °C. Each point represents the mean value, with error bars indicating the standard error of the mean. Statistical significance between different treatments at each time point is denoted by different letters, with a significance level set at p < 0.05. The arrangement of letters from top to bottom indicates the statistical ranking from highest to lowest value at each storage time. The notation “ns” indicates non-significant.
3.6. Effect of BL irradiation on the cell wall components of postharvest strawberry at different storage temperatures
Cell wall components, particularly polysaccharides such as pectin and CEL, play essential roles in maintaining firmness of postharvest fruits. Pectin is divided into SP and PP, with PP primarily located in the intercellular layer where it contributes to the adhesion and arrangement of fruit cells (Song et al., 2022). During storage, PP is gradually hydrolyzed to SP, resulting in decreased adhesion between adjacent cells and increased fruit softening (Huang et al., 2019). Previous studies indicated that the breakdown of strawberry cell walls involves substantial changes in pectin composition (Zhao et al., 2024). In this study, regardless of the storage temperatures, BL irradiation not only preserved PP content but also inhibited the conversion PP to SP (Fig. 6A and B), thereby delaying the softening process in strawberries, which was consistent with findings in postharvest kiwifruit (Xu et al., 2024). Moreover, the CEL serves as the structural framework of plant cell walls, and its degradation is a key factor in the softening of postharvest fruits (Cosgrove, 2024). This results showed that the CEL and HCEL contents of both BL-irradiated and dark-treated strawberries initially increased, followed by a decrease throughout the room- and low-temperature storage period (Fig. 6C and D). However, the effect of BL irradiation on CEL and HCEL levels in postharvest strawberries showed a slightly significant increase compared to the dark-treated control, which is consistent with findings in postharvest kiwifruit (Xu et al., 2024). Therefore, BL irradiation not only maintained cell wall components but also further delayed strawberry fruit softening. ANOVA results indicate that three-factor interactions among BL, temperature, and time significantly influenced the PP, SP, and CEL contents in postharvest strawberries (Table 1).
Fig. 6.
Effects of BL irradiation on the content of PP (A), SP (B), CEL (C), and HCEL (D) of postharvest strawberries at different storage temperatures over a 10-day period. Treatments include darkness and BL irradiation at two temperatures: 22 °C and 8 °C. Each point represents the mean value, with error bars indicating the standard error of the mean. Statistical significance between different treatments at each time point is denoted by different letters, with a significance level set at p < 0.05. The arrangement of letters from top to bottom indicates the statistical ranking from highest to lowest value at each storage time. The notation “ns” indicates non-significant.
Table 1.
ANOVA results showing the effects of light (L), temperature (Tr), and time (T) on 25 physiological and biochemical indicators in postharvest strawberries.
| Categories | Traits | Light (L) | Temperature (Tr) | Time (T) | L × Tr | L × T | Tr × T | L × Tr × T |
|---|---|---|---|---|---|---|---|---|
| Firmness | 45.62 *** | 145.71 *** | 11.03 *** | 2.23 ns | 3.17 * | 6.94 *** | 1.90 ns | |
| Soluble sugar content | 132.13 *** | 77.69 *** | 27.61 *** | 0.04 ns | 29.99 *** | 36.46 *** | 3.11 * | |
| Total phenol content | 12.14 ** | 41.21 *** | 26.17 *** | 0.07 ns | 12.74 *** | 10.37 *** | 3.12 * | |
| Respiratory rate | 132.48 *** | 909.74 *** | 43.36 *** | 5.89 * | 31.10 *** | 36.61 *** | 3.62 ** | |
| Ethylence production | 572.50 *** | 545.15 *** | 556.13 ** | 241.71 *** | 99.53 *** | 181.56 *** | 88.60 *** | |
| Activeoxygen metabolism | MDA | 93.01 *** | 6993.01 *** | 5126.61 *** | 20.40 ** | 90.69 *** | 306.89 *** | 19.76 *** |
| O2·− | 40.14 *** | 265.04 *** | 171.87 *** | 2.76 ns | 28.16 *** | 23.91 *** | 1.90 ns | |
| H2O2 | 20.07 *** | 5.94 * | 13.67 *** | 6.37 * | 8.58 *** | 1.53 ns | 4.87 ** | |
| Ascorbate-glutathione cycle | ASA | 162.01 *** | 412.81 *** | 488.94 *** | 0.60 ns | 45.16 *** | 51.21 *** | 15.14 *** |
| GSH | 6.45 * | 33.85 *** | 15.15 *** | 0.60 ns | 1.03 ns | 4.31 ** | 1.36 ns | |
| APX | 24.35 *** | 4.95 * | 13.91 *** | 3.87 ns | 5.59 *** | 2.52 * | 5.51 * | |
| GR | 1.26 ns | 379.54 *** | 116.69 *** | 0.77 ns | 2.76 * | 82.40 *** | 2.27 ns | |
| Antioxidative metabolism | SOD | 103.19 *** | 6.30 * | 88.32 *** | 0.43 ns | 21.59 *** | 23.04 *** | 9.98 *** |
| POD | 86.92 *** | 0.01 ns | 37.28 *** | 0.81 ns | 4.77 ** | 4.01 ** | 2.61 * | |
| CAT | 172.24 *** | 6.32 * | 45.86 *** | 0.28 ns | 28.67 *** | 78.19 *** | 19.20 *** | |
| PPO | 31.47 *** | 634.86 *** | 128.69 *** | 2.15 ns | 18.54 *** | 55.07 *** | 3.52 ** | |
| PAL | 65.56 *** | 2640.73 *** | 896.69 *** | 0.22 ns | 12.37 *** | 234.16 *** | 1.22 ns | |
| Cell wall | PP | 44.34 *** | 17.02 *** | 9.04 *** | 1.81 ns | 4.13 ** | 3.52 ** | 1.46 ns |
| component | SP | 41.58 *** | 0.95 ns | 1.47 ns | 8.56 * | 14.34 *** | 14.13 *** | 4.02 ** |
| CEL | 9.46 ** | 0.68 ns | 73.11 *** | 12.28 ** | 20.77 *** | 22.53 *** | 57.30 *** | |
| HCEL | 25.53 *** | 0.14 ns | 47.27 *** | 1.077 ns | 1.40 ns | 2.80 * | 0.89 ns | |
| Cell wall | PG | 98.84 *** | 260.22 *** | 8.69 *** | 0.39 ns | 5.49 *** | 13.26 *** | 1.623 ns |
| metabolizes enzymes | β-Glu | 27.83 *** | 222.70 *** | 12.36 *** | 8.22 ** | 3.77 ** | 24.96 *** | 1.78 ns |
| PME | 61.52 *** | 616.90 *** | 190.93 *** | 5.33 * | 10.36 *** | 41.10 *** | 3.24 * | |
| Cx | 343.13 *** | 94.43 *** | 78.45 *** | 64.43 *** | 14.26 *** | 22.15 *** | 9.92 *** |
F values are presented. ns = not significant; * p ≤ 0.05; ** p ≤ 0.01; *** P ≤ 0.001.
3.7. Effect of BL on CWM enzyme activity of postharvest strawberry at different storage temperatures
The metabolism of cell wall structures is primarily influenced by several enzymes, including PG, PME, Cx, β-Glu, and β-galactosidase (β-Gal) hayride components, which hydrolyze various cell wall components (Cheng et al., 2022). During both room- and low-temperature storage, PG and Cx activities in strawberries irradiated with BL were significantly lower than those in the dark-treated control (Fig. 7A and D), suggesting that BL irradiation can further maintain their integrity of cell wall structure and extend storage time of postharvest strawberries. The result is aligned with findings in kiwifruit irradiated by BL (Xu et al., 2024), in which the several genes related to cell wall degradation (e.g. bHLH and MYB families) were inhibited by BL irradiation to delay the process of fruit softening. However, PME and β-Glu activity were slightly suppressed in BL-irradiated strawberries than that in the dark-treated control during the early days of room- and low-temperature storage (Fig. 7B and C). Therefore, we speculate that BL irradiation may inhibit cell wall degradation by mainly suppressing the activities of PG and Cx involved in CWM, thereby delaying fruit softening and enhancing the shelf life during the postharvest ripening of strawberries. However, the genes related to PG and Cx activity may respond to BL irradiation and their specific mechanism needs to be further studied.
Fig. 7.
Effects of BL irradiation on the activity of PG (A), PME (B), β-Glu (C), and Cx (D) of postharvest strawberries at different storage temperatures over a 10-day period. Treatments include darkness and BL irradiation at two temperatures: 22 °C and 8 °C. Each point represents the mean value, with error bars indicating the standard error of the mean. Statistical significance between different treatments at each time point is denoted by different letters, with a significance level set at p < 0.05. The arrangement of letters from top to bottom indicates the statistical ranking from highest to lowest value at each storage time. The notation “ns” indicates non-significant.
3.8. MFV analysis
MFV provides a more comprehensive and reliable method for assessing postharvest storage tolerance in strawberries under BL irradiation, as it is based on multiple traits rather than a single trait or just a few traits for prediction. Based on the average MFV of all measured parameters, the treatments were ranked as follows: strawberries stored at 8 °C with BL irradiation scored 0.60 (calculate the mean of the sum of all indicators' MFV under 8 °C BL irradiation, same as below), followed by those at 22 °C with BL at 0.52, 8 °C in darkness at 0.46, and 22 °C in darkness at 0.36 (Table 2). These results demonstrate that BL irradiation can enhance the storage quality of postharvest strawberries across all storage temperatures, but the BL irradiation combined with low-temperature has greater potential to extend shelf life and postharvest quality of strawberry fruits.
Table 2.
Fuzzy mathematical membership function analysis of 25 physiological and biochemical indicators for postharvest strawberries under different storage temperatures with or without BL treatment.
| Fluency indices | 22 °C (Room-temperature) |
8 °C (Low-temperature) |
||
|---|---|---|---|---|
| Darkness | Blue light | Darkness | Blue light | |
| Firmness | 0.18 | 0.33 | 0.48 | 0.70 |
| Soluble sugar | 0.38 | 0.56 | 0.52 | 0.71 |
| Total phenol | 0.45 | 0.57 | 0.25 | 0.35 |
| Respiratory rate | 0.29 | 0.40 | 0.74 | 0.79 |
| Ethylene production | 0.48 | 0.74 | 0.68 | 0.68 |
| SOD | 0.23 | 0.41 | 0.26 | 0.47 |
| POD | 0.26 | 0.56 | 0.29 | 0.54 |
| CAT | 0.27 | 0.47 | 0.22 | 0.43 |
| PPO | 0.34 | 0.40 | 0.68 | 0.78 |
| AsA | 0.52 | 0.65 | 0.30 | 0.44 |
| GSH | 0.37 | 0.53 | 0.69 | 0.77 |
| APX | 0.26 | 0.37 | 0.27 | 0.52 |
| GR | 0.31 | 0.34 | 0.04 | 0.04 |
| PP | 0.24 | 0.59 | 0.12 | 0.35 |
| MDA | 0.36 | 0.38 | 0.68 | 0.68 |
| O2− | 0.31 | 0.45 | 0.62 | 0.69 |
| H2O2 | 0.64 | 0.84 | 0.78 | 0.83 |
| SP | 0.38 | 0.57 | 0.28 | 0.65 |
| CEL | 0.61 | 0.60 | 0.54 | 0.64 |
| HCEL | 0.33 | 0.50 | 0.35 | 0.46 |
| PG | 0.22 | 0.45 | 0.58 | 0.78 |
| PME | 0.43 | 0.56 | 0.77 | 0.84 |
| β-Glu | 0.38 | 0.59 | 0.83 | 0.89 |
| Cx | 0.29 | 0.68 | 0.55 | 0.71 |
| Average mean | 0.36 | 0.52 | 0.46 | 0.60 |
4. Conclusions
This study conclusively demonstrated that the integration of blue LEDs with appropriate storage temperatures (e.g. 8 °C) offers a promising approach for further enhancing postharvest quality and prolonging self-life of postharvest strawberries. The beneficial effects of BL irradiation can be attributed to three main mechanisms. Firstly, BL irradiation effectively reduces the respiratory rate and ethylene production in strawberries, thereby inhibiting over-maturation and softening of strawberry fruit. Secondly, exposure to BL increases the activity of antioxidant enzymes (e.g. SOD, POD, PAL) and boosts levels of secondary metabolites (e.g. AsA, total phenol). These changes help mitigate the cytotoxic effects of ROS, enhancing cellular protection. Thirdly, BL irradiation contributes to maintaining firmness and extending shelf-life of postharvest strawberry fruits by suppressing the activities of enzymes involved in CWM (e.g. PG, PME, Cx). Considering the multifaceted protective action of BL irradiation in the postharvest strawberry, further investigations will be required to understand the each antioxidant defense or cell wall metabolic pathway and its precise molecular mechanisms of the interactions between BL responsive factors and metabolites especially during low-temperature storage period.
CRediT authorship contribution statement
Wei Lu: Writing – original draft, Investigation, Data curation. Wanqing Li: Writing – original draft, Investigation. Keke Zhao: Validation, Methodology. Xiaofeng Bai: Data curation. Yuchang Zhang: Data curation. Qingyun Li: Investigation, Funding acquisition. Zhanjun Xue: Writing – review & editing, Supervision, Methodology. Xin-Xin Wang: Writing – review & editing, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the earmarked fund for CARS—Specialty Vegetable (grant number CARS-24-G-03).
Contributor Information
Zhanjun Xue, Email: xzj_0117@126.com.
Xin-Xin Wang, Email: xinxinwang.wur@qq.com.
Data availability
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
References
- Brehe J.E., Burch H.B. Enzymatic assay for glutathione. Analytical Biochemistry. 1976;74(1):189–197. doi: 10.1016/0003-2697(76)90323-7. [DOI] [PubMed] [Google Scholar]
- Chai J., Yang B., Xu N., Jiang Q., Gao Z., Ren X., Liu Z. Effects of low temperature on postharvest ripening and starchiness in 'Cuixiang' kiwifruit. LWT. 2024;209:116795. doi: 10.1016/J.LWT.2024.116795. [DOI] [Google Scholar]
- Chen T., Ji D., Zhang Z., Li B., Qin G., Tian S. Advances and strategies for controlling the quality and safety of postharvest fruit. Engineering. 2021;7(8):1177–1184. doi: 10.1016/J.ENG.2020.07.029. [DOI] [Google Scholar]
- Cheng C., Liu J., Wang X., Wang Y., Yuan Y., Yang S. PpERF/ABR1 functions as an activator to regulate PpPG expression resulting in fruit softening during storage in peach (Prunus persica) Postharvest Biology and Technology. 2022;189 doi: 10.1016/J.POSTHARVBIO.2022.111919. [DOI] [Google Scholar]
- Cosgrove D.J. Structure and growth of plant cell walls. Nature Reviews Molecular Cell Biology. 2024;25(5):340–358. doi: 10.1038/s41580-023-00691-y. [DOI] [PubMed] [Google Scholar]
- Dhakal R., Baek K.H. Short period irradiation of single blue wavelength light extends the storage period of mature green tomatoes. Postharvest Biology and Technology. 2014;90:73–77. doi: 10.1016/j.postharvbio.2013.12.007. [DOI] [Google Scholar]
- Du Y., Sun J., Tian Z., Cheng Y., Long C. Effect of blue light treatments on Geotrichum citri-aurantii and the corresponding physiological mechanisms of citrus. Food Control. 2023;145 doi: 10.1016/J.FOODCONT.2022.109468. [DOI] [Google Scholar]
- Freche E., Gieng J., Pignotti G., Ibrahim S.A., Feng X. Applications of lemon or cinnamon essential oils in strawberry fruit preservation: A review. Journal of Food Processing and Preservation. 2022;46(9) doi: 10.1111/JFPP.16526. [DOI] [Google Scholar]
- Ghate V., Kumar A., Kim M.J., Bang W.S., Zhou W.B., Yuk H.G. Effect of 460 nm light emitting diode illumination on survival of Salmonella spp. on fresh-cut pineapples at different irradiances and temperatures. Journal of Food Engineering. 2017;196:130–138. doi: 10.1016/j.jfoodeng.2016.10.013. [DOI] [Google Scholar]
- Gong D., Cao S., Sheng T., Shao J., Song C., Wo F., Chen W., Yang Z. Effect of blue light on ethylene biosynthesis, signalling and fruit ripening in postharvest peaches. Scientia Horticulturae. 2015;197:657–664. doi: 10.1016/j.scienta.2015.10.034. [DOI] [Google Scholar]
- Guo B., Liu G., Ye W., Xu Z., Li W., Zhuang J., Zhang X., Wang L., Lei B., Hu C., Liu Y., Dong H. Multifunctional carbon dots reinforced gelatin-based coating film for strawberry preservation. Food Hydrocolloids. 2024;147 doi: 10.1016/J.FOODHYD.2023.109327. [DOI] [Google Scholar]
- Hassan H., Amin M., Rajwana I., Ullah S., Razzaq K., Faried H., Akhtar G., Naeem-Ullah U., Qayyum M., Aslam M. Nutritional functions and antioxidative enzymes in juice extract from two different maturity stages of low temperature stored phalsa (Grewia subinaequalis DC) fruit. LWT. 2021;153 doi: 10.1016/J.LWT.2021.112552. [DOI] [Google Scholar]
- Huang J., Xu F., Zhou W. Effect of LED irradiation on the ripening and nutritional quality of postharvest banana fruit. Journal of the Science of Food and Agriculture. 2018;98(14):5486–5493. doi: 10.1002/jsfa.9093. [DOI] [PubMed] [Google Scholar]
- Huang W., Zhu N., Zhu C., Wu D., Chen K. Morphology and cell wall composition changes in lignified cells from loquat fruit during postharvest storage. Postharvest Biology and Technology. 2019;157:110975. doi: 10.1016/j.postharvbio.2019.110975. [DOI] [Google Scholar]
- Huo X., Tian X., Liu Z., Wang L., Kong Q., Wang D., Ren X. Combination of LED blue light with peppermint essential oil emulsion for the postharvest storage of Shine Muscat grape to control aspergillus carbonarius. Postharvest Biology and Technology. 2024;218:113175. doi: 10.1016/J.POSTHARVBIO.2024.113175. [DOI] [Google Scholar]
- Jiang Y., Yu L., Hu Y., Zhu Z., Zhuang C., Zhao Y., Zhong Y. The preservation performance of chitosan coating with different molecular weight on strawberry using electrostatic spraying technique. International Journal of Biological Macromolecules. 2020;151:278–285. doi: 10.1016/j.ijbiomac.2020.02.169. [DOI] [PubMed] [Google Scholar]
- Kampfenkel K., Vanmontagu M., Inzé D. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Analytical Biochemistry. 1995;225(1):165–167. doi: 10.1006/abio.1995.1127. [DOI] [PubMed] [Google Scholar]
- Kang C., Yoon E.K., Muthusamy M., Kim J.A., Jeong M.-J., Lee S.I. Blue LED light irradiation enhances L-ascorbic acid content while reducing reactive oxygen species accumulation in Chinese cabbage seedlings. Scientia Horticulturae. 2020;261:108924. doi: 10.1016/j.scienta.2019.108924. [DOI] [Google Scholar]
- Kazufumi Z., Chisato S., Minori S. Effect of light intensity and wavelengths on ascorbic acid content and the antioxidant system in tomato fruit grown in vitro. Scientia Horticulturae. 2020;274 doi: 10.1016/j.scienta.2020.109673. [DOI] [Google Scholar]
- Kokalj D., Zlatić E., Cigić B., Vidrih R. Postharvest light-emitting diode irradiation of sweet cherries (Prunus avium L.) promotes accumulation of anthocyanins. Postharvest Biology and Technology. 2019;148:192–199. doi: 10.1016/j.postharvbio.2018.11.011. [DOI] [Google Scholar]
- Kong D., Zhao W., Ma Y., Wen Y., Liang H., Zhao X. Effects of light-emitting diode (LED) illumination on the quality and antioxidant capacity of fresh-cut tomatoes during refrigerated storage.(In chinese) Food Science and Technology. 2021;46(05):45–52. doi: 10.13684/j.cnki.spkj.2021.05.008. [DOI] [Google Scholar]
- Lauria G., Piccolo E.L., Ceccanti C., Guidi L., Bernardi R., Araniti F.…Giordani T. Supplemental red LED light promotes plant productivity,“photomodulates” fruit quality and increases Botrytis cinerea tolerance in strawberry. Postharvest Biology and Technology. 2023;198 doi: 10.1016/J.POSTHARVBIO.2023.112253. [DOI] [Google Scholar]
- Li Y., Yuan D., Chen F. Effects of different wavelength LED light source irradiation on physicochemical quality of postharvest refrigerated blueberry.(In chinese). Modern. Food Science and Technology. 2024;40(05):119–126. doi: 10.13982/j.mfst.1673-9078.2024.5.0552. [DOI] [Google Scholar]
- Liu B., Jiao W., Wang B., Shen J., Zhao H., Jiang W. Near freezing point storage compared with conventional low temperature storage on apricot fruit flavor quality (volatile, sugar, organic acid) promotion during storage and related shelf life. Scientia Horticulturae. 2019;249:100–109. doi: 10.1016/j.scienta.2019.01.048. [DOI] [Google Scholar]
- Liu R., Wang J., Huang C., Su H., Huang H., Luo W., An J., Zhao H., Xu Y., Wang S. Chlorine dioxide gas slow-release film for strawberry preservation. LWT. 2023;177 doi: 10.1016/J.LWT.2023.114516. [DOI] [Google Scholar]
- Liu W., Zhang L., Ma L., Yuan H., Wang A. The HY5 transcription factor negatively regulates ethylene production by inhibiting ACS1 expression under blue light conditions in pear. Horticultural Plant Journal. 2023;9(5):920–930. doi: 10.1016/j.hpj.2022.10.007. [DOI] [Google Scholar]
- Liu Y., Schouten R.E., Tikunov Y., Liu X., Visser R.G.F., Tan F.…Marcelis L.F.M. Blue light increases anthocyanin content and delays fruit ripening in purple pepper fruit. Postharvest Biology and Technology. 2022;192:11224. doi: 10.1016/J.POSTHARVBIO.2022.112024. [DOI] [Google Scholar]
- MarMaría T.L., Paco R., Ana-Rosa B. Coordinated activation of the metabolic pathways induced by LED blue light in citrus fruit. Food Chemistry. 2020;341:128050. doi: 10.1016/j.foodchem.2020.128050. [DOI] [PubMed] [Google Scholar]
- Mesa T., Romero A., Bosch S.M. Blue LED light improves the antioxidant composition of Valencia oranges during postharvest: Impact on orange juice, pulp portion and peel residue. Scientia Horticulturae. 2024;338:113679. doi: 10.1016/J.SCIENTA.2024.113679. [DOI] [Google Scholar]
- Ning M., Zhang Q., Shu N., Qin Z., Peng R. Effects of different forms of SO2 preservative on fruit firmness and cell wall metabolism of'shine muscat'grape during storage. (In chinese) Food and Fermentation Industries. 2022;48(48):169–177. doi: 10.13995/j.cnki.11-1802/ts.028992. [DOI] [Google Scholar]
- Park H., Eo H.J., Kim C.-W., Stewart J.E., Lee U., Lee J. Physiological disorders in cold-stored ‘autumn Sense’hardy kiwifruit depend on the storage temperature and the modulation of targeted metabolites. Food Chemistry. 2024;460 doi: 10.1016/J.FOODCHEM.2024.140730. [DOI] [PubMed] [Google Scholar]
- Pérez A.A., JGuerrero B.J., Aparicio F.X., Ávila S.R., Hernández C.P., Cid P.S., Ochoa V.C. Effect of blue and ultraviolet-C light irradiation on bioactive compounds and antioxidant capacity of habanero pepper (Capsicum chinense) during refrigeration storage. Postharvest Biology and Technology. 2018;135:19–26. doi: 10.1016/j.postharvbio.2017.08.023. [DOI] [Google Scholar]
- Piechowiak T., Błaszczyk K.G., Sójka M., Antos P. Postharvest nicotinamide treatment modifies the redox status in highbush blueberry (Vaccinum corymbosum L.) fruit during storage. Postharvest Biology and Technology. 2024;217:113105. doi: 10.1016/J.POSTHARVBIO.2024.113105. [DOI] [Google Scholar]
- Qin J., Chen X., Tang X., Shao X., Lai D., Xiao W.…Dong T. Near-freezing temperature suppresses avocado (Persea americana Mill.) fruit softening and chilling injury by maintaining cell wall and reactive oxygen species metabolism during storage. Plant Physiology and Biochemistry. 2024;210 doi: 10.1016/J.PLAPHY.2024.108621. [DOI] [PubMed] [Google Scholar]
- Ratna R., Sri A., Nasrul A., Arip M.A. Effect of edible film gelatin nano-biocomposite packaging and storage temperature on the store quality of strawberry (Fragaria x ananassa var.Duchesne) Future Foods. 2023;8 doi: 10.1016/J.FUFO.2023.100276. [DOI] [Google Scholar]
- Shi L., Cao S., Shao J., Chen W., Yang Z., Zheng Y. Chinese bayberry fruit treated with blue light after harvest exhibit enhanced sugar production and expression of cryptochrome genes. Postharvest Biology and Technology. 2016;111:197–204. doi: 10.1016/j.postharvbio.2015.08.013. [DOI] [Google Scholar]
- Song Y., Hu C., Xue Y., Gu J., He J., Ren Y. 24-epibrassinolide enhances mango resistance to Colletotrichum gloeosporioides via activating multiple defense response. Scientia Horticulturae. 2022;303 doi: 10.1016/J.SCIENTA.2022.111249. [DOI] [Google Scholar]
- Song Y., Qiu K., Gao J., Kuai B. Molecular and physiological analyses of the effects of red and blue LED light irradiation on postharvest senescence of pak choi. Postharvest Biology and Technology. 2020;164 doi: 10.1016/j.postharvbio.2020.111155. [DOI] [Google Scholar]
- Sun J., Tan X., Liu B., Battino M., Meng X., Zhang F. Blue light inhibits gray mold infection by inducing disease resistance in cherry tomato. Postharvest Biology and Technology. 2024;215 doi: 10.1016/J.POSTHARVBIO.2024.113006. [DOI] [Google Scholar]
- Suthaparan A., Zhou G., Veerabagu M., Zhu P. Blue lighting combined with cold storage temperature can suppress gray mold in strawberry fruit. Postharvest Biology and Technology. 2024;218 doi: 10.1016/J.POSTHARVBIO.2024.113148. [DOI] [Google Scholar]
- Tao J., Zuo J., Watkins C.B., Bai C., He X., Liu S.…Zheng Y. Low storage temperature affects quality and volatile compounds in fresh tomatoes. Food Chemistry. 2024;460 doi: 10.1016/J.FOODCHEM.2024.140400. [DOI] [PubMed] [Google Scholar]
- Thamyres C.D.A.S., Igor H.D.L.C., Eliezer A.G., Adriana D.M. Use of edible coatings as a new sustainable alternative to extend the shelf life of strawberries (Fragaria ananassa): A review. Journal of Stored Products Research. 2024;108 doi: 10.1016/J.JSPR.2024.102375. [DOI] [Google Scholar]
- Wang Y., Lu Q., Li B., Si Y., Wang Y., Sun J., Yuan H., Wang A. LED white light-activated transcription factor MdHY5 inhibits ethylene biosynthesis during apple fruit ripening. Postharvest Biology and Technology. 2023;202 doi: 10.1016/J.POSTHARVBIO.2023.112372. [DOI] [Google Scholar]
- Wu Q., Zhou Y., Zhang Z., Li T., Jiang Y., Gao H., Yun Z. Effect of blue light on primary metabolite and volatile compound profiling in the peel of red pitaya. Postharvest Biology and Technology. 2020;160:111059. doi: 10.1016/j.postharvbio.2019.111059. [DOI] [Google Scholar]
- Xia M., Zhang S., Zhao Y., Wang Y., He F., Chen F.…Chen F. Exogenous γ-aminobutyric acid delays chilling injury of harvested kiwifruit and relates with reactive oxygen species metabolism. Food Science. 2023;44:192–206. doi: 10.7506/spkx1002-6630-20230110-066. [DOI] [Google Scholar]
- Xu F., Shi L., Chen W., Cao S., Su X., Yang Z. Effect of blue light treatment on fruit quality, antioxidant enzymes and radical-scavenging activity in strawberry fruit. Scientia Horticulturae. 2014;175:181–186. doi: 10.1016/j.scienta.2014.06.012. [DOI] [Google Scholar]
- Xu Y., Yang C., Zhang Y., Cao Q., Wan C., Chen C., Chen J., Huang Z., Gan Z. Blue light treatment delays postharvest ripening of kiwifruit by suppressing ethylene biosynthesis, starch degradation, and cell wall metabolism. LWT. 2024;199 doi: 10.1016/J.LWT.2024.116105. [DOI] [Google Scholar]
- Zhang R., He Q., Pan Q., Feng Y., Shi Y., Li G., Zhang Y., Liu Y., Khan A. Blue-green light treatment enhances the quality and nutritional value in postharvest Chinese cabbage (Brassica rapa L. ssp. pekinensis) Food Chemistry: X. 2024;24:102004. doi: 10.1016/J.FOCHX.2024.102004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Zhang M., Xu B., Mujumdar A.S., Guo Z. Light-emitting diodes (below 700 nm): Improving the preservation of fresh foods during postharvest handling, storage, and transportation. Comprehensive Reviews in Food Science and Food Safety. 2022;21(1):106–126. doi: 10.1111/1541-4337.12887. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Li S., Deng M., Gui R., Liu Y., Chen X., Lin Y., Li M., Wang Y., He W. Blue light combined with salicylic acid treatment maintained the postharvest quality of strawberry fruit during refrigerated storage. Food Chemistry: X. 2022;15 doi: 10.1016/J.FOCHX.2022.100384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Brummell D.A., Lin Q., Duan Y. Abscisic acid treatment prolongs the postharvest life of strawberry fruit by regulating sucrose and cell wall metabolism. Food Bioscience. 2024;59 doi: 10.1016/J.FBIO.2024.104054. [DOI] [Google Scholar]
- Zhou F., Yue X., Xu D., Shi J., Fang S., Yuan S., Jiang A., Zuo J., Wang Q. LED irradiation delays postharvest senescence in pakchoi by regulating amino acid metabolism. Postharvest Biology and Technology. 2022;194 doi: 10.1016/J.POSTHARVBIO.2022.112047. [DOI] [Google Scholar]
- Zhu X., Luo J., Li Q., Li J., Liu T., Wang R., Chen W., Li X. Low temperature storage reduces aroma-related volatiles production during shelf-life of banana fruit mainly by regulating key genes involved in volatile biosynthetic pathways. Postharvest Biology and Technology. 2018;146:68–78. doi: 10.1016/j.postharvbio.2018.08.015. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.