Abstract
We here analyzed changes in the proportion and content of chiral isomers of linalool and its derivatives in “Hainan dayezhong” throughout its life cycle from tea tree growth and tea manufacturing to brewing. The chiral isomers of aromatic compounds present in fresh tea leaves were found to undergo substantial diurnal and seasonal changes during tea tree growth, and their proportions varied slightly across different leaf positions. The chiral isomer content of linalool and its derivatives was consistently higher in stems than in leaves. Pest and disease stress significantly increased the proportion and content of type-R aroma. The proportion of chiral isomers underwent no considerable change during black tea manufacturing. However, their content varied dramatically among different processes. Diversity in the proportion and content of chiral isomers was observed in the wild tea tree. Further research should focus on breeding “Hainan dayezhong” wild resources to generate clones with high aroma quality.
Keywords: Tea aroma, Chiral isomers, Dynamic change, Linalool
Highlights
-
•
Aroma enantiomers exhibited remarkable diurnal and seasonal fluctuation.
-
•
Pest infestation and plant disease increased the type-R aroma proportion.
-
•
Aroma enantiomers content dramatically fluctuated during black tea manufacturing.
-
•
Brewing impact the ratio of aroma enantiomers.
-
•
Rich diversity of aroma enantiomers presented in wild tea tree.
1. Introduction
Tea is among the most popular non-alcoholic beverages consumed globally, with the aroma of a tea variety being directly linked to and inseparable from its popularity. Aroma is also a critical quality evaluation indicator for tea, making tea aroma research a crucial topic in tea science. Tea aroma is chiefly derived from the fatty acid derivation pathway, terpenoid synthesis pathway, and phenylalanine metabolism pathway. Precursors such as linolenic acid and linoleic acid generate aroma compounds such as 2-hexenal, 3-hexenol, jasmine lacton, and methyl jasmonate through the fatty acid derivatization pathway. The terpenoid synthesis pathway synthesizes linalool and its derivatives, nerolidol and geraniol, and other monoterpenoids and sesquiterpenoids. With phenylalanine as a precursor, the phenylalanine metabolic pathway produces phenylacetaldehyde, benzyl alcohol, and phenylethyl alcohol. These aroma compounds have varying odor and olfactory thresholds and contribute to the aromas of grass, flowers, fruits, honey, and so on. Detailed studies have been conducted on the tea aroma composition (Chaturvedula & Prakash, 2011), changes in tea aroma during processing (Chen et al., 2022), biosynthesis and regulatory pathways of aroma compounds (Zhou et al., 2020), and function of aroma under tea plant stress (Zhao et al., 2020).
Some aroma compounds in tea have different chiral configurations; their chiral isomers have the same chemical structure but are mirror images of each other. All the three major synthesis pathways of tea aroma produce aroma substances with a chiral structure, such as linalool, linalool oxide, 1-octen-3-ol, and methyl jasmonate. The odor and olfactory thresholds of different chiral isomers of these aroma compounds exhibit considerable variations. For example, 3R-(−)-linalool has a floral woody lavender note, with a threshold of 0.8 ppb in water, whereas 3S-(+)-linalool has a fresh, floral, and petitgrain-like odor, with a threshold of 7.4 ppb in water. Despite the aroma composition being a major determinant of tea flavor, the chiral isomer proportion in the aroma also plays a major role. Therefore, an increasing number of studies are focusing on the chiral isomers of tea aromatic compounds. Researchers have analyzed aroma chiral isomers in the fresh tea leaves of different varieties and in different finished tea products. They have also examined changes in the ratio of these isomers during tea processing and tea storage (Mu et al., 2018; Zhu et al., 2017; Zhu et al., 2020; Zhu et al., 2021). The ratio of aroma chiral isomers in tea varies markedly among different tea varieties. For example, the ratio of R-linalool in some Camellia sinensis var. assamica varieties exceeds 90 %, whereas it is less than 10 % in other varieties of C. sinensis var. sinensis (Mu et al., 2018). Raw tea leaves play a decisive role in the proportion of aroma isomers in finished tea (Zhu et al., 2017). However, different processing technologies also exert a significant impact on the proportion (Ma et al., 2021). During the storage of tea leaves, the ratio of chiral isomers of aroma compounds changes. For example, in white tea, the proportion of (1R, 2S)-methyl epijasmonate increases as the storage time increases (Zhu et al., 2020).
However, changes in the aroma chiral isomers of tea during the process from fresh tea leaves to the finished cup of tea have not been completely unveiled. For example, in the growth stage of tea plants, the effects of season, circadian rhythm, plant diseases, and insect pests on the ratio of aroma chiral isomers remain unclear. The effect of the tea brewing process on aroma chiral isomers is also unknown. A comprehensive understanding of the influence of various factors on the chiral isomers of tea aroma in the process from tea leaves to a cup of good tea will aid in better utilization of environmental factors for improving tea aroma. C. sinensis var. assamica “Hainan dayezhong” is a tea tree unique to Hainan Province, China, and is suitable for making black tea. Our previous study revealed that linalool and its derivatives were ubiquitous and the main aroma components in “Hainan dayezhong” (Zhou, He, He, et al., 2023). Therefore, in this study, the dynamic change in aroma chiral isomers during tea plant growth, the black tea manufacturing process, and the brewing process were analyzed in detail. A deeper understanding of the chiral aroma of tea may be useful in producing tea with high aroma quality.
2. Material and methods
2.1. Chemicals
(±)-Linalool, R-linalool, and (±)-linalool oxide furanoid (mixture of A and B) were purchased from Sigma (St. Louis, MO, USA). (±)-Linalool oxide pyranoid (mixture of C and D) and (±)-2,6-dimethyl-3,7-octadiene-2,6-diol (diendiol I, 96 %) were procured from TCI (Tokoyo, Japan) and BioBioPha (Yunnan China), respectively. Dichloromethane was purchased from Aladin (Shanghai, China).
2.2. Tea samples
Tea leaves of the cultivated tea plant C. sinensis var. assamica cv. “Hainan dayezhong” were sampled from the tea plantation of Jianfengling National Forest Park, Hainan Province, China. One bud and two leaves were harvested every 2 h from the tea plantation to study the diurnal dynamic of aroma chiral isomers. To determine the impact of diseases on tea aroma isomers, tender leaves with tea blister blight (infected with Exobasidium vexans Massee) and healthy tea leaves were harvested. To investigate aroma chiral isomers present in different tissues, the harvested tea leaves were segregated into various compartments including the bud, first leaf, second leaf, third leaf, fourth leaf, and stem. To understand the effect of herbivory attack on the aroma chiral isomer of tea, 50 tea green leafhoppers (Empoasca (Matsumurasca) onukii Matsuda) were reared with 10 tea shoots sampled in tea plantation in a glass beaker. Tea plant shoots not infested with insects were used as the control. One bud and two leaves were harvested 48 h after infestation. To examine the effect of tea processing on the aroma isomer, tender tea leaves were treated according to the standard black tea manufacturing process including withering, rolling, fermentation, and drying. The samples were collected after each process. The tender leaves of wild tea tree (C. sinensis var. assamica cv. “Hainan dayezhong”) were harvested in the Wuzhishan National Nature Reserve and Jianfengling National Forest Park in Hainan Province. All the experiments were performed in three replicates. All samples were quickly frozen in liquid nitrogen and stored at −80 °C for aroma analysis.
2.3. Tea leaf aroma extraction
First, finely powdered tea leaves (weighing 300 mg) were extracted with 4 mL CH2Cl2 containing 5 nmol ethyl decanoate as an internal standard for 6 h at room temperature. The extracts were passed through an anhydrous Na2SO4 column and concentrated to approximately 200 μL under N2. Then, 1 μL of the concentrated samples was subjected to gas chromatography–mass spectrometry (GC–MS) analysis.
2.4. Tea infusion aroma extraction
To study the effect of brewing on aroma isomers, samples of black tea manufactured from “Hainan dayezhong” were brewed with boiling water. The stir bar sportive extraction (SBSE) method was adopted to extract volatile compounds from tea infusions. After the tea was brewed, tea infusion (10 mL) was added to a sealed container containing a stir bar coated with polydimethylsiloxane (length: 10 mm, thick: 1.0 mm, PDMS, Gerstel GmbH & Co. KG, Mülheim an der Ruhr, Germany). Aroma extraction from the tea infusion was performed at 1000 rpm by using a magnetic stirrer, and the extract was incubated at 25 °C for 1.5 h. The absorbed aroma was desorbed using autothermal desorption TD3.5 (Gerstel GmbH & Co. KG, Mülheim an der Ruhr, Germany) and analyzed through GC–MS. The parameter settings for the thermal desorption unit were as follows: the initial temperature was held at 30 °C for 1 min, increased to 240 °C at a rate of 100 °C/min, and then again held for 5 min. The cooling injection system was maintained at −30 °C (held for 1 min), and the temperature was increased to 280 °C (held for 3 min) at the rate of 12 °C/s from −30 °C after the volatile compounds were desorbed.
2.5. Volatile enantiomer analysis through GC–MS
The Agilent 8890 GC system, coupled with the 5977B mass spectrometer, was used to analyze volatile compounds in extracted samples in the splitless injection mode. To segregate the different isomers of linalool, linalool oxides (furanoid), and diendiol I, the Astec® CHIRALDEX ™ B-DM column (30 m × 0.25 mm × 0.12 μm) was used. The Astec® CHIRALDEX ™ B-PM column (30 m × 0.25 mm × 0.12 μm) was used for separating the different isomers of linalool oxides (pyranoid). The GC program was set as follows: 40 °C for 3 min, 2 °C/min to 180 °C, and 180 °C for 30 min. The flow rate of the carrier gas helium and the ion source temperature were 1.5 mL/min and 250 °C, respectively. The mass scan range was 30–300 amu. Volatile compounds were identified by comparing their retention times with those of commercial standards. Enantiomers of the volatile compounds were determined based on previous studies (Ito et al., 2002; Mu et al., 2018; Y. Zhu et al., 2017).
3. Results and discussion
3.1. Diurnal and seasonal variations in the chiral isomers of linalool and its derivatives
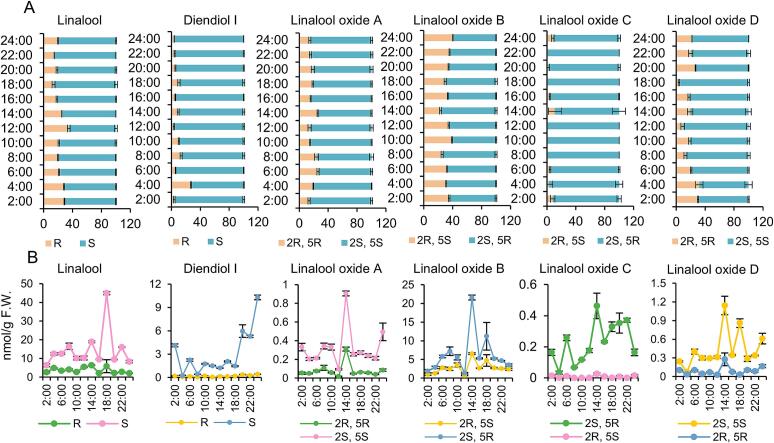
Linalool and its derivatives including linalool oxide A, linalool oxide B, linalool oxide C, linalool oxide D and diendiol I (Fig. 1) are the major aroma component in “Hainan dayezhong” tea leaves. To analyze the changes in their aroma isomers at different times of the day, one bud and two leaves were collected every 2 h during the day for aroma analysis. The proportion of isomers changed dynamically at different times of the day (Fig. 2A). The R-linalool ratio was the highest (35 %) at approximately 12:00 and lowest (14 %) at 18:00. The proportion of R-diendiol I was the highest (27 %) at 4:00 and the lowest (3 %) at 12:00. The proportion of (2R, 5R)-linalool oxide A was the highest (26 %) at 6:00 and the lowest (13 %) at 2:00. The proportion of (2R, 5S)-linalool oxide B was the highest (40 %) at 24:00 and the lowest (23 %) at 14:00. The proportion of (2R, 5S)-linalool oxide C was very low in one bud and two leaves. (2R, 5S)-Linalool oxide C was not detected from 8:00 to 12:00 and at 18:00 and 22:00, which indicated its ratio was dropped to almost 0 % at these time points. The proportion of (2R, 5S)-linalool oxide C was the highest (10 %) at 14:00. Type-(2S, 5S) was the dominant linalool oxide D configuration. Its proportion was more than 50 % at each time point of the day.
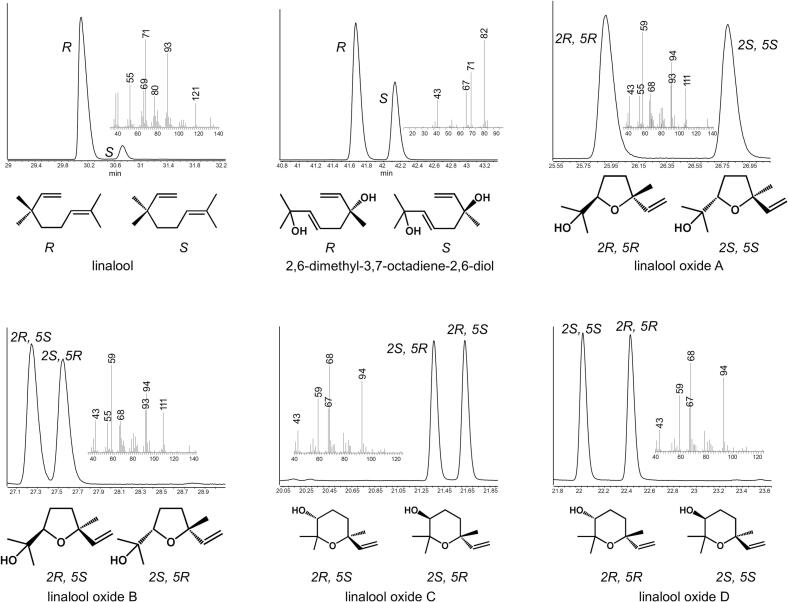
Fig. 1.
Chromatogram and Mass spectrum of commercial standards and chemical structure of chiral isomers of linalool and its derivatives.
Astec® CHIRALDEX ™ B-PM column (30 m × 0.25 mm × 0.12 μm) was used to separate chiral isomer of linalool oxide C and linalool oxide D. Others were separated using Astec® CHIRALDEX ™ B-DM column (30 m × 0.25 mm × 0.12 μm). Enantiomers of the volatile compounds were determined based on previous studies (Ito et al., 2002; Mu et al., 2018; Zhu et al., 2017).
Fig. 2.
Diurnal fluctuation of linalool and its derivatives in one bud and two leaves of cultivated ‘Hainan dayezhong’ tea tree.
A, dynamics of chiral isomer ratio. Statistically significant difference between time point was analyzed by One-way ANOVA. linalool: F = 101.323, P < 0.001; diendiol I: F = 143.361, P < 0.001; linalool oxide A: F = 24.311, P = 0.073; linalool oxide B: F = 79.598, P < 0.001; linalool oxide C: F = 4.428, P = 0.001; linalool oxide D: F = 35.250, P < 0.001. B, dynamics of chiral isomer content. Statistically significant difference between time point was analyzed by One-way ANOVA. S-linalool: F = 502.776, P < 0.001; R-linalool: F = 56.740, P < 0.001; S-diendiol I: F = 362.727, P < 0.001; R-diendiol I: F = 50.997, P < 0.001; (2S, 5S)-linalool oxide A: F = 104.209, P < 0.001; (2R, 5R)-linalool oxide A: F = 127.812, P < 0.001; (2S, 5R)-linalool oxide B: F = 65.803, P < 0.001; (2R, 5S)-linalool oxide B: F = 29.612, P < 0.001; (2S, 5R)-linalool oxide C: F = 45.993, P < 0.001; (2R, 5S)-linalool oxide C: F = 21.394, P < 0.001; (2S, 5S)-linalool oxide D: F = 80.769, P < 0.001; (2R, 5R)-linalool oxide D: F = 14.216, P < 0.001. All the statistical analysis were performed using SPSS 29.
The contents of varying chiral configurations of the same aroma compound also varied throughout the day with no obvious rhythm (Fig. 2B). However, their content changes roughly exhibited the same trend. Higher diendiol I levels were detected at night. The contents of chiral isomers of four linalool oxides all peaked at 14:00. Among the four linalool oxides, linalool oxide B exhibited the highest content, consistent with our previous findings (Zhou, He, He, et al., 2023; Zhou, He, & Zhu, 2023).
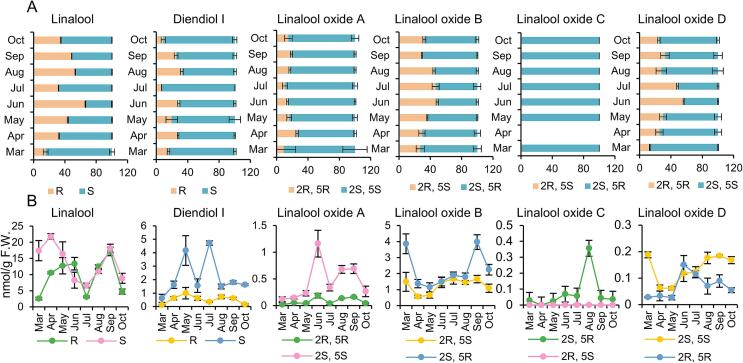
The chiral isomer ratios of linalool and its derivatives varied markedly across different months. The R-linalool ratio ranged from 15 % to 66 % from March to October (Fig. 3A). Its proportion exceeded 50 % only in June and August. Diendiol I, a specific volatile compound induced by tea green leafhopper infestation, was detected from March to October. This is consistent with the fact that Hainan has relatively high winter temperatures, and tea green leafhoppers occur year-round. From March to October, the proportion of R-diendiol I was 6.57 %–32.44 %, with the proportions in July and October being significantly lower than those in the other months. (2S, 5S)-Linalool oxide A was the major linalool oxide A, with its proportion fluctuating between 73.82 % and 91.10 % from March to October. Type-(2S, 5R) was the main linalool oxide B, with its ratio fluctuating between 27.17 % and 49.07 % from March to October. The ratios of the two chiral configurations of linalool oxide B in June, July, and August were similar. Linalool oxide C was not detected in April. Only (2S, 5R)-linalool oxide C was detected in the samples collected in different months, possibly because all the samples were collected at approximately 11:00 am. During the analysis of aroma chiral isomer ratios at different time points throughout the day, only (2S, 5R)-linalool oxide C was detected between 8:00 and 12:00 in the morning. Type-(2S, 5S) was the major linalool oxide D chiral configuration. Except in June (43.83 %), the proportion of type-(2S, 5S) was more than 50 % in all months, reaching the highest (87.08 %) in March.
Fig. 3.
Seasonal variation of linalool and its derivatives in one bud and two leaves of cultivated ‘Hainan dayezhong’ tea tree.
A, dynamics of chiral isomer ratio. Statistically significant difference between months was analyzed by One-way ANOVA. linalool: F = 517.367, P < 0.001; diendiol I: F = 29.209, P < 0.001; linalool oxide A: F = 2.361, P = 0.073; linalool oxide B: F = 25.802, P < 0.001; linalool oxide D: F = 15.282, P < 0.001. B, dynamics of chiral isomer content. Statistically significant difference between months was analyzed by One-way ANOVA. S-linalool: F = 8.525, P < 0.001; R-linalool: F = 18.568, P < 0.001; S-diendiol I: F = 25.592, P < 0.001; R-diendiol I: F = 8.569, P < 0.001; (2S, 5S)-linalool oxide A: F = 32.921, P < 0.001; (2R, 5R)-linalool oxide A: F = 26.999, P < 0.001; (2S, 5R)-linalool oxide B: F = 27.932, P < 0.001; (2R, 5S)-linalool oxide B: F = 6.242, P < 0.001; (2S, 5R)-linalool oxide C: F = 571.696, P < 0.001; (2R, 5S)-linalool oxide C: F = 101.812, P < 0.001; (2S, 5S)-linalool oxide D: F = 145.164, P < 0.001; (2R, 5R)-linalool oxide D: F = 14.223, P < 0.001. All the statistical analysis were performed using SPSS 29.
The contents of different aroma chiral isomers also varied considerably in different months (Fig. 3B). Changes in the trends of the contents of the two chiral isomers of the same compound were inconsistent in some months, which accounted for the large difference in the proportion of chiral isomers between months. For example, from April to June, the R-linalool content increased slightly, whereas the S-linalool content decreased significantly. This increased the R-linalool proportion. S-diendiol I content exhibited two obvious peaks in May and July. Whether these peaks corresponded to the peak occurrence of tea green leafhoppers needs to be investigated.
Limited studies have explored dynamic changes in the ratio of chiral isomers of plant aroma, or the so-called essential oil, over time. Changes in the ratio of the two chiral isomers of α-pinene in different months have been reported in Picea abies and Abies Sachalinensis (Kamaityte-Bukelskiene et al., 2021; Satou et al., 2009). This may be related to the temperature variations across different months. In a study, the proportion of R-α-pinene in A. sachalinensis exhibited a clear positive correlation with the average temperature of the sampling month (Satou et al., 2009).
The content of plant intrinsic aroma exhibits diurnal and seasonal variations (Dhouioui et al., 2016). Linalool and linalool oxide contents of the essential oils of many plants have been reported to fluctuate during a 24-h period and different months. According to some researchers, changes in luminosity and ultraviolet-B solar radiation within a day may affect the terpene aroma content (Gil et al., 2013). Seasonal changes in aroma are possibly related to climatic conditions such as precipitation, humidity, and temperature at the sampling time (Karalija et al., 2022). Similar to intrinsic aroma, the volatile aroma emitted by plants also exhibits obvious time rhythm. For example, volatile phenylpropanoids/benzenoids released by petunias and some volatile compounds emitted by tea leaves following insect damage displayed obvious circadian rhythms (Cai et al., 2013; Fenske et al., 2015). Changes in the expression of synthetic genes are believed to be the possible cause of changes in the aroma content (Fenske et al., 2015). The changing trends of the R/S chiral isomer content in tea leaves over time were inconsistent, indicating that dynamic changes in the expression levels of their specific synthesis genes may differ.
Our results indicated that the sampling time affects the content and proportion of aroma chiral isomers in tea. Therefore, to accurately describe the content and proportion of aroma chiral isomers in tea tree varieties, we need to state the specific sampling time. The essential oil content at different time points often exerts a decisive influence on the plant harvesting time. For example, damask roses are usually harvested in the early morning when their essential oil content is the highest (Kumar et al., 2013). In our results, it seemed that relatively high content of linalool and its derivatives was found in tea leaves picked in the afternoon. Considering that linalool and its derivatives are the main aroma substances in tea leaves of “Hainan dayezhong”, perhaps in terms of aroma, afternoon is a more suitable time for tea picking, which may make the aroma of tea more floral and sweet. Of course, the presentation of tea aroma is a very complex result of the interaction of various aroma substances, which requires our detailed evaluation.
3.2. Tissue-specific distribution of chiral isomers of linalool and its derivatives
Tea shoots were divided into the bud, first leaf, second leaf, third leaf, fourth leaf, and stem. Then, tissue-specific distribution of chiral isomers of linalool and its derivatives was analyzed.
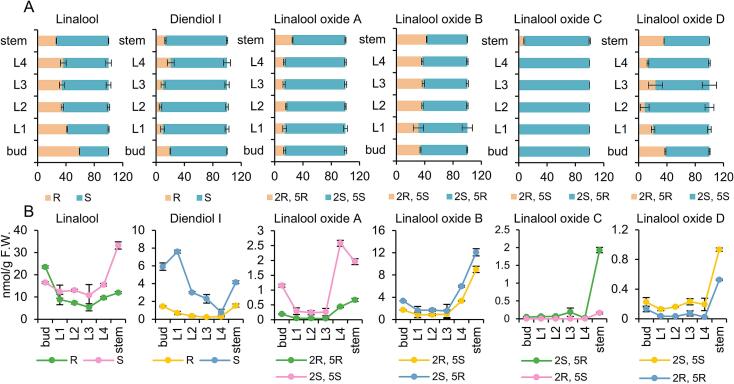
Fig. 4A showed the changes in the ratio of chiral isomers in different tissues. The R-linalool proportion was significantly higher in the bud than in the other leaf positions at 58.73 %, followed by that in the first leaf. This indicated that young leaves possessed a relatively high proportion of R-linalool. The R-linalool proportion in the stem was only 26.43 %. The R-diendiol I proportion was highest in the bud and fourth leaf, followed by the stem. No obvious pattern of diendiol I distribution was noted among various organizations. The proportion of (2R, 5R)-linalool oxide A in each leaf position was similar. However, its proportion in the stem was nearly twice that in the leaf, reaching 25.29 %. The ratio of chiral isomers of linalool oxide B exhibited no significant change in each tissue, with type-(2S, 5R) being the main configuration. (2R, 5S)-Linalool oxide C was only detected in the stem, with the proportion being 7.57 %. The proportion of linalool oxide D chiral isomers varied among the tissues. The second leaf had the lowest ratio of (2R, 5R)-linalool oxide D (8.67 %), whereas the bud had the highest ratio (37.89 %).
Fig. 4.
Tissue specific of linalool and its derivatives in cultivated ‘Hainan dayezhong’ tea tree.
A, tissue specific of chiral isomer ratio. Statistically significant difference between tissues was analyzed by One-way ANOVA. linalool: F = 70.922, P < 0.001; diendiol I: F = 14.257, P < 0.001; linalool oxide A: F = 28.431, P < 0.001; linalool oxide B: F = 4.659, P = 0.013; linalool oxide C: F = 194.508, P < 0.001; linalool oxide D: F = 17.315, P < 0.001. B, tissue specific of chiral isomer content. Statistically significant difference between tissues was analyzed by One-way ANOVA. S-linalool: F = 22.846, P < 0.001; R-linalool: F = 36.127, P < 0.001; S-diendiol I: F = 12.835, P < 0.001; R-diendiol I: F = 64.213, P < 0.001; (2S, 5S)-linalool oxide A: F = 232.124, P < 0.001; (2R, 5R)-linalool oxide A: F = 274.154, P < 0.001; (2S, 5R)-linalool oxide B: F = 109.947, P < 0.001; (2R, 5S)-linalool oxide B: F = 177.071, P < 0.001; (2S, 5R)-linalool oxide C: F = 114.350, P < 0.001; (2S, 5S)-linalool oxide D: F = 145.164, P < 0.001; (2R, 5R)-linalool oxide D: F = 672.331, P < 0.001. L1, the first leaf; L2, the second leaf; L3, the third leaf; L4, the fourth leaf. All the statistical analysis were performed using SPSS 29.
Fig. 4B showed the differences in content of chiral isomers in different tissues. The stem contained a considerably higher content of S-linalool than the leaves, whereas the R-linalool content was higher in the bud than in the other tissues. From the first leaf to fourth leaf, the content of two linalool enantiomers did not fluctuate much. The R or S-diendiol I content was higher in tender leaves (the bud and first leaf) and stem. From the second leaf to fourth leaf, the content of diendiol I decreased gradually. The content of each linalool oxide chiral isomer was significantly higher in the stems than in the leaves. For linalool oxide A and linalool oxide B, the contents in old leaves (the fourth leaf) were higher than those in young leaves.
Differences in the proportions of aroma chiral isomers in various tissues have been reported in many plants (Dangol et al., 2023; Poudel et al., 2021). For example, obvious ratio differences between the aroma chiral isomers of the rhizome, flowers, leaves, and fruits were observed in Hedychium coronarium and Zingiber roseum (Pragadheesh et al., 2013; Shanmugam et al., 2015). A study conducted using Jasminum grandiflorum revealed that the primary configuration of linalool varied in different flower stages (Pragadheesh et al., 2017). In the bud stage, R-linalool was the dominant configuration, whereas in the mature flower, S-linalool was the dominant configuration (Pragadheesh et al., 2017). The authors believed that the change in the expression level of the R-linalool synthase gene at different stages may have led to variations in the configurations. Whether the difference in the ratios of aroma chiral isomers among tea tissues is related to the expression level of synthesis genes requires to be further explored.
The great contribution of stem to tea aroma was found in our study. Expect for diendiol I, the other aroma substances were most abundant in stem, especially the four linalool oxides. Raw materials with stems obviously could help to enhance tea aroma. This is consistent with the previously reported results, which revealed a higher linalool oxide content in the stems than in the leaves (Zeng et al., 2017). In a previous study, the linalool oxides (furanoid) content in the fresh Longjing of one bud and three leaves or one bud and four leaves was higher than that in the tender buds (Shao et al., 2022). This was possibly related to the high content of these aroma compounds in the stems. The high content of diendiol I in tender leaves is possibly because that tea green leafhoppers preferably cause damage to young leaves (Zhao et al., 2019). The high content of diendiol I in stem may be a result of the damage caused by the laying of eggs by the tea green leafhoppers in the young stems (Wang et al., 2023).
3.3. Change in the chiral isomers of linalool and its derivatives under biotic stress
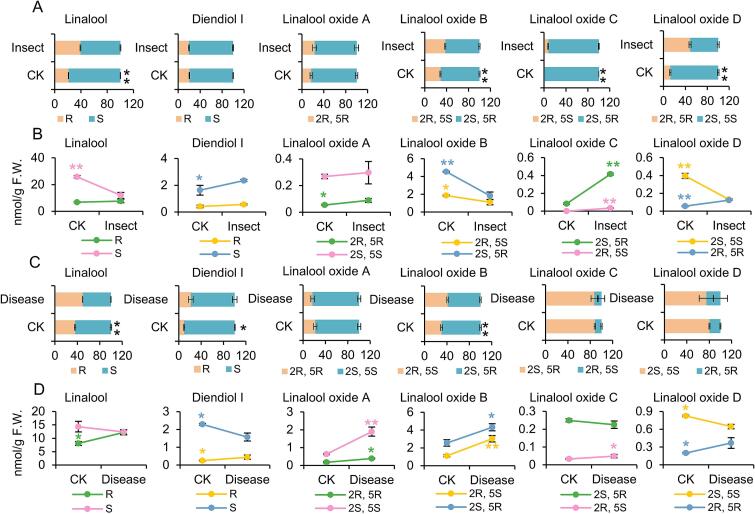
Changes in the aroma chiral configuration of tea leaves after tea green leafhopper (E. (Matsumurasca) onukii Matsuda) infestation were investigated (Fig. 5A and B). The proportions of R-linalool and type-R of linalool oxides increased to varying degrees after pest infestation (Fig. 5A). The ratio of chiral isomers of linalool oxide C and linalool oxide D changed most significantly. The proportion of (2R, 5S)-linalool oxide C increased from 0 % to 7.54 % and that of (2R, 5R)-linalool oxide D increased from 12.43 % to 48.62 % (Fig. 5A). On the other hand, tea green leafhopper infestation had no effect on the ratio of diendiol I chiral isomers (Fig. 5A).
Fig. 5.
Impact of tea green leafhopper infestation and tea blister blight on chiral configuration of linalool and its derivatives.
A, change in chiral isomer ratio upon pest infestation; B, change in chiral isomer content upon pest infestation. C, change in chiral isomer ratio upon plant disease; D, change in chiral isomer content upon plant disease. Statistically significant difference was analyzed by student's t-test. *, P < 0.05; **, P < 0.01. All the statistical analysis were performed using SPSS 29.
The content measurement results revealed that pest infestation increased the content of internal R-linalool but reduced the content of internal S-linalool (Fig. 5B). Although insect damage simultaneously increased the expression of R-linalool synthase and S-linalool synthase genes (Zhou et al., 2020), the massive release of S-linalool after insect damage possibly reduced the intrinsic content (Mei et al., 2017). Tea green leafhopper infestation simultaneously increased the content of R-diendiol I significantly (Fig. 5B). The (2R, 5R)-linalool oxide A content increased due to pest damage, whereas the type-(2S, 5S) content exhibited no significant elevation (Fig. 5B). The contents of both isomers of linalool oxide B were inhibited by the pest, with a more dramatic decrease noted in the content of the S form (Fig. 5B). The pest simultaneously triggered an increase in the content of both chiral isomers of linalool oxide C (Fig. 5B). The changes in the content of the two chiral isomers of linalool oxide D were opposite. The (2R, 5R)-linalool oxide D content increased after insect infestation, whereas the (2S, 5S)-linalool oxide D content decreased significantly (Fig. 5B).
Tea blister blight is an epidemic disease of “Hainan dayezhong” in the Jiangfenling National Forest Park. Although tea blister blight and tea green leafhopper are both biotic stresses, their effects on the chiral isomers of linalool and its derivatives were not exactly the same. The ratios of R-linalool and (2R, 5S)-linalool oxide B significantly increased in the diseased leaves (Fig. 5C), which was the same as the changes caused by the pests. Different from the impact of pests, disease incidence increased the R-diendiol ratio in the leaves but did not significantly affect the chiral isomer ratios of other linalool oxides (Fig. 5C).
Similar to the effect of pests, disease incidence increased the R-linalool content significantly in the leaves but decreased the S-linalool content (Fig. 5D). The change in the diendiol I chiral isomer content of the diseased leaves was similar as the trend observed in linalool content (Fig. 5D). The contents of each chiral isomer of linalool oxides A and B increased in the diseased leaves (Fig. 5D). The content of linalool oxide C chiral isomers did not change considerably after pathogen infection. Like the changes caused by the pests, the (2R, 5R)-linalool oxide D content increased in the diseased leaves, whereas the (2S, 5S)-linalool oxide D content decreased (Fig. 5D).
In a previous study, changes in aroma isomers affected the ability of plants to repel pests (Allmann & Baldwin, 2010). Insect attack could change the ratio of emitted R/S 1-phenylethanol in tea flowers (Zhou, Zeng, Liao, et al., 2017). Studies are needed to understand whether pests affect the proportion and content of aroma chiral isomers released by tea leaves and to explore the ecological significance of changes in these isomers in the interaction between tea plant and pests. Moreover, plant aroma exerts a certain inhibitory effect on plant pathogen, and different chiral isomers may exert varying effects. For example, (S)-limonene, but not (R)-limonene, inhibited the growth of Xanthomonas oryzae pv. oryzae (Lee et al., 2016). In tea leaves, changes in the ratio and content of aroma chiral isomers may be a mechanism to defend against tea blister blight disease.
Changes in the ratio of plant aroma configuration under biotic stress may be a mechanism used by plants to adapt to environmental stress. In our study, under pest and disease stresses, the proportion of R-type aroma compounds did not decrease, whereas that of some compounds increased significantly. Moreover, the contents of most R-type aroma chiral isomers increased under the disease stress and pest infestation. The role of R-type tea aroma in plant defense to biotic stress needs to be further explored.
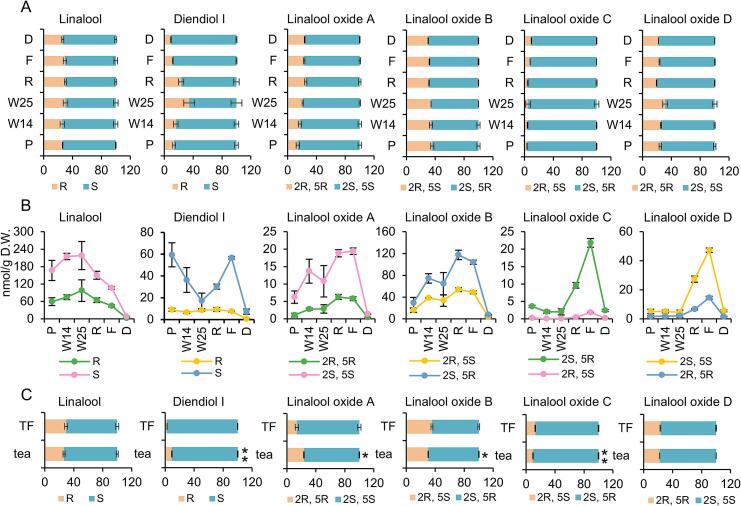
3.4. Change in the chiral isomers of linalool and its derivatives in black tea manufacturing and tea brewing
The black tea manufacturing process includes five stages: picking, withering, rolling, fermentation, and drying. The chiral isomer ratio of linalool and its derivatives exhibited no dramatic change at different stages of black tea processing (Fig. 6A). The proportion of R-diendiol and (2R, 5R)-linalool oxide A increased significantly during withering. The proportion of (2R, 5S)-linalool oxide C gradually increased with the manufacturing process.
Fig. 6.
Dynamic change of chiral configuration of linalool and its derivatives in black tea manufacturing process and brewing.
A, change in chiral isomer ratio in manufacture processing. Statistically significant difference between processes was analyzed by One-way ANOVA. linalool: F = 2.708, P = 0.073; diendiol I: F = 18.306, P < 0.001; linalool oxide A: F = 20.447, P < 0.001; linalool oxide B: F = 8.068, P = 0.002; linalool oxide C: F = 3.866, P = 0.026; linalool oxide D: F = 19.083, P < 0.001. B, change in chiral isomer content in manufacture processing. Statistically significant difference between processes was analyzed by One-way ANOVA. S-linalool: F = 8.296, P = 0.001; R-linalool: F = 7.966, P = 0.002; S-diendiol I: F = 22.379, P < 0.001; R-diendiol I: F = 10.787, P < 0.001; (2S, 5S)-linalool oxide A: F = 8.528, P = 0.001; (2R, 5R)-linalool oxide A: F = 14.650, P < 0.001; (2S, 5R)-linalool oxide B: F = 13.679, P < 0.001; (2R, 5S)-linalool oxide B: F = 40.576, P < 0.001; (2S, 5R)-linalool oxide C: F = 178.898, P < 0.001; (2R, 5S)-linalool oxide C: F = 141.296, P < 0.001; (2S, 5S)-linalool oxide D: F = 271.872, P < 0.001; (2R, 5R)-linalool oxide D: F = 98.613, P < 0.001. C, impact of brewing on chiral isomer ratio. Statistically significant difference was analyzed by student's t-test. P, picking; W14, withering for 14 h; W25, withering for 25 h; R, rolling; F, fermentation; D, drying; TF, tea infusion. All the statistical analysis were performed using SPSS 29.
From picking to drying, the aroma chiral isomer content changed significantly (Fig. 6B). The content change trends of the two chiral isomers of the same compound were relatively consistent. During withering, the contents of both chiral isomers of linalool steadily increased. S-diendiol I contents decreased dramatically during withering (−71.1 %), whereas R-diendiol I contents remained stable. Withering aided in increasing the contents of linalool oxides A and B, but exerted no significant effect on the contents of linalool oxides C and D. During the processing stage after withering, the contents of the two linalool isomers displayed a significant decreasing trend. Except for linalool, the contents of the other five aromas increased to varying degrees during rolling and fermentation. Although drying caused no significant change in the proportion of aroma chiral isomers, the content of free aroma in black tea reduced significantly.
The increase in the ratio of R-diendiol I during withering is due to the sharp decrease in the content of S-diendiol. Of the six compounds we studied, diendiol I was the only one whose content decreased significantly during withering stage. It is possible that its biosynthesis is inhibited or many free diendiol I is transformed into glycoside during withering. Different enantiomers of linalool oxide C has completely different odor. (2R, 5S)-Linalool oxide C has an earthy odor, while (2S, 5R)-linalool oxide C exhibits a sweet, floral, creamy odor (Zhou, He, & Zhu, 2023). The increasing proportion of (2R, 5S)-linalool oxide C during tea manufacturing process definitely affect tea aroma. However, whether these differences are perceptible to humans requires further research.
Our results showed that withering helped increase the content of both chiral isomers of linalool, linalool oxide A and linalool oxide B. Similar to our results, another study reported that the content of the two chiral isomers of linalool increased significantly during the spreading stage of green tea processing (Shao et al., 2022). It has been confirmed that picking-induced injury leads to the increase in linalool content during withering. Genes corresponding to R-linalool and S-linalool biosynthesis are known to be induced by mechanical damage (Zhou et al., 2020). It is possible that P450 genes responsible for the synthesis of linalool oxides (furanoid) also be induced by mechanical injury. The decrease in the content of two linalool chiral isomers after withering stage may be related to the large emission of free linalool during rolling, fermentation, and drying. Our results suggested that rolling and fermentation may contribute greatly to the improvement of the aroma quality of black tea, as the content of the four linalool derivatives all increased sharply. Cell disruption-induced hydrolysis of aroma glycosides may be responsible for the increase in the content of linalool derivatives during rolling and fermentation. A large number of aroma glycosides are stored in the vacuole (Chen & Quek, 2023). Because of rolling, glycosides in the vacuole come in contact with glycoside hydrolase distributed outside the vacuole. Fermentation provides suitable conditions for this glycoside hydrolase-catalyzed hydrolysis to produce a large amount of free aroma (Zhou, Zeng, Gui, et al., 2017). Drying causes a dramatically drop in the content of aroma substances. Consistent with our results, pan-frying and drying significantly reduced the free aroma content of green tea leaves (Flaig et al., 2020). The loss of aroma during pan-frying and drying may be related to the high temperature that causes volatilization of a large amount of free aroma. Therefore, optimizing drying conditions may help improve the aroma quality of black tea.
Brewing significantly reduced the R-diendiol proportion from 9.08 % to 2.47 % (Fig. 6C). The (2R, 5R)-linalool oxide A proportion decreased from 23.47 % to 13.94 %. No significant change was observed in the ratio of chiral isomers of linalool and its other derivatives. Based on these results, we concluded that hot water brewing has a certain impact on the ratio of aroma chiral isomers. If we want to accurately determine the distribution of these isomers in dry tea, we should avoid using tea soup brewed with hot water for the measurement. In addition, because the aroma extraction efficiency may differ between organic reagent extraction and the SBSE method, we did not compare the effect of brewing on the aroma chiral isomer content.
3.5. Chiral isomers of linalool and its derivatives in wild tea tree leaves
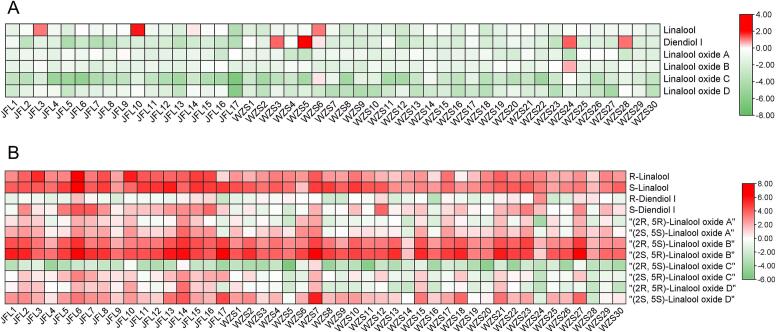
“Hainan dayezhong” has abundant wild tea tree resources. These wild tea trees exhibit enormous diversity in terms of the appearance of their leaves. Samples of multiple wild tea plants were collected from Wuzhishan and Jianfengling districts, the main areas of the distribution of wild tea trees, and differences in their aromas were analyzed.
The proportions of aroma chiral isomers in different individual plants of wild tea trees varied. Fig. 7A presents the R/S ratio of each aroma compound. Type-S was possibly the primary chiral configuration of linalool and its derivatives. Among the 17 wild tea trees collected from Jianfengling, the lowest proportion of R-linalool was 6.15 %, whereas the highest proportion was 78.61 %. Among the 30 wild tea leaves collected from Wuzhishan, the lowest proportion of R-linalool was 16.58 %, whereas the highest proportion was 67.72 %. Among the samples collected from Jianfengling, the R-diendiol I proportion was <50 %, with the highest being 39.46 %. Among the samples collected from Wuzhishan, the R-diendiol I proportion in four tea plants was approximately 70 % (70.19 %, 80.53 %, 70.80 %, and 68.71 %) and that in the other plants was <50 %. The proportion of (2S, 5S)-linalool oxide A was between 49.55 % and 91.37 %. Only one wild tea tree in Wuzhishan had a (2S, 5R)-linalool oxide B ratio of <50 % (38.85 %). The (2S, 5R)-linalool oxide B ratio in other trees was between 51.19 % and 79.78 %. Interestingly, tea trees with a lower (2R, 5R)-linalool oxide A proportion often had a higher (2R, 5S)-linalool oxide B proportion. For example, JFL16 from Jianfengling and WZS24 from Wuzhishan had the lowest (2R, 5R)-linalool oxide A proportions, respectively, but they contained the highest (2R, 5S)-linalool oxide B proportions. (2R, 5S)-Linalool oxide C could be detected in all wild tea leaves, but its proportion was low in most leaves. Only in two samples from Wuzhishan, its proportion reached approximately 50 % (WZS6 and WZS24, 53.44 % and 46.04 %, respectively). (2S, 5S)-Linalool oxide D was the primary linalool oxide D configuration in wild tea trees. Its proportion in all samples was >50 %.
Fig. 7.
Diversity of linalool and its derivatives in individual wild tea tree.
A, diversity in the ratio of chiral isomers of linalool and its derivatives; B, diversity in the content of chiral isomers of linalool and its derivatives. To better display the differences, the raw data were treated with log2 transformation. JFL, tea tree grown in Jianfengling National Park; WZS, tea tree grown in Wuzhishan National Nature Reserve.
Regarding aroma content, great differences were observed among wild tea trees (Fig. 7B). In general, the content of aroma chiral isomers of tea leaves was slightly higher in the Jianfengling area than in the Wuzhishan area. The content of each chiral isomer of linalool and linalool oxide B was significantly higher than that of the other aroma substances. Leaves with a higher linalool content also had a higher content of its derivatives, such as JFL6.
The aroma differences in individual wild tea trees completely illustrate the rich genetic diversity of “Hainan dayezhong.” According to previous studies, the linalool chiral configuration of C. sinensis var. assamica is predominantly type-R (Zhou et al., 2020; Zhu et al., 2017). Notably, in “Hainan dayezhong,” the linalool configuration is primarily type-S, unlike the proportion of linalool chiral configuration reported in other C. sinensis var. assamica cultivars. This may be because “Hainan dayezhong” evolved independently in Hainan Island and has a genetic background different from that of traditional cultivated tea (Guo et al., 2024). Metabolite differences among individual plants of wild tea trees have been reported in Jianghua Kucha (Wu et al., 2022). Significant differences were observed in the contents of various catechins, theacrine, theobromine, and other metabolites in 11 individual Jianghua Kucha tea plants. A rich genetic diversity of wild tea trees may explain individual differences in specialized metabolites (Li et al., 2024). Next, the content of nonvolatile, quality-related metabolites can be analyzed in individuals of “Hainan dayezhong.” By selecting individual plants with optimal metabolite contents and through clonal breeding, the quality of tea aroma can be optimized, and “Hainan dayezhong” wild resources can be more effectively used.
4. Conclusion
This study analyzed dynamic changes in the chiral isomers of linalool and its derivatives in “Hainan dayezhong” tea leaves. Diurnal and seasonal changes in these isomers, their tissue-specific distribution, impact of pest infestation and disease, and their change during tea manufacturing processes were studied in detail (Fig. 8). In general, type-S was the dominant chiral configuration of linalool and its derivatives in “Hainan dayezhong” tree leaves. The proportions and contents of these chiral isomers fluctuated considerably throughout the day and across different months. This offered us a reference for deciding the tea harvesting time. Except for diendiol I, the content of most aroma chiral isomers was higher in the stem than in the leaves. Pest infestation and disease incidence increased the type-R aroma proportion. Although the proportion of different chiral isomers of linalool and its derivatives did not exhibit considerable variations during black tea manufacturing, their contents dramatically fluctuated. The R-diendiol I proportion reduced significantly after tea brewing, whereas the proportion of other aroma chiral isomers was slightly affected. Chiral isomers of linalool and its derivatives in the wild tea tree “Hainan dayezhong” presented a rich diversity in their proportion and content. Selecting superior clones from these wild tea tree resources for molecular breeding can be a promising approach for improving aroma quality of “Hainan dayezhong.”
Fig. 8.
Conclusion of dynamics of chiral isomers of linalool and its derivatives.
Funding
This work was supported by the National Natural Science Foundation of China (32260785), Hainan Provincial Natural Science Foundation of China (323RC521), Hainan Province Science and Technology Special Fund (ZDYF2021XDNY302).
CRediT authorship contribution statement
Ying Zhou: Writing – review & editing, Writing – original draft, Investigation, Conceptualization. Junjie Tian: Investigation. Hainuo Hong: Investigation. Yang Gao: Investigation. Yunchuan He: Investigation. Zeng-Rong Zhu: Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
Data will be made available on request.
References
- Allmann S., Baldwin I.T. Insects betray themselves in nature to predators by rapid isomerization of green leaf volatiles. Science. 2010;329(5995):1075–1078. doi: 10.1126/science.1191634. [DOI] [PubMed] [Google Scholar]
- Cai X.-M., Sun X.-L., Dong W.-X., Wang G.-C., Chen Z.-M. Herbivore species, infestation time, and herbivore density affect induced volatiles in tea plants. Chemoecology. 2013;24:1–14. doi: 10.1007/s00049-013-0141-2. [DOI] [Google Scholar]
- Chaturvedula V.S.P., Prakash I. The aroma, taste, color and bioactive constituents of tea. Journal of Medicinal Plant Research. 2011;5(11):2110–2124. doi: 10.5897/JMPR.9001187. [DOI] [Google Scholar]
- Chen Q., Zhu Y., Liu Y., Liu Y., Dong C., Lin Z., Teng J. Black tea aroma formation during the fermentation period. Food Chemistry. 2022;374 doi: 10.1016/j.foodchem.2021.131640. [DOI] [PubMed] [Google Scholar]
- Chen X., Quek S.Y. Free and glycosidically bound aroma compounds in fruit: Biosynthesis, transformation, and practical control. Critical Reviews in Food Science and Nutrition. 2023;63(28):9052–9073. doi: 10.1080/10408398.2022.2064422. [DOI] [PubMed] [Google Scholar]
- Dangol S., Poudel D.K., Ojha P.K., Maharjan S., Poudel A., Satyal R.…Setzer W.N. Essential oil composition analysis of cymbopogon species from eastern Nepal by GC-MS and chiral GC-MS, and antimicrobial activity of some major compounds. Molecules. 2023;28(2) doi: 10.3390/molecules28020543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhouioui M., Boulila A., Chaabane H., Zina M.S., Casabianca H. Seasonal changes in essential oil composition of Aristolochia longa L. ssp. paucinervis batt. (Aristolochiaceae) roots and its antimicrobial activity. Industrial Crops and Products. 2016;83:301–306. doi: 10.1016/j.indcrop.2016.01.025. [DOI] [Google Scholar]
- Fenske M.P., Hewett Hazelton K.D., Hempton A.K., Shim J.S., Yamamoto B.M., Riffell J.A., Imaizumi T. Circadian clock gene LATE ELONGATED HYPOCOTYL directly regulates the timing of floral scent emission in Petunia. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(31):9775–9780. doi: 10.1073/pnas.1422875112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaig M., Qi S.C., Wei G., Yang X., Schieberle P. Characterisation of the key aroma compounds in a Longjing green tea infusion (Camellia sinensis) by the sensomics approach and their quantitative changes during processing of the tea leaves. European Food Research and Technology. 2020;246:2411–2425. doi: 10.1007/s00217-020-03584-y. [DOI] [Google Scholar]
- Gil M., Bottini R., Berli F., Pontin M., Silva M.F., Piccoli P. Volatile organic compounds characterized from grapevine (Vitis vinifera L. cv. Malbec) berries increase at pre-harvest and in response to UV-B radiation. Phytochemistry. 2013;96:148–157. doi: 10.1016/j.phytochem.2013.08.011. [DOI] [PubMed] [Google Scholar]
- Guo D., Li D., Wang Z., Li D., Zhou Y., Xiang G., Zhang W., Wang W., Fang Z., Hao T., Zheng D., Lei Y., Yang L., Zhang W., Tang S., Zheng L., Cao Y., Huang Y., Duan S. Genome resequencing reveals an independently originated Camellia sinensis variety – Hainan tea. Agrobiodiversity. 2024;3:3–11. doi: 10.48130/abd-0024-0003. [DOI] [Google Scholar]
- Ito Y., Sugimoto A., Kakuda T., Kubota K. Identification of potent odorants in Chinese jasmine green tea scented with flowers of Jasminum sambac. Journal of Agricultural and Food Chemistry. 2002;50(17):4878–4884. doi: 10.1021/jf020282h. [DOI] [PubMed] [Google Scholar]
- Kamaityte-Bukelskiene L., Loziene K., Labokas J. Dynamics of isomeric and enantiomeric fractions of pinene in essential oil of Picea abies annual needles during growing season. Molecules. 2021;26(8) doi: 10.3390/molecules26082138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karalija E., Dahija S., Tarkowski P., Zeljkovic S.C. Influence of climate-related environmental stresses on economically important essential oils of Mediterranean Salvia sp. Frontiers in Plant Science. 2022;13 doi: 10.3389/fpls.2022.864807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R., Sharma S., Sood S., Agnihotri V.K., Singh B. Effect of diurnal variability and storage conditions on essential oil content and quality of damask rose (Rosa damascena Mill.) flowers in north western Himalayas. Scientia Horticulturae. 2013;154:102–108. doi: 10.1016/j.scienta.2013.02.002. [DOI] [Google Scholar]
- Lee G.W., Chung M.S., Kang M., Chung B.Y., Lee S. Direct suppression of a rice bacterial blight (Xanthomonas oryzae pv. oryzae) by monoterpene (S)-limonene. Protoplasma. 2016;253(3):683–690. doi: 10.1007/s00709-015-0904-4. [DOI] [PubMed] [Google Scholar]
- Li M.M., Meegahakumbura M.K., Wambulwa M.C., Burgess K.S., Moller M., Shen Z.F.…Gao L.M. Genetic analyses of ancient tea trees provide insights into the breeding history and dissemination of Chinese Assam tea (Camellia sinensis var. assamica) Plant Diversity. 2024;46(2):229–237. doi: 10.1016/j.pld.2023.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W., Zhu Y., Shi J., Wang J., Wang M., Shao C., Yan H., Lin Z., Lv H. Insight into the volatile profiles of four types of dark teas obtained from the same dark raw tea material. Food Chemistry. 2021;346 doi: 10.1016/j.foodchem.2020.128906. [DOI] [PubMed] [Google Scholar]
- Mei X., Liu X., Zhou Y., Wang X., Zeng L., Fu X., Li J., Tang J., Dong F., Yang Z. Formation and emission of linalool in tea (Camellia sinensis) leaves infested by tea green leafhopper (Empoasca (Matsumurasca) onukii Matsuda) Food Chemistry. 2017;237:356–363. doi: 10.1016/j.foodchem.2017.05.124. [DOI] [PubMed] [Google Scholar]
- Mu B., Zhu Y., Lv H.P., Yan H., Peng Q.H., Lin Z. The enantiomeric distributions of volatile constituents in different tea cultivars. Food Chemistry. 2018;265:329–336. doi: 10.1016/j.foodchem.2018.05.094. [DOI] [PubMed] [Google Scholar]
- Poudel D.K., Rokaya A., Ojha P.K., Timsina S., Satyal R., Dosoky N.S.…Setzer W.N. The chemical profiling of essential oils from different tissues of Cinnamomum camphora L. and their antimicrobial activities. Molecules. 2021;26(17) doi: 10.25135/rnp.11.17.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pragadheesh V.S., Chanotiya C.S., Rastogi S., Shasany A.K. Scent from Jasminum grandiflorum flowers: Investigation of the change in linalool enantiomers at various developmental stages using chemical and molecular methods. Phytochemistry. 2017;140:83–94. doi: 10.1016/j.phytochem.2017.04.018. [DOI] [PubMed] [Google Scholar]
- Pragadheesh V.S., Yadav A., Singh M., Chanotiya C.S. Characterization of volatile components of Zingiber roseum essential oil using capillary GC on modified cyclodextrins. Natural Product Communications. 2013;8(2):221–224. doi: 10.1177/1934578X1300800223. [DOI] [PubMed] [Google Scholar]
- Satou T., Matsuura M., Murakami S., Hayashi S., Koike K. Composition and seasonal variation of the essential oil from Abies sachalinensis from Hokkaido, Japan. Natural Product Communications. 2009;4(6):845–848. doi: 10.1177/1934578X0900400621. [DOI] [PubMed] [Google Scholar]
- Shanmugam P.V., Yadav A., Chanotiya C.S. Enantiomer differentiation of key volatile constituents from leaves, stems, rhizome and flowers of cultivated Hedychium coronarium Koenig from India. Journal of Essential Oil Research. 2015;27:101–106. doi: 10.1080/10412905.2014.987929. [DOI] [Google Scholar]
- Shao C.Y., Zhang Y., Lv H.P., Zhang Z.F., Zeng J.M., Peng Q.H.…Lin Z. Aromatic profiles and enantiomeric distributions of chiral odorants in baked green teas with different picking tenderness. Food Chemistry. 2022;388 doi: 10.1016/j.foodchem.2022.132969. [DOI] [PubMed] [Google Scholar]
- Wang M., Han S., Wu Y., Lin J., Zhou J., Han B. Tea green leafhopper-induced synomone attracts the egg parasitoids, mymarids to suppress the leafhopper. Pest Management Science. 2023;79(10):3785–3795. doi: 10.1002/ps.7563. [DOI] [PubMed] [Google Scholar]
- Wu W., Lu M., Peng J., Lv H., Shi J., Zhang S.…Lin Z. Nontargeted and targeted metabolomics analysis provides novel insight into nonvolatile metabolites in Jianghua Kucha tea germplasm (Camellia sinensis var. assamica cv. Jianghua) Food Chemistry: X. 2022;13 doi: 10.1016/j.fochx.2022.100270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L., Zhou Y., Fu X., Mei X., Cheng S., Gui J., Dong F., Tang J., Ma S., Yang Z. Does oolong tea (Camellia sinensis) made from a combination of leaf and stem smell more aromatic than leaf-only tea? Contribution of the stem to oolong tea aroma. Food Chemistry. 2017;237:488–498. doi: 10.1016/j.foodchem.2017.05.137. [DOI] [PubMed] [Google Scholar]
- Zhao M., Wang L., Wang J., Jin J., Zhang N., Lei L., Gao T., Jing T., Zhang S., Wu Y., Wu B., Hu Y., Wan X., Schwab W., Song C. Induction of priming by cold stress via inducible volatile cues in neighboring tea plants. Journal of Integrative Plant Biology. 2020;62(10):1461–1468. doi: 10.1111/jipb.12937. [DOI] [PubMed] [Google Scholar]
- Zhao X., Chen S., Wang S., Shan W., Wang X., Lin Y.…Yu X. Defensive responses of tea plants (Camellia sinensis) against tea green leafhopper attack: A multi-omics study. Frontiers in Plant Science. 2019;10:1705. doi: 10.3389/fpls.2019.01705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Deng R., Xu X., Yang Z. Enzyme catalytic efficiencies and relative gene expression levels of (R)-linalool synthase and (S)-linalool synthase determine the proportion of linalool enantiomers in Camellia sinensis var. sinensis. Jouranl of Agricultural and Food Chemistry. 2020;68(37):10109–10117. doi: 10.1021/acs.jafc.0c04381. [DOI] [PubMed] [Google Scholar]
- Zhou Y., He W., He Y., Chen Q., Gao Y., Geng J., Zhu Z.R. Formation of 8-hydroxylinalool in tea plant Camellia sinensis var. assamica “Hainan dayezhong”. Food Chemistry: Molecular Sciences. 2023;6 doi: 10.1016/j.fochms.2023.100173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., He Y., Zhu Z.R. Understanding of formation and change of chiral aroma compounds from tea leaf to tea cup provides essential information for tea quality improvement. Food Research International. 2023;167 doi: 10.1016/j.foodres.2023.112703. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Zeng L., Gui J., Liao Y., Li J., Tang J., Meng Q., Dong F., Yang Z. Functional characterizations of beta-glucosidases involved in aroma compound formation in tea (Camellia sinensis) Food Research International. 2017;96:206–214. doi: 10.1016/j.foodres.2017.03.049. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Zeng L., Liao Y., Dong F., Peng Q., Li J.…Yang Z. Insects (Thrips hawaiiensis (Morgan)) change the stereochemical configuration of 1-phenylethanol emitted from tea (Camellia sinensis) flowers. RSC Advances. 2017;7:32336–32343. doi: 10.1039/C7RA03219F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Zhu Y., Wang K., Niu Y., Xiao Z. Characterization of key aroma compounds and enantiomer distribution in Longjing tea. Food Chemistry. 2021;361 doi: 10.1016/j.foodchem.2021.130096. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Kang S., Yan H., Lv H.P., Zhang Y., Lin Z. Enantiomeric distributions of volatile lactones and terpenoids in white teas stored for different durations. Food Chemistry. 2020;320 doi: 10.1016/j.foodchem.2020.126632. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Shao C.Y., Lv H.P., Zhang Y., Dai W.D., Guo L.…Lin Z. Enantiomeric and quantitative analysis of volatile terpenoids in different teas (Camellia sinensis) Journal of Chromatography A. 2017;1490:177–190. doi: 10.1016/j.chroma.2017.02.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.