Abstract
Prolactinomas are the most prevalent subtype of pituitary adenomas and represent one of the leading etiological factors responsible for amenorrhea and infertility in women. The primary therapeutic approach entails the use of dopamine agonists, which effectively restore fertility. In cases of microprolactinomas, the likelihood of experiencing a symptomatic enlargement of the tumor during pregnancy is exceptionally low, estimated at a mere 2.4%. Consequently, once pregnancy is successfully achieved, the administration of dopamine agonists is discontinued, with ongoing clinical monitoring of the patient's condition. The incidence of pituitary apoplexy during pregnancy is exceedingly rare. We present a case of a 29-year-old patient with microprolactinoma, treated with cabergoline, which was discontinued upon achieving pregnancy. However, at the 16th week of gestation, she presented with persistent headaches and compromised visual acuity, manifesting as left temporal hemianopia. A noncontrast magnetic resonance imaging (MRI) revealed an enlargement of the adenoma, accompanied by evidence of hemorrhage. She was referred to our hospital at 26 weeks of gestation. Cabergoline treatment was reinstated and well tolerated by the patient. Doses were increased to 2 mg per week because bitemporal hemianopia was not improving. A subsequent noncontrast MRI scan performed at 35 weeks of gestation demonstrated a further increase in adenoma size, measuring 17 × 21 × 13 mm, with signs of intratumoral bleeding. A planned cesarean section was performed at 39 weeks of gestation, without encountering maternal–fetal complications. Breastfeeding was not initiated due to the adenoma's compression of the chiasm. Accordingly, dopamine agonist therapy was continued. During the postpartum follow-up, the patient experienced a resumption of menstrual cycles, normalization of prolactin levels, and a reduction in tumor size. Ultimately, the diagnosis was established as a microprolactinoma, which had enlarged during pregnancy due to a pituitary tumor apoplexy. Although microprolactinomas typically carry a low risk of symptomatic tumor growth during pregnancy, this case emphasizes the critical importance of vigilant clinical monitoring to swiftly detect and manage this rare complication. This instance serves as an educational example of an uncommon event—a microprolactinoma experiencing apoplexy during pregnancy.
1. Introduction
Prolactinomas are tumors derived from pituitary lactotroph cells and represent the most common pituitary adenomas, accounting for 40%–50% of cases. They have a higher incidence in women aged between 25 and 35, with the majority being microprolactinomas (less than 10 mm) [1, 2]. These tumors often manifest as infertility since hyperprolactinemia affects the secretion of gonadotropin-releasing hormone (GnRH), follicle-stimulating hormone (FSH), and luteinizing hormone (LH). Moreover, elevated prolactin levels hinder corpus luteum formation and the secretion of progesterone. All this leads to amenorrhea, infertility, and hypogonadism [2–5]. Effective treatment with dopamine agonists such as bromocriptine and cabergoline can reverse hyperprolactinemia and hypogonadism while promoting a reduction in tumor size in most cases, thus facilitating pregnancy [4–8]. In microprolactinomas, the risk of symptomatic tumor enlargement during pregnancy is very low (2.4%), with minimal growth in the maximum adenoma diameter, typically not exceeding 10 mm. After achieving pregnancy, dopamine agonists are discontinued, and the patient is closely monitored clinically. Pituitary apoplexy during pregnancy is exceedingly rare, with a reported prevalence of 1 in 10,000 pregnancies [9–11]. It presents as an acute clinical syndrome resulting from tumor infarction or hemorrhage, causing a sudden and transient increase in size. Here, we present the case of a patient with a microprolactinoma who experienced significant growth during pregnancy due to intratumoral bleeding (apoplexy).
2. Case Presentation
A 29-year-old woman from Venezuela had a medical history of hyperprolactinemia diagnosed in her country of origin, with no prior imaging or treatment. Since relocating to Uruguay 5 years ago, she has not undergone any follow-up care. She presented herself at another hospital with a 3-year history of amenorrhea and a strong desire for pregnancy. She denied experiencing galactorrhea or headaches and was not taking any medications at the time of consultation. Her prolactin levels were elevated at 157.4 ng/ml (normal reference range: 4.8–23.3 ng/ml). Her liver and renal function, as well as TSH levels, were within normal limits. A pituitary magnetic resonance imaging (MRI) scan revealed a hypointense area on T1-weighted images following gadolinium administration, measuring 3 × 4.6 × 3.7 mm, consistent with a microadenoma. The pituitary stalk showed a slight deviation to the left (Figure 1).
Figure 1.
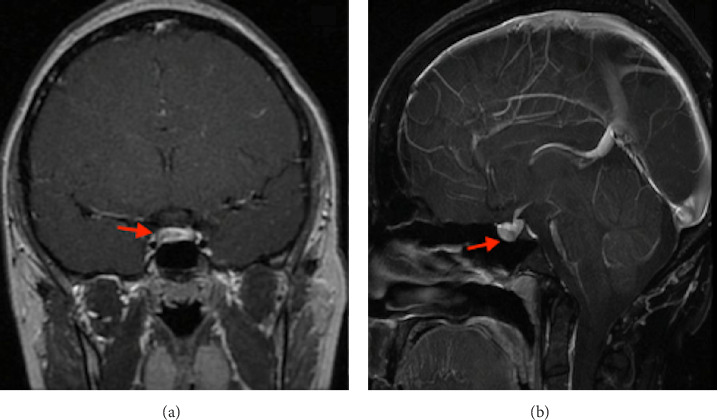
Magnetic resonance imaging, T1 post-gadolinium. (A) Coronal view and (B) Sagittal view. A 3 × 4.6 × 3.7 mm microadenoma is observed (red arrow).
Initial treatment with 5 mg of bromocriptine was initiated; however, her prolactin levels continued to rise, reaching 212 ng/ml. Subsequently, she was transitioned to cabergoline in incremental doses, ultimately reaching 1 mg per week. Pregnancy was successfully achieved with prolactin levels decreasing to 49 ng/ml, leading to the discontinuation of cabergoline. At 17 weeks of gestation, she presented with persistent headaches and altered visual acuity. A confrontational visual field examination revealed left temporal hemianopsia. A pituitary MRI without contrast demonstrated an enlargement of the pituitary adenoma, measuring 12 × 19 × 11 mm, with extension into the suprasellar cistern and compression of the optic chiasm. The pituitary stalk was no longer visible, and there was no evidence of cavernous sinus invasion; however, intratumoral bleeding was observed (Figure 2). The patient was referred to our hospital.
Figure 2.
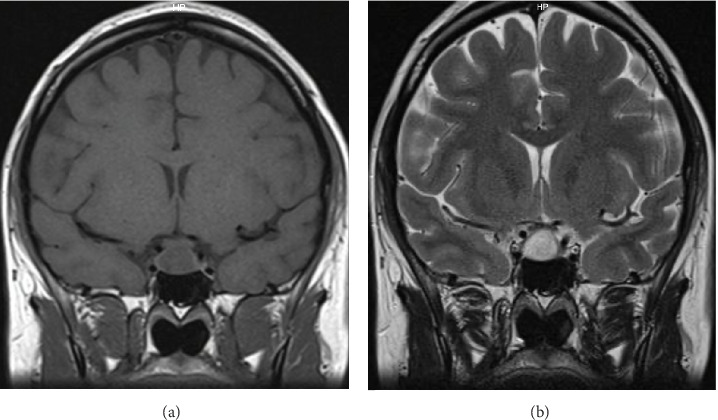
MRI, (A) T1 coronal view without contrast and (B) T2. An increase in tumor size is observed, measuring 12 × 19 × 11 mm. with intratumoral bleeding.
Her initial evaluation at our facility took place at 26 weeks of gestational age. Cortisol and thyroid hormone levels were within normal limits. Treatment with cabergoline was reinstated, with the dosage progressively increased to 2 mg per week. Ophthalmological evaluation confirmed bilateral temporal hemianopsia.
Given the persistence of headaches and the absence of significant improvement in visual acuity, a follow-up MRI scan, conducted without contrast, was scheduled at 35 weeks of gestation. This scan revealed further enlargement of the adenoma to 17 × 21 × 13 mm, accompanied by hemorrhage within the pituitary lesion (Figure 3).
Figure 3.
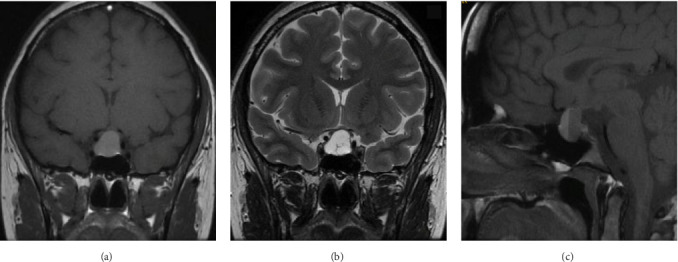
MRI without contrast. (A) T1 coronal view, (B) T2 and (C) T1 sagittal view. An increase in the size of the prolactinoma is observed, measuring 17 × 21 × 13 mm, compressing the chiasm. Presents fluid level and is hyperintense on T1 compatible with bleeding.
Thyroid function tests and cortisol levels remained within the normal range.
In collaboration with the obstetrics team, it was decided to proceed with a scheduled cesarean section upon completion of 39 weeks of gestation. The male newborn had a birth weight of 3686 g, a length of 51 cm, and a head circumference of 36.5 cm, all appropriate for gestational age. No perinatal complications were observed, and they were discharged 72 h after birth. Due to the chiasm compression, breastfeeding was not initiated, and dopamine agonist therapy was continued. Subsequently, the patient resumed regular menstrual cycles, prolactin levels normalized, and there was no evidence of tumor remnant on MRI (Figure 4).
Figure 4.
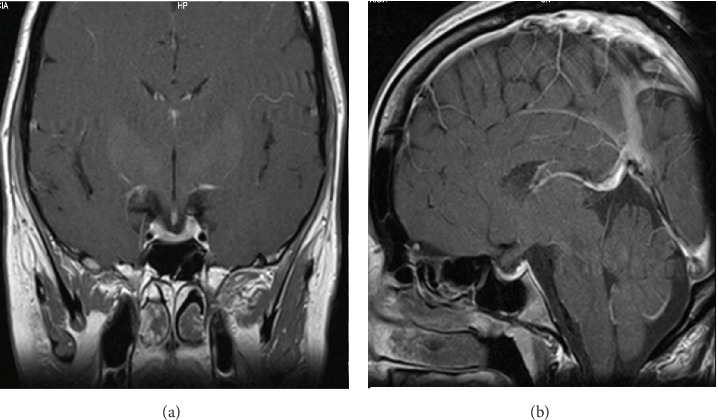
Magnetic resonance imaging, T1 post-gadolinium. (A) Coronal view and (B) Sagittal view. No evidence of the prolactinoma is seen.
3. Discussion
The occurrence of hemorrhage or infarction within a pituitary tumor, known as pituitary apoplexy, represents a rare and potentially life-threatening complication, often carrying serious consequences, including deficiencies in hormonal axes originating from the pituitary gland [2]. The estimated prevalence of this condition is ~6.2 cases per 100,000 individuals in the general population, with a slightly higher occurrence of 1 per 10,000 in pregnant women [9–11]. Within the subgroup of prolactinomas, the prevalence of pituitary apoplexy is roughly 6.8%, with a higher incidence observed in macroprolactinomas (20.3%) compared to microprolactinomas (3.1%) [12].
Pregnancy represents a recognized risk factor for the development of pituitary apoplexy. This heightened risk can be attributed to increased blood flow and hormonal stimulation of the pituitary gland, resulting in an enlargement of both the pituitary gland itself and any preexisting tumors [13]. Furthermore, during pregnancy, the blood supply to the pituitary gland may be compromised due to the expansion of lactotroph cells and the consequent compression of blood vessels [14]. It is a matter of debate whether treatment with dopamine agonists can induce pituitary apoplexy [15]. Other predisposing factors encompass arterial hypertension, diabetes, discontinuation of dopamine agonist therapy, head trauma, use of estrogen therapy, and anticoagulant medications [9].
There have been 48 documented cases of pituitary apoplexy occurring during pregnancy, with only nine of these cases involving patients with microprolactinomas, as in our case (Table 1) [9, 16, 17].
Table 1.
Cases of pituitary apoplexy in microprolactinomas.
| Reference | Age | Parity | Gestational age (weeks) | Adenoma size (mm) | PRL at diagnosis (ng/ml) | PRL before pregnancy (ng/ml) |
Prior DA treatment | Clinical presentation | Therapeutic management | Birth | Lactation | Post partum treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gondim et al. [22] | 29 | 1 | 30 | 3 × 4 | 177 | 5 | BC | Headache, decreased AV LE: central scotoma RE: Ptosis, diplopia, 3rd CN paralysis |
BC for 2 weeks, then TSS, T4, HC |
VD, term, healthy NB | No data | Did not require |
|
| ||||||||||||
| Couture et al. [23] | 37 | 1 | 16 | 7 × 7 | 105 | 35.4 | CB | Headache, vomiting, blurred vision VF: normal |
CB (up to 4 weeks after birth) | CS, term, healthy NB | Yes | Did not require |
|
| ||||||||||||
| Hayes et al. [24] | 41 | 1 | 18 | 6 × 9 | 61a | No data | CB | Headache, decreased VA VF: bitemporal deficit |
TSS, HC | VD, term, healthy NB | Yes (2 weeks) | Did not require |
|
| ||||||||||||
| Annamalai et al. [25] | 25 | 1 | 37 | 4 | 96 | No data | CB | Headache VF: normal |
CB, HC | CS, term, healthy NB | Yes (CB suspended) | Did not require |
|
| ||||||||||||
| Kuhn et al. [16] | 31 | 2 | 36 | No data | 149 | 17 | CB | Headache, photophobia VF: hemianopsia bitemporal |
CB, TSS | CS, term, healthy NB | No data | No data |
|
| ||||||||||||
| Kuhn et al. [16] | 21 | 1 | 26 | 7 | 80 | No data | BC | Fatigue, Polyurodipsia VF: normal |
T4, HC, Desmopressin | VD, healthy NB | No data | TSS Desmopressin (DA) |
|
| ||||||||||||
| Kuhn et al. [16] | 32 | 3 | 33 | 10 | 240 | No data | CB | Headache, decreased VA in LE | CB, HC | PV, term, healthy NB | No | CB, HC (SAI) |
|
| ||||||||||||
| Kuhn et al. [16] | 23 | 1 | 16 | 5 | 160 | No data | CB | Headache, VF: quadrantopsia RE |
CB | CS, term, healthy NB | Yes | TSS |
|
| ||||||||||||
| Kuhn et al. [16] | 25 | 2 | 24 | No data | No data | No data | CB | Headache, VF: no data |
CB | VD, healthy NB | No | TSS |
|
| ||||||||||||
| Our case | 29 | 1 | 16 | 3 × 4, 6 × 3.7 | 157.4 | 49 | CB | Headache VF: temporal hemianopsia LE |
CB | Cs, term, healthy | No | CB |
Abbreviations: BC, bromocriptine; CB, cabergoline; CS, caesarean section; DA, dopamine agonist; HC, hydrocortisone; LE, left eye; NB, new born; RE, right eye; SAI, secondary adrenal insufficiency; T4, levothyroxine; TSS, transsphenoidal surgery; VA, visual acuity; VD, vaginal delivery; VF, visual field.
a1300 mUI/L.
Notably, it has been reported that pituitary apoplexy tends to occur more frequently during the second and third trimesters of pregnancy [9, 16]. In all instances involving microprolactinomas, dopamine agonists were discontinued upon pregnancy confirmation. The most common initial symptoms were sudden severe headaches, often followed by visual disturbances (present in seven cases of microprolactinomas, including our case).
In cases of severe and intractable headaches, it is advisable to perform an MRI without contrast and hormonal studies to assess for potential pituitary hormone deficiencies. In our patient's case, despite the detection of pituitary hemorrhage, other pituitary hormonal axes remained unaffected, as indicated by normal cortisol and free thyroxin levels, obviating the need for exogenous supplementation.
In situations where neurological status deteriorates, surgical decompression may become necessary, typically during the second trimester [2, 5]. Presently, there are no established clinical guidelines for the management of pituitary apoplexy during pregnancy. Optimal management should be guided by a multidisciplinary team, including an endocrinologist, ophthalmologist, and neurosurgeon, and tailored to the patient's individual characteristics [13]. The primary objective is to alleviate symptoms and relieve compression of the optic pathway.
In the United Kingdom, guidelines for managing pituitary apoplexy recommend surgical intervention in cases exhibiting significant ophthalmic signs or a decreased level of consciousness. Additionally, they propose the initiation of steroid therapy empirically for hemodynamically unstable patients and consider steroid therapy for stable patients with morning cortisol levels below 19 mcg/dl [18]. Surgical procedures during pregnancy have been documented in 12 cases (48%), while the remaining cases, including ours, were managed conservatively. Conservative management typically includes the use of dopamine agonists in eight cases and hormone replacement therapy when deemed necessary. Importantly, most reported cases, including ours, culminated in full-term pregnancies, with all newborns exhibiting good health [9, 16].
Regarding the management of microprolactinomas during pregnancy, current guidelines recommend discontinuing treatment with dopamine agonists upon confirming pregnancy. This is due to the fact that these drugs can cross the placental barrier, potentially exposing the developing fetus to their effects [1]. Among the available dopamine agonists, bromocriptine has undergone extensive safety studies and has demonstrated a favorable safety profile. Studies have reported rates of spontaneous abortions, fetal malformations, and alterations in postnatal development comparable to those observed in healthy women [2, 4]. Cabergoline, which boasts a longer half-life, also appears to be safe for use during pregnancy. However, there is a relative paucity of studies compared to bromocriptine regarding its safety in pregnancy.
A recent study conducted in Brazil, involving 223 pregnancies in patients with prolactinomas, reported a spontaneous abortion rate of 11%, a figure similar to that in the general population. Interestingly, the study revealed higher rates of spontaneous abortion in patients who continued cabergoline treatment after pregnancy detection compared to those who discontinued treatment (38% vs., 7.5%) [8]. Notably, there were no significant differences in terms of other maternal–fetal outcomes. Importantly, current evidence does not suggest harm to fetuses exposed to dopamine agonists [1, 2]. However, it is worth noting that more research is needed to comprehensively assess the safety of dopamine agonists during pregnancy.
Regarding the follow-up of prolactinomas during pregnancy, clinical monitoring of patients in each trimester is recommended. Measurement of prolactin levels during pregnancy is not advisable since physiologically, these levels can increase up to 10 times the normal range, with levels at the end of pregnancy reaching ~200 ng/ml [1, 2, 6]. This physiological increase is attributed to the proliferation of lactotroph cells and their hyperplasia, which leads to an enlargement of the pituitary gland, with dimensions reaching up to 11.8 mm at the end of pregnancy and immediately postpartum. This hormonal surge is mediated by placental estrogen stimulating the mitotic activity of lactotroph cells and the synthesis of prolactin [5, 6].
Routine MRI studies are also not recommended unless the patient presents with symptoms suggestive of adenoma growth, such as headaches or impaired visual acuity, as symptomatic growth of microprolactinomas is uncommon during pregnancy [1, 3]. The risk of a significant increase in tumor size is low, with complications in microprolactinomas, such as headaches or compression of the optic chiasm or pituitary stalk, occurring in less than 5% of cases [5, 6]. In contrast, macroprolactinomas carry a higher risk of symptomatic growth, with rates of up to 30% [4, 5].
In cases where clinical suspicion arises regarding tumor growth, an MRI without contrast should be performed. If tumor growth is confirmed, treatment with bromocriptine is typically recommended [1, 4]. In our specific case, cabergoline was reinstated as the patient had not responded satisfactorily to bromocriptine prior to pregnancy. Importantly, current literature does not indicate any additional harm to the fetus in such cases. If, following the restart of dopamine agonist treatment, the tumor size fails to decrease or symptoms due to mass effect persist, surgical intervention in the second trimester is a viable option, or delivery may be considered if the pregnancy is near term [1, 10].
Regarding breastfeeding, it is considered safe for patients who are not receiving dopamine agonists. However, it is not recommended for patients with prolactinomas that compress the optic chiasm or in cases where the use of dopamine agonists during pregnancy has been deemed necessary, as was the case in our situation [4, 5]. Following childbirth, prolactin levels decline rapidly, although they remain elevated during the lactation period. Importantly, breastfeeding has not been associated with an increase in tumor size, despite the fact that nipple stimulation can lead to increased prolactin secretion [5].
A recommended follow-up includes prolactin and MRI studies 2 months postpartum [4]. Interestingly, pregnancy has been shown to induce remission of hyperprolactinemia in microprolactinomas, likely due to autoinfarction of the tumor [19, 20]. In cases where remission is achieved, and dopamine agonist treatment is discontinued, regular monitoring is essential for at least 5 years [3]. Factors favoring remission include older maternal age, lower prolactin levels at diagnosis and postpartum, and smaller adenoma size at diagnosis [21].
4. Conclusion
While the likelihood of experiencing a symptomatic increase in tumor size in microprolactinomas during pregnancy is notably low, it remains imperative to maintain clinical follow-up to promptly detect and assess this potential complication. Furthermore, this case serves as a noteworthy illustration of a rare occurrence: a prolactinoma apoplexy during pregnancy.
Acknowledgments
During the preparation of this manuscript, the author(s) utilized ChatGPT to enhance the readability and language of the text. Following its use, the author(s) thoroughly reviewed and refined the content to ensure its accuracy and appropriateness, taking full responsibility for the final published version of the article.
Data Availability Statement
All relevant data underlying the findings of this case report are included within the article. No additional source data are available. The case information has been appropriately anonymized to protect patient confidentiality in accordance with ethical guidelines.
Ethics Statement
The patient provided written informed consent for the publication of this case and any related images, as well as for the publication of indirectly identifiable data (age, gender, and medical history).
Disclosure
No persons or third-party services were involved in the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Lucia Introini drafted the manuscript. Maria M. Pineyro contributed to the writing. Lucia Introini, Jenifer Silva, Mariana Risso, Beatriz Mendoza, and Maria M. Pineyro contributed to acquiring clinical data, agreed with the manuscript's results and conclusions. Lucia Introini and Maria M. Pineyro made critical revisions and approved the final version. All authors revised and approved the final manuscript and agreed to be accountable for the content and conclusion of the article
Funding
There was no funding for this manuscript.
References
- 1.Melmed S., Casanueva F. F., Hoffman A. R., et al. Diagnosis and Treatment of Hyperprolactinemia: An Endocrine Society Clinical Practice Guideline, the. Journal of Clinical Endocrinology & Metabolism . 2011;96(2):273–288. doi: 10.1210/jc.2010-1692. [DOI] [PubMed] [Google Scholar]
- 2.Galvão A., Gonçalves D., Moreira M., Inocêncio G., Silva C., Braga J. Prolactinoma and Pregnancy - a Series of Cases Including Pituitary Apoplexy. Journal of Obstetrics and Gynaecology . 2017;37(3):284–287. doi: 10.1080/01443615.2016.1233946. [DOI] [PubMed] [Google Scholar]
- 3.Yalin G. Y., Dogansen S. C., Yarman S. Review of Clinical Recommendations on Prolactinoma and Pregnancy. The Turkish Journal of Endocrinology and Metabolism . 2018;22(1):54–56. doi: 10.25179/tjem.2017-57578. [DOI] [Google Scholar]
- 4.Valassi E. Pituitary Disease and Pregnancy. Endocrinología, Diabetes y Nutrición . 2021;68(3):184–195. doi: 10.1016/j.endinu.2020.07.007. [DOI] [Google Scholar]
- 5.Synder P. J. Management of Lactotroph Adenoma (Prolactinoma) Before and During Pregnancy. In: Connor R. F., editor. Up To Date . Wolkers Kluker; [Google Scholar]
- 6.Naliato E. C. O., Neiva G., Palhano R., Violante A. H. D. Prolactinoma and Gestation: A Reality. Critical Care Obstetrics and Gynecology . 2018;04(3):p. 10. doi: 10.21767/2471-9803.1000163. [DOI] [Google Scholar]
- 7.Huang W., Molitch M. E. Pituitary Tumors in Pregnancy. Endocrinology and Metabolism Clinics of North America . 2019;48(3):569–581. doi: 10.1016/j.ecl.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Anna B. G. S, Musolino N. R. C., Gadelha M. R., et al. A Brazilian Multicentre Study Evaluating Pregnancies Induced by Cabergoline in Patients Harboring Prolactinomas. Pituitary . 2020;23(2):120–128. doi: 10.1007/s11102-019-01008-z. [DOI] [PubMed] [Google Scholar]
- 9.Oguz S. H., Soylemezoglu F., Dagdelen S., Erbas T. A Case of Atypical Macroprolactinoma Presenting With Pituitary Apoplexy During Pregnancy and Review of the Literature. Gynecological Endocrinology . 2020;36(2):109–116. doi: 10.1080/09513590.2019.1650339. [DOI] [PubMed] [Google Scholar]
- 10.Glezer A., Bronstein M. D. Prolactinomas in Pregnancy: Considerations Before Conception and During Pregnancy. Pituitary . 2020;23(1):65–69. doi: 10.1007/s11102-019-01010-5. [DOI] [PubMed] [Google Scholar]
- 11.Orrego A., Jácome A. Diagnóstico y Manejo de la Apoplejía Hipofisaria. Revista Colombiana de Endocrinología, Diabetes & Metabolismo . 2019;6(3):188–197. doi: 10.53853/encr.6.3.533. [DOI] [Google Scholar]
- 12.Sarwar K. N., Huda MSB., Van de Velde V., et al. The Prevalence and Natural History of Pituitary Hemorrhage in Prolactinoma. The Journal of Clinical Endocrinology & Metabolism . 2013;98(6):2362–2367. doi: 10.1210/jc.2013-1249. [DOI] [PubMed] [Google Scholar]
- 13.Khaldi S., Saad G., Elfekih H., et al. Pituitary Apoplexy of a Giant Prolactinoma During Pregnancy. GynecolEndocrinol . 2021;37(9)863 [Google Scholar]
- 14.deHeide L. J. M., van Tol K. M., Doorenbos B. Pituitary Apoplexy Presenting during Pregnancy. Netherlands Journal of Medicine . 2004:62393–62396. [Google Scholar]
- 15.Briet C., Salenave S., Bonneville J.-F.çois, Laws E. R., Chanson P. Pituitary Apoplexy. Endocrine Reviews . 2015;36(6):622–645. doi: 10.1210/er.2015-1042. [DOI] [PubMed] [Google Scholar]
- 16.Kuhn E., Weinreich A. A., Biermasz N. R., Jorgensen J. O. L., Chanson P. Apoplexy of Microprolactinomas During Pregnancy: Report of Five Cases and Review of the Literature. European Journal of Endocrinology . 2021;185(1):99–108. doi: 10.1530/EJE-21-0145. [DOI] [PubMed] [Google Scholar]
- 17.Gheorghe A. M., Trandafir A. I., Stanciu M., Popa F. L., Nistor C., Carsote M. Challenges of Pituitary Apoplexy in Pregnancy. Journal of Clinical Medicine . 2023;12(10) doi: 10.3390/jcm12103416.3416 [DOI] [Google Scholar]
- 18.Rajasekaran S., Vanderpump M., Baldeweg S., et al. UK Guidelines for the Management of Pituitary Apoplexy. Clinical Endocrinology . 2011;74(1):9–20. doi: 10.1111/j.1365-2265.2010.03913.x. [DOI] [PubMed] [Google Scholar]
- 19.Colao A., Di Sarno A., Cappabianca P., Di Somma C., Pivonello R., Lombardi G. Withdrawal of Long-Term Cabergoline Therapy for Tumoral and Nontumoral Hyperprolactinemia. New England Journal of Medicine . 2003;349(21):2023–2033. doi: 10.1056/NEJMoa022657. [DOI] [PubMed] [Google Scholar]
- 20.Auriemma R. S., Perone Y., Di Sarno A., et al. Results of a Single-Center Observational 10-Year Survey Study on Recurrence of Hyperprolactinemia After Pregnancy and Lactation. The Journal of Clinical Endocrinology & Metabolism . 2013;98(1):372–379. doi: 10.1210/jc.2012-3039. [DOI] [PubMed] [Google Scholar]
- 21.O’Sullivan S. M., Farrant M. T., Ogilvie C. M., Gunn A. J., Milsom S. R. An Observational Study of Pregnancy and Post-Partum Outcomes in Women With Prolactinoma Treated With Dopamine Agonists. Australian and New Zealand Journal of Obstetrics and Gynaecology . 2020;60(3):405–411. doi: 10.1111/ajo.13070. [DOI] [PubMed] [Google Scholar]
- 22.Gondim J., Ramos Júnior F., Pinheiro I., Schops M., TellaJúnior O. I. Minimally Invasive Pituitary Surgery in a Hemorrhagic Necrosis of Adenoma During Pregnancy. Minimally Invasive Neurosurgery . 2003;46(3):173–176. doi: 10.1055/s-2003-40734. [DOI] [PubMed] [Google Scholar]
- 23.Couture N., Aris-Jilwan N., Serri O. Apoplexy of a Microprolactinoma During Pregnancy: Case Report and Review of Literature. Endocrine Practice . 2012;18(6):e147–e150. doi: 10.4158/EP12106.CR. [DOI] [PubMed] [Google Scholar]
- 24.Hayes A. R., O’Sullivan A. J., Davies M. A. A Case of Pituitary Apoplexy in Pregnancy. Endocrinology, Diabetes & Metabolism Case Reports . 2014;2014 doi: 10.1530/EDM-14-0043.140043 [DOI] [Google Scholar]
- 25.Annamalai A. K., Jeyachitra G., Jeyamithra A., Ganeshkumar M., Srinivasan K. G., Gurnell M. Gestational Pituitary Apoplexy. Indian Journal of Endocrinology and Metabolism . 2017;21(3):484–485. doi: 10.4103/ijem.IJEM_8_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data underlying the findings of this case report are included within the article. No additional source data are available. The case information has been appropriately anonymized to protect patient confidentiality in accordance with ethical guidelines.


