Abstract
Insulin regulates glucose uptake into fat and muscle by modulating the distribution of the GLUT4 glucose transporter between the surface and interior of cells. The GLUT4 trafficking pathway overlaps with the general endocytic recycling pathway, but the degree and functional significance of the overlap are not known. In this study of intact adipocytes, we demonstrate, by using a compartment-specific fluorescence-quenching assay, that GLUT4 is equally distributed between two intracellular pools: the transferrin receptor-containing endosomes and a specialized compartment that excludes the transferrin receptor. These pools of GLUT4 are in dynamic communication with one another and with the cell surface. Insulin-induced redistribution of GLUT4 to the surface requires mobilization of both pools. These data establish a role for the general endosomal system in the specialized, insulin-regulated trafficking of GLUT4. Trafficking through the general endosomal system is regulated by rab11. Herein, we show that rab11 is required for the transport of GLUT4 from endosomes to the specialized compartment and for the insulin-induced translocation to the cell surface, emphasizing the importance of the general endosomal pathway in the specialized trafficking of GLUT4. Based on these findings we propose a two-step model for GLUT4 trafficking in which the general endosomal recycling compartment plays a specialized role in the insulin-regulated traffic of GLUT4. This compartment-based model provides the framework for understanding insulin-regulated trafficking at a molecular level.
INTRODUCTION
Insulin plays a major role in regulating the disposal of dietary glucose by modulating glucose uptake into muscle and fat. In the basal state glucose uptake into these cells is low, and insulin stimulates a rapid and reversible increase in glucose uptake of 5–15-fold (Czech, 1995). Insulin modulates glucose uptake by regulating the distribution of GLUT4, a glucose transporter isoform primarily expressed in fat and muscle, between the interior and surface of cells (Cushman and Wardzala, 1980; Suzuki and Kono, 1980; for reviews see, Czech and Corvera, 1999; Pessin et al., 1999; Simpson et al., 2001). In the absence of insulin, GLUT4 is predominantly localized to intracellular compartments because it is internalized ∼10 times as rapidly as it is returned to the cell surface. Insulin increases the recycling of GLUT4 without increasing internalization, thereby resulting in a net shift in the distribution of GLUT4 to the cell surface (Holman and Cushman, 1994). Although the phenomenon of GLUT4 retention and redistribution is well established, the intracellular GLUT4 retention compartment(s) have not been characterized in detail, and the trafficking step(s) modulated by insulin have not been identified. GLUT4-containing vesicles have been isolated and the protein composition has been determined biochemically (Cain et al., 1992; Thoidis et al., 1993; Kandror and Pilch, 1994a; Mastick et al., 1994; Kandror et al., 1995; Morris et al., 1998). In more recent studies, proteins in GLUT4-containing vesicles have been analyzed using immunoelectron microscopy (Ramm et al., 2000). Finally, it has been shown that ∼50% of the GLUT4 in low-density membrane fraction of fractionated adipocytes is segregated from the transferrin receptor (TR) (Livingstone et al., 1996; Martin et al., 1996). In sum these studies indicate a significant overlap between specialized GLUT4 vesicles and proteins found in endosomes (for example, TR) and proteins that traffic between endosomes and the trans-Golgi network (TGN) (mannose 6 phosphate receptor). These important studies however do not address the dynamic overlap between specialized GLUT4-containing compartments and other intracellular compartments nor do they address the functional significance of multiple GLUT4 compartments.
The insulin-regulated amino peptidase (IRAP) is the only other protein known to traffic like GLUT4 (Kandror and Pilch, 1994b; Keller et al., 1995; Ross et al., 1996). IRAP is expressed in fat and muscle, where it is predominately located intracellularly in compartments that contain GLUT4 (Malide et al., 1997; Martin et al., 1997; Sumitani et al., 1997; Garza and Birnbaum, 2000). Insulin induces a large redistribution of IRAP to the cell surface, indicating that GLUT4 and IRAP are cargo proteins of the insulin-regulated membrane trafficking pathway. Unlike GLUT4, IRAP is expressed in tissues other than fat and muscle (Keller et al., 1995). The physiological role of IRAP is unknown, and therefore the significance of IRAP's expression in many tissues is not clear.
We have analyzed insulin-regulated trafficking using a chimera between IRAP and the human TR (Johnson et al., 1998). This chimera, vpTR, contains the cytoplasmic domain of IRAP fused to the transmembrane and extracellular sequences of the TR. In 3T3-L1 adipocytes vpTR is trafficked by the insulin-regulated pathway (Subtil et al., 2000). Recent data demonstrate that the specialized traffic of IRAP and GLUT4 is not restricted to differentiated cell types (Johnson et al., 1998; Lampson et al., 2000, 2001; Bogan et al., 2001). The insulin-regulated trafficking pathway in undifferentiated cells is, in general terms, similar to the pathway in fat cells. In undifferentiated cells GLUT4 and vpTR are recycled at ∼1/10th the rate at which they are internalized, and insulin specifically stimulates their recycling back to the cell surface (Johnson et al., 1998; Lampson et al., 2000, 2001; Bogan et al., 2001). The net effect of insulin on the distribution of GLUT4 and IRAP in undifferentiated cells is smaller (∼3-fold increase) than in differentiated cells (Lampson et al., 2000). The more modest effect of insulin on specialized trafficking in undifferentiated cells could be due to differences in the retention mechanism, or in the signal transduction pathway, or differences in both.
In studies of Chinese hamster ovary (CHO) cells we have shown that GLUT4 and vpTR are returned to the cell surface directly from the TR-containing endosomal recycling compartment. In these cells GLUT4 and vpTR return to the plasma membrane more slowly than the TR because they are transported to the cell surface in specialized vesicles. GLUT4 and IRAP are sorted from the TR-containing general endosomal system at the late step of formation of recycling vesicles that mediate transport to the plasma membrane (Lampson et al., 2001). This is in contrast to differentiated cells, in which there is evidence for a distinct GLUT4/IRAP recycling compartment (Livingstone et al., 1996; Martin et al., 1996).
In this report, to further understand the relationship between the endosomal system and the insulin-regulated pathway in insulin target cells, we use quantitative fluorescence microscopy and horseradish peroxidase (HRP)-mediated fluorescence quenching to compare the trafficking of GLUT4 and the TR in 3T3-L1 adipocytes. We find in intact adipocytes that a fraction of GLUT4 is segregated to a specialized endosomal compartment that excludes the TR. The increased intracellular retention of GLUT4 and the increased magnitude of insulin-induced translocation in adipocytes, relative to undifferentiated cells, both correlate with the formation of the specialized compartment. These data indicate that a key step in establishing the specialized insulin response of adipocytes is the sorting of GLUT4 from the general endocytic recycling compartment. We propose a model in which there are two slow, insulin-regulated trafficking steps in adipocytes: transport of GLUT4 from the TR-endosomal compartment to a specialized compartment, and transport from this specialized compartment back to the cell surface.
The GTPase rab11 regulates traffic from the TR-containing endocytic recycling compartment (Ullrich et al., 1996; Chen et al., 1998; Sonnichsen et al., 2000). Rab11 function is required in the trafficking of the TR from the general endosomal recycling compartment to the cell surface as well as in traffic between endosomes and the TGN (Ren et al., 1998; Wilcke et al., 2000). We find that rab11 function is required for sorting of GLUT4 from TR-containing endosomes to the specialized compartment in adipocytes. Failure to sort GLUT4 to the specialized compartment results in a blunting of insulin-induced translocation to the cell surface, emphasizing that transport from the endosomes is a key step in the insulin-induced translocation of GLUT4 to the surface of adipocytes.
MATERIALS AND METHODS
Ligands and Chemicals
Human transferrin (Tf) was purchased from Sigma-Aldrich (St. Louis, MO) and further purified by Sephacryl S-300 gel filtration. HRP was conjugated to iron-loaded Tf as described previously (Mayor et al., 1998). An anti-hemagglutinin (HA) monoclonal antibody (mAb) (HA.11) was purchased from Berkley Antibody (Richmond, CA). A rabbit anti-glutathione S-transferase (GST) polyclonal antibody (Z-5) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). B3/25, an anti human TR mAb was purchased from Roche Applied Science (Indianapolis, IN). Tf and the anti-HA antibody were labeled with fluorescent dye Cy3 (Biological Detection Systems, Pittsburgh, PA) according to the manufacturer's instructions.
Electroporation
DNA coding for the human TR and vpTR were subcloned into a pCMV vector (Johnson et al., 1998). Construction of HA-GLUT4-GFP was described previously (Dawson et al., 2001). The rab11BP(334–504) construct was created using standard recombinant cloning procedures. 3T3-L1 cells were cultured in DMEM with 10% calf serum and penicillin-streptomycin. Cells were differentiated as described previously (Subtil et al., 2000). Four days after beginning differentiation, cells were electroporated at 180 V and 950 μF with 45 μg of plasmid DNA and plated onto coverslip-bottom dishes (Kanzaki and Pessin, 2001). Cells were used 24 h after electroporation. For each plasmid, 45 μg was used when cells were electroporated with multiple constructs.
Down-Regulation of Endogenous Mouse TR
The mouse TR of 3T3-L1 adipocytes was down-regulated by incubating cells for 18 h with a rat mAb that recognizes the extracellular domain of the mouse TR (Subtil et al., 2000). Neither the human TR nor vpTR is down-regulated by this treatment.
Fluorescence-quenching Assay
The HRP-mediated fluorescence quenching assay has been described previously (Mayor et al., 1998; Lampson et al., 2001). 3T3-L1 adipocytes were electroporated with HA-GLUT4-GFP and either vpTR or TR 24 h before the experiment. On the day of the experiment, cells were incubated with 170 nM insulin in DMEM medium with 220 mM bicarbonate and 20 mM HEPES pH 7.4 (med 1) for 30 min to increase recycling of HA-GLUT4-GFP. The cells were washed with 150 mM NaCl, 20 mM HEPES, 1 mM CaCl2, 5 mM KCl, and 1 mM MgCl2 pH 7.4 (med 2), incubated for 3 min with 200 mM NaCl, 50 mM 2-(N-morpholino)ethanesulfonic acid pH 5.0, and washed three times with med 2 to remove insulin. The cells were incubated for 4 h with Cy3-labeled anti-HA antibody, 20 μg/ml HRP-Tf, and 1 mg/ml ovalbumin in med 1. During this 4-h incubation the HA-GLUT4-GFP was bound by the anti-HA antibody. The cells also return to the basal state during this period. Control cells were incubated for the same period of time with the anti-HA antibody but without the HRP-Tf. After this incubation the cells were chilled to 4°C and washed twice with med 2. Surface-bound HRP-Tf was removed by incubating cells for 5 min in ice-cold citrate buffer (20 mM sodium citrate and 150 mM NaCl, pH 5.0), with one exchange of buffer, followed by two 5-min washes with ice-cold med 2. For the quenching reaction, cells were incubated with 250 μg/ml diaminobenzidine (DAB) and 0.0025% H2O2 in the dark for 30 min on ice, followed by two washes with ice-cold med 2. Finally, cells were fixed for 20 min with 3.7% formaldehyde in med 2.
To establish that HRP restricted to the surface of cells can quench fluorescence on the plasma membrane, 3T3-L1 adipocytes, electroporated with HA-GLUT4-GFP and the TR, were incubated in serum-free media for 3 h, stimulated with 170 nM insulin for 20 min, and chilled to 4°C. The cells were incubated with Cy3-anti-HA antibody on ice with or without HRP-Tf for 1 h. The cells were washed with med 2 (4°C) to remove antibody and Tf-HRP that had not bound to the cells (surface HA-GLUT4-GFP and TR, respectively). The cells were incubated with DAB and H2O2 for 30 min at 4°C. In these experimental conditions the Cy3-anti-HA antibody and the HRP-Tf are restricted to the surface of cells.
Fluorescence microscopy was performed with a DMIRB inverted microscope (Leica, Deerfield, IL), with a cooled charge-coupled device camera (Princeton Instruments, Trenton, NJ). Images were collected with a 40 × 1.25 numerical aperture oil immersion objective. MetaMorph software (Universal Imaging, West Chester, PA) was used for image processing and quantification. Cells expressing HA-GLUT4-GFP were selected manually based upon green fluorescent protein (GFP) fluorescence. The total GFP and Cy3 fluorescence intensity per cell was calculated, and the average fluorescence intensity per pixel was determined by dividing the total intensity by the area of the cell measured in pixels. To correct for background fluorescence the same measurements were made for cells that did not express HA-GLUT4-GFP. The background fluorescence intensity per pixel (for both the Cy3 and GFP fluorescence) were subtracted from the experimental data. The Cy3/GFP ratio was calculated for each cell and averaged over multiple cells for each experiment.
Recycling Assay
Recycling of TR and vpTR was measured as described previously (Lampson et al., 2001). Time points of 0 and 60 min for TR, and 0 and 120 min for vpTR were used because the vpTR is more slowly recycled than the TR. To test rab11 function cells were coelectroporated with rab11BP(334–504) and either TR or vpTR.
Translocation Assay
The insulin-stimulated translocation was measured as described previously (Lampson et al., 2000).
RESULTS
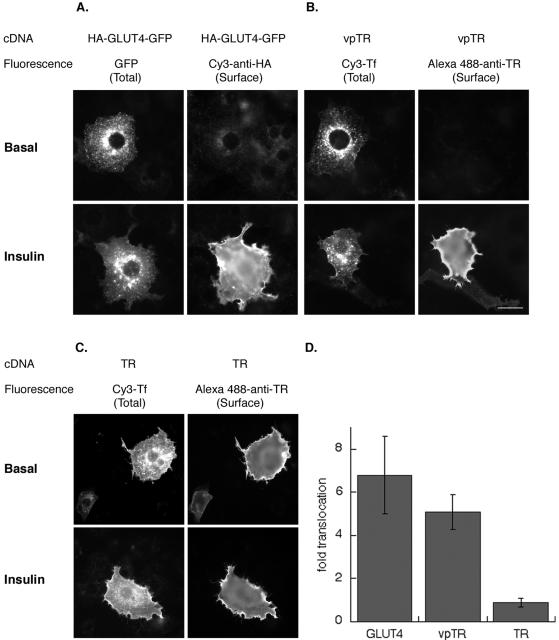
In CHO cells, GLUT4 and IRAP are trafficked by an insulin-responsive recycling pathway that is kinetically distinct from the general endocytic recycling pathway yet does not involve traffic through a specialized insulin-regulated compartment (Lampson et al., 2001). In this study we used the TR as a marker of general trafficking, and vpTR and HA-GLUT4-GFP, a GLUT4 construct with an HA epitope in the first exofacial loop and a GFP fused to GLUT4's cytoplasmic C terminus, as reporters for insulin-regulated traffic (Lampson et al., 2000). To extend this analysis to adipocytes, we transiently transfected 3T3-L1 adipocytes, by electroporation, with the cDNAs of these reporters and determined the effect of insulin on their distributions by using a quantitative single-cell fluorescence assay (Lampson et al., 2001). In HA-GLUT4-GFP, GFP fluorescence is a measure of total expression of the construct, and the amount of HA-GLUT4-GFP on the surface of intact cells is determined with a fluorescent-labeled antibody against the HA epitope (Figure 1A). In the basal state HA-GLUT4-GFP was mainly localized to the peri-centriolar region, with some HA-GLUT4-GFP distributed in punctuate structures throughout the cell. HA-GLUT4-GFP was predominantly sequestered intracellularly in the absence of insulin. The distribution of the HA-GLUT4-GFP was identical to the distribution of endogenous GLUT4 in 3T3-L1 adipocytes. There was a dramatic increase in HA-GLUT4-GFP expression on the surface of cells incubated with 170 nM insulin for 15 min. This increase in surface expression of GLUT4 was most clear in the anti-HA epitope staining; however, the redistribution to the surface was also evident as an increase in diffuse GFP fluorescence (Figure 1A).
Figure 1.
Insulin stimulated translocation of HA-GLUT4-GFP, vpTR, and the TR in 3T3-L1 adipocytes. Cells were transiently transfected by electroporation with the cDNAs of these reporters, and the effect of insulin (170 nM for 15 min) on their distribution was determined using a quantitative single-cell fluorescence assay. (A) Example of cells expressing HA-GLUT4-GFP. GFP fluorescence is a measure of total expression. The amount of HA-GLUT4-GFP on the surface is determined with a fluorescent antibody against the HA-epitope in fixed unpermeabilized cells. (B) Example of cells expressing vpTR or the TR (C). The total amount of the constructs expressed was determined by incubating cells with Cy3-Tf for 4 h at 37°C. Where noted, insulin was added to half of the samples for the last 15 min. The amount of the constructs on the surface was detected with an antibody against the extracellular domain of the human TR. Bar, 30 μm. (D) Summary of the translocation measured in five independent experiments (mean ± SEM). In each experiment the data from ∼20 cells per condition were quantified. The insulin-induced translocation was quantified by comparing surface-to-total ratio in the presence and absence of insulin. The surface-to-total ratio normalizes the data for the level of each construct expressed.
To compare the behaviors of vpTR and the TR in response to insulin, cells were incubated with Cy3-Tf for 4 h at 37°C to label the total cycling pool of vpTR or TR (Subtil et al., 2000). Insulin was added to half the samples for the last 15 min of incubation. The Cy3-Tf fluorescence is a measure of the total expression per cell of vpTR or TR. After fixation, vpTR or TR on the surface of intact cells was stained using an antibody against the extracellular domain of the human TR (Lampson et al., 2000). In the absence of insulin, vpTR, like HA-GLUT4-GFP, was concentrated in the peri-centriolar region of cells and very little was detected on the surface (Figure 1B). After treatment with insulin, vpTR was dramatically redistributed to the surface. TR was also concentrated in the peri-centriolar region of cells, yet unlike HA-GLUT4-GFP and vpTR, a significant fraction of the TR was on the surface in the absence of insulin and the effect of insulin on its distribution was much less pronounced (Figure 1C).
The insulin-induced translocation was quantified by comparing the surface fluorescence to the total fluorescence measured in the presence and absence of insulin. The surface-to-total ratio normalizes the surface expression data from individual cells to the total amount expressed in that cell. A summary of a number of independent translocation experiments demonstrates that insulin induced a large redistribution of HA-GLUT4-GFP and vpTR to the cell surface in adipocytes, whereas it had a negligible effect on the TR (Figure 1D). These results document the validity of the quantitative single-cell fluorescence microscopy assay for studies of insulin-regulated trafficking in 3T3-L1 adipocytes.
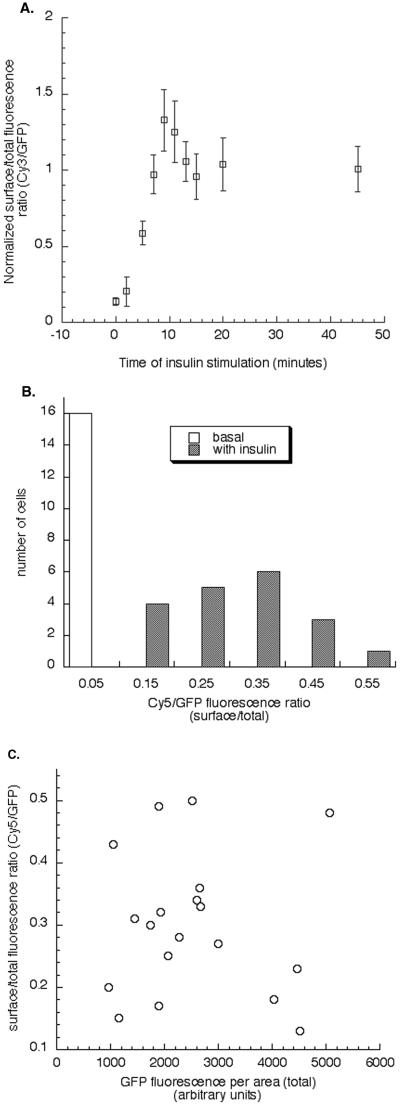
To establish the time required to reach a new steady state in the presence of insulin, we measured surface expression as a function of time in the presence of insulin (Figure 2A). We found that the amount of GLUT4 on the surface at 10 min after insulin stimulation was ∼40% higher than in the insulin steady state, which was reached at ∼15 min after insulin treatment. A similar “overshoot” in GLUT4 surface expression has been reported previously (Bogan et al., 2001). In all studies we use 15-min incubation for analysis of GLUT4 behavior in the presence of insulin.
Figure 2.
Analysis of single cell data. (A) Analysis of the extent of translocation of HA-GLUT4-GFP to the cell surface of day 5 differentiated 3T3-L1 cells stimulated with insulin for various lengths of time. 3T3-L1 cells were electroporated with HA-GLUT4-GFP cDNA and were stimulated with 170 nM insulin for 0 (basal), 2, 5, 7, 9, 11, 13, 15, 20, and 45 min. The proportion of surface HA-GLUT4-GFP was determined after each time point as described in MATERIALS AND METHODS. The data from 20 individual cells is averaged for each time point and normalized to the steady-state level. Data from a representativeexperiment are shown. The same results were seen in two additional experiments. (B) Distribution of HA-GLUT4-GFP surface-to-total ratios among cells in the basal and insulin stimulated state. Data from a representative experiment are shown. The data from individual cells are grouped according to the surface-to-total ratio. The bin size was chosen arbitrarily. The surface and total fluorescence values are in arbitrary fluorescence units and therefore the ratio value is proportional to, but not an absolute measure of, the fraction of the construct on the surface. An increase in this ratio indicates an increase of the construct on the surface. The ratios in the insulin-stimulated conditions are normally distributed. The same is true for the basal ratios. C) Distribution of HA-GLUT4-GFP surface-to-total ratio as a function of HA-GLUT4-GFP expression per cell in the insulin stimulated state. The data are from the same experiment as shown in B. The variability in the surface-to-total distribution of individual cells does not correlate with the amount of HA-GLUT4-GFP expressed.
An advantage of this fluorescence method is that we can analyze translocation in individual cells. A histogram of the surface-to-total ratio of HA-GLUT4-GFP from a representative experiment is shown in Figure 2B. In this experiment 16 cells in the basal state were examined, and the surface-to-total ratios for HA-GLUT4-GFP were all <0.05. This is a ratio of Cy5 and GFP arbitrary fluorescence units, and although changes in distribution are determined by comparing ratio values, the ratio values are not a direct measure of the percentage of the constructs on the surface (i.e., the 0.05 value does not indicate that 5% is on the surface). The 19 cells examined in the insulin-treated sample were distributed between 0.15 and 0.55. This variability in the surface-to-total distributions among the cells is not correlated with the amount of HA-GLUT4-GFP expressed. These data indicate that the HA-GLUT4-GFP is not expressed at a level that saturates the GLUT4 retention mechanism, because the distribution of the surface-to-total ratios at the highest levels of expression are indistinguishable from those at the lowest level of expression (Figure 2C). Similarly, neither vpTR nor the TR was expressed at a level that saturate their trafficking in any of the experiments presented. In all cases, the ratio values for the population of cells in the basal and insulin-treated samples were normally distributed.
Half of GLUT4 Is in TR-containing Endosomes in Basal State
To quantitatively examine the overlap between the TR and GLUT4 pathways, we used the technique of HRP-mediated fluorescence quenching (Mayor et al., 1998; Lampson et al., 2001). The HRP-catalyzed DAB polymerization reaction product quenches fluorescence, and this method can be used to establish that a fluorescent probe and HRP are within the same compartment. We have used this technique to show that HA-GLUT4-GFP is in TR-containing endosomes in CHO cells (Johnson et al., 2001; Lampson et al., 2001).
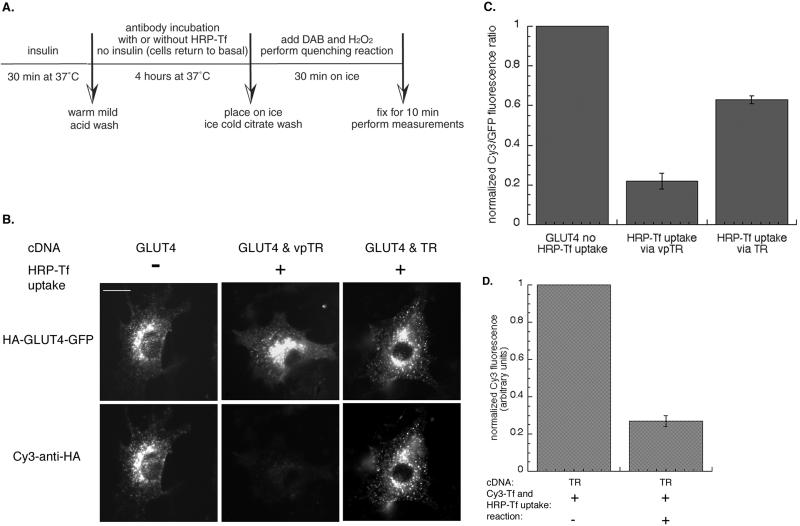
Adipocytes were double transfected with HA-GLUT4-GFP and the TR, or HA-GLUT4-GFP and vpTR, so that HRP-Tf could be targeted to either the normal endocytic pathway or to the specialized insulin-regulated pathway, respectively. The cells were stimulated with insulin for 30 min to increase the trafficking of HA-GLUT4-GFP to the surface. The insulin was removed and the cells were incubated with Cy3-labeled anti-HA antibody for 4 h in the absence of insulin. During this 4-h incubation the lumen of compartments containing GLUT4 become labeled with Cy3 as the HA-GLUT4-GFP bound by antibody on the surface is reinternalized (Figure 3A). The cells return to the basal state during this incubation. The cells were chilled to 4°C and incubated with DAB and H2O2 for 30 min, washed, fixed, and examined.
Figure 3.
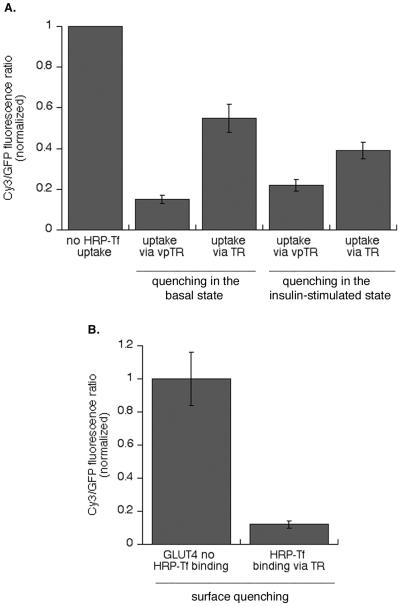
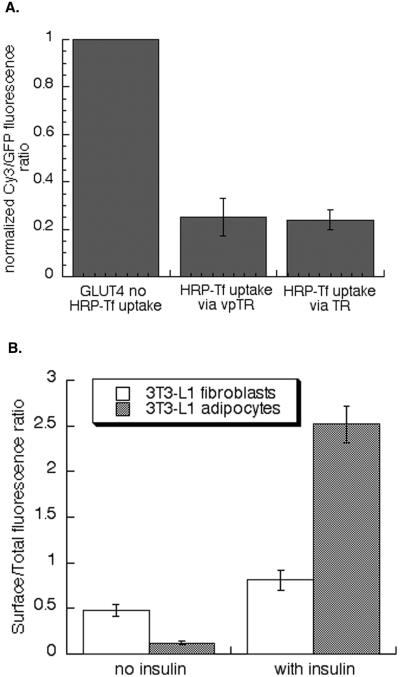
Compartment-specific fluorescence quenching in adipocytes. (A) Scheme of designed fluorescence-quenching experiment in 3T3-L1 adipocytes. Cells were stimulated with insulin for 30 min to increase recycling of HA-GLUT4-GFP. Insulin was removed by a mild-acid wash, and the cells were incubated with Cy3-anti-HA antibody, with or without HRP-Tf (20 μg/ml) for 4 h. During this incubation all of the HA-GLUT4-GFP is labeled with Cy3 anti-HA antibody, thereby placing a fluorescent probe within the lumen of these compartments. The compartments containing vpTR or the TR contain HRP-Tf. The cells were chilled to 4°C and the HRP catalyzed DAB polymerization initiated by adding DAB and H2O2 for 30 min on ice. The samples are fixed and then examined. (B) Images from a representative experiment. Cells were transfected with HA-GLUT4-GFP and the TR, or HA-GLUT4-GFP and vpTR. The top panels are the GFP fluorescence and the bottom panels are the Cy3 fluorescence from the same cells. There is complete overlap of the two probes in control cells (no HRP-Tf uptake), indicating all the intracellular compartments of HA-GLUT4-GFP are in dynamic communication with the cell surface. Bar, 20 μm. (C) Summary of the fluorescence quenching measured in 15 independent experiments (means ± SEM). The GFP fluorescence controls for expression level of HA-GLUT4-GFP. To compare the results of the independent experiments, the data from the individual experiments were normalized to the Cy3/GFP ratio in control cells (no HRP-Tf uptake) for that experiment. (D) Summary of data from five independent experiments measuring the Cy3-Tf fluorescence quenched by HRP-Tf in cells expressing the TR (means ± SEM). The data from the individual experiments were normalized to the Cy3 fluorescence in control cells (no reaction) for that experiment. These data establish that the maximum quenching for this assay as 80% of control.
Binding of the antibody to the HA epitope did not alter the behavior of HA-GLUT4-GFP. HA-GLUT4-GFP bound by the anti-HA antibody was retained in the basal state and insulin stimulated its translocation to the surface to the same extent as HA-GLUT4-GFP not bound by the antibody, consistent with previous studies (Lampson et al., 2001). The antibody is restricted to compartments containing GLUT4 because it remains bound to the HA epitope as GLUT4 cycles through the intracellular compartments. In control condition (no HRP-Tf uptake), the Cy3–anti-HA labeling colocalized completely with the GFP, indicating that all the intracellular compartments that contain HA-GLUT4-GFP were accessible to Cy3–anti-HA internalized from the medium (Figure 3B). In cells where HRP-Tf was taken up by vpTR, the Cy3 fluorescence was quenched by the HRP reaction product, demonstrating that vpTR delivered HRP-Tf to all the compartments that contain HA-GLUT4-GFP (Figure 3B).
The Cy3 fluorescence was only partially quenched when HRP-Tf was taken by the TR, with some structures appearing to be near totally quenched and others only partially quenched (Figure 3B). The partial quenching is difficult to detect visually because GLUT4 is concentrated in the peri-centriolar region of the cells. The degree of overlap between the TR-containing endosomes and the specialized GLUT4/IRAP compartments that exclude the TR was determined by quantifying the degree of Cy3–anti-HA quenching. The Cy3 fluorescence was normalized to the GFP fluorescence, which controls for the amount of HA-GLUT4-GFP expressed per cell. The GFP fluorescence is not affected by the HRP reaction within the lumen of the endosomes (Lampson et al., 2001). The Cy3/GFP ratio was decreased by ∼80% when HRP-Tf was delivered by vpTR. When the TR was used to deliver HRP-Tf only 40% was quenched, indicating that a significant fraction of HA-GLUT4-GFP was in a compartment inaccessible to the TR (Figure 3C). The degree of quenching was not increased when cells were incubated with twice the amount of HRP-Tf, indicating that the partial quenching in cells expressing the TR was not a result of partial occupancy of the TR with HRP-Tf. The same degrees of quenching were observed when cells were incubated with the Cy3-labeled anti-HA antibody for 4 h, washed, and then incubated with HRP-Tf for an additional 4 h in the presence of the labeled antibody.
The 80% quenching observed when HRP-Tf was delivered by vpTR was the maximum quenching achievable by the assay. When Cy3-Tf and HRP-Tf were cointernalized via the TR (or vpTR), so that both the fluorescence and HRP were in the same compartments, we found ∼80% quenching of the Cy3 fluorescence (Figure 3D). We do not know why the maximum quenching was less than complete. Regardless, our data indicate that HRP-Tf taken up by the TR has access to only half of the GLUT4 accessible to vpTR. These data demonstrate that in intact adipocytes there is a significant pool of intracellular GLUT4 that, although derived from the general endosomal system, is not accessible to proteins that traffic through the general recycling pathway (i.e., TR).
Two Intracellular Trafficking Steps Are Regulated by Insulin
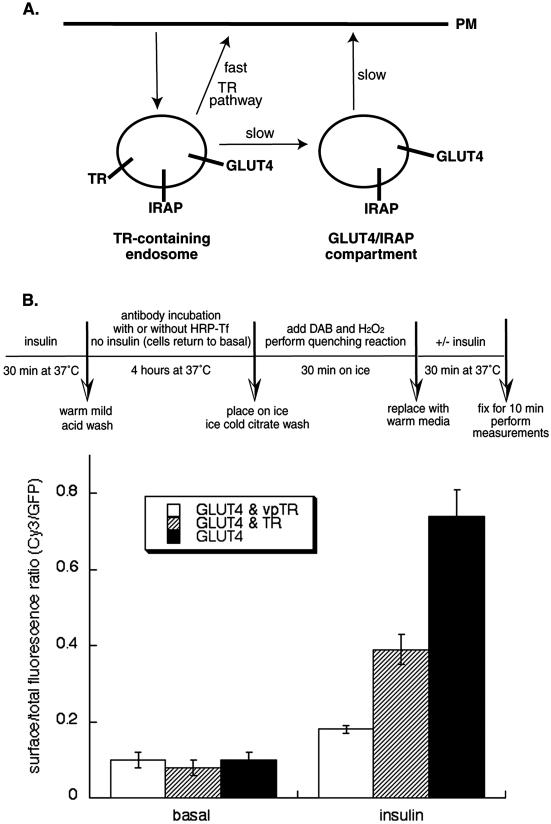
In previous studies of 3T3-L1 adipocytes, we found that vpTR traffics back to the cell surface as a single kinetic pool with no detectable amount returning to the plasma membrane via the rapid TR recycling pathway (Subtil et al., 2000). Similarly, the majority GLUT4 also traffics back to the cell surface as a single kinetic pool. Kinetic modeling experiments indicate that >95% of GLUT4 is returned to the cell surface by the slow recycling pathway (Holman et al., 1994, for discussion of previous GLUT4 modeling studies). The rate of internalization of GLUT4 in the basal state is fast relative to the rate of return to the plasma membrane (Holman and Cushman, 1994; Rea and James, 1997). Therefore, the nearly equal distribution of GLUT4 between the TR-containing endosomes and the specialized compartment indicates that the rate constant for GLUT4's transport from the TR-containing endosomes is similar to that for GLUT4's movement from the specialized compartment to the cell surface. A model consistent with these observations is that the pronounced intracellular concentration of GLUT4 and IRAP in adipocytes is achieved by two consecutive, slow intracellular transport steps: from the TR-containing endosomes to the specialized GLUT4/IRAP compartment and from this compartment to the cell surface (Figure 4A).
Figure 4.
Effect of HRP-mediated compartment ablation on insulin-stimulated translocation of HA-GLUT4-GFP. (A) Schematic illustrating that the two nearly equal pools of intracellular GLUT4 in the basal state indicate that the rate of transport from endosomes must be similar to the rate of transport from the specialized compartment. (B) Schema of the designed fluorescence quenching experiment after insulin stimulation. Insulin recruits GLUT4 from both pools. Cells were incubated with HRP-Tf (20 μg/ml) for 3.5 h, incubated with DAB and H2O2 on ice for 30 min, treated with or without insulin for 30 min at 37°C, and then fixed. The control cells were not incubated with HRP-Tf but were treated with DAB and H2O2 on ice for 30 min. The HA-GLUT4-GFP translocation was measured using quantitative fluorescence microscopy. The data are from a representative experiment (means ± SEM).
To determine whether both of these transport steps are stimulated by insulin, the effect of functionally inactivating the TR-containing endosomes or the vpTR-containing compartments by HRP-mediated ablation was determined. Cells coexpressing HA-GLUT4-GFP and TR, or HA-GLUT4-GFP and vpTR were incubated with HRP-Tf for 4 h at 37°C, chilled to 4°C, and the HRP reaction was performed. The cells were washed and warmed to 37°C for 30 min with or without insulin. At the end of this incubation the cells were fixed and the surface-to-total distribution of HA-GLUT4-GFP determined as described in Figure 1. When the compartments containing vpTR were ablated, the translocation of HA-GLUT4-GFP was almost completely abrogated, indicating that the compartments that contain HA-GLUT4-GFP have been rendered nonfunctional by the HRP reaction product (Figure 4B). However, when the TR-containing compartments were ablated, insulin-stimulated translocation of HA-GLUT4-GFP was reduced by approximately half (Figure 4B). These data demonstrate that full insulin-induced translocation requires mobilization of GLUT4 in the specialized compartment as well as GLUT4 in the TR-containing endosomes, and thereby support the hypothesis that transport from TR-containing endosomes is insulin regulated.
Overlap between TR and GLUT4 Is Greater in Presence of Insulin
In the above-mentioned experiments the codistribution of the TR and HA-GLUT4-GFP was determined in the basal state. We next used the fluorescence-quenching assay to determine whether insulin affects the segregation of GLUT4 from the TR. Cells were incubated with Cy3-labeled anti-HA antibody and HRP-Tf at 37°C for 4 h. Insulin was added during the final 30 min. The samples were chilled to 4°C and the HRP reaction performed without removing surface bound HRP-Tf (Figure 5). Insulin did not alter the ability of HRP-Tf internalized by vpTR to quench the anti-HA Cy3 fluorescence, documenting that vpTR traffics with GLUT4 in the presence of insulin as well as in the basal state (Figure 5A). The overlap between the TR and GLUT4 was increased by insulin, but was not as extensive as that between vpTR and GLUT4. In this experiment HRP-Tf on the surface was not removed, and the HRP reaction product formed on the surface of cells is able to efficiently quench Cy3 fluorescence on the surface (Figure 5B). Thus, in this experiment a fraction of the increased codistribution of TR and GLUT4 can be accounted for by the insulin-induced increase of HA-GLUT4-GFP on the surface of cells. The difference between the quenching in the cells expressing TR or vpTR reflects the size of the pool of GLUT4 that is inaccessible to TR in the presence of insulin. The observation that HRP-Tf delivered by the TR is unable to quench the anti-HA Cy3 fluorescence to the same degree as vpTR indicates that in the presence of insulin GLUT4 is transported back to the cell surface by a pathway that only partially overlaps with the TR recycling pathway.
Figure 5.
Effect of insulin on the codistribution of GLUT4, the TR, and vpTR. (A) Cells were incubated with Cy3-labeled anti-HA antibody and HRP-Tf for 4 h at 37°C. Insulin was added for the last 30 min of incubation. The cells were incubated with DAB and H2O2 for 30 min on ice. The degree of quenching when HRP-Tf is taken up by the TR is increased compared with basal quenching, but is still not as efficient than quenching via vpTR. The data for the quenching in the presence of insulin are a summary of six independent experiments, and the data for the quenching in the basal state, shown for the sake of comparison, are from a representative experiment. (B) Surface quenching of the Cy3–anti-HA antibody. Cells were stimulated with insulin for 15 min, and the Cy3-anti-HA antibody as well as HRP-Tf were bound on ice for 1 h. The cells were incubated with DAB and H2O2 as described previously. The data are from a representative experiment (means ± SEM) and were normalized to the Cy3/GFP ratio in control cells (no HRP-Tf uptake).
Rab11 Regulates Trafficking of GLUT4 from TR-containing Endosomes
The above-mentioned studies in intact adipocytes demonstrate that GLUT4 is partially retained within TR-containing endosomes, and that the insulin-induced increase in surface GLUT4 requires mobilization of this endosomal pool. Transport through the general endosomal compartment is known to be regulated by a variety of factors. One of these is rab11, which regulates traffic from endosomes to the plasma membrane and from endosomes to the TGN (Ullrich et al., 1996; Ren et al., 1998; Wilcke et al., 2000). Previous studies have shown that rab11 functions in intracellular pathways, and there is no evidence that rab11 modulates internalization from the plasma membrane (Ullrich et al., 1996). An effector of rab11, rab11BP, has been identified that upon a conformational change interacts with rab11 in association with membranes (Mammoto et al., 1999; Zeng et al., 1999) and disruption of this interaction inhibits the TR recycling (Zeng et al., 1999). The region of rab11BP that binds rab11 is contained between residues 336 and 504, and expression of this domain [rab11BP(334–504)] in CHO cells also inhibits TR recycling (Ren et al., unpublished data). We have therefore used this fragment as a dominant inhibitor of rab11 function to examine the role of the TR-containing endosomes in the trafficking of GLUT4 in adipocytes.
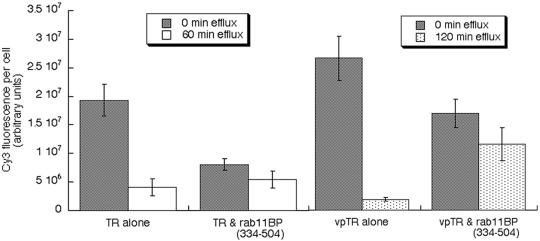
We first examined the effect of rab11BP(334–504) on TR and vpTR trafficking in adipocytes by using a fluorescent-Tf recycling assay (Figure 6). In control cells expressing the TR, 80% of Tf was released from cells in 60 min, whereas only 33% was released in 60 min when rab11BP(334–504) was coexpressed with the TR. In control cells expressing vpTR, 95% of Tf was released in 120 min, whereas only 30% was released when rab11BP(334–504) was coexpressed with vpTR. (Figure 6). In both cases the amount of Tf taken up during the 3.5-h preincubation was reduced relative to control, another indication that rab11BP(334–504) slows recycling in adipocytes. These data demonstrate that rab11 function is required for the basal trafficking of both the TR and vpTR in adipocytes.
Figure 6.
Effect of rab11BP(334–504) on the recycling of the TR and vpTR. Cells were incubated for 3.5 h with Cy3-Tf, washed, and incubated for an additional 60 min (for the TR) or 120 min (for vpTR) in medium without Cy3-Tf. At the end of the incubation the cells were fixed and the amount of Cy3-Tf remaining was determined. The different efflux times were chosen because the vpTR is recycled more slowly than the TR (Subtil et al., 2000). Data are from a representative experiment (means ± SEM).
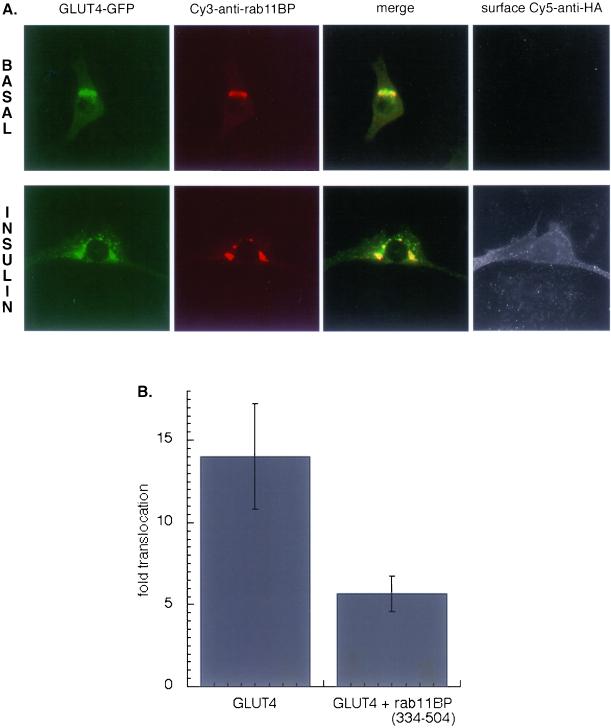
In cells expressing rab11BP(334–504) the GLUT4 expression pattern was altered to an intense peri-nuclear concentration that colocalizes with rab11BP(334–504) (Figure 7A). There was some translocation of HA-GLUT4-GFP to the surface, but the magnitude of the response to insulin in the presence of rab11BP(334–504) was one-half of that of matched control cells, demonstrating that inhibition of rab11 function by expression of rab11BP(334–504) severely inhibits insulin-stimulated translocation (Figure 7B).
Figure 7.
Effect of rab11BP(334–504) on the translocation of HA-GLUT4-GFP in 3T3-L1 adipocytes. (A) Rab11BP(334–504), a GST-fusion construct, is stained with a Cy3-anti-GST, and the surface expression of HA-GLUT4-GFP is detected with a Cy5-anti-HA. Images are from a representative experiment. (B) Summary of data from four independent experiments measuring the insulin-induced translocation of HA-GLUT4-GFP in control cells and cells expressing rab11BP(334–504) (means ± SEM).
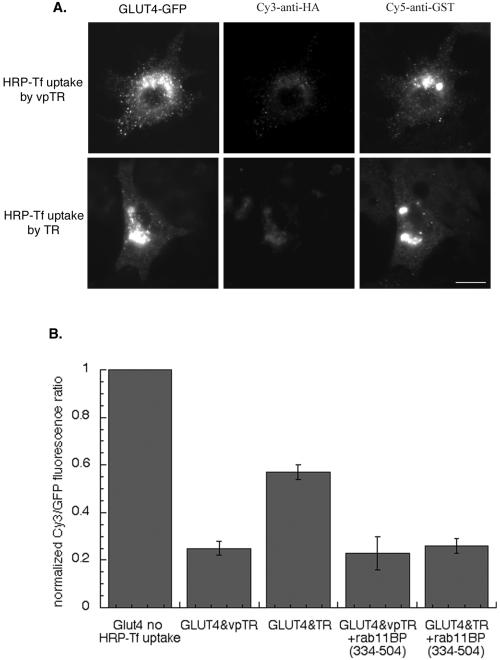
These findings support a functional role for rab11 in the trafficking of GLUT4 in adipocytes. To determine whether rab11BP(334–504) affects traffic of GLUT4 from endosomes, we assessed, by using the fluorescence-quenching assay, the extent to which the distributions of TR and HA-GLUT4-GFP was affected by expression of rab11BP(334–504) (Figure 8). When rab11BP(334–504) was expressed, the Cy3-anti-HA fluorescence was quenched equally well by HRP-Tf internalized via the TR and vpTR. These data demonstrate that the expression of rab11BP(334–504), which inhibits rab11 function, causes a redistribution of GLUT4 from a compartment inaccessible to the TR to one accessible to TR. This implies that the pool of GLUT4 molecules in the specialized compartment is directly derived, by an rab11-dependent process, from GLUT4 molecules in the TR-containing endosomes. That the rab11BP segment causes both a redistribution of GLUT4 to TR-containing endosomes and a decrease in its insulin-stimulated translocation to the plasma membrane indirectly demonstrates the importance of the specialized compartment in the large translocation of GLUT4 characteristic of adipocytes.
Figure 8.
Effect of rab11BP on the codistribution of GLUT4, the TR, and vpTR. (A) Experiment has been performed as described in Figure 3. Rab11BP(334–504) is stained with a Cy5-anti-GST. Images are from a representative experiment. Bar, 20 μm. (B) Summary of data from five independent fluorescence-quenching experiments determining the codistribution of the TR and GLUT4, or vpTR and GLUT4 (means ± SEM). The data from the individual experiments were normalized to the Cy3/GFP ratio in control cells (no HRP-Tf uptake) for that experiment.
Increased Translocation of GLUT4 in Adipocytes Correlates with Its Sorting to a Compartment That Is Not Accessible to TR
The magnitude of the insulin-induced redistribution of GLUT4 in undifferentiated cells is smaller than the translocation in adipocytes. We have shown in CHO cells that GLUT4 is retained within the TR-containing endosomal system (Lampson et al., 2001). Thus, the partial segregation of GLUT4 to a specialized compartment in adipocytes correlates with the more robust translocation in those cells. To more specifically examine this correlation, we characterized the behavior of GLUT4 in 3T3-L1 fibroblasts. HRP-Tf internalized by vpTR or the TR quenched the Cy3-labeled anti-HA antibody fluorescence equally, demonstrating that in preadipocytes GLUT4 is in the TR-containing endosomes (Figure 9A). In addition to finding GLUT4 within general endosomes, the ability of insulin to stimulate translocation of GLUT4 in these cells was decreased relative to adipocytes (Figure 9B). Thus, both the increase in retention in the basal state (less on the surface) and the increased insulin-induced translocation correlate with GLUT4 being targeted to a specialized compartment in adipocytes. Based on these data we propose that the specialized, insulin-responsive GLUT4 compartment in 3T3-L1 adipocytes arises from the GLUT4/IRAP-containing vesicles that also are formed from TR-endosomes in undifferentiated cells.
Figure 9.
Fluorescence quenching and translocation in 3T3-L1 fibroblasts. (A) Summary of the data from four independent experiments measuring the Cy3-Tf fluorescence quenched by HRP-Tf in cells expressing HA-GLUT4-GFP and the TR, or HA-GLUT4-GFP and vpTR. The data are means ± SEM. The data from the individual experiments were normalized to the Cy3/GFP ratio in control cells (no HRP-Tf uptake) for that experiment. Fibroblasts are preconfluent 3T3-L1 cells. (B) HA-GLUT4-GFP translocation in adipocytes and fibroblasts. Data are from a representative experiment (means ± SEM).
DISCUSSION
In this study we used an HRP-mediated fluorescence-quenching assay to characterize the insulin-regulated trafficking pathway in intact adipocytes. As reporters for this specialized endocytic pathway we used an HA-GLUT4-GFP construct and the vpTR chimera (Lampson et al., 2000; Dawson et al., 2001). Quantitative measurements of insulin-stimulated translocation and intracellular codistribution demonstrate that these constructs are valid reporters for studies of this specialized pathway in adipocytes.
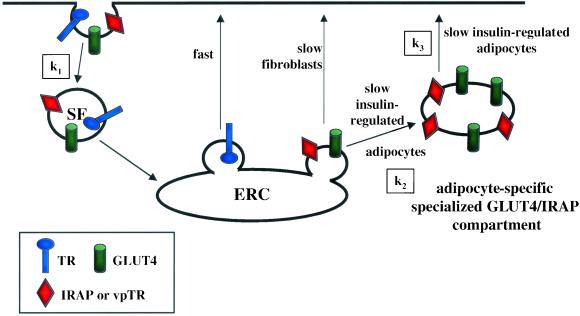
Based on the findings of this study and those published previously, we propose a two-step model for the insulin-regulated trafficking in adipocytes (Figure 10). In this model there are two intracellular compartments that contain GLUT4 and IRAP. One compartment is the TR-containing endosomal compartment, and the other is an adipocyte specific compartment that excludes the TR. These two compartments are in dynamic communication with the cell surface, and both compartments are required for the retention of GLUT4 and IRAP. Insulin stimulates a redistribution of GLUT4 and IRAP to the surface by recruiting GLUT4 and IRAP from both intracellular compartments, and consequently, in adipocytes there are two insulin-regulated transport steps. The predominant effect of insulin is on the transport of GLUT4 from intracellular compartments to the surface. There is evidence that insulin causes a small decrease in internalization, which would augment insulin's large effect by further increasing the amount of GLUT4 on the surface (Czech and Buxton, 1993; Yang and Holman, 1993; Huang et al., 2001). In this report, we have focused on the intracellular trafficking steps; however, an effect of insulin on internalization does not alter the interpretation of our data nor is it incompatible with our two-step model.
Figure 10.
Two-step model for insulin-regulated traffic in adipocytes. The first retention step occurs at the TR-containing endosomes. GLUT4 and IRAP are sorted from the rapid TR recycling pathway because they are sequestered in specialized transport vesicles that bud slowly from endosomes. We propose that this step is common to differentiated and undifferentiated cells, and therefore not adipocyte specific. In adipocytes these vesicles do not fuse directly with the plasma membrane but there is an additional insulin-regulated retention step. GLUT4 and IRAP in this postendosomal pool constitute the adipocyte-specific compartment. This specialized post endosomal compartment may be a stable fusion/fission competent compartment or it may be a collection of tethered vesicles. The available data do not distinguish between these two possibilities. Proper insulin-induced redistribution of GLUT4 and IRAP to the cell surface involves the recruitment of GLUT4/IRAP from both endosomes and the adipocyte-specific compartment.
The overshoot in surface expression of GLUT4 illustrates the complexity of the effect of insulin on GLUT4 redistribution to the plasma membrane in adipocytes. The overshoot has been observed when translocation of GLUT4 was measured directly (Bogan et al., 2001). An overshoot in GLUT4 surface expression is consistent with a number of models for GLUT4 trafficking, including the one proposed herein, and therefore does not distinguish among various kinetic models for GLUT4 trafficking. In this study we focus on the basal and insulin-stimulated steady states, and future studies of the transition between these states are required to understand the overshoot.
The two intracellular pools of GLUT4 are supported by the studies of intact cells described in this report and by previous biochemical studies showing that a significant amount of GLUT4 in the low-density membrane fraction of adipocytes is inaccessible to the TR (Livingstone et al., 1996). The experiments in intact cells extend our understanding of insulin-regulated trafficking in adipocytes by demonstrating that the GLUT4 in the specialized compartment is in dynamic communication with the cell surface and therefore it is not a static storage pool. In the fluorescence-quenching experiments the lumen of compartments containing GLUT4 are labeled as GLUT4 cycles to the cell surface, where it is bound by the fluorescent antibody against the HA epitope engineered into an extracellular domain of GLUT4. Thus, the Cy3–anti-HA antibody not accessible to the TR was internalized from the cell surface. The observation that all of the GLUT4 is accessible to vpTR internalized from the plasma membrane provides additional evidence that the GLUT4/IRAP specialized compartment is in dynamic communication with the cell surface. From our studies we conclude that GLUT4 and IRAP traffic from the surface through the TR-containing endosomes to the specialized compartment.
A number of previous studies have proposed that GLUT4 is localized to multiple intracellular compartments (Holman et al., 1994; Yeh et al., 1995). Our studies provide an important advance in the understanding of GLUT4 trafficking by demonstrating that the TR-endosomal compartment is specifically involved in the retention and insulin-induced translocation of GLUT4 in adipocytes, a classic insulin-target tissue. The conclusion that GLUT4 is specifically retained within endosomes is based on two observations. One, vpTR and GLUT4 are recycled back to the cell surface as a single kinetic pool, demonstrating that the vpTR and GLUT4 in endosomes are efficiently diverted from the rapid TR recycling pathway to the slow, insulin regulated-pathway (Subtil et al., 2000). Two, the near equal distribution of GLUT4 between the TR-endosomes and the specialized compartment indicates that the rate constant for the transport of GLUT4 and IRAP from endosomes to the specialized compartment (k2) is similar to that rate constant for transport from the specialized compartment to the plasma membrane (k3), because the rate constant for internalization (k1) is fast relative to the rate constant for return to the cell surface (Figure 10) (Robinson et al., 1992; Czech and Buxton, 1993; Yang and Holman, 1993; Holman and Cushman, 1994). Consequently, the return of GLUT4 to the cell surface involves two slow intracellular trafficking steps.
The hypothesis that both transport from endosomes and from the specialized compartment are regulated by insulin is supported by the observations that ablation of the TR-containing endosomes reduces insulin-stimulated translocation of GLUT4 by about half, whereas ablation of the vpTR-containing compartments essentially abrogates translocation. These results are in agreement with a previous study that found that HRP-mediated endosome ablation blunted GLUT4 translocation by 30% (Millar et al., 1999).
This two step model proposes that transport of GLUT4 from the general TR-containing endosomal compartment is a key regulated step in the insulin-induced redistribution of GLUT4. In addition to the data summarized above, the results demonstrating a requirement for rab11 function in insulin-stimulated translocation emphasize the importance of this trafficking step. The small GTPase rab11 regulates transport from endosomes back to the plasma membrane and from endosomes to the TGN (Ren et al., 1998; Trischler et al., 1999; Wilcke et al., 2000). Our data indicate that rab11 function is also required for transport from the TR-containing endosomes to the specialized GLUT4/IRAP compartment. This conclusion is based on studies using mutants of the rab11 effector protein rab11BP(334–504). Expression of this inhibitory construct reduces recycling of the TR and vpTR in adipocytes, suggesting that the function of rab11 is required in the basal state trafficking of GLUT4 in these specialized cells. This dominant inhibitory construct blunts insulin-stimulated translocation of GLUT4 by inhibiting GLUT4 transport to the specialized compartment. These data provide direct evidence that GLUT4 traffics through the general endosomal compartments on its way to the specialized compartment. It is important to stress that these data do not indicate that rab11 is a direct target of insulin action, but rather that rab11 function is required for insulin-stimulated translocation. Rab11 has previously been localized to GLUT4 vesicles isolated from fat cells, although the functional significance of this localization was not examined (Kessler et al., 2000).
It is becoming increasingly clear that the general endosomal pathway is an important component of the specialized GLUT4 pathway. Further support for the functional role of the endocytic pathway in the specialized pathway is the finding that undifferentiated cells have an insulin-regulated trafficking mechanism (Lampson et al., 2000; Bogan et al., 2001; Johnson et al., 2001). One mechanistic difference between adipocytes and the undifferentiated cells is that undifferentiated cells do not have a specialized intracellular compartment that contains GLUT4 yet excludes the TR. We propose that in fibroblasts GLUT4 is trafficked from the TR-containing endosomes directly to the plasma membrane, and that the specialized transport vesicles that traffic GLUT4 to the plasma membrane bud slowly from the endosomes (Lampson et al., 2001).
The findings that GLUT4 is dynamically retained within the TR-containing endosomes in undifferentiated cells and that the TR-containing endosomes play a significant role in the retention of GLUT4 in adipocytes raise the question of the relationship between insulin-regulated trafficking in different cell types. We propose that in differentiated cells there is an additional slow step in the return of GLUT4 to the cell surface, and it is this additional step that gives rise to the pool of GLUT4 inaccessible to TR in adipocytes. The concept that the specialized compartment develops early in differentiation, possibly from endosomes, has been discussed previously (El-Jack et al., 1999). The nature of the specialized compartment is not known. It could be a collection of tethered vesicles or it could be a stable compartment to which transport vesicles fuse and bud. Although the finding that GLUT4 is still partially segregated from the TR in the presence of insulin is more in line with the specialized compartment being a stable compartment through which GLUT4 traffics in the basal and insulin-stimulated states, it is not incompatible with the compartment being tethered vesicles. Further studies are required to rigorously address this question.
Recent studies indicate that GLUT4 trafficking in muscle cells and cardiomyocytes may also be described by a two-step model (Becker et al., 2001; Foster et al., 2001), further linking the development of a specialized, postendosomal GLUT4 retention compartment to physiologically relevant target tissues.
The two-step model we propose provides a framework for studies of insulin-regulated trafficking at a molecular level by providing a description of this pathway at a compartment level. It is likely that different proteins regulate the two intracellular transport steps and it is therefore important to consider that proteins identified as potential regulators of GLUT4 trafficking may be functioning at one of two distinct intracellular trafficking steps.
ACKNOWLEDGMENTS
We thank Fred Maxfield, Tim Ryan, and members of the McGraw laboratory for helpful comments and discussion. Anja Zeigerer is a Ph.D. student from the University of Heidelberg, Germany. This work was supported by grants DK-52852 and DK-57689 (to T.E.M.), and a fellowship from the W.M. Keck Foundation (to M.A.L.).
Abbreviations used:
- HPR
horseradish peroxidase
- Tf
transferrin
- TR
transferrin receptor
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–02–0071. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–02–0071.
REFERENCES
- Becker C, Sevilla L, Tomas E, Palacin M, Zorzano A, Fischer Y. The endosomal compartment is an insulin-sensitive recruitment site for GLUT4 and GLUT1 glucose transporters in cardiac myocytes. Endocrinology. 2001;142:5267–5276. doi: 10.1210/endo.142.12.8555. [DOI] [PubMed] [Google Scholar]
- Bogan J, McKee A, Lodish H. Insulin-responsive compartments containing GLUT4 in 3T3–L1 and CHO cells: regulation by amino acid concentrations. Mol Cell Biol. 2001;21:4785–4806. doi: 10.1128/MCB.21.14.4785-4806.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain CC, Trimble WS, Lienhard GE. Members of the VAMP family of synaptic vesicle proteins are components of glucose transporter-containing vesicles from rat adipocytes. J Biol Chem. 1992;267:11681–11684. [PubMed] [Google Scholar]
- Chen W, Feng Y, Chen D, Wandinger-Ness A. Rab11 is required for trans-golgi network-to-plasma membrane transport and a preferential target for GDP dissociation inhibitor. Mol Biol Cell. 1998;9:3241–3257. doi: 10.1091/mbc.9.11.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman SW, Wardzala LJ. Potential mechanism of insulin action on glucose transport in the isolated rat adipose cell. Apparent translocation of intracellular transport systems to the plasma membrane. J Biol Chem. 1980;255:4758–4762. [PubMed] [Google Scholar]
- Czech MP. Molecular actions of insulin on glucose transport. Annu Rev Nutr. 1995;15:441–471. doi: 10.1146/annurev.nu.15.070195.002301. [DOI] [PubMed] [Google Scholar]
- Czech MP, Buxton JM. Insulin action on the internalization of the GLUT4 glucose transporter in isolated rat adipocytes. J Biol Chem. 1993;268:9187–9190. [PubMed] [Google Scholar]
- Czech MP, Corvera S. Signaling mechanisms that regulate glucose transport. J Biol Chem. 1999;274:1865–1868. doi: 10.1074/jbc.274.4.1865. [DOI] [PubMed] [Google Scholar]
- Dawson K, Aviles-Hernandez A, Cushman S, Malide D. Insulin-regulated trafficking of dual-labeled glucose transporter 4 in primary rat adipose cells. Biochem Biophys Res Commun. 2001;287:445–454. doi: 10.1006/bbrc.2001.5620. [DOI] [PubMed] [Google Scholar]
- El-Jack AK, Kandror KV, Pilch PF. The formation of an insulin-responsive vesicular cargo compartment is an early event in 3T3–L1 adipocyte differentiation. Mol Biol Cell. 1999;10:1581–1594. doi: 10.1091/mbc.10.5.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster LJ, Li D, Randhawa VK, Klip A. Insulin accelerates inter-endosomal GLUT4 traffic via phosphatidylinositol 3-kinase and protein kinase B. J Biol Chem. 2001;276:44212–44221. doi: 10.1074/jbc.M102964200. [DOI] [PubMed] [Google Scholar]
- Garza L, Birnbaum M. Insulin-responsive aminopeptidase trafficking in 3T3–L1 adipocytes. J Biol Chem. 2000;275:2560–2567. doi: 10.1074/jbc.275.4.2560. [DOI] [PubMed] [Google Scholar]
- Holman GD, Cushman SW. Subcellular localization and trafficking of the GLUT4 glucose transporter isoform in insulin-responsive cells. Bioassays. 1994;16:753–759. doi: 10.1002/bies.950161010. [DOI] [PubMed] [Google Scholar]
- Holman GD, Lo Leggio L, Cushman SW. Insulin-stimulated GLUT4 glucose transporter recycling. A problem in membrane protein subcellular trafficking through multiple pools. J Biol Chem. 1994;269:17516–17524. [PubMed] [Google Scholar]
- Huang J, Imamura T, Olefsky JM. Insulin can regulate GLUT4 internalization by signaling to Rab5 and the motor protein dynein. Proc Natl Acad Sci USA. 2001;98:13084–13089. doi: 10.1073/pnas.241368698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A, Lampson M, McGraw T. A di-leucine sequence and a cluster of acidic amino acids are required for dynamic retention in the endosomal recycling compartment of fibroblasts. Mol Biol Cell. 2001;12:367–381. doi: 10.1091/mbc.12.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LS, Presley JF, Park JC, McGraw TE. Slowed receptor trafficking in mutant CHO lines of the End1 and End2 complementation groups. J Cell Physiol. 1994;158:29–38. doi: 10.1002/jcp.1041580105. [DOI] [PubMed] [Google Scholar]
- Johnson AO, Subtil A, Petrush R, Kobylarz K, Keller SR, McGraw TE. Identification of an insulin-responsive, slow endocytic recycling mechanism in Chinese hamster ovary cells. J Biol Chem. 1998;273:17968–17977. doi: 10.1074/jbc.273.28.17968. [DOI] [PubMed] [Google Scholar]
- Kandror KV, Coderre L, Pushkin AV, Pilch PF. Comparison of glucose-transporter-containing vesicles from rat fat and muscle tissues: evidence for a unique endosomal compartment. Biochem J. 1995;307:383–390. doi: 10.1042/bj3070383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandror K, Pilch PF. Identification and isolation of glycoproteins that translocate to the cell surface from GLUT4-enriched vesicles in an insulin-dependent fashion. J Biol Chem. 1994a;269:138–142. [PubMed] [Google Scholar]
- Kandror KV, Pilch PF. gp160, a tissue-specific marker for insulin-activated glucose transport. Proc Natl Acad Sci USA. 1994b;91:8017–8021. doi: 10.1073/pnas.91.17.8017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanzaki M, Pessin J. Insulin-stimulated GLUT4 translocation in adipocytes is dependent upon cortical actin remodeling [In Process Citation] J Biol Chem. 2001;276:42436–42444. doi: 10.1074/jbc.M108297200. [DOI] [PubMed] [Google Scholar]
- Keller SR, Scott HM, Mastick CC, Aebersold R, Lienhard GE. Cloning and characterization of a novel insulin-regulated membrane aminopeptidase from Glut4 vesicles [published erratum appears in J. Biol. Chem. (1995) 270, 30236] J Biol Chem. 1995;270:23612–23618. doi: 10.1074/jbc.270.40.23612. [DOI] [PubMed] [Google Scholar]
- Kessler A, Tomas E, Immler D, Meyer H, Zorzano A, Eckel J. Rab11 is associated with GLUT4-containing vesicles and redistributes in response to insulin. Diabetologia. 2000;43:1518–1527. doi: 10.1007/s001250051563. [DOI] [PubMed] [Google Scholar]
- Lampson M, Racz A, Cushman S, McGraw T. Demonstration of insulin-responsive trafficking of GLUT4 and vpTR in fibroblasts. J Cell Sci. 2000;113:4065–4076. doi: 10.1242/jcs.113.22.4065. [DOI] [PubMed] [Google Scholar]
- Lampson M, Schmoranzer J, Zeigerer A, Simon S, McGraw T. Insulin-regulated release from the endosomal recycling compartment is regulated by budding of specialized vesicles [In Process Citation] Mol Biol Cell. 2001;12:3489–3501. doi: 10.1091/mbc.12.11.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone C, James DE, Rice JE, Hanpeter D, Gould GW. Compartment ablation analysis of the insulin-responsive glucose transporter (GLUT4) in 3T3–L1 adipocytes. Biochem J. 1996;315:487–495. doi: 10.1042/bj3150487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malide D, St-Denis JF, Keller SR, Cushman SW. Vp165 and GLUT4 share similar vesicle pools along their trafficking pathways in rat adipose cells. FEBS Lett. 1997;409:461–468. doi: 10.1016/s0014-5793(97)00563-2. [DOI] [PubMed] [Google Scholar]
- Mammoto A, Ohtsuka T, Hotta I, Sasaki T, Takai Y. Rab11BP/Rabphilin-11, a downstream target of rab11 small G protein implicated in vesicle recycling. J Biol Chem. 1999;274:25517–25524. doi: 10.1074/jbc.274.36.25517. [DOI] [PubMed] [Google Scholar]
- Martin S, Rice JE, Gould GW, Keller SR, Slot JW, James DE. The glucose transporter GLUT4 and the aminopeptidase vp165 colocalise in tubulo-vesicular elements in adipocytes and cardiomyocytes. J Cell Sci. 1997;110:2281–2291. doi: 10.1242/jcs.110.18.2281. [DOI] [PubMed] [Google Scholar]
- Martin S, Tellam J, Livingstone C, Slot JW, Gould GW, James DE. The glucose transporter (GLUT-4) and vesicle-associated membrane protein-2 (VAMP-2) are segregated from recycling endosomes in insulin-sensitive cells. J Cell Biol. 1996;134:625–635. doi: 10.1083/jcb.134.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastick CC, Aebersold R, Lienhard GE. Characterization of a major protein in GLUT4 vesicles. Concentration in the vesicles and insulin-stimulated translocation to the plasma membrane. J Biol Chem. 1994;269:6089–6092. [PubMed] [Google Scholar]
- Mayor S, Sabharanjak S, Maxfield FR. Cholesterol-dependent retention of GPI-anchored proteins in endosomes. EMBO J. 1998;17:4626–4638. doi: 10.1093/emboj/17.16.4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar C, Shewan A, Hickson G, James D, Gould G. Differential regulation of secretory compartments containing the insulin-responsive glucose transporter 4 in 3T3–L1 adipocytes. Mol Biol Cell. 1999;10:3675–3688. doi: 10.1091/mbc.10.11.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris NJ, Ross SA, Lane WS, Moestrup SK, Petersen CM, Keller SR, Lienhard GE. Sortilin is the major 110-kDa protein in GLUT4 vesicles from adipocytes. J Biol Chem. 1998;273:3582–3587. doi: 10.1074/jbc.273.6.3582. [DOI] [PubMed] [Google Scholar]
- Pessin J, Thurmond D, Elmendorf J, Coker K, Okada S. Molecular basis of insulin-stimulated GLUT4 vesicle trafficking. Location! Location! Location! J Biol Chem. 1999;274:2593–2596. doi: 10.1074/jbc.274.5.2593. [DOI] [PubMed] [Google Scholar]
- Ramm G, Slot J, James D, Stoorvogel W. Insulin recruits GLUT4 from specialized VAMP2-carrying vesicles as well as from the dynamic endosomal/trans-Golgi network in rat adipocytes. Mol Biol Cell. 2000;11:4079–4091. doi: 10.1091/mbc.11.12.4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea S, James DE. Moving GLUT4: the biogenesis and trafficking of GLUT4 storage vesicles. Diabetes. 1997;46:1667–1677. doi: 10.2337/diab.46.11.1667. [DOI] [PubMed] [Google Scholar]
- Ren M, Xu G, Zeng J, De Lemos-Chiarandini C, Adesnik M, Sabatini DD. Hydrolysis of GTP on rab11 is required for the direct delivery of transferrin from the pericentriolar recycling compartment to the cell surface but not from sorting endosomes. Proc Natl Acad Sci USA. 1998;95:6187–6192. doi: 10.1073/pnas.95.11.6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson LJ, Pang S, Harris DS, Heuser J, James DE. Translocation of the glucose transporter (GLUT4) to the cell surface in permeabilized 3T3–L1 adipocytes: effects of ATP insulin, and GTP gamma S and localization of GLUT4 to clathrin lattices. J Cell Biol. 1992;117:1181–1196. doi: 10.1083/jcb.117.6.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross SA, Scott HM, Morris NJ, Leung WY, Mao F, Lienhard GE, Keller SR. Characterization of the insulin-regulated membrane aminopeptidase in 3T3–L1 adipocytes. J Biol Chem. 1996;271:3328–3332. doi: 10.1074/jbc.271.6.3328. [DOI] [PubMed] [Google Scholar]
- Simpson F, Whitehead J, James D. GLUT4–at the cross roads between membrane trafficking and signal transduction. Traffic. 2001;2:2–11. doi: 10.1034/j.1600-0854.2001.020102.x. [DOI] [PubMed] [Google Scholar]
- Sonnichsen B, De RS, Nielsen E, Rietdorf J, Zerial M. Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J Cell Biol. 2000;149:901–914. doi: 10.1083/jcb.149.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subtil A, Lampson MA, Keller SR, McGraw TE. Characterization of the insulin-regulated endocytic recycling mechanism in 3T3–L1 adipocytes using a novel reporter molecule. J Biol Chem. 2000;275:4787–4795. doi: 10.1074/jbc.275.7.4787. [DOI] [PubMed] [Google Scholar]
- Sumitani S, Ramlal T, Somwar R, Keller SR, Klip A. Insulin regulation and selective segregation with glucose transporter-4 of the membrane aminopeptidase vp165 in rat skeletal muscle cells. Endocrinology. 1997;138:1029–1034. doi: 10.1210/endo.138.3.5010. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Kono T. Evidence that insulin causes translocation of glucose transport activity to the plasma membrane from an intracellular storage site. Proc Natl Acad Sci USA. 1980;77:2542–2545. doi: 10.1073/pnas.77.5.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoidis G, Kotliar N, Pilch PF. Immunological analysis of GLUT4-enriched vesicles. Identification of novel proteins regulated by insulin and diabetes. J Biol Chem. 1993;268:11691–11696. [PubMed] [Google Scholar]
- Trischler M, Stoorvogel W, Ullrich O. Biochemical analysis of distinct Rab5- and Rab11-positive endosomes along the transferrin pathway. J Cell Sci. 1999;112:4773–4783. doi: 10.1242/jcs.112.24.4773. [DOI] [PubMed] [Google Scholar]
- Ullrich O, Reinsch S, Urbe S, Zerial M, Parton R. Rab11 regulates recycling through the pericentriolar recycling endosome. J Cell Biol. 1996;135:913–924. doi: 10.1083/jcb.135.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcke M, Johannes L, Galli T, Mayau V, Goud B, Salamero J. Rab11 regulates the compartmentalization of early endosomes required for efficient transport from early endosomes to the trans-golgi network. J Cell Biol. 2000;151:1207–1220. doi: 10.1083/jcb.151.6.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Holman GD. Comparison of GLUT4 and GLUT1 subcellular trafficking in basal and insulin-stimulated 3T3–L1 cells. J Biol Chem. 1993;268:4600–4603. [PubMed] [Google Scholar]
- Yeh JI, Verhey KJ, Birnbaum MJ. Kinetic analysis of glucose transporter trafficking in fibroblasts and adipocytes. Biochemistry. 1995;34:15523–15531. doi: 10.1021/bi00047a018. [DOI] [PubMed] [Google Scholar]
- Zeng J, et al. Identification of a putative effector protein for rab11 that participates in transferrin recycling. Proc Natl Acad Sci USA. 1999;96:2840–2845. doi: 10.1073/pnas.96.6.2840. [DOI] [PMC free article] [PubMed] [Google Scholar]