Abstract
Purpose
Autism spectrum disorder (ASD) is a complex neurodevelopmental disorder increasingly recognized for its strong association with chronic inflammation. Adipose tissue functions as an endocrine organ and can secrete inflammatory cytokines to mediate inflammation. However, its involvement in ASD-related inflammation remains unclear. The present study aimed to clarify the role of adipose tissue in inducing inflammatory responses associated with ASD.
Methods
A total of 36 children with ASD and 18 unrelated healthy controls, aged 2–14.5 years, were enrolled in the study. The up-regulated differentially expressed genes from the GSE18123 dataset were subjected to gene ontology (GO) enrichment analysis to explore ASD-associated pathways. Plasma cytokines and adipokines levels were quantified using Milliplex MAP immunoaffinity technology. The BTBR T + Itprtf/J (BTBR) mice that are known for their core ASD behavioral traits and inflammatory phenotypes were employed as an animal ASD model to verify the key clinical findings.
Results
GO enrichment analyses revealed immune dysfunction in ASD. Symptom analysis showed that the recruited individuals had typical autistic symptoms. Plasma analysis showed no significant difference in adipokines levels, including adiponectin, leptin, resistin, adipsin, and lipocalin-2, between the ASD and control groups. However, markedly elevated levels of IL-6, IL-8, and tumor necrosis factor (TNF-α) were detected in children with ASD, suggesting that the inflammatory state is independent of adipokines. Similar results were also observed in BTBR autistic mice. Notably, levels of insulin, which are closely related to the exertion of adipokines function, also showed no significant changes.
Conclusions
Our findings suggest that inflammation in ASD likely originates from non-adipocyte sources, implying that adipose tissue may not play a major role in inflammatory pathogenesis of ASD. Consequently, targeting adipose-related inflammation may not be an effective treatment approach, providing new directions for the development of targeted interventions.
Keywords: Inflammation, Adipokines, Autism spectrum disorder, Inflammatory cytokines, Insulin
Highlights
-
•
Definitely clear that ASD is an inflammatory state.
-
•
ASD patients only exhibit elevated inflammatory cytokines, with no significant changes in adipokine levels.
-
•
Identical patterns of inflammatory cytokine and adipokines were observed in the BTBR autistic mouse model.
-
•
Adipose tissue may not be a key contributor to the inflammatory pathogenesis of Autism Spectrum Disorder.
1. Introduction
Autism spectrum disorder (ASD) is a complex neurodevelopment condition characterized by impaired social interactions, restricted interests and stereotyped behaviors. The prevalence of ASD has been rising significantly (Park et al., 2016), affecting about 1% of the global population (Lord et al., 2018). The etiology of ASD is multifactorial, encompassing genetic, environmental, and neurological factors. Increasing evidence suggests that immune dysregulation and chronic inflammation play a critical role in ASD pathophysiology (Bhandari et al., 2020). Many studies have demonstrated that individuals with ASD typically exhibit elevated levels of pro-inflammatory cytokines, such as interleukin 1 beta (IL-1β), interleukin 6 (IL-6), and tumor necrosis factor (TNF-α), in their peripheral blood (Al-Ayadhi, 2005). Levels of some of the pro-inflammatory factors, such as monocyte chemoattractant protein-1 (MCP-1) and IL-6, are also elevated in the cerebrospinal fluid (CSF) of ASD patients (Masi et al., 2015; Theoharides et al., 2016). These inflammatory cytokines can affect synaptic plasticity, promote abnormal cortical development, and potentially contribute to the formation of ASD-like phenotypes (Choi et al., 2016; Bhandari et al., 2020).
Adipose tissue is traditionally regarded as an energy storage organ to regulate energy balance. It has emerged as a key player in regulating systemic inflammation by secreting adipokines that affect the activity of immune cells residing within the tissue (Guerreiro et al., 2022). The involvement of adipose tissue is linked to various mental illnesses (Simon et al., 2006). However, the role of adipose tissue in ASD has not been extensively investigated. Scattered studies have reported that human adipose-derived stem cells and the exosomes they secrete can ameliorate ASD-like behaviors in mice with autism (Ha et al., 2017; Fu et al., 2024). This seems to suggest a potential beneficial link between adipose tissue and autism. However, it is noteworthy that adipose tissue has been found to contain almost all types of immune cells (Mancuso, 2016), increasing the likelihood of its involvement in the inflammatory pathogenesis of ASD. The underlying mechanism likely involve the activation of immune cells residing in adipose tissue, which in turn triggers the release of inflammatory factors. In this process, macrophage phenotype shifts from the anti-inflammatory M2 state to the pro-inflammatory M1 state, leading to the secretion of inflammatory cytokines such as IL-6, IL-18, and TNF-α (Mancuso, 2016). Higher IL-6 levels in cord blood of newborns have been linked to abnormal neurodevelopmental outcome at 12 months after postpartum (Walsh et al., 2013). The inflammatory cascade triggered by these cytokines may influence neurodevelopmental pathways implicated in ASD. Nevertheless, the relationship between adipokine levels and ASD remains controversial. For instance, it has been reported that adiponectin serum levels in children with ASD are either decreased or remain unchanged (Blardi et al., 2010; Fujita-Shimizu et al., 2010; Rodrigues et al., 2014). Furthermore, elevated leptin levels have been observed in some ASD studies (Ashwood et al., 2008), while another adipokine resistin has shown contradictory results, with reports of both decreased and increased levels in ASD (Rodrigues et al., 2014; Ghaffari et al., 2016). These adipokines, which can function as either anti- or pro-inflammatory factors, play critical roles in the immune system (Fasshauer and Blüher, 2015; Mancuso, 2016). Therefore, there is a pressing need for detailed comparative studies to determine the exact contribution of adipose tissue to ASD-related inflammation.
In light of the link between adipokines and inflammation, the present study aimed to enhance our understanding of the inflammatory pathogenesis of ASD through examining adipokines levels in both clinical and animal model settings. Notably, previous studies have not specifically explored the role of adipose tissue in ASD etiology. The alterations in adipokine secretion observed in other studies may have been influenced by confounding factors, such as obesity, which is not a core ASD symptoms. The present study hypothesis is that adipose tissue may not play a central role in the inflammatory pathogenesis of ASD if adipokines are not significantly altered, potentially redirecting therapeutic efforts toward other inflammation sources.
2. Materials and methods
2.1. Subjects
A total of 36 children with ASD aged 2–14.5 years and 18 healthy control individuals aged 2–14 years were enrolled in the study. Children were diagnosed with ASD based on the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) criteria (Association, 2013). Clinical information was gathered based on a comprehensive observation scale designed in our previously published work (Gao et al., 2017). It includes 66 traits in three domains of development: language (A, 14 traits), social communication (B, 17 traits), and stereotyped interests and behaviors (C, 19 traits). Symptom manifestations are categorized into three types: persistent, incidental, and absent. Persistent symptoms refer to those that occur frequently and continuously over time, reflecting a more stable and severe condition. Incidental symptoms, on the other hand, are those that appear sporadically or occur only under specific conditions, potentially representing less severe or occasional clinical presentations. This classification aims to encompass the diversity of symptom expression in autism and reveal the potential relationship between clinical symptom severity and functional impairments associated with ASD. Even though language impairment has been removed from the core symptoms of autism in the DSM-5, it is still presented here as one of the developmental features of patients with autism. The following exclusion criteria were applied: having genetic defects that have been linked to ASD (such as Rett syndrome, fragile X syndrome, tuberous sclerosis, focal epilepsy, etc.); having chronic systemic disorders or neurological diseases (such as diabetes mellitus, heart failure, hypertension, cerebral palsy, etc.); having severe head trauma; and having a history of inflammatory conditions or having an active infection within the past month. Patients who had received psychopharmacologic treatment were also excluded. Healthy children with normal development, matched to the ASD group based on age, gender, and geographic region (specifically Shenzhen City), were selected and diagnosed by a pediatric endocrinologist. Exclusion criteria comprised any history of developmental, neurological, or psychiatric disorders, as well as chronic systemic illnesses or recent infections. All of the study procedures complied with the standards of the Declaration of Helsinki and the current ethical guidelines, and were approved by the ethics committee of the Shenzhen Maternity and Child Healthcare Hospital, Shenzhen, China. Informed consent was obtained from the parents of all pediatric participants.
2.2. Blood sample collection
Approximately 5 mL of venous blood was collected from participants in the morning after overnight fasting. The samples were placed into tubes containing EDTA as an anticoagulant and processed using centrifugation at 4000 rpm for 10 min at 4 °C. The upper plasma was then harvested for cytokine and adipokine measurement. IL-6, IL-8, TNF-α, leptin, and insulin levels were measured using the Milliplex MAP human adipokine magnetic bead panel 2 (Cat.# HADK2MAG-61K; Millipore, Germany) with plasma loading of 25 μL per well. Adiponectin, resistin, adipsin, and lipocalin-2 levels were measured using the Milliplex MAP human adipokine magnetic bead panel 1 (Cat.#HADK1MAG-61K; Millipore, Germany) with 1:400 diluted plasma loading of 25 μL per well. Each plasma sample was assayed once. Data were analyzed using Milliplex Analyst 5.1 software (Millipore, Germany).
2.3. Gene ontology (GO) analysis
Differentially expressed genes (DEGs) between the ASD and control groups were identified using the online tool GEO2R based on previously published datasets GSE18123 and GSE28521, respectively. Functional enrichment analysis for genes up-regulated in the ASD group was performed based on GO annotation using the clusterProfiler package implemented in the R language (R 3.6.3) (Yu et al., 2012).
2.4. ASD mouse model and cytokine measurement
BTBR T + Itpr3tf/J (BTBR) mice commonly used as an ASD animal model were obtained from Jackson Laboratory (Bar Harbor, ME, USA). BTBR mice have severely reduced interhemispheric communication due to the absence of the corpus callosum and diminished hippocampal commissure. They display behaviors similar to those seen in humans with ASD (Ruskin et al., 2013). Healthy C57BL/6 mice were used as a control and were obtained from GemPharmatech Company (Jiangsu, China). All animal experiments were conducted with the approval of the Animal Ethics Committee of Peking University Shenzhen Hospital. Mice were maintained at 22 ± 2 °C in a 12 h light/dark cycle, housed in a specific pathogen-free environment, and provided water and libitum with standard rodent chow. Peripheral blood was collected via retro-orbital venous sampling. Mouse plasma levels of IL-6, IL-1β, and TNF-α were measured using the Mouse Premixed Multi-Analyte kit (Cat. LXSAMSM-03; R&D Systems, USA) with 50 μl of 1:2 diluted plasma per well. Adipsin and resistin levels were measured using the Mouse Premixed Multi-Analyte kit (Cat. LXSAMSM-02; R&D Systems, USA) with 50 μl of 1:200 diluted plasma per well. Adiponectin levels were measured using the Mouse Premixed Multi-Analyte kit (Cat. LXSAMSM-01; R&D Systems, USA) with 50 μl of 1:4000 diluted plasma per well. Each sample were tested in three technical replicates. Data were analyzed using xPONENT 3.1 software (Luminex, Austin, TX, USA).
2.5. Behavioral testing
Eighteen-week-old male mice were used to abolish the impact of menstrual cycle on animal behaviors in behavior experiments. For all experiments, a camera was mounted above the arena and video footage was transmitted to a computer running Smart v3.0 software (Panlab, USA). A series of behavioral tests (social interaction, self-grooming, novel object recognition (NOR), and open field (OF)) were performed on a total of 20 mice (10 controls and 10 BTBR mice).
Three-chamber social (TCS) interaction test. Social interaction was assessed using the conventional TCS interaction test (60 cm × 40 cm × 40 cm). Animals were habituated in the arena on day one before the initial testing. During the habituation, two empty cups were placed in the back left and right corner of the arena. The mice were allowed to explore for 10 min before being returned to their home cages. During the sociability measurement, an inanimate toy was placed inside one cup, and a social target mouse inside the other cup. The test mouse was placed in the center area and allowed to freely explore the arena for 10min. Interaction time was measured based on the time the test animal spent in the areas surrounding the cups (≤3.5 cm). Sociability was defined as time spent with the social target divided by total interaction time and expressed as a percentage. To evaluate reciprocal social interactions reflecting social affiliation, social recognition and social memory, social preference tests were performed by replacing the inanimate toy with a novel mouse. Social interactions were recorded for 10 min. Social preference was defined as time spent with the novel mouse divided by total interaction time and expressed as a percentage. The arena was cleaned with 70% ethanol between different mouse tests.
Self-grooming. Mice were observed for spontaneous grooming behaviors when placed individually in a clean cage. The animals were allowed to habituate to the novel environment for 10 min before the testing period. Each mouse was observed for 20 min to record the time spent in grooming, which was defined as licking paws, washing nose or face, or scratching fur with any foot. Grooming frequency was calculated as grooming counts divided by total observed time. Grooming duration was defined as total grooming time divided by total counts of grooming during the 20-min experiment.
NOR test. The NOR test consisted of three steps: habituation, training session (10 min), and test session (10 min). Mice were individually habituated in a square wooden box (40 cm × 40 cm × 40 cm). During the training session, the box contained two identical objects, referred to as ‘familiar objects’. In the NOR test session, one familiar object (F) was replaced with a novel object (N). The exploration time was recorded and defined as time spent in sniffing or touching the object with the nose and/or forepaws. The ‘recognition index’ was calculated as the time spent in exploring object N compared to the total time spent exploring both object (N + F).
OF test. Mice were placed in a square wooden box (40 cm × 40 cm × 40 cm) for the locomotor activity test. The amount of time the animal spent in the center (20 cm × 20 cm) was recorded. Mice were allowed to acclimate in the box for 5 min, after which they were placed in the central area and their movements were monitored with a video camera for 10 min. The open field was thoroughly cleaned with 70% ethanol between trails. The traveled distance, time spent in the center and mean speed in the center were analyzed automatically using Smart software.
2.6. Gene expression
Genes expression levels were evaluated using quantitative reverse-transcription polymerase chain reaction (qRT-PCR). Reverse transcription was performed on 0.5 μg of total RNA extracted from mouse blood cells using oligo (dT) primers provided in the kit (RR091A, Takara). Quantitative amplification was conducted utilizing a LightCycler480 apparatus (Roche, Basel, Switzerland). The relative quantification for gene expression was determined using the 2-△△Ct method with normalization to GAPDH levels. The primers used for qRT-PCR are listed in Table 1.
Table 1.
Primers for qRT-PCR.
| Target | Forward primer (5′-3′) | Reverse primer (5′-3′) |
|---|---|---|
| IL-6 | CAACGATGATGCACTTGCAGA | CTCCAGGTAGCTATGGTACTCCAGA |
| IL-1β | TGGATGCTCTCATCAGGACAG | GAAATGCCACCTTTTGACAGTG |
| TNF-α | ACGTGGAACTGGCAGAAGAG | GGTCTGGGCCATAGAACTGA |
| Retn (Resistin) | CATGCCACTGTGTCCCATCG | GCTCAAGACTGCTGTGCCTTC |
| Lcn2 (Lipocalin-2) | ACATTTGTTCCAAGCTCCAGGGC | CATGGCGAACTGGTTGTAGTCCG |
| Adipoq (Adiponectin) | AATCCTGCCCAGTCATGCCGAAG | TCTCCAGGAGTGCCATCTCTGCCATC |
| GAPDH | TGTGTCCGTCGTGGATCTGA | TTGCTGTTGAAGTCGCAGGAG |
2.7. Statistical analysis
The Shapiro-Wilk test or the Kolmogorov-Smirnov test was used to determine whether the variables were normally distributed. Differences between two groups were evaluated using the Student's t-test or Mann-Whitney U test according to their distribution properties, and p-value was corrected by the Benjamini-Hochberg False Discovery Rate (FDR) procedure. The corrected p-values were considered significant if they were <0.05. Statistical power in human sample size was calculated at 0.87 with an alpha error rate of 0.05.
3. Results
3.1. Demographic characteristics of enrolled individuals
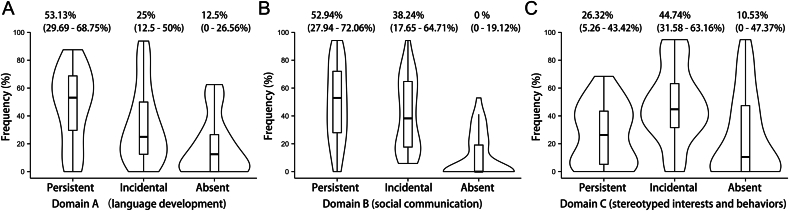
A total of 54 children were included in the study, comprising 36 patients with ASD (24 boys, 12 girls) and 18 controls (12 boys, 6 girls). The mean age was 6.4 ± 3.0 years in the ASD group and 6.8 ± 3.5 years in the control group (p = 0.6303). The clinical manifestations of symptoms in an autistic individual were described as persistent, incidental, or asymptomatic phenotype to reflect the symptom burden. The frequency of these symptom burdens was then calculated separately for each subject with ASD across the three core ASD domains: language development, social interaction, and stereotyped behaviors. Most symptoms were persistent in the language development domain, with a median occurrence frequency of 53.13% (Fig. 1A), indicating language impairment. Similar deficits were observed in the social domain (Fig. 1B). Incidental symptoms were more common for stereotyped behaviors, with a median frequency of 44.74% (Fig. 1C). Overall, ASD patients in the study exhibited the three core symptoms of ASD, meeting the symptom criterion for ASD and typically manifesting as persistent or incidental phenotypes.
Fig. 1.
Frequency distribution of clinical symptom manifestations in all ASD patients across three domains: A) Domain A (language development); B) Domain B (social communication); C) Domain C (stereotyped interests and behaviors). The symptom levels were categorized as persistent, incidental or absent. Violin plot width represents data density, with an embedded boxplot showing quantiles and outliers.
3.2. GO analysis indicates inflammation linked to ASD
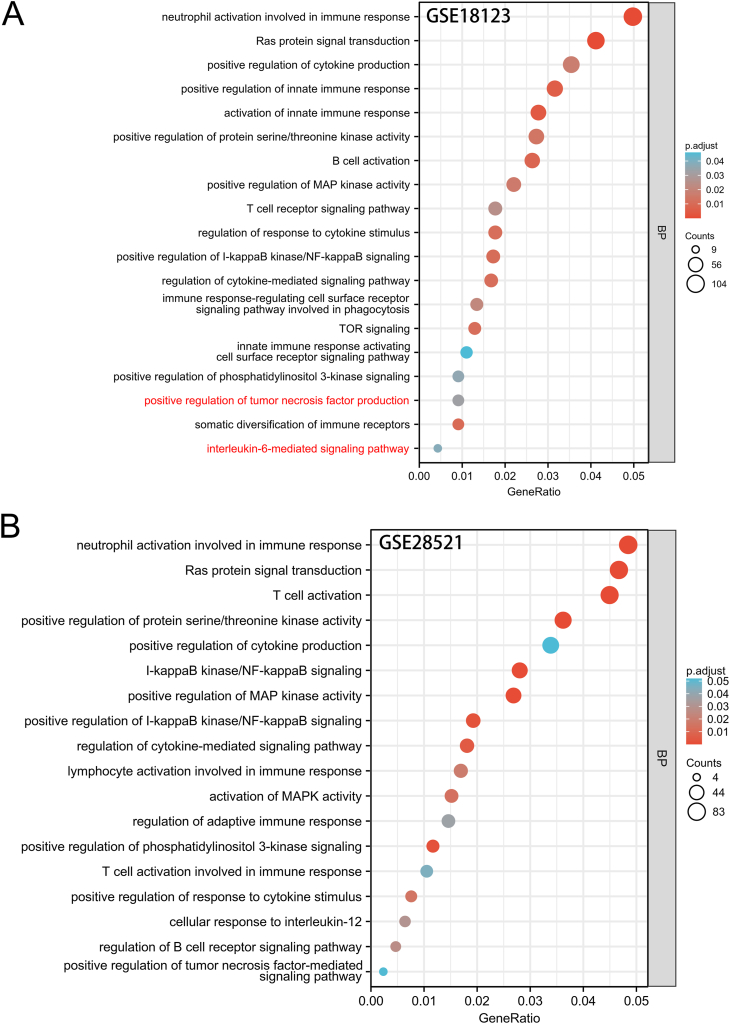
A total of 4452 genes were identified to be differentially expressed from the GSE18123 dataset, with 3135 up-regulated genes. This dataset, which includes 31 ASD cases and 31 controls, was generated from peripheral blood tissue. Further analysis of these up-regulated genes revealed that the biological processes related to immune dysfunction were heavily enriched in autism, including neutrophil activation involved in immune response, positive regulation of innate immune response, activation of innate immune response and somatic diversification of immune receptors (Fig. 2A). Of note, the inflammation signaling pathway of nuclear factor-κB (NF-κB) was activated, suggesting that pro-inflammatory mediators may be induced in individuals with ASD. Pathways such as positive regulation of tumor necrosis factor (TNF) production and positive regulation of cytokine production were enriched. In addition, their downstream signaling pathways were also found to be aberrantly activated. For example, regulation of cytokine-mediated signaling pathway, immune response-regulating cell surface receptor signaling pathway involved in phagocytosis, regulation of response to cytokine stimulus and somatic diversification of immune receptors, were all enriched. However, biological processes linked to adipokines dysfunction were not observed in ASD. Similar GO terms were enriched by up-regulated DEGs in the brain tissue of ASD patients from another dataset GSE28521 (Fig. 2B), which included 39 autistic and 40 normal brain samples. Consistent inflammatory dysregulation acquired in both the blood and brain datasets, suggest that the inflammatory responses in blood may somehow imply an inflammatory state of the brain. In summary, an abnormal activation of the inflammatory response occurred in ASD, but no signs of adipokines dysfunction were observed in autism.
Fig. 2.
Functional enrichment analysis based on gene ontology (GO) for differentially expressed genes up-regulated in ASD patients. Biological processes (BPs) significantly enriched in autism are related to immunity or inflammation. GO analyses based on A) GSE18123 and B) GSE28521 datasets. Counts: number of up-regulated differentially expressed genes (DEGs) annotated to the GO term; p.adjust: adjusted p-value obtained using hypergeometric test with FDR correction; GeneRatio: number of up-regulated DEGs annotated to the GO term/total number of up-regulated DEGs.
3.3. Circulating plasma levels of inflammatory cytokines
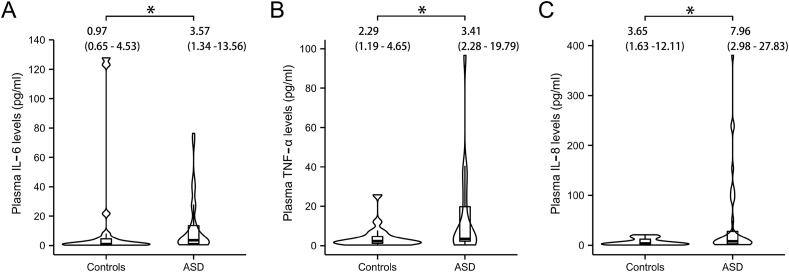
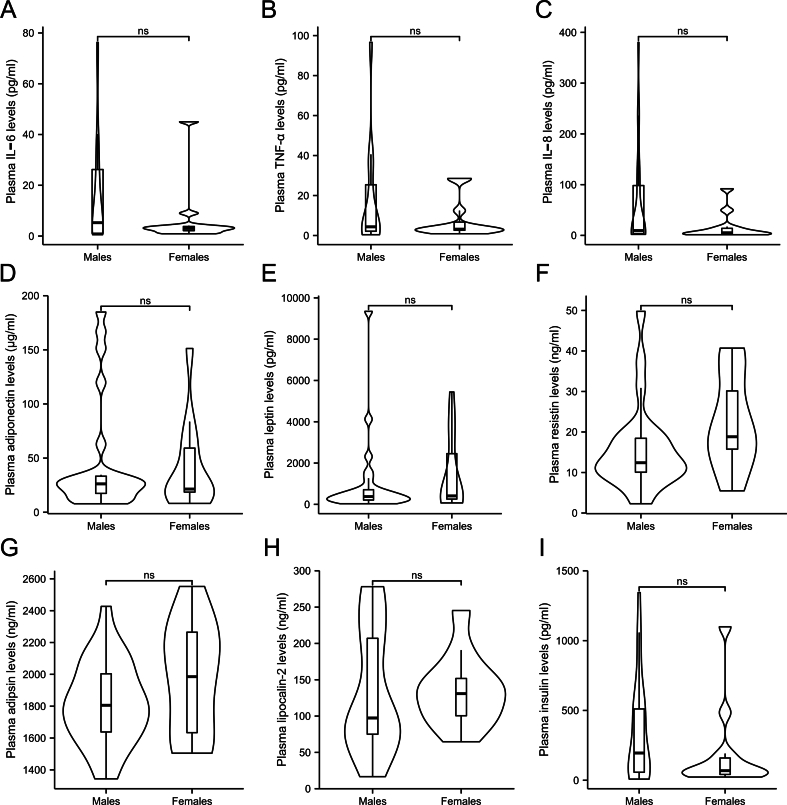
Chronic inflammation has been documented in ASD based on the GO analyses results mentioned above. Clinically, elevated levels of inflammatory cytokines were also observed in the plasma of autistic children. Specifically, IL-6 and TNF-α levels were significantly higher in the ASD group (3.57 pg/mL and 3.41 pg/mL, respectively) compared to those in the control group (0.97 pg/mL and 2.29 pg/mL, p = 0.024 and p = 0.024, respectively; Fig. 3A and B). These findings align with the results shown in Fig. 2A, which highlights the activation of the IL6-mediated signaling pathway and TNF-α production pathway. Additionally, increased levels of IL-8 were noted in the plasma of children with ASD (7.96 pg/mL in ASD and 3.65 pg/mL in controls, p = 0.024; Fig. 3C). All of these indicate a systematic inflammatory response involved in ASD development. It has been reported that ASD prevalence is four times higher in boys than in girls, raising the question of whether male patients with ASD might exhibit more server inflammation. However, no discernible differences in plasma levels of IL-6, TNF-α and IL-8 were identified between male and female pediatric patients with ASD (p > 0.1; Supplementary Figs. 1A–C), suggesting no gender sensitivity to inflammation cause in ASD.
Fig. 3.
Comparison of inflammatory cytokine plasma levels between healthy controls and ASD children. A) IL-6; B) TNF-α; C) IL-8. Violin plot width represents data density, with an embedded boxplot showing quantiles and outliers. Statistical analyses were performed using non-parametric Mann-Whitney U test. The tests were one-tailed and corrected for multiple comparisons using Benjamin-Hochberg method. Statistical significance is denoted by ∗p < 0.05.
3.4. Circulating plasma levels of adipokines
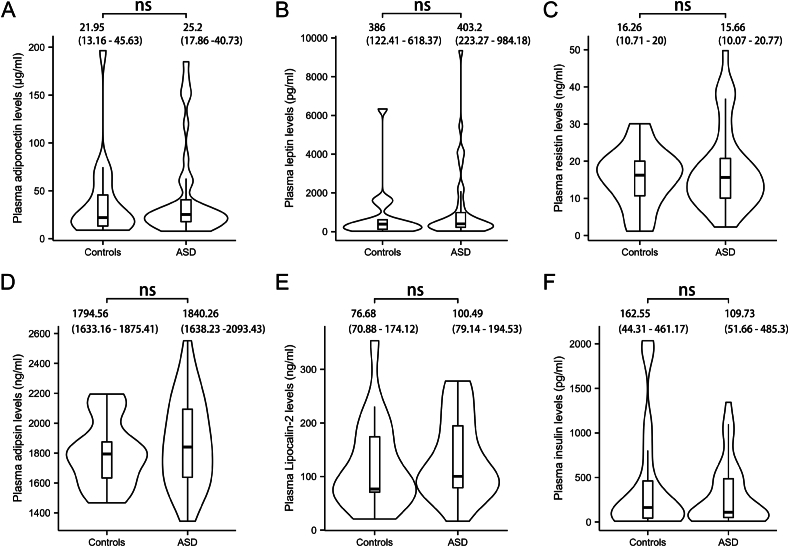
To evaluate the roles of adipokines in autism, we examined the alterations in adipokines levels in the plasma. However, no significant differences were found between the ASD and control group in the levels of adiponectin (25.2 μg/mL and 21.95 μg/mL, respectively, p = 0.982; Fig. 4A), leptin (403.2 pg/mL and 386 pg/mL, respectively, p = 0.892; Fig. 4B), resistin (15.66 ng/mL and 16.26 ng/mL, respectively, p = 0.982; Fig. 4C), adipsin (1840.26 ng/mL and 1794.56 ng/mL, respectively, p = 0.892; Fig. 4D) and lipocalin-2 (100.49 ng/mL and 76.68 ng/mL, respectively, p = 0.892; Fig. 4E) levels. We also measured plasma insulin levels and found no significant difference between the groups (Fig. 4F), suggesting that inflammatory response in ASD is not associated with insulin. Furthermore, we compared the plasma levels of adipokines between male and female pediatric patients with ASD to assess whether potential gender difference could be a confounding factor. As shown in Supplementary Figs. 1D–I, no statistically significant differences were observed across all measured adipokines (p > 0.05).
Fig. 4.
Comparison of adipokine plasma levels between healthy controls and ASD children. A) Adiponectin; B) Leptin; C) Resistin; D) Adipsin; E) Lipocalin-2; F) Insulin. Violin plot width represents data density, with an embedded boxplot showing quantiles and outliers. Statistical analyses were performed using non-parametric Mann-Whitney U test. The tests were two-tailed and corrected for multiple comparisons using Benjamin-Hochberg method. ns, not significant.
3.5. ASD behaviors in BTBR mice
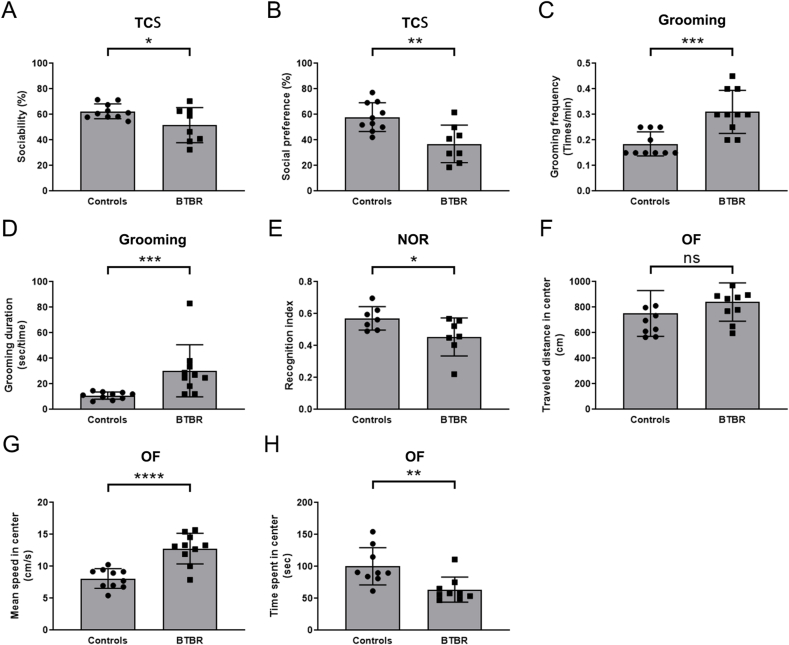
In humans, ASD is characterized in terms of behavior deficits. In phase 1 of the TCS assay, BTBR autistic mice spent less time with the target mouse compared to health control mice. For BTBR mice, they took a similar amount of time to interact with the live mouse and an inanimate toy, indicating impaired sociability (Fig. 5A). When the toy was replaced with a novel mouse, BTBR mice displayed a clear impairment in preference for social novelty (Fig. 5B). Additionally, BTBR mice exhibited pronounced repetitive and stereotyped behaviors, such as increased frequency and duration of grooming (Fig. 5C and D). We also conducted an NOR test to assess the cognitive deficit in BTBR mice, which is based on the rodent's innate preference for novel versus familiar objects. As expected, BTBR mice showed limited exploration of the novel objects compared to controls (Fig. 5E), indicating a recognition memory deficit. Moreover, BTBR mice displayed significant anxiety-related phenotypes in the OF test. When there was no difference in the distance traveled in the center of open filed, an increase in movement speed means a decrease in residence time in the center area (Fig. 5F–H), suggesting the presence of anxiety-like behaviors in BTBR mice. Collectively, these results indicate that BTBR mice exhibit ASD-relevant behaviors, supporting the use of BTBR mice as a representative ASD model for investigating the role of immunology-related factors in vivo.
Fig. 5.
ASD-like behavioral phenotypes of BTBR mice. A) Social behaviors were evaluated using the three-chamber social (TCS) interaction test, measuring sociability as the percentage of time spent with the social mouse object/total time spent with both social and inanimate objects. B) Social preference was assessed by measuring the percentage of time spent with the novel social object/total time spent interacting with both mice. C, D) Stereotyped behaviors were evaluated by measuring self-grooming frequency (counts of grooming/total observed time) and grooming duration (total time spent in grooming/total counts of grooming). E) Cognitive behavior was assessed using the novel object recognition (NOR) test and evaluated based on the recognition index (time spent in exploring the novel object/total time spent in exploring both objects). F-H) Anxiety-like behaviors were assessed using the open-field (OF) test. Two-tailed unpaired t-test was used for statistical analysis. Statistical significance was indicated by ∗p<0.05; ∗∗p<0.01; ∗∗∗p<0.001; ∗∗∗∗p<0.0001.
3.6. Expression of inflammatory cytokines and adipokines in BTBR mice
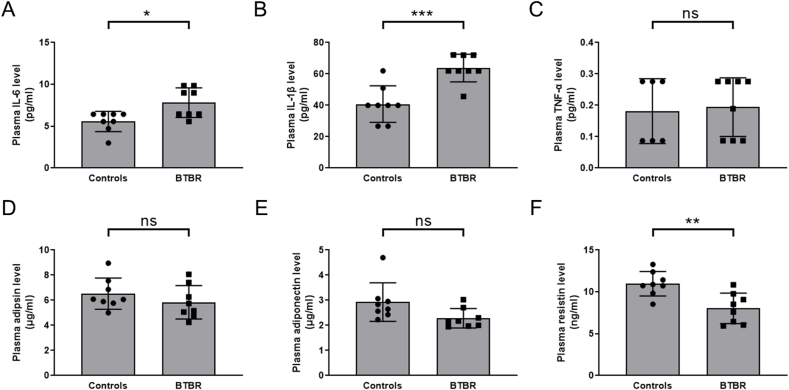
To verify the inflammatory status of BTBR mice, we isolated plasma from the blood samples and measured the plasma levels of commercially available cytokines. Consistent with human data, BTBR mice exhibited elevated levels of inflammatory cytokines, including those of IL-6 and IL-1β (Fig. 6A and B). TNF-α levels showed no significant difference, likely due to its low plasma concentration (Fig. 6C). Notably, plasma adipokines levels, such as those of adipsin and adiponectin, showed no difference between the groups (Fig. 6D and E), although the body weight of BTBR mice was heavier than that of health control mice (36.13 ± 1.90 g for BTBR and 29.56 ± 2.16 g for control mice, respectively, p<0.0001). Resistin levels were significantly lower in BTBR mice (Fig. 6F). As an inflammatory stimulator, its reduction further suggests that the elevated inflammatory levels in BTBR mice may not be dependent on adipose tissue.
Fig. 6.
Comparison of plasma cytokines levels between healthy control and BTBR autistic mice. A) IL-6; B) IL-1β; C) TNF-α; D) Adipsin; E) Adiponectin; F) Resistin. Two-tailed unpaired t-test was used for statistical analysis. Multiple comparison correction was performed using Benjamin-Hochberg method. Statistical significance was indicated by ∗p<0.05; ∗∗p<0.01; ∗∗∗p<0.001; ns, not significant.
Gene expressions levels of the cytokines were further examined in mouse peripheral blood cells. BTBR mice exhibited increased mRNA expression levels of inflammatory genes, including IL-6, IL-1β and TNF-α (Fig. 7A–C). However, the expression levels of adipokines remained unchanged between BTBR mice and controls (Fig. 7D–F). Taken together, these findings do not only confirm the presence of inflammation in ASD, but also further suggest that the elevated inflammatory response may originate from blood immune cells rather than adipose tissue.
Fig. 7.
mRNA levels of cytokines in mouse peripheral blood cells were analyzed by qRT-PCR. A) IL-6; B) IL-1β; C) TNF-α; D) Adiponectin; E) Resistin; F) Lipocalin-2. Two-tailed unpaired t-test was used for statistical analysis. Statistical significance was indicated by ∗p<0.05; ∗∗∗∗p<0.001; ns, not significant.
4. Discussion
ASD is a multifactorial disorder resulting from interactions between genetic, environmental, and immunological factors. Brain inflammation has been closely associated with behavioral deficits in ASD and is possibly derived from microglial activation, anti-brain antibody reactivity, and astroglial activation (Greene et al., 2019). Elevated levels of inflammatory markers such as MCP-1, IL-6, IL-10, and transforming growth factor β1 (TGF-β1), have been observed in postmortem brain tissues of ASD patients (Vargas et al., 2005). Additionally, increased levels of interferon-ɣ, IL-8, MCP-1, TGF-β2, and TNF-α have been reported in the CSF of ASD patients (Chez et al., 2007; Shen et al., 2020). These aberrant elevations of inflammatory mediator levels have been linked to impaired verbal communication and abnormal behavioral outcomes (Ashwood et al., 2011). The current study corroborates these findings, revealing elevated circulating levels of pro-inflammatory factors, such as IL-1β, IL-6, and TNF-α. These cytokines, including IL-12, can act on NMDAR, AMPAR, and metabotropic glutamate receptors of excitatory glutamatergic synapses, disrupting the balance of excitatory and inhibitory pathway and altering synaptic plasticity, ultimately leading to the behavioral changes observed in ASD (Pickering et al., 2005; Fang et al., 2013; Goines and Ashwood, 2013; Ohja et al., 2018; Taoro-Gonzalez et al., 2018). Propionic acid is produced in the gut of individuals with ASD. It induces neuro-inflammation and leads to significant microglial over-proliferation, which may disturb neuro-circuitry by constituting a physical barrier via over-growth of those glial cells (Shultz et al., 2008; Abdelli et al., 2019). Interestingly, although the incidence of ASD is significantly higher in boys than in girls, no significant gender differences were observed in the levels of inflammatory factors. This may be because estrogen is thought to have anti-inflammatory effects and can decrease of pro-inflammatory factor expression by regulating the immune system (Duncan, 2020), thereby protecting female patients against ASD.
The immune response involved in inflammation is modulated by various immune cells, including those residing in adipose tissue. Adipose tissue is not only composed of adipocytes but also a broad array of immune cells (Mancuso, 2016). The cross-talk between adipocytes and immune cells contributes to adipokines secretion and maintains tissue inflammation homeostasis. Unlike the classic inflammatory response, adipose tissue inflammation is triggered by intrinsic signals resulting from a positive energy balance, which can lead to obesity (Cavalcanti-de-Albuquerque et al., 2019). Chronic positive energy balance induces a phenotypic switch in adipose tissue-resident macrophage from an anti-inflammatory M2 phenotype to a pro-inflammatory M1 phenotype (Francisco et al., 2018), leading to the production of large amounts of pro-inflammatory cytokines (Ashwood et al., 2011; Vieira-Potter, 2014). This is consistent with the current findings of elevated IL-6, IL-8 and TNF-α plasma levels in children with ASD. However, no clear correlation between ASD severity and obesity has been documented (Buro et al., 2022; van der Lubbe et al., 2024), and there is a lack of evidence suggesting that ASD is a physiological condition related to chronic positive energy balance. Another study further indicated that adipose tissue may not be a potential key pathogenic factor in ASD. The CHD8 gene is known to be a common pathogenic gene in ASD. Research has shown that CHD8 deficiency significantly decreases adipose tissue in mice, but this reduction does not impact the occurrence of ASD-like behaviors (Kita et al., 2018). Thus, whether adipose tissue is involved in the inflammatory pathogenesis of ASD still requires further investigation.
Given that inflammatory cytokines secreted from adipose tissue were typically accompanied by abnormal adipokine release (Jeon, 2016; Mancuso, 2016), detecting adipokine levels could provide insights into whether adipose tissue is involved in ASD inflammation. Leptin is a well-known pro-inflammatory adipokine implicated in behavioral regulation that can directly enhance the production of pro-inflammatory cytokines, such as IL-6, IL-12, IL-18, IL-8 and TNF-α (Mancuso, 2016). However, the present study found no significant difference in plasma leptin levels between autistic children and controls. Adiponectin is primarily produced by adipocytes and is associated with insulin sensitivity and has anti-inflammatory effects (Fasshauer and Blüher, 2015; Ali et al., 2024). While meta-analysis has shown a higher adiponectin level in Alzheimer's disease (AD) patients compared to controls (Ma et al., 2016), its role in autism is less well-established (Ali et al., 2024). The present study confirms that adiponectin levels are not significantly altered in ASD, including BTBR autistic mice. Resistin is predominantly expressed in macrophages and stromal cells of adipose tissue (Ayala-Fontánez et al., 2016). It is capable of inducing insulin resistance and promoting chronic inflammation (Jeon, 2016). Despite its pro-inflammatory effect (Lee et al., 2014), resistin levels were lower in BTBR mice, further suggesting that elevated inflammation in ASD may not be adipose tissue-dependent. Adipsin is a circulating serine protease mainly produced by resident macrophages and adipocytes. It is involved in inflammatory responses by activating the complement alternative pathway (Giralt et al., 2016). However, there were no significant difference in its concentration in either ASD or BTBR mice. Lipocalin-2 is a glycoprotein involved in the transport of hydrophobic molecules that is correlated with inflammation (Mancuso, 2016; Kim and Choi, 2020). It also showed no significant difference in plasma concentration between children with ASD and healthy controls. In summary, the alteration in these adipokine levels could be reflective of perturbations in immune homeostasis within adipose tissue. However, the present data indicate that there are no significant differences in the levels of almost all tested adipokines between the ASD and control groups, suggesting that adipocytes may not be involved in the inflammatory response observed in ASD.
Some controversial reports about the association of adipokines with ASD may be attributed to obesity-related traits in certain patients. However, the relationship between obesity and autism remains inconclusive. Studies have found that the prevalence of obesity among children with ASD is likely similar to that seen in typically-developing children, suggesting that risk of obesity for autistic children is comparable to that of normal children (Curtin et al., 2014; Buro et al., 2022). Additionally, the functional roles of the discussed adipokines are closely tied to insulin regulation. For instance, adiponectin increases insulin sensitivity, adipsin promotes insulin secretion, leptin improves insulin sensitivity, and both lipocalin-2 and resistin are associated with insulin resistance (Golubović et al., 2013; Choi and Cohen, 2017). This strongly suggests that insulin levels should be affected by adipokine level changes. however, the present study found no significant alteration in insulin levels in ASD patients compared to those in the controls. Therefore, there is no sign that adipokine levels significantly change or contribute to the inflammatory response in ASD.
Several limitations in the present study should be clarified. First, this is a single-center study with a relatively small sample size, which may result in lower statistical confidence. Although the BTBR autism mouse model supports the study results, validation in a larger cohort is essential for consolidating these finding. Second, clinical information that affects adipokines expression patterns, such as weight, height, body mass index percentile, and blood glucose level, was very little collected in autistic children. Future studies should explore the correlation between adipokines and ASD-related inflammation in more detail, taking these confounding factors into account. Third, the present study did not consider the dynamic changes of adipokines and inflammatory cytokines in the body. Longitudinal studies tracking the changes in inflammatory cytokines, adipokine levels, and behavioral outcomes over time can further elucidate the temporal relationships between inflammation, adipokine levels and ASD symptoms. In summary, addressing these limitations through multi-center studies with larger sample sizes can enhance the generalizability of the results. These efforts will also help to refine therapeutic strategies for ASD.
In conclusion, the major study findings are that circulating adipokine levels in children with ASD are similar to those of age-matched controls, suggesting that adipokines may not play a significant role in ASD pathogenesis. These results contribute to a more nuanced understanding of the inflammatory mechanisms in ASD and may help to guide future research and therapeutic approaches.
CRediT authorship contribution statement
Baojiang Wang: Writing – original draft, Supervision, Project administration, Methodology, Funding acquisition, Data curation, Conceptualization. Yueyuan Qin: Validation, Methodology, Data curation. Yong Chen: Writing – review & editing, Methodology. Xiujie Zheng: Validation, Data curation. Yanjuan Chen: Data curation. Juan Zhao: Methodology. Feng Zhang: Validation. Shan Duan: Writing – review & editing, Supervision, Resources, Formal analysis.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank all the participants in this study. The study was generously supported by Shenzhen Science and Technology Innovation Commision Funded Project for Basic Research (JCYJ20220530155201004), Guangdong Provincial Natural Science Foundation (2023A1515010151), and Shenzhen Key Laboratory of Maternal and Child Health and Diseases (ZDSYS20230626091559006).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2024.100929.
Contributor Information
Baojiang Wang, Email: wangcell@163.com.
Shan Duan, Email: shanduan@hotmail.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Supplementary Fig. 1.
Comparison of cytokine plasma levels between autistic male and female pediatric patients. A) IL-6; B) TNF-α; C) IL-8; D) Adiponectin; E) Leptin; F) Resistin; G) Adipsin; H) Lipocalin-2; I) Insulin. Violin plot width represents data density, with an embedded boxplot showing quantiles and outliers. Statistical analyses were performed using non-parametric Mann-Whitney U test. The tests were two-tailed and corrected for multiple comparisons using Benjamin-Hochberg method. ns, not significant.
Data availability
Data will be made available on request.
References
- Abdelli L.S., Samsam A., Naser S.A. Propionic acid induces gliosis and neuro-inflammation through modulation of PTEN/AKT pathway in autism spectrum disorder. Sci. Rep. 2019;9(1):8824. doi: 10.1038/s41598-019-45348-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Ayadhi L.Y. Pro-inflammatory cytokines in autistic children in central Saudi Arabia. Neurosciences. 2005;10(2):155–158. [PubMed] [Google Scholar]
- Ali M., Kamran M., Talha M., Shad M.U. Adiponectin blood levels and autism spectrum disorders: a systematic review. BMC Psychiatr. 2024;24(1):88. doi: 10.1186/s12888-024-05529-1. [DOI] [Google Scholar]
- Ashwood P., Krakowiak P., Hertz-Picciotto I., Hansen R., Pessah I.N., Van de Water J. Associations of impaired behaviors with elevated plasma chemokines in autism spectrum disorders. J. Neuroimmunol. 2011;232(1–2):196–199. doi: 10.1016/j.jneuroim.2010.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P., Kwong C., Hansen R., Hertz-Picciotto I., Croen L., Krakowiak P., Walker W., Pessah I.N., Van de Water J. Brief report: plasma leptin levels are elevated in autism: association with early onset phenotype? J. Autism Dev. Disord. 2008;38(1):169–175. doi: 10.1007/s10803-006-0353-1. [DOI] [PubMed] [Google Scholar]
- Association A.P. fifth ed. American Psychiatry Association; Arlington, VA: 2013. Diagnostic and Statistical Manual of Mental Disorders. (DSM-5) [Google Scholar]
- Ayala-Fontánez N., Soler D.C., McCormick T.S. Current knowledge on psoriasis and autoimmune diseases. Psoriasis (Auckl) 2016;6:7–32. doi: 10.2147/ptt.S64950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari R., Paliwal J.K., Kuhad A. Neuropsychopathology of autism spectrum disorder: complex interplay of genetic, epigenetic, and environmental factors. Adv. Neurobiol. 2020;24:97–141. doi: 10.1007/978-3-030-30402-7_4. [DOI] [PubMed] [Google Scholar]
- Blardi P., de Lalla A., Ceccatelli L., Vanessa G., Auteri A., Hayek J. Variations of plasma leptin and adiponectin levels in autistic patients. Neurosci. Lett. 2010;479(1):54–57. doi: 10.1016/j.neulet.2010.05.027. [DOI] [PubMed] [Google Scholar]
- Buro A.W., Salinas-Miranda A., Marshall J., Gray H.L., Kirby R.S. Correlates of obesity in adolescents with and without autism spectrum disorder: the 2017-2018 National Survey of Children's Health. Disabil. Health J. 2022;15(2) doi: 10.1016/j.dhjo.2021.101221. [DOI] [Google Scholar]
- Cavalcanti-de-Albuquerque J.P., Bober J., Zimmer M.R., Dietrich M.O. Regulation of substrate utilization and adiposity by Agrp neurons. Nat. Commun. 2019;10(1):311. doi: 10.1038/s41467-018-08239-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chez M.G., Dowling T., Patel P.B., Khanna P., Kominsky M. Elevation of tumor necrosis factor-alpha in cerebrospinal fluid of autistic children. Pediatr. Neurol. 2007;36(6):361–365. doi: 10.1016/j.pediatrneurol.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Choi C.H.J., Cohen P. Adipose crosstalk with other cell types in health and disease. Exp. Cell Res. 2017;360(1):6–11. doi: 10.1016/j.yexcr.2017.04.022. [DOI] [PubMed] [Google Scholar]
- Choi G.B., Yim Y.S., Wong H., Kim S., Kim H., Kim S.V., Hoeffer C.A., Littman D.R., Huh J.R. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science. 2016;351(6276):933–939. doi: 10.1126/science.aad0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin C., Jojic M., Bandini L.G. Obesity in children with autism spectrum disorder. Harv. Rev. Psychiatr. 2014;22(2):93–103. doi: 10.1097/hrp.0000000000000031. [DOI] [Google Scholar]
- Duncan K.A. Estrogen Formation and inactivation following TBI: what we know and where we could go. Front. Endocrinol. 2020;11:345. doi: 10.3389/fendo.2020.00345. [DOI] [Google Scholar]
- Fang X.X., Jiang X.L., Han X.H., Peng Y.P., Qiu Y.H. Neuroprotection of interleukin-6 against NMDA-induced neurotoxicity is mediated by JAK/STAT3, MAPK/ERK, and PI3K/AKT signaling pathways. Cell. Mol. Neurobiol. 2013;33(2):241–251. doi: 10.1007/s10571-012-9891-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasshauer M., Blüher M. Adipokines in health and disease. Trends Pharmacol. Sci. 2015;36(7):461–470. doi: 10.1016/j.tips.2015.04.014. [DOI] [PubMed] [Google Scholar]
- Francisco V., Pino J., Gonzalez-Gay M.A., Mera A., Lago F., Gómez R., Mobasheri A., Gualillo O. Adipokines and inflammation: is it a question of weight? Br. J. Pharmacol. 2018;175(10):1569–1579. doi: 10.1111/bph.14181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y., Zhang Y.L., Liu R.Q., Xu M.M., Xie J.L., Zhang X.L., Xie G.M., Han Y.T., Zhang X.M., Zhang W.T., Zhang J., Zhang J. Exosome lncRNA IFNG-AS1 derived from mesenchymal stem cells of human adipose ameliorates neurogenesis and ASD-like behavior in BTBR mice. J. Nanobiotechnol. 2024;22(1):66. doi: 10.1186/s12951-024-02338-2. [DOI] [Google Scholar]
- Fujita-Shimizu A., Suzuki K., Nakamura K., Miyachi T., Matsuzaki H., Kajizuka M., Shinmura C., Iwata Y., Suda S., Tsuchiya K.J., Matsumoto K., Sugihara G., Iwata K., Yamamoto S., Tsujii M., Sugiyama T., Takei N., Mori N. Decreased serum levels of adiponectin in subjects with autism. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2010;34(3):455–458. doi: 10.1016/j.pnpbp.2009.12.020. [DOI] [Google Scholar]
- Gao J., Duan Y.H., Lin S., Yang P., Song H., Jia Q.L., Duan F.L., Zheng K.F., Wang B.J., Zhang L.H., Gu X.Y., Wang H.Y., Zeng X.C., Shao H., Duan S. Combined symptoms of social communication impairments: promising signs for early screening of autism. Biomed. Res. 2017;28(11):4942–4945. [Google Scholar]
- Ghaffari M.A., Mousavinejad E., Riahi F., Mousavinejad M., Afsharmanesh M.R. Increased serum levels of tumor necrosis factor-alpha, resistin, and visfatin in the children with autism spectrum disorders: a case-control study. Neurol. Res. Int. 2016;2016 doi: 10.1155/2016/9060751. [DOI] [Google Scholar]
- Giralt M., Cereijo R., Villarroya F. Adipokines and the endocrine role of adipose tissues. Handb. Exp. Pharmacol. 2016;233:265–282. doi: 10.1007/164_2015_6. [DOI] [PubMed] [Google Scholar]
- Goines P.E., Ashwood P. Cytokine dysregulation in autism spectrum disorders (ASD): possible role of the environment. Neurotoxicol. Teratol. 2013;36:67–81. doi: 10.1016/j.ntt.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golubović M.V., Dimić D., Antić S., Radenković S., Djindjić B., Jovanović M. Relationship of adipokine to insulin sensitivity and glycemic regulation in obese women--the effect of body weight reduction by caloric restriction. Vojnosanit. Pregl. 2013;70(3):284–291. doi: 10.2298/vsp1303284v. [DOI] [PubMed] [Google Scholar]
- Greene R.K., Walsh E., Mosner M.G., Dichter G.S. A potential mechanistic role for neuroinflammation in reward processing impairments in autism spectrum disorder. Biol. Psychol. 2019;142:1–12. doi: 10.1016/j.biopsycho.2018.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro V.A., Carvalho D., Freitas P. Obesity, adipose tissue, and inflammation answered in questions. J. Obes. 2022 doi: 10.1155/2022/2252516. 2022. [DOI] [Google Scholar]
- Ha S., Park H., Mahmood U., Ra J.C., Suh Y.H., Chang K.A. Human adipose-derived stem cells ameliorate repetitive behavior, social deficit and anxiety in a VPA-induced autism mouse model. Behav. Brain Res. 2017;317:479–484. doi: 10.1016/j.bbr.2016.10.004. [DOI] [PubMed] [Google Scholar]
- Jeon S.M. Regulation and function of AMPK in physiology and diseases. Exp. Mol. Med. 2016;48(7):e245. doi: 10.1038/emm.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.A., Choi K.M. Newly discovered adipokines: pathophysiological link between obesity and cardiometabolic disorders. Front. Physiol. 2020;11 doi: 10.3389/fphys.2020.568800. [DOI] [Google Scholar]
- Kita Y., Katayama Y., Shiraishi T., Oka T., Sato T., Suyama M., Ohkawa Y., Miyata K., Oike Y., Shirane M., Nishiyama M., Nakayama K.I. The autism-related protein CHD8 cooperates with C/EBPβ to regulate adipogenesis. Cell Rep. 2018;23(7):1988–2000. doi: 10.1016/j.celrep.2018.04.050. [DOI] [PubMed] [Google Scholar]
- Lee S., Lee H.C., Kwon Y.W., Lee S.E., Cho Y., Kim J., Lee S., Kim J.Y., Lee J., Yang H.M., Mook-Jung I., Nam K.Y., Chung J., Lazar M.A., Kim H.S. Adenylyl cyclase-associated protein 1 is a receptor for human resistin and mediates inflammatory actions of human monocytes. Cell Metabol. 2014;19(3):484–497. doi: 10.1016/j.cmet.2014.01.013. [DOI] [Google Scholar]
- Lord C., Elsabbagh M., Baird G., Veenstra-Vanderweele J. Autism spectrum disorder. Lancet. 2018;392(10146):508–520. doi: 10.1016/s0140-6736(18)31129-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Zhang W., Wang H.F., Wang Z.X., Jiang T., Tan M.S., Yu J.T., Tan L. Peripheral blood adipokines and insulin levels in patients with Alzheimer's disease: a replication study and meta-analysis. Curr. Alzheimer Res. 2016;13(3):223–233. doi: 10.2174/156720501303160217111434. [DOI] [PubMed] [Google Scholar]
- Mancuso P. The role of adipokines in chronic inflammation. ImmunoTargets Ther. 2016;5:47–56. doi: 10.2147/itt.S73223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masi A., Quintana D.S., Glozier N., Lloyd A.R., Hickie I.B., Guastella A.J. Cytokine aberrations in autism spectrum disorder: a systematic review and meta-analysis. Mol. Psychiatr. 2015;20(4):440–446. doi: 10.1038/mp.2014.59. [DOI] [Google Scholar]
- Ohja K., Gozal E., Fahnestock M., Cai L., Cai J., Freedman J.H., Switala A., El-Baz A., Barnes G.N. Neuroimmunologic and neurotrophic interactions in autism spectrum disorders: relationship to neuroinflammation. NeuroMolecular Med. 2018;20(2):161–173. doi: 10.1007/s12017-018-8488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H.R., Lee J.M., Moon H.E., Lee D.S., Kim B.N., Kim J., Kim D.G., Paek S.H. A short review on the current understanding of autism spectrum disorders. Exp Neurobiol. 2016;25(1):1–13. doi: 10.5607/en.2016.25.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering M., Cumiskey D., O'Connor J.J. Actions of TNF-alpha on glutamatergic synaptic transmission in the central nervous system. Exp. Physiol. 2005;90(5):663–670. doi: 10.1113/expphysiol.2005.030734. [DOI] [PubMed] [Google Scholar]
- Rodrigues D.H., Rocha N.P., Sousa L.F., Barbosa I.G., Kummer A., Teixeira A.L. Changes in adipokine levels in autism spectrum disorders. Neuropsychobiology. 2014;69(1):6–10. doi: 10.1159/000356234. [DOI] [PubMed] [Google Scholar]
- Ruskin D.N., Svedova J., Cote J.L., Sandau U., Rho J.M., Kawamura M., Jr., Boison D., Masino S.A. Ketogenic diet improves core symptoms of autism in BTBR mice. PLoS One. 2013;8(6) doi: 10.1371/journal.pone.0065021. [DOI] [Google Scholar]
- Shen L., Liu X., Zhang H., Lin J., Feng C., Iqbal J. Biomarkers in autism spectrum disorders: current progress. Clin. Chim. Acta. 2020;502:41–54. doi: 10.1016/j.cca.2019.12.009. [DOI] [PubMed] [Google Scholar]
- Shultz S.R., MacFabe D.F., Ossenkopp K.P., Scratch S., Whelan J., Taylor R., Cain D.P. Intracerebroventricular injection of propionic acid, an enteric bacterial metabolic end-product, impairs social behavior in the rat: implications for an animal model of autism. Neuropharmacology. 2008;54(6):901–911. doi: 10.1016/j.neuropharm.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Simon G.E., Von Korff M., Saunders K., Miglioretti D.L., Crane P.K., van Belle G., Kessler R.C. Association between obesity and psychiatric disorders in the US adult population. Arch. Gen. Psychiatr. 2006;63(7):824–830. doi: 10.1001/archpsyc.63.7.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taoro-Gonzalez L., Arenas Y.M., Cabrera-Pastor A., Felipo V. Hyperammonemia alters membrane expression of GluA1 and GluA2 subunits of AMPA receptors in hippocampus by enhancing activation of the IL-1 receptor: underlying mechanisms. J. Neuroinflammation. 2018;15(1):36. doi: 10.1186/s12974-018-1082-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theoharides T.C., Tsilioni I., Patel A.B., Doyle R. Atopic diseases and inflammation of the brain in the pathogenesis of autism spectrum disorders. Transl. Psychiatry. 2016;6(6):e844. doi: 10.1038/tp.2016.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Lubbe A., Swaab H., Vermeiren R., van den Akker E., Ester W. Novel insights into obesity in preschool children with autism spectrum disorder. Child Psychiatr. Hum. Dev. 2024 doi: 10.1007/s10578-024-01679-1. [DOI] [Google Scholar]
- Vargas D.L., Nascimbene C., Krishnan C., Zimmerman A.W., Pardo C.A. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann. Neurol. 2005;57(1):67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- Vieira-Potter V.J. Inflammation and macrophage modulation in adipose tissues. Cell Microbiol. 2014;16(10):1484–1492. doi: 10.1111/cmi.12336. [DOI] [PubMed] [Google Scholar]
- Walsh B.H., Boylan G.B., Livingstone V., Kenny L.C., Dempsey E.M., Murray D.M. Cord blood proteins and multichannel-electroencephalography in hypoxic-ischemic encephalopathy. Pediatr. Crit. Care Med. 2013;14(6):621–630. doi: 10.1097/PCC.0b013e318291793f. [DOI] [PubMed] [Google Scholar]
- Yu G., Wang L.G., Han Y., He Q.Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16(5):284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.