Abstract
Members of the highly conserved serine/arginine-rich (SR) protein family are nuclear factors involved in splicing of metazoan mRNA precursors. In mammals, two nuclear import receptors, transportin (TRN)-SR1 and TRN-SR2, are responsible for targeting SR proteins to the nucleus. Distinctive features in the nuclear localization signal between Drosophila and mammalian SR proteins prompted us to examine the mechanism by which Drosophila SR proteins and their antagonist repressor splicing factor 1 (RSF1) are imported into nucleus. Herein, we report the identification and characterization of a Drosophila importin β-family protein (dTRN-SR), homologous to TRN-SR2, that specifically interacts with both SR proteins and RSF1. dTRN-SR has a broad localization in the cytoplasm and the nucleus, whereas an N-terminal deletion mutant colocalizes with SR proteins in nuclear speckles. Far Western experiments established that the RS domain of SR proteins and the GRS domain of RSF1 are required for the direct interaction with dTRN-SR, an interaction that can be modulated by phosphorylation. Using the yeast model system in which nuclear import of Drosophila SR proteins and RSF1 is impaired, we demonstrate that complementation with dTRN-SR is sufficient to target these proteins to the nucleus. Together, the results imply that the mechanism by which SR proteins are imported to the nucleus is conserved between Drosophila and humans.
INTRODUCTION
Serine-arginine-rich (SR) proteins are required for constitutive pre-mRNA splicing and also regulate alternative splice site selection in a concentration-dependent manner. SR proteins have a modular structure that consists of one or two RNA recognition motifs (RRMs) and a C-terminal arginine-serine repeat of varying length (RS domain) (for reviews, see Manley and Tacke, 1996; Graveley, 2000). Functionally, many of the SR proteins are able to bind several classes of specific RNA motifs known as exonic splicing elements, which play a key role in both alternative and constitutive splice site selection in several systems (for reviews, see Tacke and Manley, 1999; Blencowe, 2000). Recently it has been shown that some of the functions of SR proteins can be antagonized by RSF1, a splicing repressor isolated from Drosophila that also exhibits a modular organization with a N-terminal RRM-type RNA binding domain and a C-terminal part enriched in glycine (G), arginine (R), and serine (S) residues (GRS domain) (Labourier et al., 1999a,b). The RRM domain of these splicing factors mediates specific recognition of RNA sequences, whereas the RS or GRS domains are responsible for specific protein–protein interactions, which are instrumental for the assembly of the spliceosome (Wu and Maniatis, 1993; Amrein et al., 1994; Kohtz et al., 1994; Zuo and Maniatis, 1996; Labourier et al., 1999b) as well as for the regulation of SR proteins and RSF1 subcellular localization (Li and Bingham, 1991; Hedley et al., 1995; Caceres et al., 1997; this study).
SR proteins can be organized in the interphase nucleus in a characteristic speckled pattern (Fu and Maniatis, 1992; Mintz and Spector, 2000), and also shuttle rapidly and continuously between the nucleus and the cytoplasm (Caceres et al., 1998). This distribution between cellular compartments is expected to alter the steady-state concentrations of SR proteins and thus affect the pattern of alternative splicing. Recently, new members of the importin β (impβ) family, termed transportin (TRN)-SR1 and TRN-SR2, have been shown to interact with human SR proteins (Kataoka et al., 1999; Lai et al., 2000, 2001). TRN-SR1 and TRN-SR2 have almost identical sequences except that TRN-SR1 contains additional unique regions in the central and C-terminal parts of the protein. Interaction between TRN-SR1/2 and SR proteins involves the RS domain and is abolished by RanGTP (Lai et al., 2000, 2001). Accordingly, a truncated TRN-SR2 that is defective in Ran binding, colocalizes with SR proteins in nuclear speckles (Lai et al., 2000). It is known that direct interaction of impβ with specific nucleoporins mediates docking of the cargo complex to the cytoplasmic face of the nuclear pore complexes, whereas its interaction with RanGTP releases the cargo in the nucleus (for review, see Mattaj and Englmeier, 1998; Izaurralde and Adam, 1998; Pemberton et al., 1998; Gorlich and Kutay, 1999).
The mechanisms regulating the cellular localization of SR proteins are determined, at least in part, by the phosphorylation status of the RS domain. Remarkably, TRN-SR2 has a strong preference for phosphorylated RS domains and mediates nuclear import of phosphorylated, but not unphosphorylated, SR proteins (Lai et al., 2000, 2001). Phosphorylation of serine residues in the RS domain releases these factors from storage/assembly loci (nuclear speckles) and recruits them to the sites of active transcription (Misteli et al., 1998). Furthermore, overexpression of either SR protein kinase (SRPK) or Clk/Sty, a prototypical kinase with dual specificity, capable of phosphorylating tyrosines as well as serines and threonines (Colwill et al., 1996), causes cytoplasmic accumulation of SR proteins (Gui et al., 1994; Colwill et al., 1996). The Drosophila homolog of human SF2/ASF (dASF), which lacks phosphorylation sites for Drosophila SRPK (dSRPK) in the RS domain, was unable to shuttle between the nucleus and the cytoplasm, although it was imported to the nucleus (Allemand et al., 2001). Because several Drosophila SR proteins have distinctive features in their RS domain compared with their human ortholog proteins (Allemand et al., 2001), it is unknown whether the phosphorylation-mediated cellular localization is conserved between the two species. It is also unknown whether the GRS domain of RSF1, like the RS domain, mediates import of RSF1 to the nucleus. In this study, we demonstrate that both the RS domain of Drosophila SR proteins and the GRS domain of RSF1 serve as unique nuclear localization signals. We identified a Drosophila impβ family protein (dTRN-SR) homologous to human TRN-SR2 that specifically interacts with both human and Drosophila SR proteins as well as RSF1 and serves as the nuclear import receptor for many SR proteins and their antagonist RSF1 in Drosophila.
MATERIALS AND METHODS
Computer Analysis
BLAST search in the Berkeley Drosophila Genome Project (BDGP) database for expressed sequence tags (Rubin et al., 2000) that have homologies with human TRN-SRs revealed a cDNA clone (LD21546) encoding dTRN-SR (GenBank accession number AAF51214). This cDNA is encoded by single-copy gene annotated in the GadFly genomic sequences of the BDGP database as CG2848. The cytological position of this gene is 23B1 on the left arm of chromosome II.
Expression of GFP Fusion Proteins in HeLa and Drosophila S2 Cells
Humanized GFP (pEGFP-C1; CLONTECH, GenBank accession number “U55762”) was fused in frame to the NH2 terminus of cDNAs corresponding to dTRN-SR [BamHI-EcoRI fragment from pBluescript II (KS+)-dTRN-SR plasmid, described below, inserted between the BglII and EcoRI sites of the vector] and dTRN-SRΔN217 (PmlI-EcoRI fragment from pBluescript II (KS+)-dTRN-SR, inserted between HindIII-blunt ended with Klenow enzyme and EcoRI sites of the vector).
The pEGFP-hSF2/ASF, pEGFP-hSF2/ASF-ΔRS, pEGFP-dASF, pEGFP-dASF-ΔRS, pEGFP-Rbp1, and pEGFP-B52 constructs were described previously (Allemand et al., 2001). To allow for expression of GFP-B52-ΔRS and GFP-Rbp1-ΔRS, cDNA fragments spanning codons 1–186 and 1–81, respectively, were generated by polymerase chain reaction (PCR) from pUAS-B52 and pGST-Rbp1 plasmids (Labourier et al., 1999b) and cloned in pEGFP-C1 vector between BglII and ApaI sites for B52 and between EcoRI and BamHI sites for Rbp1. The pEGFP-hSC35 and pEGFP-hSC35-ΔRS constructs were generated by subcloning the EcoRI-BamHI fragments of pUHD-HA-SC35 and pUHD-HA-SC35ΔRS plasmids (Sureau et al., 2001) into pEGFP-C1 vector. The pEGFP-dSC35 and pEGFP-dSC35-ΔRS constructs were obtained by inserting the cDNA fragments spanning the full-length coding sequence or codons 1–106 of dSC35 (Drosophila SC35; generated by PCR amplification of cDNA clone LD32469) between EcoRI and BamHI sites of pEGFP-C1 plasmid. The pEGFP-d9G8 and pEGFP-d9G8-ΔRS constructs were obtained by inserting the cDNA fragments spanning the full-length coding sequence or codons 1–81 of d9G8 (Drosophila 9G8; generated by PCR amplification of cDNA clone LD02483) in the XhoI site of pEGFP-C1 vector. The pEGFP-RSF1 and pEGFP-RSF1ΔRS were obtained by inserting the cDNA fragments spanning the full-length coding sequence or codons 1–80 of RSF1 (generated by PCR amplification of pGST-RSF1 plasmid (Labourier et al., 1999b) between XhoI-SalI and XhoI-HindIII of the pEGFP-C1 vector, respectively.
The pEGFP-RS(SF2/ASF) and pEGFP-RS(dASF) were described previously (Allemand et al., 2001). To produce green fluorescent protein (GFP) fusions with the RS domains of B52 (aa 187–350), 9G8 (aa 82–259), dSC35 (aa 105–196), and Rbp1 (aa 82–136), and GRS domain of RSF1 (aa 82–201), fragments containing HindIII (5′ end) and BamHI (3′ end) restriction sites (XhoI-HindIII sites for RSF1) were generated by PCR amplification from appropriate pEGFP constructs described above, and inserted into the pEGFP-C1 plasmid. The RS domain of hSC35 (aa 89–221) was generated by PCR as an EcoRI-BamHI fragment and cloned into the appropriate sites of the pEGFP-C2 vector.
All open reading frames and in-frame fusions were entirely sequenced to verify their integrity. Sequences of the oligonucleotides used for all PCR amplifications are available upon request.
Monolayer Drosophila S2 or HeLa cells were grown in Schneider's Drosophila medium (Invitrogen, Carlsbad, CA) or in RPMI 1640 medium (Invitrogen), respectively, supplemented with 10% fetal calf serum on 3-cm-diameter dishes (Nalge Nunc, Naperville, IL) to 70–80% confluence. They were transfected with 1 μg of the indicated plasmids and 19 μg carrier DNA, by using FUGENE 6 transfection reagent (Roche Applied Science, Indianapolis, IN) for HeLa cells or as indicated by the manufacturer (Kit DES; Invitrogen) for S2 cells. Twenty-four hours posttransfection, cells were washed with phosphate-buffered saline (PBS) and fixed for 15 min at room temperature with 4% paraformaldehyde. The fixed cells were then permeabilized with PBS/0.5% Triton X-100 during 5 min, washed two times with PBS, and then stained either with 4,6-diamidino-2-phenylindole (DAPI) or with a monoclonal antibody (mAb) against SC35 as described previously (Fu and Maniatis, 1992). Each experiment was reproduced in multiple independent transfections, and the cells shown in Figures 1 and 3 are representative of the large effects observed under each set of conditions.
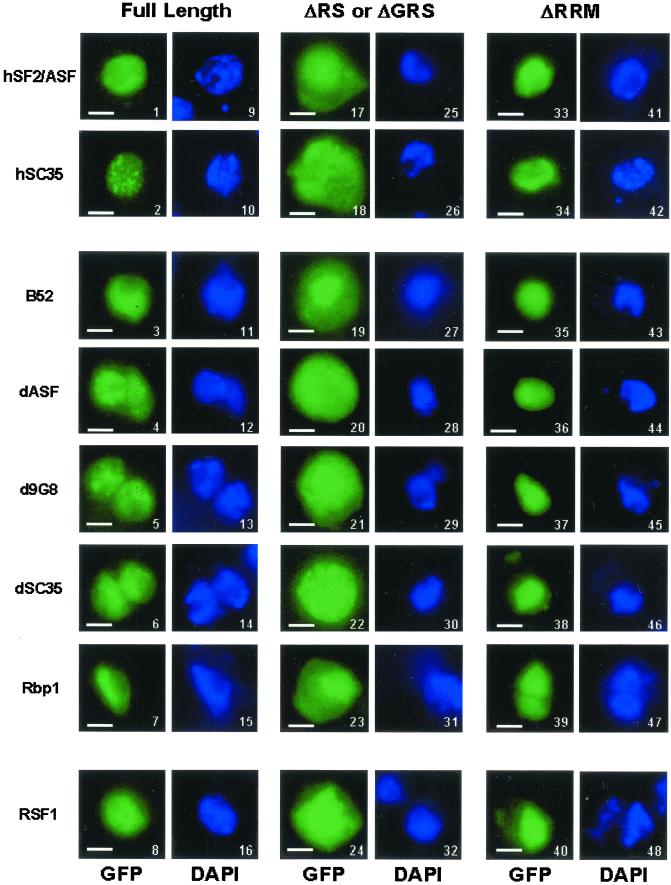
Figure 1.
Cellular localization of GFP fusion proteins in Drosophila S2 Schneider cells. Direct fluorescence of GFP-hSF2ASF (1), GFP-hSC35 (2), GFP-B52 (3), GFP-dASF (4), GFP-d9G8 (5), GFP-dSC35 (6), GFP-Rpb1 (7), and GFP-RSF1 (8); GFP-hSF2ASFΔRS (17), GFP-hSC35ΔRS (18), GFP-B52ΔRS (19), GFP-dASFΔRS (20), GFP-d9G8ΔRS (21), GFP-dSC35ΔRS (22), GFP-Rbp1ΔRS (23), and GFP-RSF1ΔGRS (24) deleted of the RS domain or GRS domain; GFP-hSF2ASFΔRRM (33), GFP-hSC35ΔRRM (34), GFP-B52ΔRRM (35), GFP-dASFΔRRM (36), GFP-d9G8ΔRRM (37), GFP-dSC35ΔRRM (38), GFP-Rbp1ΔRRM (39), and GFP-RSF1ΔRRM (40) deleted of the RRM domain. Fusion proteins were analyzed 20 h after transfection. Expression of fusion proteins was confirmed by immunoblot analysis by using an anti-GFP antibody (our unpublished data). The position of nuclei was confirmed by DAPI staining of the same cells transfected with GFP fusion proteins shown in 1–8 (9–16, respectively), 17–24 (25–32, respectively), and 33–40 (41–48, respectively). Bar, 10 μm.
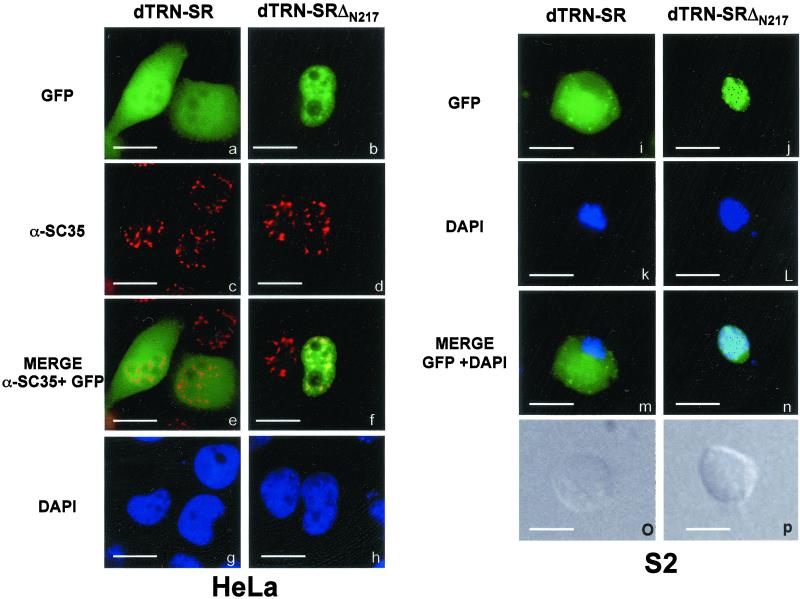
Figure 3.
Cellular localization of dTRN-SR GFP fusion proteins in HeLa cells (a–h) and in Drosophila S2 Schneider cells (i–p). Direct fluorescence of GFP-dTRN-SR (a and i) and GFP-dTRN-SRΔN217 (b and j) fusion proteins were analyzed 20 h posttransfection. Expression of fusion proteins was confirmed by immunoblot analysis by using an anti-GFP antibody (our unpublished data). The position of nuclei was confirmed by DAPI staining of the same transfected HeLa (g and h) and S2 (k and l) cells with GFP-dTRN-SR (g and k) or GFP-dTRN-SRΔN217 (h and l). Indirect immunofluorescence staining of HeLa cells in a and b with the αSC35 mAb (c and d, respectively) showed the cellular localization of endogenous SR protein SC35. A merge between a and c (e) and between b and d (f) shows that dTRN-SRΔN217 and SC-35 colocalize in the speckles. The Normarski interference-contrast of the same S2 cells transfected with GFP-dTRN-SR (o) and GFP-dTRN-SRΔN217 (p) are shown. Bar, 10 μm
Yeast Strains, Plasmids, and Transformation
To express mutant recombinant proteins deleted in their C-terminal RS or GRS domain by using the yeast pB42AD vector (CLONTECH, Palo Alto, CA), fragments of cDNAs spanning amino acid positions 1–195 for hSF2/ASF (from pEGFP-hSF2/ASF plasmid), 1–189 for dASF (from pEGFP-dASF plasmid), 1–186 for B52 (from pUAS-B52 plasmid; Labourier et al., 1999b), 1–104 for dSC35 (cDNA clone LD32469), 1–81 for d9G8 (cDNA clone LD02483), 1–81 for Rbp1 (from the pEGFP-Rbp1 plasmid) and 1–80 for RSF1 (from the pGST-RSF1 plasmid; Labourier et al., 1999a) were generated by PCR amplification. Except for B52 and RSF1, all DNA fragments contained the EcoRI site joined to the ATG start codon at the 5′ end and the XhoI site joined to the TAA stop codon at the 3′ end. Each full-length cDNA or corresponding ΔRS fragment was inserted between EcoRI and XhoI sites of the pB42AD vector. B52 cDNA and its ΔRS fragment were amplified with oligonucleotides possessing the SalI site both at the 5′ and 3′ ends. RSF1 cDNA and its ΔRS fragment were generated using primers containing the XhoI site at both ends. B52 and RSF1 full-length cDNA and their ΔRS fragments were inserted into pB42AD vector digested by XhoI.
To express GFP fusions in yeast, EcoRI-XhoI fragments obtained from appropriate pB42AD constructs containing full-length cDNAs encoding hSF2/ASF, dASF, dSC35, d9G8, RBP1, and dU1–70K were inserted between the EcoRI and XhoI sites of pUG36 (URA3 CEN) vector (Bordonne, 2000). RSF1 was inserted as an XhoI/XhoI fragment. All GFP-fusions were expressed from an inducible MET25 promoter. To avoid overexpression, yeast cells were grown under repressing conditions (presence of methionine in the medium) because repression of the MET25 promoter results in a rest activity of 10–20% (Mumberg et al., 1994).
To obtain a c-myc–tagged dTRN-SR (pRS422-dTRN-SR), a PCR fragment containing BamHI and XhoI sites at its 5′ and 3′ ends, respectively, was amplified by PCR and inserted in frame downstream of the c-myc–tag sequence (10 amino acids) into the pRS422 expression vector (ADE2, 2 μ) under the control of the MET25 promoter.
The yeast strain mtr10::HIS3 (matα, ade2, his3, leu2, trp1, ura3, mtr10::HIS3) was described previously (Senger et al., 1998). Wild-type (EGY48) yeast strain was a gift from J. Yeakley (University of California at San Diego, San Diego, CA). Yeast strains were transformed following the lithium acetate method (Hill et al., 1991). Yeast cells expressing recombinant proteins from pB42AD plasmids were grown on appropriate liquid media containing galactose.
mtr 10::HIS3 cells expressing recombinant proteins from pUG36 plasmids were allowed to grow on −His, −Ura-selective plates. Resulting strains were complemented with dTRN-SR expressed from pRS422 plasmid and selected on −His, −Ura, −Ade-selective plates. Yeast strains were propagated in yeast extract-peptone-dextrose and SD media by standard methods.
Western and Far Western Blotting
To generate pBluescript II(KS+)-dTRN-SR, a BamHI-EcoRI fragment was amplified by PCR from the cDNA clone LD21546, by using Pfu DNA polymerase (Promega, Madison, WI) and inserted between the corresponding sites of pBluescript II (KS+) vector. The pBluescript II (SK−)-dTRN was the generous gift of A. Norvell (HHMI, Princeton University, Princeton, NJ).
Hexahistidine-tagged SF2/ASF and dASF proteins were produced and purified from Escherichia coli (eSF2/ASF and edASF) or from baculovirus infected Sf9 cells (bSF2/ASF and bdASF) as described previously (Allemand et al., 2001).
Yeast whole-cell extracts for Western blot analysis were prepared essentially as described previously (Camasses et al., 1998). HeLa nuclear extracts or Drosophila Kc nuclear extracts were prepared under standard conditions as described previously (Labourier et al., 1999a; Allemand et al., 2001). Purified SR proteins, whole-cell extracts or nuclear extracts were separated by SDS-PAGE gel, transferred to nitrocellulose by electroblotting for 2 h in 10 mM 3-(cyclohexylamino)-1-propanesulfonic acid, pH 11, transfer buffer containing 10% methanol. For Western analysis, filters were probed either with anti-hemagglutinin (HA) antibodies (Rat mAb, 3F10; Roche Applied Science) followed by incubation with secondary antibody Rat IgG conjugated to peroxidase (Sigma-Aldrich, St. Louis, MO) or anti-c-myc antibodies 9E10 (kindly provided by D. Mathieu (Institut de Génétique Moléculaire, Montpellier, France) followed by incubation with anti-mouse IgG conjugated to peroxidase (Amersham Biosciences, Piscataway, NJ).
For Far Western analysis, the filters were treated as described previously (Kohtz et al., 1994) and probed with 35S-labeled dTRN-SR or dTRN, produced by TNT reticulocyte transcription translation system (Promega) in the presence of [35S]methionine (Amersham Biosciences). Before incubation with the filters, 35S-labeled probes were treated with RNase A (1 mg/ml) for 30 min.
RESULTS
RS Domain of Drosophila SR Proteins and GRS Domain of RSF1 Are Sufficient to Mediate Nuclear Import
To examine the cellular distribution of Drosophila SR proteins and their antagonist RSF1, GFP was fused in frame to the amino terminus of each of B52/SRp55, dASF, d9G8, dSC35, Rbp1/SRp20, and RSF1 and the fusion proteins were transiently expressed in Drosophila S2 cells (Figure 1). All Drosophila SR fusion proteins (Figure 1, 3–7), as well as RSF1 (Figure 1, 8) and human SR proteins SF2/ASF (hASF/SF2; Figure 1, 1) and SC35 (hSC35; Figure 1, 2), were localized in the nucleus of S2 cells. In contrast to mammalian cells, where SR proteins colocalized with both speckles and diffuse pools of splicing factors excluding the nucleoli (Allemand et al., 2001), only a diffuse pattern was seen in S2 cells (Figure 1, 1–8). Fusion proteins lacking the RS or GRS domain (Figure 1, 17–24), like GFP alone, had a broader cellular distribution both in the nucleus and cytoplasm. In contrast, the GFP fusions that harbor the RS or the GRS domain alone (Figure 1, 33–40) displayed the same nuclear distribution as full-length fusion proteins, demonstrating that the GRS domain and the RS domain of all SR proteins from Drosophila act as nuclear localization signals. None of these fusions, however, were directed to nuclear speckles. Thus, the GRS domain of RSF1 and the RS domain of Drosophila SR proteins are necessary and sufficient for nuclear localization.
Figure 2.
Amino acid sequence alignments of dTRN-SR with TRN-SR1, TRN-SR2, and Mtr10p as obtained by the ClustalW program. Identical amino acid residues are marked in dark and conservative substitutions such as RKH, IVLM, ED, FY, and ST are marked in gray. Gaps are introduced to optimize amino acid sequence alignments.
Identification and Characterization of Drosophila Transportin SR
The RS domains of human SR proteins interact directly with two different nuclear import receptors, TRN-SR1 (Kataoka et al., 1999) and TRN-SR2 (Lai et al., 2000). A BLAST homology search with full-length TRN-SR1 and 2 in the fly database (BDGP database) revealed a 914-amino acid protein that bears significant homology to these nuclear import receptors (39/59% identity vs. similarity) and was therefore named dTRN-SR. Because TRN-SR1 has additional amino acids both in the middle and the C terminus (Figure 2), dTRN-SR was more similar to hTRN-SR2 than hTRN-SR1. Both TRN-SR2 and dTRN-SR have significant similarity with the Saccharomyces cerevisiae protein Mtr10p (Kadowaki et al., 1994; 25/44% identity vs. similarity for dTRN-SR), a nuclear import receptor for the mRNA-binding protein Npl3p (Pemberton et al., 1997; Senger et al., 1998).
Comparisons of the cDNA and genomic sequences revealed that dTRN-SR is a single copy gene composed of six exons spaced by five introns. Thus, unlike mammals, D. melanogaster seems to have only one nuclear import receptor, dTRN-SR, which is probably responsible for the nuclear localization of all SR proteins.
A Truncated dTRN-SR Mutant Exhibits a Speckled Pattern in Nuclei of HeLa Cells
A transiently expressed TRN-SR2 mutant lacking the N-terminal 281-amino acids, ΔN281, localized predominantly in the nucleoplasm and accumulated in speckled domains in the nuclei of HeLa cells (Lai et al., 2000). To test whether deletion of the N-terminal domain of dTRN-SR could similarly affect its cellular distribution, we fused GFP in frame to the N terminus of full-length dTRN-SR or its truncated version lacking the first N-terminal 217-amino acids, ΔN217, and transiently expressed fusion proteins in either HeLa cells (Figure 3, a–h) or Drosophila S2 cells (Figure 3, i–p). In both cell lines, full-length dTRN-SR fusion protein was detected both in the nucleus and the cytoplasm (Figure 3, a and i). In contrast, ΔN217 was localized predominantly in the nucleus (Figure 3, b and j). Immunofluorescence with anti-SC35 antibodies clearly established that a dTRN-SR mutant lacking the N-terminal region localized to the nucleus of HeLa cells in a speckled pattern that coincides with the distribution of the SR protein SC35 (Figure 3, b, d, and f). Altogether, the results show that dTRN-SR has the same localization properties as hTRN-SR2, suggesting that dTRN-SR is the import receptor for Drosophila SR proteins.
dTRN-SR Recognizes Both Human and Drosophila SR Proteins
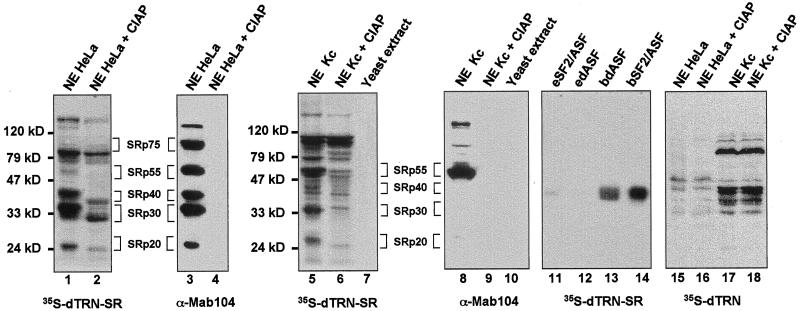
To determine the specificity of binding of dTRN-SR to target proteins, we used Far Western blotting (Kohtz et al., 1994). This method was successfully applied to reveal specific interactions between members of the SR protein family and other splicing factors (Wu and Maniatis, 1993; Kohtz et al., 1994) as well as for the study of interactions of yeast ribosomal proteins with their specific karyopherins (Rout et al., 1997). Proteins from HeLa and Drosophila Kc nuclear extracts were separated by SDS-PAGE gels, transferred to filters, renatured, and probed with 35S-labeled dTRN-SR produced in rabbit reticulocyte lysate (Figure 4, lanes 1 and 5). In HeLa nuclear extracts, dTRN-SR labeled predominantly protein species with apparent molecular weights of ∼20, 30, 40, 55, 75, 85, and 130 kDa (Figure 4, lane 1). Except for the 85- and 130-kDa proteins, which were only seen with dTRN-SR, protein bands with identical mobilities also appeared when filters were probed with mAb 104 (Figure 4, lane 3), a mAb specific for the phosphoepitope present in a subset of SR proteins. These data suggested that the 20-, 30-, 40-, 55-, and 75-kDa bands are probably SRp20, SRp30, SRp40, SRp55, and SRp75, respectively. Based on the migration, the 85-kDa band is probably the recently identified serine-arginine-rich splicing regulatory protein, SRrp86, which contains a single N-terminal RRM and two C-terminal RS domains, but is not recognized by mAb 104 (Barnard and Patton, 2000). However, further studies are required to confirm this possibility. Binding of dTRN-SR to human SR proteins correlated with their abundance in the extract, suggesting that dTRN-SR had no preference for binding any of the SR proteins. There was, however, a weak signal at the level of SRp55 and SRp75, probably due to a less efficient renaturation than for the other SR proteins.
Figure 4.
Far Western analysis showing a physical interaction between dTRN-SR and SR proteins from both HeLa and Drosophila Kc cells. Proteins from HeLa (lanes 1–4, 15, and 16), Drosophila Kc (lanes 5, 6, 8, 9, 17, and 18) nuclear extracts or yeast S. cerevisae total extracts (lanes 7 and 10) either untreated (lanes 1, 3, 5, 7, 8, 10, 15, and 17) or treated (lanes 2, 4, 6, 9, 16, and 18) with calf intestinal alkaline phosphatase (CIAP) as well as purified recombinant dASF and SF2/ASF 100 ng each, expressed either in a baculovirus system (bdASF, lane 13, and bSF2/ASF, lane 14, receptively) or in E. coli (edASF, lane 12, and eSF2/ASF, lane 11, respectively) were separated on a SDS-PAGE, transferred to nitrocellulose, renatured, and probed either with 35-S-labeled dTRN-SR (lanes 1, 2, 5–7, and 11–14) or 35-S-labeled dTRN (lanes 15–18). Proteins (lanes 3, 4, 8, and 9) were subjected to Western blot analysis by using mAb 104. Molecular weight markers are indicated on the left of the Far Western panels, and SR protein species are indicated on the right.
Given that TRN-SR2 can directly interact with phosphorylated but not unphosphorylated SR proteins (Lai et al., 2001), we tested whether interactions between dTRN-SR and proteins from HeLa extracts are sensitive to phosphorylation. HeLa nuclear extracts were subjected to phosphatase treatment before Far Western blotting. As expected, mAb 104 failed to detect any protein from treated extracts, implying that dephosphorylation of SR proteins was complete (Figure 4, lane 4). In agreement with the fact that dephosphorylation of SR proteins alters their electrophoretic mobility, SR protein bands detected with 35S-labeled dTRN-SR from treated extracts, had faster mobilities than those from untreated extracts (Figure 4, compare lanes 1 and 2). Dephosphorylated SR proteins, as well as the 85- and 130-kDa bands showed weaker binding signals, indicating that dTRN-SR preferentially bound phosphorylated versions of these proteins (Figure 4, lane 2).
Immunoblotting of mAb 104 to nuclear extracts from Drosophila (Kc) cells revealed that SRp55/B52 is the prominent immunoreactive polypeptide (Figure 4, lane 8), whereas 35S-labeled dTRN-SR revealed several protein bands with molecular weights of ∼26, 34, 55, 79, and 100 kDa. Detection of proteins corresponding to 26–79 kDa was strongly diminished after dephosphorylation (Figure 4, lane 6), demonstrating that dTRN-SR recognized weakly unphosphorylated proteins. Consistent with this result, purified recombinant hSF2/ASF and dASF expressed in a baculovirus system (bSF2/ASF and bdASF), where the phosphorylation of recombinant proteins is expected to take place, bind more efficiently dTRN-SR (Figure 4, lanes 13 and 14) than unphosphorylated versions expressed in bacteria (eSF2/ASF and edASF; Figure 4, lanes 11 and 12). However, dephosphorylation did not alter the intensity of the 100-kDa band, whose identity is presently unknown (Figure 4, compare lanes 5 and 6). The 26–79-kDa protein bands have sizes consistent with those of recently identified SR proteins from Drosophila, and therefore are likely to be Drosophila SR proteins (see below). The interaction between dTRN-SR and Drosophila SR proteins is specific, because probing yeast S. cerevisiae whole-cell extracts with 35S-labeled dTRN-SR did not reveal any reactive protein (Figure 4, lane 7). Indeed, yeast S.cerevisiae does not have SR proteins and mAb 104 does not cross-react with any protein in yeast (Figure 4, lane10).
To test whether another member of impβ family was capable of interacting with SR proteins, we performed a Far Western experiment with dTRN, an ortholog to human TRN1 and S. cerevisiae Kap104p. TRN1 binds the M9 sequence of hnRNP A1 and is responsible for targeting this protein to the nucleus (Pollard et al., 1996), whereas Kap104p plays a role in the nuclear import of a yeast hnRNP-like protein Nab2p (Aitchison et al., 1996). Our data (lanes 15–18) clearly show that protein bands revealed by 35S-labeled dTRN were different from those detected by dTRN-SR in HeLa and Kc extracts. In Drosophila extracts, for example, there were two sets of proteins that interacted with dTRN, one at ∼75 kDa, and others between 30 and 40 kDa (Figure 4, lane 17). The latter proteins are probably Drosophila hnRNPs, Squid (SqdB and SqdS)/hp40, known to bind dTRN (Norvell et al., 1999). Unlike the interaction between dTRN-SR and SR proteins, the interaction between dTRN and its target proteins was not sensitive to dephosphorylation (Figure 4, lanes 16 and 18). Thus, only dTRN-SR, but not dTRN, binds Drosophila SR proteins.
dTRN-SR Binds Specifically to RS Domain of SR Proteins
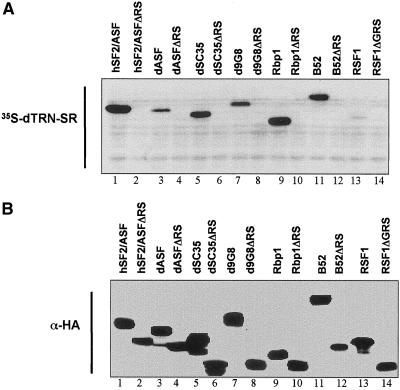
The Far Western experiments described above demonstrated that dTRN-SR does not bind proteins from yeast whole-cell extracts. This finding prompted us to use yeast S. cerevisiae as model system to examine the potential requirement of different SR proteins domains and eventually RSF1 for the interaction with dTRN-SR. To this end, both wild-type and deletion mutants deleted in the RS domain (ΔRS) of hSF2/ASF and Drosophila SR proteins and GRS domain (ΔGRS) of RSF1 were expressed from a multicopy plasmid under control of the GAL10 promoter in yeast. The recombinant proteins contained an N-terminal HA-tag to facilitate their detection with an anti-HA antibody. Western blotting (Figure 5B) of total yeast extracts with an anti-HA antibody confirmed the expression of full-length and deletion mutants in a wild-type yeast strain. Probing duplicated filters with 35S-labeled dTRN-SR revealed that dTRN-SR bound efficiently to hSF2/ASF, dASF, dSC35, d9G8, Rbp1, and B52, but only weakly to RSF1 (Figure 5A, compare lanes 1, 3, 5, 7, 9, 11, and lane 13). In sharp contrast, none of the mutant proteins deleted in the RS domain (hSF2/ASFΔRS, dASFΔRS, dSC35ΔRS, d9G8ΔRS, Rbp1ΔRS, and B52ΔRS) or GRS domain (RSF1ΔGRS) were detected with 35S-labeled dTRN-SR, showing that the RS domain is required for interaction between dTRN-SR and SR proteins. The lack of recognition between dTRN-SR and proteins deleted in the RS domain or GRS domain provides further evidence that the observed interaction between dTRN-SR and the SR proteins or RSF1 is specific.
Figure 5.
Binding of dTRN-SR to SR proteins, RSF1 and deletion mutants expressed in wild-type yeast strains. Total extracts prepared from yeast expressing indicated recombinant proteins (lanes 1–14) were assayed by either Far Western analysis by using 35-S-labeled dTRN-SR (A) or Western blot analysis by using 3F10 anti-HA antibody (B). Both panels represent two independent migrations.
dTRN-SR Serves as an Import Receptor for Drosophila SR Proteins and RSF1 In Vivo
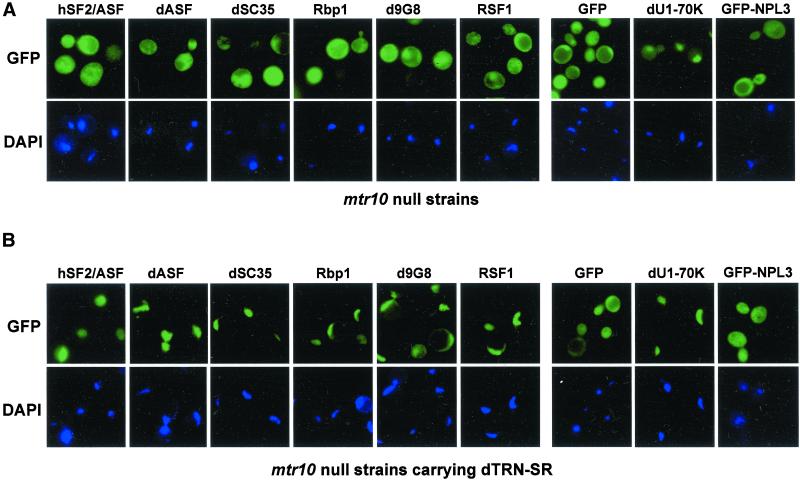
The RNA binding protein Npl3p that has been implicated in mRNA transport (Lee et al., 1996) is one of the yeast proteins, that resembles both hnRNP and SR proteins from mammalian cells (Siebel et al., 1999; Gilbert et al., 2001). Curiously, the nuclear import receptor for Npl3p was identified as Mtr10p, the yeast relative of dTRN-SR (Senger et al., 1998). Thus, it is possible that SR proteins in yeast use Mtr10p as a nuclear import receptor (Yeakley et al., 1999). Given that deletion of MTR10 resulted in cytoplasmic accumulation of Npl3p (Pemberton et al., 1997; Senger et al., 1998), the latter scenario predicts that in the absence of Mtr10p, SR proteins and potentially RSF1 should also accumulate in the cytoplasm. We tested this hypothesis by expressing GFP-SR fusion proteins in an MTR10 deletion mutant. To avoid overexpression of these fusion proteins, they were expressed using a centromeric plasmid without induction (see MATERIALS AND METHODS). The levels of expression were monitored by Western blot analysis with an anti-GFP antibody (our unpublished data). When living cells were analyzed by fluorescence microscopy, GFP-SF2/ASF, GFP-dASF, GFP-dSC35, GFP-Rbp1, GFP-d9G8, GFP-RSF1, and GFP accumulated in the cytoplasm of the mtr10 mutant (Figure 6A). Similar cytoplasmic localizations were observed in wild-type cells (our unpublished data), indicating that the lack of Mtr10p was not responsible for the cytoplasmic accumulation of SR proteins. As expected, GFP-Npl3 also had a cytoplasmic distribution in the mtr10 mutant (Figure 6A), whereas it had a nuclear localization in wild-type cells (our unpublished data). As a control, we analyzed the localization of Drosophila U1–70K fused to GFP (Figure 6A). dU1–70K, a specific protein associated with U1 snRNP, contains a classical nuclear localization signal (Romac et al., 1994), and as such its localization should not be affected by the MTR10 deletion. As expected, no inhibition of nuclear import of this protein was observed in either the mtr10 mutant (Figure 6A) or wild-type cells (our unpublished data), again showing that Mtr10p could not replace dTRN-SR for nuclear import of Drosophila SR proteins exogenously expressed in yeast. These results are also consistent with the idea that other nuclear import receptors, like Kap104, the functional homolog of dTRN (Aitchison et al., 1996; Siomi et al., 1998), cannot replace dTRN-SR to mediate nuclear localization of SR proteins in yeast. Moreover, none of the SR proteins expressed in yeast were able to bind dTRN, as revealed by Far Western (our unpublished data).
Figure 6.
Nuclear import of SR proteins and RSF1 requires dTRN-SR in yeast cells. Direct fluorescence of GFP-hSF2ASF, GFP-dASF, GFP-dSC35, GFP-Rpb1, GFP-d9G8, GFP-RSF1, GFP, GFP-dU1–70K, and GFP-NPL3 was analyzed after transformation in either mtr 10::HIS3 cells (A) or mtr 10::HIS3 cells complemented with c-myc-dTRN-SR (B). The position of nuclei was confirmed by DAPI staining of the transformed cells. Bar, 5 μm.
To test whether mislocalization of GFP-SR protein fusions in yeast could be corrected by dTRN-SR, mtr10 mutants expressing Drosophila SR proteins, hSF2/ASF as well as RSF1 were transformed with plasmids expressing c-myc–tagged dTRN-SR. Immunobloting with anti-c-myc antibodies revealed that similar levels of the c-myc–dTRN-SR fusion were expressed (our unpublished data). As shown in Figure 6B, expression of c-myc-dTRN-SR in mtr10 mutant strains induced dramatic changes in the localization of GFP-Drosophila SR proteins (Figure 6B), hSF2/ASF (Figure 6B, 1) as well as RSF1 (Figure 6B), which exhibited an exclusive intranuclear location in the complemented strains. However, the distribution of GFP (Figure 6B), dU1–70K (Figure 6B) and Npl3 (Figure 6B) remained unchanged. Taken together, these data show that dTRN-SR specifically mediates the nuclear import of Drosophila SR proteins as well as their antagonist RSF1.
DISCUSSION
In this report we identify the Drosophila dTRN-SR, homologous to human TRN-SR2, as a nuclear import receptor for both Drosophila SR proteins and their antagonist RSF1. The extensive conservation between vertebrate and Drosophila dTRN-SR supports the hypothesis that an important event in the regulation of splicing takes place at the level of nuclear uptake of SR proteins and their antagonist. Considering that the SR- and RSF1-related proteins can affect splice site selection in a concentration-dependent manner, the regulation of this nuclear traffic of splicing factors may play an important role in the regulation of alternative splicing. Previous studies showed that protein kinases that modify the RS domain of SR proteins may contribute to their spatial and temporal regulation (Tazi et al., 1997), as well as to the modulation of their activity (Wang and Manley, 1997). In this context, it is significant that phosphorylation of these factors alters their interaction with dTRN-SR. Using far Western experiments, we have been able to show that both human and Drosophila SR proteins interact with dTRN-SR in a phosphorylation-dependent manner. Like the mammalian TRN-SR2, dTRN-SR preferentially associates with phosphorylated SR proteins. Thus, these results demonstrate the high degree of evolutionary conservation of function of the transportin pathway and corroborate previous work in mammalian cells showing that phosphorylation of the RS domain is an important determinant in the nuclear uptake of SR proteins (Yeakley et al., 1999; Lai et al., 2000, 2001).
Although TRN-SR2 was only shown to interact with SR proteins, this study provides the first evidence that the Drosophila homolog dTRN-SR is able to bind both SR proteins and the splicing repressor RSF1. The most prominent feature of the GRS domain of RSF1, which mediates interaction with dTRN-SR, is its abundance in Gly, Ser, and Arg, but its primary sequence is not significantly similar to the RS domain of the SR protein. The observed interaction of dTRN-SR with the GRS domain strengthens the idea that dTRN-SR recognizes its import substrates not only via a primary sequence but also by secondary and/or tertiary structural features. Consistent with this idea, the RS domains of individual vertebrate SR proteins are conserved only in terms of their overall composition and the presence of many consecutive RS or SR dipeptides, whereas several Drosophila SR proteins have a glycine hinge region between the RNA binding domain [with one or two RRM(s)] and the RS repeats. Significantly, the Drosophila homologue of hSF2/ASF, dASF, which lacks the RS repeats and instead has a G-rich region at the RS domain (Allemand et al., 2001), interacted with dTRN-SR and was efficiently imported in yeast complemented with dTRN-SR (Figures 4 and 6). Furthermore, although phosphorylation of the RS domain by SRPK1 has been shown to be critical for efficient nuclear import of SR fusion proteins via TRN-SR2, this does not seem to apply for dTRN-SR. RSF1 and dASF, for instance, which are not substrates for SRPK kinases from different organisms (Allemand et al., 2001; our unpublished data), interacted with dTRN-SR in a RS- or GRS-domain–dependent manner and were efficiently imported both in S2 and yeast cells, provided that the cells contained dTRN-SR. This result is consistent with recent reports showing that phosphorylation by SRPKs promotes shuttling of SR proteins between the nucleus and cytoplasm (Allemand et al., 2001; Gilbert et al., 2001). Thus, the mechanism by which dTRN-SR recognizes its target proteins is of considerable interest and remains to be clarified. The yeast system may be useful for analyzing the phosphorylation sites of RS or GRS domains at different levels of SR kinases and for examining whether differentially phosphorylated RS or GRS domains exhibit different affinities to dTRN-SR.
Another striking result of our analysis was the finding that the RS domain of all SR proteins tested, was sufficient to trigger nuclear localization in S2 cells, whereas in mammalian cells SR proteins containing two RRMs, such as SF2/ASF, dASF and B52/SRp55, did not require the RS domain for proper nuclear localization (Allemand et al., 2001). It is therefore possible that in mammalian cells, there are at least two nuclear import pathways for SR proteins: RS-dependent and RS-independent pathways. This could explain why mammalian cells contain two transportins, TRN-SR1 and TRN-SR2, mediating nuclear import of SR proteins. Given that TRN-SR2 is capable of targeting phosphorylated but not unphosphorylated SR proteins to the nucleus (Lai et al., 2001) and that TRN-SR1 does not seem to have such a requirement (Kataoka et al., 1999), it is tempting to speculate that TRN-SR1 is responsible for the import of SR proteins regardless of phosphorylation and/or presence or absence of the RS domain. In contrast, Drosophila cells only have one transportin for both SR proteins and RSF1, which do not have a classical RS domain.
During initial steps of the spliceosome assembly, the RS or GRS domain of SR proteins and RSF1 are used for a variety of protein–protein interactions, which play crucial roles in splice site selection. Herein, we have shown that these domains can also mediate a specific interaction with dTRN-SR. Our Far Western experiments demonstrate that dTRN-SR has the ability to interact with multiple RS-containing proteins from two highly divergent species, Drosophila and human. However, we noted a weak binding of dTRN-SR to human SRp75, when this protein was abundant. A similar result was also obtained with TRN-SR1 (Kataoka et al., 1999), suggesting that SRp75 either has a different receptor or is difficult to renature under the conditions used for Far Western analysis. We favor the latter possibility because Far Western experiments performed with labeled SR proteins also showed weaker binding to SRp75 compared with other SR proteins (Labourier et al., 1999b; Barnard and Patton, 2000).
Thus, this work paves the way toward a molecular genetic analysis of the biological role of dTRN-SR, which may allow the elucidation of factors modifying the activity of SR proteins. The interaction between dTRN-SR and target proteins is so robust that it can be used to screen expression libraries with 35S-labeled dTRN-SR. Among potential candidates, are the 100- and the 85-kDa proteins identified from Drosophila Kc (Figure 4, lanes 5 and 6) and HeLa (Figure 4, lanes 1 and 2) nuclear extracts, respectively. Given that SR proteins and their antagonist RSF1 are specific targets for dTRN-SR, we may identify novel factors that regulate splice site selection by regulating the effects of some SR proteins. Further studies designed to determine whether the 85-kDa band corresponds to SRrp86, which has such a function in splicing, should confirm this prediction.
ACKNOWLEDGMENTS
We are grateful to J. Soret, H.M. Bourbon, E. Kremer, and N. Taylor for helpful discussions. This work was supported by a grant from the Association pour la Recherche sur le Cancer and Center National de Recherche Scientifique. E.A. was supported by a graduate fellowship from the Ministère de l' Education Nationale, de la Recherche et de la Technologie, and benefited from graduate training fellowships from the Association pour la Recherche sur le Cancer and FRM. S.D. benefited from a Center National de Recherche Scientifique “poste rouge” and postdoctoral fellowship from the FRM.
Abbreviations used:
- dTRN
Drosophila transportin
- dTRN-SR
Drosophila transportin-SR
- dU1–70K
Drosophila U1 small nuclear ribonucleoprotein (snRNP)-70K specific protein
- GFP
green fluorescent protein
- GRS
glycine-, arginine-, and serine-rich
- GST
glutathione S-transferase
- HnRNP
heterogenous nuclear ribonucleoprotein
- RRM
RNA recognition motif
- RS
arginine- and serine-rich
- SR
serine- and arginine-rich
- SRPK
SR protein-specific kinase
- TRN1
transportin 1
- TRN-SR
transportin-SR
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–02–0102. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–02–0102.
REFERENCES
- Aitchison JD, Blobel G, Rout MP. Kap104p: a karyopherin involved in the nuclear transport of messenger RNA binding proteins. Science. 1996;274:624–627. doi: 10.1126/science.274.5287.624. [DOI] [PubMed] [Google Scholar]
- Allemand E, Gattoni R, Bourbon HM, Stevenin J, Caceres JF, Soret J, Tazi J. Distinctive features of Drosophila alternative splicing factor RS domain: implication for specific phosphorylation, shuttling, and splicing activation. Mol Cell Biol. 2001;21:1345–1359. doi: 10.1128/MCB.21.4.1345-1359.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrein H, Hedley ML, Maniatis T. The role of specific protein-RNA and protein-protein interactions in positive and negative control of pre-mRNA splicing by Transformer 2. Cell. 1994;76:735–746. doi: 10.1016/0092-8674(94)90512-6. [DOI] [PubMed] [Google Scholar]
- Barnard DC, Patton JG. Identification and characterization of a novel serine-arginine-rich splicing regulatory protein. Mol Cell Biol. 2000;20:3049–3057. doi: 10.1128/mcb.20.9.3049-3057.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blencowe BJ. Exonic splicing enhancers: mechanism of action, diversity and role in human genetic diseases. Trends Biochem. 2000;25:106–110. doi: 10.1016/s0968-0004(00)01549-8. [DOI] [PubMed] [Google Scholar]
- Bordonne R. Functional characterization of nuclear localization signals in yeast Sm proteins. Mol Cell Biol. 2000;20:7943–7954. doi: 10.1128/mcb.20.21.7943-7954.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caceres JF, Misteli T, Screaton GR, Spector DL, Krainer AR. Role of the modular domains of SR proteins in subnuclear localization and alternative splicing specificity. J Cell Biol. 1997;138:225–238. doi: 10.1083/jcb.138.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caceres JF, Screaton GR, Krainer AR. A specific subset of SR proteins shuttles continuously between the nucleus and the cytoplasm. Genes Dev. 1998;12:55–66. doi: 10.1101/gad.12.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camasses A, Bragado-Nilsson E, Martin R, Seraphin B, Bordonne R. Interactions within the yeast Sm core complex: from proteins to amino acids. Mol Cell Biol. 1998;18:1956–1966. doi: 10.1128/mcb.18.4.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwill K, Pawson T, Andrews B, Prasad J, Manley JL, Bell JC, Duncan PI. The Clk/Sty protein kinase phosphorylates SR splicing factors and regulates their intranuclear distribution. EMBO J. 1996;15:265–275. [PMC free article] [PubMed] [Google Scholar]
- Fu XD, Maniatis T. Isolation of a complementary DNA that encodes the mammalian splicing factor SC35. Science. 1992;256:535–538. doi: 10.1126/science.1373910. [DOI] [PubMed] [Google Scholar]
- Gilbert W, Siebel CW, Guthrie C. Phosphorylation by Sky1p promotes Npl3p shuttling and mRNA dissociation. RNA. 2001;7:302–313. doi: 10.1017/s1355838201002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorlich D, Kutay U. Transport between the cell nucleus and the cytoplasm. Annu Rev Cell Dev Biol. 1999;15:607–660. doi: 10.1146/annurev.cellbio.15.1.607. [DOI] [PubMed] [Google Scholar]
- Graveley BR. Sorting out the complexity of SR protein functions. RNA. 2000;6:1197–1211. doi: 10.1017/s1355838200000960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui JF, Lane WS, Fu XD. A serine kinase regulates intracellular localization of splicing factors in the cell cycle. Nature. 1994;369:678–682. doi: 10.1038/369678a0. [DOI] [PubMed] [Google Scholar]
- Hedley ML, Amrein H, Maniatis T. An amino acid sequence motif sufficient for subnuclear localization of an arginine/serine-rich splicing factor. Proc Natl Acad Sci USA. 1995;92:11524–11528. doi: 10.1073/pnas.92.25.11524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J, Donald KA, Griffiths DE, Donald G. DMSO-enhanced whole cell yeast transformation. Nucleic Acids Res. 1991;19:5791. doi: 10.1093/nar/19.20.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaurralde E, Adam S. Transport of macromolecules between the nucleus and the cytoplasm. RNA. 1998;4:351–364. [PMC free article] [PubMed] [Google Scholar]
- Kadowaki T, Chen S, Hitomi M, Jacobs E, Kumagai C, Liang S, Schneiter R, Singleton D, Wisniewska J, Tartakoff AM. Isolation and characterization of Saccharomyces cerevisiaemRNA transport-defective (mtr) mutants. J Cell Biol. 1994;126:649–659. doi: 10.1083/jcb.126.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka N, Bachorik JL, Dreyfuss G. Transportin-SR, a nuclear import receptor for SR proteins. J Cell Biol. 1999;145:1145–1152. doi: 10.1083/jcb.145.6.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohtz JD, Jamison SF, Will CL, Zuo P, Luhrmann R, Garcia-Blanco MA, Manley JL. Protein-protein interactions and 5′-splice-site recognition in mammalian mRNA precursors. Nature. 1994;368:119–124. doi: 10.1038/368119a0. [DOI] [PubMed] [Google Scholar]
- Labourier E, Allemand E, Brand S, Fostier M, Tazi J, Bourbon HM. Recognition of exonic splicing enhancer sequences by the Drosophila splicing repressor RSF1. Nucleic Acids Res. 1999a;27:2377–2386. doi: 10.1093/nar/27.11.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labourier E, Bourbon HM, Gallouzi I, Fostier M, Allemand E, Tazi J. Antagonism between RSF1 and SR proteins for both splice site recognition in vitro and Drosophila development. Genes Dev. 1999b;13:740–753. doi: 10.1101/gad.13.6.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai MC, Lin RI, Huang SY, Tsai CW, Tarn WY. A human importin-β family protein, transportin-SR2, interacts with the phosphorylated RS domain of SR proteins. J Biol Chem. 2000;275:7950–7957. doi: 10.1074/jbc.275.11.7950. [DOI] [PubMed] [Google Scholar]
- Lai MC, Lin RI, Tarn WY. Transportin-SR2 mediates nuclear import of phosphorylated SR proteins. Proc Natl Acad Sci USA. 2001;98:10154–10159. doi: 10.1073/pnas.181354098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MS, Henry M, Silver PA. A protein that shuttles between the nucleus and the cytoplasm is an important mediator of RNA export. Genes Dev. 1996;10:1233–1246. doi: 10.1101/gad.10.10.1233. [DOI] [PubMed] [Google Scholar]
- Li H, Bingham PM. Arginine/serine-rich domains of the su(wa) and tra RNA processing regulators target proteins to a subnuclear compartment implicated in splicing. Cell. 1991;67:335–342. doi: 10.1016/0092-8674(91)90185-2. [DOI] [PubMed] [Google Scholar]
- Manley JL, Tacke R. SR proteins and splicing control. Genes Dev. 1996;10:1569–1579. doi: 10.1101/gad.10.13.1569. [DOI] [PubMed] [Google Scholar]
- Mattaj IW, Englmeier L. Nucleocytoplasmic transport: the soluble phase. Annu Rev Biochem. 1998;67:265–306. doi: 10.1146/annurev.biochem.67.1.265. [DOI] [PubMed] [Google Scholar]
- Mintz PJ, Spector DL. Compartmentalization of RNA processing factors within nuclear speckles. J Struct Biol. 2000;129:241–251. doi: 10.1006/jsbi.2000.4213. [DOI] [PubMed] [Google Scholar]
- Misteli T, Caceres JF, Clement JQ, Krainer AR, Wilkinson MF, Spector DL. Serine phosphorylation of SR proteins is required for their recruitment to sites of transcription in vivo. J Cell Biol. 1998;143:297–307. doi: 10.1083/jcb.143.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumberg D, Muller R, Funk M. Regulatable promoters of Saccharomyces cerevisiae: comparison of transcriptional activity and their use for heterologous expression. Nucleic Acids Res. 1994;22:5767–5768. doi: 10.1093/nar/22.25.5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norvell A, Kelley RL, Wehr K, Schupbach T. Specific isoforms of squid, a Drosophila hnRNP, perform distinct roles in Gurken localization during oogenesis. Genes Dev. 1999;13:864–876. doi: 10.1101/gad.13.7.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemberton LF, Blobel G, Rosenblum JS. Transport routes through the nuclear pore complex. Curr Opin Cell Biol. 1998;10:392–399. doi: 10.1016/s0955-0674(98)80016-1. [DOI] [PubMed] [Google Scholar]
- Pemberton LF, Rosenblum JS, Blobel G. A distinct and parallel pathway for the nuclear import of a mRNA-binding protein. J Cell Biol. 1997;139:1645–1653. doi: 10.1083/jcb.139.7.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard VW, Michael WM, Nakielny S, Siomi MC, Wang F, Dreyfuss G. A novel receptor-mediated nuclear protein import pathway. Cell. 1996;86:985–994. doi: 10.1016/s0092-8674(00)80173-7. [DOI] [PubMed] [Google Scholar]
- Romac JM, Graff DH, Keene JD. The U1 small nuclear ribonucleoprotein (snRNP) 70K protein is transported independently of U1 snRNP particles via a nuclear localization signal in the RNA-binding domain. Mol Cell Biol. 1994;14:4662–4670. doi: 10.1128/mcb.14.7.4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rout MP, Blobel G, Aitchison JD. A distinct nuclear import pathway used by ribosomal proteins. Cell. 1997;89:715–725. doi: 10.1016/s0092-8674(00)80254-8. [DOI] [PubMed] [Google Scholar]
- Rubin GM, Hong L, Brokstein P, Evans-Holm M, Frise E, Stapleton M, Harvey DA. A Drosophilacomplementary DNA resource. Science. 2000;287:2222–2224. doi: 10.1126/science.287.5461.2222. [DOI] [PubMed] [Google Scholar]
- Senger B, Simos G, Bischoff FR, Podtelejnikov A, Mann M, Hurt E. Mtr10p functions as a nuclear import receptor for the mRNA-binding protein Npl3p. EMBO J. 1998;17:2196–2207. doi: 10.1093/emboj/17.8.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebel CW, Feng L, Guthrie C, Fu XD. Conservation in budding yeast of a kinase specific for SR splicing factors. Proc Natl Acad Sci USA. 1999;96:5440–5445. doi: 10.1073/pnas.96.10.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siomi MC, Fromont M, Rain JC, Wan L, Wang F, Legrain P, Dreyfuss G. Functional conservation of the transportin nuclear import pathway in divergent organisms. Mol Cell Biol. 1998;18:4141–4148. doi: 10.1128/mcb.18.7.4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sureau A, Gattoni R, Dooghe Y, Stevenin J, Soret J. SC35 autoregulates its expression by promoting splicing events that destabilize its mRNAs. EMBO J. 2001;20:1785–1796. doi: 10.1093/emboj/20.7.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacke R, Manley JL. Determinants of SR protein specificity. Curr Opin Cell Biol. 1999;11:358–362. doi: 10.1016/S0955-0674(99)80050-7. [DOI] [PubMed] [Google Scholar]
- Tazi J, Rossi F, Labourier E, Gallouzi I, Brunel C, Antoine E. DNA topoisomerase I: customs officer at the border between DNA and RNA worlds? J Mol Med. 1997;75:786–800. doi: 10.1007/s001090050168. [DOI] [PubMed] [Google Scholar]
- Vetter IR, Arndt A, Kutay U, Gorlich D, Wittinghofer A. Structural view of the Ran-Importin β interaction at 2.3 A resolution. Cell. 1999;97:635–646. doi: 10.1016/s0092-8674(00)80774-6. [DOI] [PubMed] [Google Scholar]
- Wang J, Manley JL. Regulation of pre-mRNA splicing in metazoa. Curr Opin Genet Dev. 1997;7:205–211. doi: 10.1016/s0959-437x(97)80130-x. [DOI] [PubMed] [Google Scholar]
- Wu JY, Maniatis T. Specific interactions between proteins implicated in splice site selection and regulated alternative splicing. Cell. 1993;75:1061–1070. doi: 10.1016/0092-8674(93)90316-i. [DOI] [PubMed] [Google Scholar]
- Yeakley JM, Tronchere H, Olesen J, Dyck JA, Wang HY, Fu XD. Phosphorylation regulates in vivo interaction and molecular targeting of serine/arginine-rich pre-mRNA splicing factors. J Cell Biol. 1999;145:447–455. doi: 10.1083/jcb.145.3.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo P, Maniatis T. The splicing factor U2AF35 mediates critical protein-protein interactions in constitutive and enhancer-dependent splicing. Genes Dev. 1996;10:1356–1368. doi: 10.1101/gad.10.11.1356. [DOI] [PubMed] [Google Scholar]