Abstract
Researchers have documented that exposure to different kinds of psychosocial stressors can lead to emotional difficulties and, further, that heightened reactivity to stress can moderate these associations. Recently, investigators have distinguished among threat, deprivation, and unpredictability as different dimensions of early life stress (ELS). It is not clear, however, whether reactivity in specific stress response systems functions as a diathesis to lead to emotional difficulties following exposure to these dimensions of ELS. In this study (N = 154) we examined whether stress reactivity, assessed across different psychobiological systems during the Trier Social Stress Test, is a unitary or multidimensional construct, and if reactivity differentially moderates the associations between ELS dimensions and adolescents’ susceptibility to emotional and behavioral problems two years later. A factor analysis conducted on stress reactivity measures yielded two factors: one composed of reactivity in heart rate, heart rate variability, and cortisol, and one composed of reactivity in skin conductance and self-reported mood. These two factors independently moderated the associations between early unpredictability and subsequent emotional problems. For each factor, the combination of higher unpredictability and higher stress reactivity predicted higher emotional problems; stress reactivity factors were not significant moderators of the effects of threat and deprivation. Our findings suggest that increased stress reactivity, assessed across several domains of functioning, functions as a diathesis that interacts with ELS characterized by unpredictability to predict subsequent mental health difficulties in adolescents and, further, that low stress reactivity buffers against mental health difficulties in adolescents who have experienced unpredictability early in life.
Keywords: Stress reactivity, Early life stress, Diathesis-stress, Adolescent development
Highlights (85 characters each, including spaces)
-
•
We factor-analyzed five measures of reactivity to a psychosocial stressor.
-
•
Heart rate, heart rate variability, and cortisol reactivity formed one factor.
-
•
Skin conductance reactivity and mood reactivity formed another factor.
-
•
Both factors moderated the association of unpredictability with emotional problems.
-
•
Lower reactivity buffered the effects of early life unpredictability.
1. Introduction
Over the past several decades, researchers have consistently documented associations between exposure to early life stress (ELS) and the subsequent emergence of maladaptive behaviors (e.g., Alastalo et al., 2013; Chahal et al., 2022). It is important to recognize, however, that not everyone who experiences ELS develops emotional and behavioral problems (Hostinar et al., 2023). Daníelsdóttir (2024) recently demonstrated in a large twin study that although ELS exposure increases risk for developing psychopathology, there is substantial variability in this risk that is likely attributable to such factors as the home environment (e.g., socioeconomic status) and genetic predisposition. In this context, the diathesis-stress hypothesis posits that some individuals have a specific vulnerability that, in the face of exposure to stressful events, leads to emotional and behavioral difficulties (Belsky and Pluess, 2009). In attempting to identify individuals at the greatest risk for developing emotional problems following ELS, a number of investigators have examined a range of measures of susceptibility, including stress reactivity (e.g. (Daches et al., 2019; Turner et al., 2020; Winiarski et al., 2018), generally defined as the body's immediate response to a stressor (e.g., (Liu et al., 2017; Mücke et al., 2018). If the diathesis-stress hypothesis is valid in the context of stress reactivity, which researchers have posited reflects a sensitivity to environmental stimuli (Boyce and Ellis, 2005), then (only) individuals with a diathesis of altered stress reactivity would be expected to develop symptoms of psychopathology following ELS exposure.
There are two important questions regarding the nature of stress reactivity that must be resolved if we are to continue to make progress in this field. The first question is whether there are meaningfully distinct patterns of stress reactivity. Investigators have begun to examine whether and how the different biological stress reactivity systems respond to a single stressor. Most commonly, researchers have examined alterations in the hypothalamic-pituitary-adrenal (HPA) axis (e.g., Juruena et al., 2020), the sympathetic and parasympathetic nervous systems (SNS and PNS, respectively) (Diamond et al., 2012; Lin et al., 2018), and self-reports or observations of affect (Gotlib et al., 2021; Swales et al., 2018) in response to experiencing a stressor. For example, Glier et al. (2022) found that adolescents respond to a stressor either with lower PNS reactivity and higher SNS and HPA-axis reactivity, or with higher PNS and low to moderate SNS and HPA-axis reactivity; however, these investigators did not examine whether or how these different patterns of reactivity were related to subsequent emotional difficulties. From a somewhat different perspective, latent profile analyses of stress reactivity, which cluster together participants who respond similarly across a number of measures, have yielded at least three groups, or profiles, of individuals: those who react to stressors primarily via the HPA-axis; those who react to stressors primarily via the autonomic nervous system (ANS); and those who react to stressors across all measured biological systems (Kupper et al., 2021; Quas et al., 2014; Rudd et al., 2021). It is noteworthy, however, that there is substantial variability in the number of profiles that are identified, in the proportion of participants in each profile, and in the characteristics of profiles beyond the three described above. Further, it is still not clear from these approaches and analyses whether patterns of stress reactivity are implicated differentially in the development of emotional and behavioral problems.
The second, related, question that we believe researchers should address is whether there is specificity in the biological stress response systems that best index vulnerability to ELS exposure and, further, if that specificity differs according to the dimension of ELS experienced. Investigators have recently distinguished among three main dimensions or types of ELS: threat, or forces that pose a risk to an individual's safety; deprivation, or a lack of expected environmental stimuli and unpredictability, or variability in the availability of resources or safety (Chahal et al., 2022; McLaughlin et al., 2021). Recent work suggests that there is some specificity in the stress response system that indexes vulnerability to the development of emotional problems. For example, Somers et al. (2017) found that young adults who experienced greater childhood family adversity had higher depressive symptoms and less positive affect only if they also had higher increases in heart rate in response to a laboratory interpersonal stressor. Similarly, Steeger et al. (2017) found that adolescents with a history of stressful family events had more internalizing and externalizing problems if they also exhibited heightened cortisol reactivity in response to a laboratory conflict task (see also (Eisenlohr-Moul et al., 2018; Kushner et al., 2016) for findings that blunted reactivity increases risk) Importantly, however, each of these studies only examined one type of ELS; none have tested whether different types of ELS may lead to or predict stress responses in different biological systems.
The present study was designed to examine patterns of stress reactivity in different psychobiological systems and test whether these patterns differentially moderate the association between dimensions of ELS and subsequent emotional and behavioral problems. Specifically, we first explored whether measures of stress reactivity assessed across different functional domains (i.e., changes in heart rate, heart rate variability, skin conductance, cortisol secretion, and self-reported affect) in response to a single stressor (the Trier Social Stress Test) cohere to form a unitary construct or factor. We then explored whether stress reactivity to the Trier Social Stress Test interacts with threat, unpredictability, and deprivation to predict emotional problems. And as a sensitivity analysis, we explored whether individual measures of stress reactivity interact with ELS to predict emotional problems. As Hostinar et al. (2023) posited, exposure to ELS does not increase individuals' risk of developing specific psychopathological disorders; rather, it seems to increase individuals’ overall risk of experiencing any form of emotional distress. Therefore, we examined general emotional and behavioral problems as our primary outcome and, as a sensitivity analysis, also explored whether early adversity and stress reactivity interact to predict more specific internalizing and externalizing problems.
2. Methods
2.1. Participants
Using flyers and online advertisements, we recruited adolescents from the California San Francisco Bay Area who were in the early stages of puberty to participate in a study of adolescent brain development. Exclusion criteria were factors that would preclude MRI scan (e.g., metal implants, braces), a history of major neurological or medical illness, and a lack of fluency in English or severe learning disabilities that would make it difficult for participants to understand the study procedures. Females who reported having started menses were excluded, and boys were matched to girls on Tanner stage in order to obtain participants in early puberty. Participants’ parents or legal guardians provided informed consent and participants provided informed assent. All study procedures were approved by the Stanford University Institutional Review Board.
In total, 229 participants were enrolled in the study. We administered the Trier Social Stress Test (TSST) to 169 participants at baseline (Mage = 12.26, SD = 1.46). Fifteen participants did not have data that were suitable for analysis due to artifact in data signal (with overlap, ten participants had unusable skin conductance data, six had unusable heart rate data, five were missing cortisol data, and one was missing a mood rating), leaving 154 participants with complete TSST data. Participants also reported on their lifetime exposure to ELS at baseline and on their emotional and behavioral problems at a follow-up visit approximately two years after they completed the TSST (Mage = 14.44, SD = 1.63). An additional 15 participants did not complete the follow-up assessment, and a further 19 were missing demographic data or ELS interview. There were no significant differences between participants who did or did not have complete TSST data or between participants who did or did not complete the follow-up visit with respect to age, race, socioeconomic status, or ELS exposure. Male participants, however, were less likely to have completed the follow-up assessment than female participants (X2(1) = 5.21, p = .022). Participants with missing data were therefore excluded from analyses.
2.2. Demographic characteristics
We collected data on participants age, sex, and race at baseline. Of the 154 participants, 54.5% were female (coded as male = 1, female = 2). Participants reported belonging to one of the following racial/ethnic categories: White (46.1%), Black (7.8%), Hispanic (7.8%), Asian (11.0%), multiple races (22.7%), or another race (4.5%). We binarized this variable for ease of interpretation in analyses given the low representation of participants in each category other than White, such that participants were coded as 0 (only White) or 1 (not only White). Participants also completed the Tanner Staging questionnaire of pubertal development (Marshall and Tanner, 1968). Measures of household socioeconomic status are described below.
2.3. Trier Social Stress Task
Participants completed a version of the Trier Social Stress Task (TSST; (Kirschbaum et al., 1993), adapted for children and adolescents, that has been shown to elicit a robust cortisol and autonomic response (Allen et al., 2017; Seddon et al., 2020). Participants fasted for at least 30 min prior to saliva sample collection; 84.1% of the TSST sessions assessments began after 13:00 h. Shortly after arriving at the laboratory, participants provided a mood rating and a saliva sample for cortisol analysis (Sample 1). An experimenter placed electrodes on participants to measure electrocardiogram and skin conductance activity throughout the TSST. Participants were instructed to relax for 5 min, and a second mood rating and saliva sample were obtained afterwards (Sample 2; the mean interval between Samples 1 and 2 was 156 min (SD = 50.20)). The experimenter told the beginning of a story to participants and instructed them to make up an exciting ending to this story within the next 5 min. They were told that a judge would be videotaping them and would evaluate their ending. After the 5-min speech preparation period, participants delivered their story ending to an impartial judge (a young adult male or female), who maintained a neutral expression and took notes on the presentation with the video camera beside them (purportedly, but not actually, recording). After 5 min, the judge asked participants to complete a serial subtraction task aloud for another 5 min. The judge interrupted the participants if they made a mistake and instructed them to start over. A third mood rating and saliva sample were obtained at the end of this 15-min interval (Sample 3, 15 min from the beginning of the TSST; the mean interval between Samples 2 and 3 was 22.98 min [SD = 3.13]). Finally, participants watched a 30-min neutral video clip, during which two more mood ratings and saliva samples were obtained (Samples 4 and 5, 15 and 30 min after the offset of the stressor). The mean intervals between Samples 3 and 4 and between Samples 3 and 5 were 18 (SD = 2.49) and 36 min (SD = 3.43).
2.3.1. Cortisol
All saliva samples were collected using SalivaBio Children's Swabs (Salimetrics, LLC) and stored in a −20 degree C freezer after the conclusion of the TSST. Samples were assayed using a high-sensitivity (0.004 μg/dL) immunoassay kit from Immuno-Biological Laboratories Inc. (Hamburg, Germany; intra- and inter-assay coefficients of variation range between 3% and 5%). Consistent with field recommendations (Stalder et al., 2016) and our prior work (Kircanski et al., 2019), we winsorized cortisol values for each sample that were >2 standard deviations above the mean. A total of 28 (4.12%) cortisol values were winsorized. We calculated the area under the curve with respect to increase (AUCi) to assess total cortisol production (Pruessner et al., 2003). Based on our previous work (Kircanski et al., 2019) showing that peak cortisol levels were observed 30 min from stressor onset (i.e., Sample 4) in the current sample, we also calculated AUCi values for baseline-to-peak stress reactivity (i.e., from Samples 2 to 4).
2.3.2. Mood ratings
Participants rated how stressed they felt (i) after the baseline period; (ii) after the serial subtraction task; and (iii) after each of the 15-min neutral videos. Participants responded to the prompt “How stressed are you feeling right now?” on a scale from 1 (not stressed at all) to 7 (very stressed). We computed a residual of the mood rating after the story telling task by regressing this rating on the baseline mood rating.
2.3.3. Physiological activity was recorded continuously at a sampling rate of 500 Hz using the Biopac MP150 system and AcqKnowledge software package (Biopac systems, Goleta, CA)
Specifically, we recorded participants' cardiovascular activity using the electrocardiogram (ECG) amplifier module and three disposable electrodes positioned in a modified lead II configuration. We also recorded skin conductance level (SCL) with the electrodermal activity (EDA) amplifier module and two disposable electrodes placed on the palm of each participant's nondominant hand.
2.3.4. Cardiac measures
We scored the physiological data in 5-min segments using ANSLAB (Wilhelm and Peyk, 2005), a physiological software package. Trained research staff inspected the ECG signal for artifacts and missing R-peaks. For each 60s, if one R-peak was missing, an R-peak was inserted at a time point halfway between the two adjacent R-peaks. If more than one R-peak was missing, that specific 60-s period was not scored (Berntson et al., 1997). We extracted heart rate and root mean squared successive differences (RMSSD) in heart rate as a measure of heart rate variability (HRV). We calculated a slope from baseline (five 60s intervals) through the story telling (five 60s intervals) and serial subtraction task (five 60s intervals) using the nlme package in R (Pinheiro et al., 2022).
2.3.5. Skin conductance
We also scored skin conductance data in 5-min segments using ANSLAB. Trained research staff visually inspected the signal for artifacts and drops in the signal, which were corrected using the “high smooth” function in ANSLAB. The average SCL for each 5-min segment was extracted. We calculated a slope from baseline through the serial subtraction task using the nlme package in R (Pinheiro et al., 2022).
2.4. Early life stress
Trained research staff administered the Traumatic Events Screening Inventory for Children (TESI-C, (Ribbe, 1996), an interview in which participants were asked about 30 different types of potentially stressful events that the participant ever experienced. Three coders blind to the subject's perspective of the event then rated the objective severity of the event using the UCLA Life Stress Interview coding system (Rudolph et al., 2000). Event severity was rated on a scale from 0 (little to no impact) to 4 (extremely severe impact). We then summed the severity ratings for each type of stressful event reported to form one ELS severity score. See King et al. (2017) for more detailed information about the administration and scoring of the TESI-C in this study. We operationalized and measured dimensions of ELS (i.e., threat, unpredictability, and deprivation) based on work from Chahal and colleagues (2022).
2.4.1. Threat
To index exposure to ELS characterized by threatening events, we summed objective severity scores for the following events: family verbal conflict (experienced directly or indirectly), bullying (verbal or physical), community conflict (violence and verbal conflict), community instability (violence or threats of violence), domestic violence (including threats of violence), emotional abuse, physical abuse (including threats of abuse), mugging or robbery, war or terrorism, sexual abuse (including witnessing abuse), and kidnapping.
2.4.2. Unpredictability
To assess exposure to ELS characterized by unpredictability, we summed the objective severity rating of the following events: moved households, death of someone close, parental divorce, separation from family related to legal issues or travel, family legal problems, witness or experience illness or injury, witness or experience an accident, experience a disaster, and family financial insecurity.
2.4.3. Deprivation
We computed a composite score for deprivation by averaging Z-scored measures of household and neighborhood socioeconomic status (SES). The following indices were standardized with a z-score transformation and averaged. Parent-reported education levels were coded ordinally: 1 = no GED/high school diploma (0.47%), 2 = GED/high school diploma (1.42%), 3 = some college (18.96%), 4 = 2-year college degree (8.53%), 5 = 4-year college degree (37.44%), 6 = master's degree (26.54%), 7 = professional degree (4.74%). Participants' parents reported their annual household income and the number of people living in their household. Income was divided by the 2019 Department of Housing and Urban Development (HUD) low-income limit for the number of inhabitants in Santa Clara County (https://www.huduser.gov/portal/datasets/il.html#2019) (King et al., 2020; Noble et al., 2015). This income-to-needs ratio (INR) was used as a covariate measuring socioeconomic status in analyses where deprivation was not a predictor.
To assess neighborhood level SES we used measures from the California Communities and Environmental Health Screening Tool 3.0 (CalEnviroScreen, https://oehha.ca.gov/calenviroscreen), a tool created by the Office of Environmental Health Hazard Assessment for the California Environmental Protection Agency (Faust et al., 2017). We included Census tract level measures of unemployment (the percentage of the eligible labor force that was not employed from 2011 to 2015), poverty (the percentage of the population living below two times the federal poverty level from 2011 to 2015), and housing burden (the percentage of household that make less than 80% of the HUD Area Median Family Income and also pay more than half of their income to housing costs from 2009 to 2013).
Finally, participants completed the Multidimensional Neglectful Behavior Scale (MNBS; (Kantor et al., 2004). Participants rated 8 items with respect to the degree to which they experienced psychosocial (e.g., “My parents comfort me when I am upset”) and material (“My parents give me enough clothes to keep me warm”) neglect on a scale from 1 (strongly disagree) to 4 (strongly agree). We calculated a total neglect score by summing the scores on items across the MNBS; higher scores indicated higher experiences of neglect. All household and neighborhood indices were standardized and averaged to create a deprivation composite measure.
2.5. Emotional and behavioral problems
Participants reported their symptoms of psychopathology at a follow-up assessment approximately two years after completing the TSST (M interval = 2.17 years; SD = 0.59). Specifically, participants completed the Youth Self-Report (YSR; (Achenbach and Rescorla, 2001), a 112-item questionnaire assessing emotional and behavioral problems. Participants rated how well items describe them on a scale from 0 (not true) to 2 (very true). To operationalize the magnitude of emotional and behavioral difficulties, in our analyses we used raw score for the “total problems” scale of the YSR, composed of the sum of scores on the anxious/depressed, withdrawn/depressed, somatic complaints rule breaking, aggressive behavior, social problems, thought problems, and attention problems subscales.
2.6. Analyses
We entered the heart rate, heart rate variability, cortisol, skin conductance, and mood reactivity variables into an exploratory factor analysis with varimax rotation and Kaiser normalization and retained factors with an eigenvalue greater than or equal to one. We then explored associations between the resulting factor scores, the ELS dimensions, and participants’ YSR total problems score. To test the diathesis-stress hypothesis, we tested interactions between the ELS dimensions and the stress reactivity factor(s) predicting total problems. Participant sex assigned at birth, ethnicity, participant baseline age subtracted from the baseline average, time between baseline and follow-up, household INR (except when testing the deprivation dimension), and hour of day (out of 24) that the TSST was conducted were included as covariates. Follow-up analyses examined the sensitivity of the results to the stress reactivity factor(s), by repeating the regression analyses with the individual reactivity measures used as moderators in place of the factor(s). We also repeated the regressions predicting internalizing and externalizing problems to examine the specificity of the diathesis-stress interaction to predict different domains of emotional and behavioral problems. Finally, we tested whether the analyses were sensitive to pubertal stage of development at baseline.
3. Results
3.1. Participant characteristics
Sociodemographic characteristics of the participants and scores on the measures of early adversity, stress reactivity, and symptoms of psychopathology are presented in Table 1. At baseline 15.3% of participants had a T-score of 65 or above for total emotional and behavioral problems, 16.9% for internalizing problems, and 6.8% for externalizing problems. At follow-up, 22.0% of participants had a T-score above of 65 or above for total emotional and behavioral problems, 21.2% for internalizing problems, and 13.6% for externalizing problems.
Table 1.
Participant characteristics.
| M/% | SD | Range | |
|---|---|---|---|
| Age at Baseline (years) | 12.26 | 1.46 | 9.47–15.85 |
| Income to Needs Ratio | 1.27 | 0.54 | 0.05–1.97 |
| Sex (% female) | 54.5% | – | – |
| Race | |||
| White | 46.1% | – | – |
| Black | 7.8% | – | – |
| Hispanic | 7.8% | – | – |
| Asian | 11.0% | – | – |
| Biracial | 22.7% | – | – |
| Other | 4.5% | – | – |
| Age at Follow-Up (years) | 14.44 | 1.63 | 11.37–19.23 |
| Time of Trier Administration (hours) | 15.40 | 2.83 | 7:00–20:00 |
| Threat | 1.14 | 1.65 | 0.00–9.50 |
| Unpredictability | 4.00 | 3.07 | 0.00–13.50 |
| Deprivation | −0.04 | 0.70 | −1.22-2.05 |
| Trier Heart Rate Reactivity | −0.01 | 0.38 | −0.72-1.43 |
| Trier Heart Rate Variability Reactivity | −0.02 | 0.56 | −1.82-2.31 |
| Trier Cortisol Reactivity | 1.44 | 2.76 | −5.35-9.00 |
| Trier Skin Conductance Reactivity | 0.002 | 0.20 | −1.23-0.65 |
| Trier Mood Stress Reactivity | −0.02 | 0.98 | −1.74-2.81 |
| YSR Total Problems Score | 51.33 | 10.79 | 4.00–118.00 |
| YSR Internalizing Problems Score | 51.48 | 11.25 | 0.00–40.00 |
| YSR Externalizing Problems Score | 48.45 | 9.39 | 0.00–51.00 |
Note: Trier = Trier Social Stress Test; YSR=Youth Self-Report questionnaire.
3.2. Factor analysis of the measures of stress reactivity
A principal axis factor analysis of the heart rate, heart rate variability, cortisol, skin conductance, and mood reactivity variables yielded a Kaiser-Meyer-Olkin measure of sampling adequacy of 0.605, indicating mediocre sampling adequacy (Kaiser and Rice, 1974). Importantly, Bartlett's test of sphericity was significant (X2(10) = 53.93, p < .001), indicating that the data are suitable for factor analysis. The factor analysis yielded two factors with an eigenvalue of one or greater. The first factor, which had an eigenvalue of 1.70 and explained 34.00% of the total variance, was composed of heart rate reactivity, heart rate variability reactivity, and cortisol reactivity (see Table 2). All three of these variables had factor loadings >0.4, with heart rate variability loading negatively. We refer to this factor as the Cardiac and Cortisol Reactivity factor. The second factor, which had an eigenvalue of 1.10 and explained 22.06% of the total variance, was composed of skin conductance and mood reactivity. Both variables had factor loadings between 0.3 and 0.4, suggesting low to moderate correlations with the factor. We refer to this factor as the SCL and Mood Reactivity factor. The two factors were positively correlated (r = 0.274, p < .05). In the supplement we present correlations among the variables in Table S1.
Table 2.
Factor analyses of stress reactivity variables.
| Heart and Cortisol Reactivity | SCL and Mood Reactivity | |
|---|---|---|
| % variance explained | 33.997% | 22.061% |
| Trier Heart Rate Reactivity | 0.779 | |
| Trier Heart Rate Variability Reactivity | −0.495 | |
| Trier Cortisol Reactivity | 0.459 | |
| Trier Skin Conductance Reactivity | 0.382 | |
| Trier Mood Stress Reactivity | 0.318 | |
Note: Trier = Trier Social Stress Test; SCL=Skin Conductance Level.
3.3. Diathesis-stress analyses
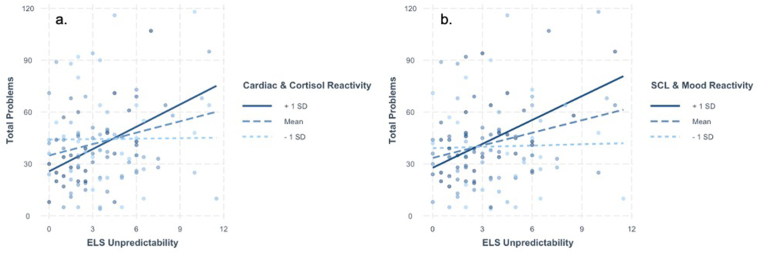
To examine whether the measures of stress reactivity fit a diathesis-stress formulation, we conducted separate regression models testing the interaction of the ELS dimensions and the two stress reactivity factors to predict YSR total problems scores. The results of these analyses are presented in Table 3, Table 4. Both models were statistically significant (Cardiac and Cortisol Reactivity model R2 = 0.097, p = .015; SCL and Mood Reactivity model R2 = 0.087, p = .023). As can be seen from the Tables, higher unpredictability was associated with higher YSR total problems scores. There was also a main effect of Cardiac and Cortisol Reactivity predicting lower total problems scores; there was no main effect of SCL and Mood Reactivity on total problems scores. The interactions between unpredictability and each factor significantly predicted total problems scores. Specifically, the association between unpredictability and total problems was positive and statistically significant at higher (+1 SD: B = 4.30, SE = 1.43, p < .001), but not lower (−1 SD: B = 0.09, SE = 1.14, p = .930) levels of Cardiac and Cortisol Reactivity (see Fig. 1a). Similarly, the association between total problems was positive and statistically significant at higher (+1 SD: B = 4.60, SE = 1.43, p < .001) but not lower (−1 SD: B = 0.25, SE = 1.17, p = .830) levels of SCL and Mood Reactivity (see Fig. 1b). There were no main effects of threat or deprivation on total problems, and threat and deprivation did not interact with either factor to predict YSR scores (see Supplemental Tables S3–6).
Table 3.
Heart and cortisol reactivity moderates the association between ELS threat and unpredictability problems.
| B | 95% CI | p | |
|---|---|---|---|
| Baseline Age | 1.915 | −1.793, 5.624 | 0.308 |
| Income to Needs Ratio | 6.514 | −2.501, 15.529 | 0.155 |
| Sex | 10.905 | 0.507, 21.304 | 0.040 |
| Race | 9.528 | −0.052, 19.108 | 0.051 |
| Years between Baseline and Follow-Up | 2.554 | −6.002, 11.109 | 0.555 |
| Time of Trier Administration | −0.602 | −2.391, 1.188 | 0.506 |
| Heart and Cortisol Reactivity | −10.947 | −19.520, −2.374 | 0.013 |
| ELS Unpredictability | 2.453 | 0.589, 4.317 | 0.010 |
| Unpredictability ∗ Heart and Cortisol Reactivity | 2.609 | 0.437, 4.780 | 0.019 |
| Adjusted R2 | 0.095 | 0.021 | |
| F | (9, 103) = 2.310 | ||
Note: Sex: 0 = Male, 1 = Female, Time of Trier Administration: hour of the day out of 24, ELS = Early Life Stress.
Table 4.
SCL and mood reactivity moderates the association between ELS unpredictability and total problems.
| B | 95% CI | p | |
|---|---|---|---|
| Baseline Age | 0.512 | −3.252, 4.276 | 0.788 |
| Income to Needs Ratio | 6.132 | −2.852, 15.116 | 0.179 |
| Sex | 6.537 | −3.595, 16.670 | 0.204 |
| Race | 8.660 | −1.007, 18.327 | 0.079 |
| Years between Baseline and Follow-Up | 5.128 | −3.391, 13.647 | 0.235 |
| Time of Trier Administration | −0.445 | −2.243, 1.353 | 0.625 |
| SCL and Mood Reactivity | −10.585 | −27.090, 5.921 | 0.206 |
| ELS Unpredictability | 2.545 | 0.717, 4.373 | 0.007 |
| Unpredictability ∗ SCL and Mood Reactivity | 4.362 | 0.367, 8.358 | 0.033 |
| Adjusted R2 | 0.080 | 0.038 | |
| F | (9, 103) = 2.080 | ||
Note: Sex: 0 = Male, 1 = Female, Time of Trier Administration: hour of the day out of 24, ELS = Early Life Stress.
Fig. 1.
a–b. Higher Early Life Stress (ELS) unpredictability was associated with more total emotional and behavioral problems for those with higher Cardiac and Cortisol Reactivity (a) and those with higher Skin Conductance Level (SCL) and Mood Reactivity (b).
3.4. Sensitivity analyses
To test the specificity and the utility of the stress reactivity factors in moderating the association between unpredictability and YSR total problems, we replaced the factors with the individual reactivity measures. There was a main effect of heart rate reactivity (B = −25.77, 95% CI = −44.63, −6.90) and heart rate variability (B = 16.88, 95% CI = 3.11, 30.66) on total problems; no other reactivity measure was associated with total problems in these models. Only heart rate reactivity was statistically significant in moderating the association between unpredictability and total problems (see Supplemental Tables S7–11): higher increases in heart rate during the TSST in the context of higher unpredictability was associated with more total problems (see Supplemental Fig. S1). Thus, heart rate reactivity may be a diathesis for the development of emotional and behavioral problems following unpredictability in early life and may be sufficient in determining risk for these problems.
We also conducted additional regression analyses predicting internalizing and externalizing problems, separately, instead of a single total problems score, from the interaction of unpredictability and scores on the two stress reactivity factors. There was a main effect of Cardiac and Cortisol Reactivity on internalizing problems (B = −3.95, 95% CI = −6.99, −0.90) and of unpredictability on externalizing problems (B = 0.92, 95% CI = 0.34, 1.50). There were no main effects of SCL and Mood Reactivity on internalizing or externalizing problems. Unpredictability interacted significantly with the Cardiac and Cortisol Reactivity factor to predict internalizing, such that greater unpredictability in the context of higher reactivity was associated with more internalizing and externalizing problems (see Supplemental Tables 12–13). The interaction did not significantly predict externalizing symptoms. Thus, there may not be specificity in the interaction of unpredictability and scores on the Cardiac and Cortisol Reactivity factor in predicting emotional and behavioral problems. Unpredictability did not interact with the SCL and Mood Reactivity factor to predict either externalizing or internalizing problems (see Supplemental Tables 14–15), suggesting that in the context of early life unpredictability, SCL and Mood Reactivity index vulnerability for emotional and behavioral problems that are not incorporated in the measurement of internalizing or externalizing problems (i.e., social, thought, and attention problems).
Finally, we entered Tanner stage of pubertal development at baseline as a covariate in the models predicting internalizing problems from the interaction of ELS Unpredictability and Heart and Cortisol Reactivity and the interaction of ELS Unpredictability and SCL and Mood Reactivity to see whether the analyses were sensitive to pubertal status. Tanner stage was not a significant predictor of internalizing problems; further, the variance explained with pubertal status included in the analysis was lower in both the model with Heart and Cortisol Reactivity (B = 0.245, 95% CI = −6.640, 7.130, R2 = 0.089) and the model with SCL and Mood Reactivity (B = 0.309, 95% CI = −6.510, 7.128, R2 = 0.078).
4. Discussion
A central aim of this study was to examine whether stress reactivity, assessed in multiple domains, is a unitary construct that functions as a diathesis or susceptibility to emotional problems in those exposed to different types of ELS. We also explored whether there is specificity between the dimension of ELS experienced and stress reactivity in predicting emotional problems. A factor analysis of HPA-axis, SNS, PNS, and mood reactivity yielded two factors: a Cardiac and Cortisol Reactivity factor and an SCL and Mood Reactivity factor. Importantly, both factors significantly moderated the association between unpredictable (but not threat- or deprivation-related) ELS and emotional and behavioral problems. Subsequent sensitivity analyses on the individual measures of stress reactivity indicated that only heart rate reactivity moderated this relation, suggesting that this single measure offers adequate predictive utility in detecting who is at risk following early life unpredictability. Further, in testing the specificity of these interactions to internalizing and externalizing problems, we found that whereas the Cardiac and Cortisol Reactivity factor interacted with unpredictability to predict both internalizing and externalizing problems, the SCL and Mood Reactivity factor did not predict either domain of problems. Thus, in this sample, unpredictability in early life interacts with greater stress reactivity to lead to more emotional problems.
Previous research that has examined associations among multiple, interacting, stress-reactivity systems has yielded mixed findings (Kupper et al., 2021; Quas et al., 2014; Rudd et al., 2021). We did not generate hypotheses about the possible structure of the factor analysis we conducted on the five reactivity variables given the paucity of research examining associations among the variables. Because self-reported stress during the TSST formed a factor with skin conductance, we posit that, at least in this study, awareness of bodily stress responses was closely aligned with the sensation of increased sweat production. We should note, however, that findings of prior research examining the association of interoception (i.e., perception of bodily sensations) with skin conductance have been equivocal (Herman et al., 2021; Zaman et al., 2020); clearly, additional research is needed to gain a more comprehensive understanding of how these constructs are related. More studies have reported positive correlations between cortisol and heart rate variability reactivity (Bennett et al., 2024; Glier et al., 2022; Michels et al., 2013) and between cortisol levels and heart rate (Thayer and Sternberg, 2006), supporting the Cardiac and Cortisol Reactivity factor structure that we also obtained. We used continuous scores rather than discrete profile groupings to capture the degree of reactivity in each measure and found that higher reactivity in both of the resultant factors was associated with more emotional problems only in the context of higher unpredictability, supporting the diathesis-stress formulation that high sensitivity interacts with specific early stress to increase risk for psychopathology. Indeed, this study is among the first to test a diathesis-stress model with different dimensions of ELS within a single sample. Although previous research has provided support for this model when examining single specific types of ELS (e.g., maltreatment, family-related stress; (Duprey et al., 2021; Somers et al., 2017), no other study to date has tested the diathesis-stress model with multiple dimensions of ELS in one sample. Our findings suggest that among adolescents who have experienced greater unpredictability in early life, heightened stress reactivity, particularly heart rate reactivity, confers vulnerability to the development of emotional problems. Life history theory posits that unpredictable environments should promote faster developmental pacing, which can lead to the development of emotional problems (Ellis et al., 2022). Individuals who are less sensitive to the environment may be more resilient to these emotional problems; future work should examine whether less sensitive individuals are also resilient to accelerated development. Although faster developmental pace is also posited in the context of threat-related ELS, the low severity rating of threat in this sample may have limited our findings. Sensitivity analyses indicated that stage of pubertal development at baseline was not associated with internalizing problems, although in prior work we have found that in females, threat and unpredictability were associated with earlier pubertal timing but not with faster pubertal pacing over time, and that faster pubertal development was associated with higher internalizing and externalizing problems (Ho et al., 2024). Future research is needed to replicate the current findings in a larger sample with greater exposure to ELS, particularly to threat-related ELS.
We also found specificity in this sample with respect to the dimension of ELS experienced and the responsiveness of different stress reactivity systems predicting the development of psychopathology. Specifically, we found that heart rate reactivity was the only individual variable that moderated the association between unpredictability and emotional problems; indeed, it is possible that heart rate reactivity drove the significant interaction of unpredictability and the Cardiac and Cortisol Reactivity factor in predicting later problems, suggesting that heart rate reactivity on its own sufficiently reflects a diathesis to unpredictability. Further, although the SCL and Mood Reactivity factor interacted with early unpredictability to predict emotional problems, neither of the individual variables that comprised this factor (SCL and mood reactivity) did so, indicating that the combination of the two reactivity systems is more informative in predicting emotional problems than is either one alone. Importantly, lower scores for both reactivity factors seemed to be protective against more emotional and behavioral problems regardless of whether early life unpredictability was experienced, supporting the formulations that reactivity reflects sensitivity to the environment and that reduced sensitivity to an unpredictable environment can promote adaptive functioning.
Finally, we examined whether the interaction of unpredictability and stress reactivity was sensitive to the domain of emotional problems by predicting internalizing and externalizing problems separately. Interestingly, Steeger et al. (2017) found that cortisol reactivity in response to a laboratory task interacted with a history of stressful family events to predict both internalizing and externalizing problems. Whereas the Cardiac and Cortisol Reactivity factor interacted with unpredictability to predict both internalizing and externalizing problems, the SCL and Mood Reactivity factor did not interact with unpredictability to predict either internalizing or externalizing problems. Therefore, SCL and Mood Reactivity may be more sensitive to risk for the development of social, thought, and/or attention problems – YSR subscales that are included in the total problems score – than for the development of internalizing or externalizing problems. There is less published work that focuses on how these three subscales relate to ELS and stress reactivity than there is research focuses on internalizing and externalizing problems; therefore, we did not further examine associations of the stress reactivity factors with thought, attention, and social problems. It will be important in future research to examine the unique variance in emotional and behavioral problems represented by these three subscales. Thus, the important issue of specificity in the associations among dimensions of ELS, patterns of stress reactivity, and types of emotional problems is not yet settled and warrants further investigation.
We should note three limitations of this study. First, we relied on adolescents’ self-reports to assess emotional and behavioral problems. While we used a structured interview to assess ELS exposure and a well-validated social stress paradigm to measure reactivity in multiple biological systems, the use of self-report may introduce bias and our findings should be replicated using more objective measures of emotional functioning. Second, although 84.1% of participants took part in the TSST between 13:00–20:00 h, we did not explicitly account for time between waking or eating and the time the TSST was administered, which are potentially confounding factors. Third, this sample was not recruited on the basis of having experienced ELS, which may have limited the range of exposure to deprivation and threat-related ELS, in particular. The higher variability of unpredictability relative to the variability of the other ELS dimensions in this sample may account for the specificity findings. Researchers should replicate our findings in a sample that has experienced more severe ELS characterized by threats to safety in order to assess their generalizability. As a related point, it is possible that early experiences of unpredictability interacted significantly with stress reactivity to the TSST because this task itself has inherent aspects of unpredictability. Thus, it will be important in future research to replicate our findings both in samples with greater exposure to different forms of ELS and using different types of stressors.
Despite these limitations, we demonstrated that stress reactivity is not a unitary construct and, further, that high levels of reactivity across stress response systems index a diathesis or vulnerability to the development of symptoms of psychopathology in adolescents, particularly in those who experienced unpredictability in early life. Exposure to unpredictability was associated with both internalizing and externalizing problems in adolescents with high heart rate, PNS, and HPA-axis reactivity, and with more overall emotional and behavioral problems in those with high SNS and affective reactivity. Lower levels of reactivity may reflect less sensitivity to unstable environments and prevent the development of emotional problems. High heart rate was a particularly salient moderator of the association between unpredictability and emotional problems, and may be a useful indicator for the identification of at-risk youth. Understanding the pathways and mechanisms by which ELS can lead to emotional and behavioral problems later in life may help to inform intervention, prevention, and screening efforts in youth who have experienced adversity.
CRediT authorship contribution statement
J.L. Buthmann: Writing – original draft, Conceptualization. C. Antonacci: Writing – review & editing, Data curation. J.P. Uy: Writing – review & editing. L.R. Borchers: Writing – review & editing. J.G. Miller: Writing – review & editing. I.H. Gotlib: Writing – original draft, Conceptualization.
Ethics approval statement
All study procedures were approved by the Stanford University Institutional Review Board; all minor participants gave written informed assent and guardians gave written informed consent.
Funding statement
Funding was provided by NIH R37MH101495 (PI: Gotlib), The Brain and Behavior Research Foundation Young Investigator Grant (PI: Buthmann), T32MH020016 (PI: Antonacci), and F32MH135657 (PI: Uy).
Declaration of competing interest
The authors have no direct or indirect conflicts of interest to report.
Acknowledgements
We thank the participating families and research staff for their time and effort.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynstr.2024.100706.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
Data availability
Data will be made available on request.
References
- Achenbach T.M., Rescorla L.A. University of Vermont (Research center for children; 2001. Manual for the ASEBA School-Age Forms & Profiles. Burling. youth and families) [Google Scholar]
- Alastalo H., Bonsdorff M.B. von, Räikkönen K., Pesonen A.-K., Osmond C., Barker D.J.P., Heinonen K., Kajantie E., Eriksson J.G. Early Life stress and physical and psychosocial functioning in late adulthood. PLoS One. 2013;8 doi: 10.1371/journal.pone.0069011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen A.P., Kennedy P.J., Dockray S., Cryan J.F., Dinan T.G., Clarke G. The trier social stress test: principles and practice. Neurobiol Stress. 2017;6:113–126. doi: 10.1016/j.ynstr.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J., Pluess M. Beyond diathesis stress: differential susceptibility to environmental influences. Psychol. Bull. 2009;135:885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Bennett M.M., Tomas C.W., Fitzgerald J.M. Relationship between heart rate variability and differential patterns of cortisol response to acute stressors in mid-life adults: a data-driven investigation. Stress Health. 2024;40 doi: 10.1002/smi.3327. [DOI] [PubMed] [Google Scholar]
- Berntson G.G., Bigger J.T., Eckberg D.L., Grossman P., Kaufmann P.G., Malik M., Nagaraja H.N., Porges S.W., Saul J.P., Stone P.H., van der Molen M.W. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Boyce W.T., Ellis B.J. Biological sensitivity to context: I. An evolutionary–developmental theory of the origins and functions of stress reactivity. Develop. Psychopathol. 2005;17 doi: 10.1017/S0954579405050145. [DOI] [PubMed] [Google Scholar]
- Chahal R., Miller J.G., Yuan J.P., Buthmann J.L., Gotlib I.H. An exploration of dimensions of early adversity and the development of functional brain network connectivity during adolescence: implications for trajectories of internalizing symptoms. Dev. Psychopathol. 2022;34:557–571. doi: 10.1017/S0954579421001814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daches S., Vine V., George C.J., Kovacs M. Adversity and depression: the moderating role of stress reactivity among high and low risk youth. J. Abnorm. Child Psychol. 2019;47:1391–1399. doi: 10.1007/s10802-019-00527-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daníelsdóttir H.B., Aspelund T., Shen Q., Halldorsdottir T., Jakobsdóttir J., Song H., Lu D., Kuja-Halkola R., Larsson H., Fall K., Magnusson P.K.E., Fang F., Bergstedt J., Valdimarsdóttir U.A. Adverse childhood experiences and adult mental health outcomes. JAMA Psychiatr. 2024;81:586–594. doi: 10.1001/jamapsychiatry.2024.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond L., Fagundes C., Cribbet M. Individual differences in adolescents' sympathetic and parasympathetic functioning moderate associations between family environment and psychosocial adjustment. Dev. Psychol. 2012;48:918–931. doi: 10.1037/a0026901. [DOI] [PubMed] [Google Scholar]
- Duprey E.B., Oshri A., Liu S., Kogan S.M., Caughy M.O. Physiological stress response reactivity mediates the link between emotional abuse and youth internalizing problems. Child Psychiatry Hum Dev. 2021;52:450–463. doi: 10.1007/s10578-020-01033-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenlohr-Moul T.A., Miller A.B., Giletta M., Hastings P.D., Rudolph K.D., Nock M.K., Prinstein M.J. HPA axis response and psychosocial stress as interactive predictors of suicidal ideation and behavior in adolescent females: a multilevel diathesis-stress framework. Neuropsychopharmacol. 2018;43:2564–2571. doi: 10.1038/s41386-018-0206-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis B.J., Sheridan M.A., Belsky J., McLaughlin K.A. Why and how does early adversity influence development? Toward an integrated model of dimensions of environmental experience. Dev. Psychopathol. 2022;34:447–471. doi: 10.1017/S0954579421001838. [DOI] [PubMed] [Google Scholar]
- Faust J., August L., Bangia K., Galaviz V., Leichty J., Prasad S., Schmitz R., Slocombe A., Welling R., Zeise L. Update to the California Communities environmental health screening tool. CalEnviroScreen. 2017;3.0 [Google Scholar]
- Glier S., Campbell A., Corr R., Pelletier-Baldelli A., Yefimov M., Guerra C., Scott K., Murphy L., Bizzell J., Belger A. Coordination of autonomic and endocrine stress responses to the Trier Social Stress Test in adolescence. Psychophysiology. 2022;59 doi: 10.1111/psyp.14056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib I.H., Borchers L.R., Chahal R., Gifuni A.J., Teresi G.I., Ho T.C. Early life stress predicts depressive symptoms in adolescents during the COVID-19 Pandemic: the mediating role of perceived stress. Front. Psychol. 2021;11:3864. doi: 10.3389/fpsyg.2020.603748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman A.M., Esposito G., Tsakiris M. Body in the face of uncertainty: the role of autonomic arousal and interoception in decision-making under risk and ambiguity. Psychophysiology. 2021;58 doi: 10.1111/psyp.13840. [DOI] [PubMed] [Google Scholar]
- Ho T.C., Buthmann J., Chahal R., Miller J.G., Gotlib I.H. Exploring sex differences in trajectories of pubertal development and mental health following early adversity. Psychoneuroendocrinology. 2024;161 doi: 10.1016/j.psyneuen.2023.106944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar C.E., Swartz J.R., Alen N.V., Guyer A.E., Hastings P.D. The role of stress phenotypes in understanding childhood adversity as a transdiagnostic risk factor for psychopathology. Journal of Psychopathology and Clinical Science. 2023;132:277–286. doi: 10.1037/abn0000619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juruena M.F., Eror F., Cleare A.J., Young A.H. In: Anxiety Disorders: Rethinking and Understanding Recent Discoveries, Advances in Experimental Medicine and Biology. Kim Y.-K., editor. Springer; Singapore: 2020. The role of early life stress in HPA Axis and Anxiety; pp. 141–153. [DOI] [PubMed] [Google Scholar]
- Kaiser H.F., Rice J. Little jiffy, mark iv. Educ. Psychol. Meas. 1974;34:111–117. doi: 10.1177/001316447403400115. [DOI] [Google Scholar]
- Kantor G.K., Holt M.K., Mebert C.J., Straus M.A., Drach K.M., Ricci L.R., Macallum C.A., Brown W. 2004. The Parent-Report Multidimensional Neglectful Behavior Scale. [DOI] [PubMed] [Google Scholar]
- King L.S., Colich N.L., LeMoult J., Humphreys K.L., Ordaz S.J., Price A.N., Gotlib I.H. The impact of the severity of early life stress on diurnal cortisol: the role of puberty. Psychoneuroendocrinology. 2017;77:68–74. doi: 10.1016/j.psyneuen.2016.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King L.S., Dennis E.L., Humphreys K.L., Thompson P.M., Gotlib I.H. Cross-sectional and longitudinal associations of family income-to-needs ratio with cortical and subcortical brain volume in adolescent boys and girls. Dev. Cogn. Neurosci. 2020;44:100796. doi: 10.1016/j.dcn.2020.100796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircanski K., Sisk L.M., Ho T.C., Humphreys K.L., King L.S., Colich N.L., Ordaz S.J., Gotlib I.H. Early life stress, cortisol, frontolimbic connectivity, and depressive symptoms during puberty. Dev. Psychopathol. 2019;31:1011–1022. doi: 10.1017/S0954579419000555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C., Pirke K.M., Hellhammer D.H. The ’Trier Social Stress Test’--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kupper N., Jankovic M., Kop W.J. Individual differences in cross-system physiological activity at rest and in response to acute social stress. Psychosom. Med. 2021;83:138. doi: 10.1097/PSY.0000000000000901. [DOI] [PubMed] [Google Scholar]
- Kushner M.R., Barrios C., Smith V.C., Dougherty L.R. Physiological and behavioral vulnerability markers increase risk to early life stress in preschool-aged children. J. Abnorm. Child Psychol. 2016;44:859–870. doi: 10.1007/s10802-015-0087-7. [DOI] [PubMed] [Google Scholar]
- Lin H.-P., Lin H.-Y., Lin W.-L., Huang A.C.-W. Effects of stress, depression, and their interaction on heart rate, skin conductance, finger temperature, and respiratory rate: sympathetic-parasympathetic hypothesis of stress and depression. J. Clin. Psychol. 2018;67:1080–1091. doi: 10.1002/jclp.20833. [DOI] [PubMed] [Google Scholar]
- Liu J.J.W., Vickers K., Reed M., Hadad M. Re-conceptualizing stress: shifting views on the consequences of stress and its effects on stress reactivity. PLoS One. 2017;12 doi: 10.1371/journal.pone.0173188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall W.A., Tanner J.M. Growth and physiological development during adolescence. Annu. Rev. Med. 1968;19:283–300. doi: 10.1146/annurev.me.19.020168.001435. [DOI] [PubMed] [Google Scholar]
- McLaughlin K.A., Sheridan M.A., Humphreys K.L., Belsky J., Ellis B.J. 2021. The Value of Dimensional Models of Early Experience: Thinking Clearly about Concepts and Categories. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels N., Sioen I., Clays E., De Buyzere M., Ahrens W., Huybrechts I., Vanaelst B., De Henauw S. Children's heart rate variability as stress indicator: association with reported stress and cortisol. Biol. Psychol. 2013;94:433–440. doi: 10.1016/j.biopsycho.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Mücke M., Ludyga S., Colledge F., Gerber M. Influence of regular physical activity and fitness on stress reactivity as measured with the Trier Social Stress Test Protocol: a systematic review. Sports Med. 2018;48:2607–2622. doi: 10.1007/s40279-018-0979-0. [DOI] [PubMed] [Google Scholar]
- Noble K.G., Houston S.M., Brito N.H., Bartsch H., Kan E., Kuperman J.M., Sowell E.R. Family income, parental education and brain structure in children and adolescents. Nat. Neurosci. 2015;18(5):773. doi: 10.1038/nn.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro J., Bates D., DebRoy S., Sarkar D., Heisterkamp S., Van Willigen B., Ranke J., R Core Team . 2022. Nlme: Linear and Nonlinear Mixed Effects Models. [Google Scholar]
- Pruessner M., Hellhammer D.H., Pruessner J.C., Lupien S.J. Self-reported depressive symptoms and stress levels in healthy young men: associations with the cortisol response to awakening. Psychosom. Med. 2003;65:92–99. doi: 10.1097/01.PSY.0000040950.22044.10. [DOI] [PubMed] [Google Scholar]
- Quas J.A., Yim I.S., Oberlander T.F., Nordstokke D., Essex M.J., Armstrong J.M., Bush N., Obradović J., Boyce W.T. The symphonic structure of childhood stress reactivity: patterns of sympathetic, parasympathetic, and adrenocortical responses to psychological challenge. Dev. Psychopathol. 2014;26:963–982. doi: 10.1017/S0954579414000480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribbe D. Measurement of Stress, Trauma, and Adaptation. 1996. Psychometric review of traumatic event screening instrument for children (TESI-C) pp. 386–387. [Google Scholar]
- Rudd K.L., Roubinov D.S., Jones-Mason K., Alkon A., Bush N.R. Developmental consequences of early life stress on risk for psychopathology: longitudinal associations with children's multisystem physiological regulation and executive functioning. Dev. Psychopathol. 2021;33:1759–1773. doi: 10.1017/S0954579421000730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph K.D., Hammen C., Burge D., Lindberg N., Herzberg D., Daley S.E. Toward an interpersonal life-stress model of depression: the developmental context of stress generation. Dev. Psychopathol. 2000;12:215–234. doi: 10.1017/S0954579400002066. [DOI] [PubMed] [Google Scholar]
- Seddon J.A., Rodriguez V.J., Provencher Y., Raftery-Helmer J., Hersh J., Labelle P.R., Thomassin K. Meta-analysis of the effectiveness of the Trier Social Stress Test in eliciting physiological stress responses in children and adolescents. Psychoneuroendocrinology. 2020;116 doi: 10.1016/j.psyneuen.2020.104582. [DOI] [PubMed] [Google Scholar]
- Somers J.A., Ibrahim M.H., Luecken L.J. Biological sensitivity to the effects of childhood family adversity on psychological well-being in young adulthood. Child. Maltreat. 2017;22:236–244. doi: 10.1177/1077559517711041. [DOI] [PubMed] [Google Scholar]
- Stalder T., Kirschbaum C., Kudielka B.M., Adam E.K., Pruessner J.C., Wüst S., Dockray S., Smyth N., Evans P., Hellhammer D.H., Miller R., Wetherell M.A., Lupien S.J., Clow A. Assessment of the cortisol awakening response: expert consensus guidelines. Psychoneuroendocrinology. 2016;63:414–432. doi: 10.1016/j.psyneuen.2015.10.010. [DOI] [PubMed] [Google Scholar]
- Steeger C.M., Cook E.C., Connell C.M. The interactive effects of stressful family life events and cortisol reactivity on adolescent externalizing and internalizing behaviors. Child Psychiatry Hum Dev. 2017;48:225–234. doi: 10.1007/s10578-016-0635-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swales D.A., Winiarski D.A., Smith A.K., Stowe Z.N., Newport D.J., Brennan P.A. Maternal depression and cortisol in pregnancy predict offspring emotional reactivity in the preschool period. Dev. Psychobiol. 2018;60:557–566. doi: 10.1002/dev.21631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer J.F., Sternberg E. Beyond heart rate variability. Ann. N. Y. Acad. Sci. 2006;1088:361–372. doi: 10.1196/annals.1366.014. [DOI] [PubMed] [Google Scholar]
- Turner A.I., Smyth N., Hall S.J., Torres S.J., Hussein M., Jayasinghe S.U., Ball K., Clow A.J. Psychological stress reactivity and future health and disease outcomes: a systematic review of prospective evidence. Psychoneuroendocrinology. 2020;114 doi: 10.1016/j.psyneuen.2020.104599. [DOI] [PubMed] [Google Scholar]
- Wilhelm F.H., Peyk P. 2005. "ANSLAB: Autonomic Nervous System Laboratory. Version 4.0. [Google Scholar]
- Winiarski D.A., Engel M.L., Karnik N.S., Brennan P.A. Early life stress and childhood aggression: mediating and moderating effects of child callousness and stress reactivity. Child Psychiatry Hum Dev. 2018;49:730–739. doi: 10.1007/s10578-018-0785-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman J., Van de Pavert I., Van Oudenhove L., Van Diest I. The use of stimulus perception to account for variability in skin conductance responses to interoceptive stimuli. Psychophysiology. 2020;57 doi: 10.1111/psyp.13494. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.