Abstract
Endothelial cells undergo branching morphogenesis to form capillary tubes. We have utilized an in vitro Matrigel overlay assay to analyze the role of the cytoskeleton and Rho GTPases during this process. The addition of matrix first induces changes in cell morphology characterized by the formation of dynamic cellular protrusions and the assembly of discrete aggregates or cords of aligned cells resembling primitive capillary-like structures, but without a recognizable lumen. This is followed by cell migration leading to the formation of a complex interconnecting network of capillary tubes with readily identifiable lumens. Inhibition of actin polymerization or actin-myosin contraction inhibits cell migration but has no effect on the initial changes in endothelial cell morphology. However, inhibition of microtubule dynamics prevents both the initial cell shape changes as well as cell migration. We find that the small GTPase Rac is essential for the matrix-induced changes in endothelial cell morphology, whereas p21-activated kinase, an effector of Rac, is required for cell motility. We conclude that Rac integrates signaling through both the actin and microtubule cytoskeletons to promote capillary tube assembly.
INTRODUCTION
Branching morphogenesis is a complex developmental program that results in the formation of networks of hollow tubes by epithelial or endothelial cell types (Metzger and Krasnow, 1999). This morphogenetic program underlies the development of many solid organs, including the lung, kidney, and the vascular system. The study of model biological systems such as Drosophila tracheal, mouse lung, and mouse vascular development has provided much information about the genetic control of branching morphogenesis (Risau, 1997; Hogan, 1999). Recently, considerable attention has focused on vascular development in particular, driven partly by the appreciation of the importance of blood vessel formation in disease states such as inflammation and cancer (Folkman, 1995).
Development of the circulatory system occurs in the main by two apparently distinct mechanisms, vasculogenesis and angiogenesis (Risau, 1997). Vasculogenesis is responsible for the formation of the major vessels, the aorta, the large vessels supplying the head and neck, and those supplying the limbs (Ambler et al., 2001). During vasculogenesis, mesoderm-derived precursor cells, angioblasts, differentiate into endothelial cells, migrate, and then assemble to form cellular cords and finally lumen-containing primitive vessels. This leads to the formation of a primary vascular plexus, which is then extensively remodeled to give rise to a vascular system with a distinct pattern of arteries and veins. It has long been thought that vasculogenesis occurred only during development, but recent evidence suggests it may also occur in the adult (Springer et al., 1998).
Angiogenesis is the process of budding and branching of new capillaries from these preexisting vessels. During development, this contributes to the expansion of the vascular plexus and is the major mechanism of vascularization of the brain and kidney and the formation of intersomitic vessels (Carmeliet, 2000). Angiogenesis accounts for the neovascularization that occurs during the female reproductive cycle and wound healing and also in pathological conditions such as retinopathy, tumor growth, and metastasis or inflammatory states such as rheumatoid arthritis (Folkman, 1995).
During vascular assembly, endothelial cells must respond to a variety of extracellular signals, including soluble factors and cell-cell and cell-matrix interactions, and a number of molecular mechanisms underlying blood vessel formation have been described. Endothelial-specific growth factors such as vascular endothelial growth factors or angiopoietins (Ang-1 and Ang-2) with their respective receptor families, the vascular endothelial growth factors and TIE receptors, have been identified (Gale and Yancopoulos, 1999). Recently, it has become clear that other families of signaling molecules more commonly associated with neuronal development such as the ephrin/EPH receptor (Wang et al., 1998; Adams et al., 1999) and semaphorin/neuropilin families (Soker et al., 1998) may also play an important role in the assembly and patterning of the vasculature. Cell-cell interactions, mediated predominately by the endothelial-specific VE-cadherin, are also required for angiogenesis both in vivo and in vitro (Bach et al., 1998; Carmeliet et al., 1999). A wide variety of extracellular matrix (ECM) and integrin receptors, which bind the ECM, most notably αvβ3 and αvβ 5, have been demonstrated to regulate endothelial function during angiogenesis (Friedlander et al., 1995; Bazzoni et al., 1999). These diverse patterning signals must be integrated by the endothelial cell to control morphogenesis.
Surprisingly, little is known of the intracellular signaling pathways that regulate the changes in cell shape and motility required for a complex process such as capillary formation. The Rho family of small GTPases are key molecules controlling the organization of the actin cytoskeleton (Hall, 1998), and in fibroblasts, Cdc42 leads to filopodia formation, Rac to membrane ruffling, lamellipodia, and focal complexes, and Rho to actin stress fibers and focal adhesions (Ridley and Hall, 1992; Ridley et al. 1992; Kozma et al. 1995; Nobes and Hall, 1995). As such, they are important regulators of cell migration, cell shape, and polarity (Nobes and Hall, 1999; Etienne-Manneville and Hall, 2001). We are interested in whether Rho GTPases play a role in capillary formation, a morphogenetic program characterized by profound changes in cell shape and migration.
MATERIALS AND METHODS
Reagents
Matrigel was purchased from Collaborative Research (Waltham, MA). Rat anti-tyr tubulin (YL1/2) was from Serotec (Oxford, UK). Monoclonal antibodies to cadherin 5 and b-catenin were from Transduction Laboratories (Lexington, KY). Fluorescein isothiocyanate-conjugated goat anti-rat, fluorescein isothiocyanate-conjugated goat anti-mouse were purchased from Jackson Immunoresearch Laboratories (West Grove, PA). Rhodamine phalloidin, taxol (paclitaxel), cytochalasin D, 2,3-butanedione monoxime (BDM), and ML7 were all from Sigma Chemicals (St. Louis, MO). C3 transferase was prepared as described (Ridley and Hall, 1992). Y 27632 was from Upstate Biochemicals. Toxin B 10463 and 1470 were the kind gifts of Dr. C. von Eichel-Streiber (Gutenberg University, Mainz, Germany).
Cell Culture
Primary human umbilical vein endothelial cells (HUVECs) were purchased from TCS (New Hope, PA) and were used from passages 2 to 8. Cells were routinely cultured in large vessel endothelial basal medium containing endothelial growth supplement according to the manufacturer's recommendations.
For experiments with all inhibitors, cell death was assessed by nuclear staining with Hoechst 33342 (10 μg/ml in phosphate-buffered saline [PBS]) for 20 min and counting apoptotic nuclei with condensed chromatin. No significant increase in cell death was noted with inhibitors at the concentrations used.
In Vitro Angiogenesis Assay
For the Matrigel overlay assay, cells (2–3 × 104) were seeded on 13-mm glass coverslips in 4-well plates and were grown in complete medium overnight. The following day, medium was aspirated and cells were overlaid with ∼25–50 μl Matrigel (without phenol red but with growth factors) diluted 1:1 with sterile, cold PBS. The Matrigel was allowed to polymerize for 1–2 h at 37°C before the addition of 1 ml of complete media. Capillary tube formation was assessed after 20–24 h using a DM IRB inverted microscope (Leica, Deerfield, IL) fitted with a Coolsnap digital camera (Photometrics, Tucson, AZ). Images were acquired and processed using Open Lab (Improvision, Coventry, United Kingdom) software. To quantify tube formation, three random areas were imaged and the length of continuous cords of three or more cells was measured using Open Lab software. Significance was determined by using unpaired t test. To identify vacuoles and lumenal structures, cells were visualized using phase contrast or Leica modulation contrast optics (based on Hoffman modulation contrast principle) and were photographed as above. To quantify lumen formation by light microscopy, six random areas on each coverslip were photographed. Lumens were defined as vacuoles or phase light spaces that traversed the length of at least one cell body. Lumen formation was assessed separately for branch points and for interconnecting segments of the network.
For microinjection experiments, patches of ∼100–150cells on each coverslip were injected with EGFP-C1(CLONTECH, Palo Alto, CA) vector (0.1 mg/ml) and the indicated GTPase mutant in the vector pRK 5 at 0.05- 0.1 mg/ml. Expression of all constructs was confirmed by staining with 9E10 monoclonal anti-myc antibody. Injected cells were identified using a inverted microscope (Leica) fitted with fluorescence and were photographed. Cells incorporated into capillary tubes were readily identified by their elongated morphology. Green fluorescent protein (GFP)-positive cells incorporated into structures at least three cells in length were regarded as positive and are expressed as a percentage of the total number of GFP-positive cells. Results represent experiments carried out in duplicate at least three times. Significance was determined by unpaired t test.
Plasmids and Microinjection
Myc-tagged N17 Rac, N17 CDC42 (G25K isotype), V12 Rac 1, V14 Rho A, and V12 Cdc42 were as described. Myc-tagged versions of Wiskott-Aldrich syndrome protein (WASP; 201–321), kinase dead full-length p21-activated kinase (Pak; K299A), a gift from Dr. Lisa Stowers, and auto-inhibitory fragment of Pak (83–149; Daub and Hall, 2001) were subcloned into pRK5 and plasmid DNA prepared using caesium chloride/ethidium bromide gradients. Cells were viewed using an inverted microscope (Zeiss, Jena, Germany) and DNA was injected into the cell nucleus using a micropipette controlled by an Eppendorf micromanipulator. Patches of ∼100–150 cells on each coverslip were injected with EGFP-C1 (0.1 mg/ml) and either empty vector (0.1 mg/ml) or the plasmid of interest in pRK 5 at 0.05–0.1 mg/ml. Expression of GFP and myc-tagged protein could be detected 2 h after injection. In all cases, coexpression of myc-tagged proteins was confirmed by staining with 9E10 monoclonal anti-myc antibody.
Electron Microscopy
Electron Microscopy was performed as described (Hopkins and Trowbridge, 1983). Briefly, cells grown in Matrigel on 13 mm diameter glass coverslips were fixed with 2% paraformaldehyde/1.5% glutaraldehyde in 0.1 M sodium cacodylate buffer for 20 min. The cells were postfixed in 1% osmium tetroxide/1.5% potassium ferricyanide and were treated with tannic acid. The cells were then dehydrated and embedded “en face” in Epon. Coverslips were removed by plunging the samples into liquid nitrogen, leaving the cells on the epon. Transverse sections (60 nm) were cut on a Reichert-Jung Ultracut E microtome. The sections were stained with lead citrate and viewed in an EM400 electron microscope (Phillips, Shelton, CT).
For light microscopy examination, cells were embedded in Epon containing methylene blue as above, and the block was trimmed and then reembedded in Epon without methylene blue. This allowed us to cut 1-μm sections perpendicular to the plane of Matrigel with a dry glass knife using a Reichert-Jung E ultratome. These sections were mounted on glass slides and were stained with 1% toluidine blue in 1% borax. To further characterize lumens, serial 1-μm sections were cut to 30 μm from a single block, and the presence of a lumen was scored in every fifth section. In all cases in which the lumen was seen en face, lumens were present throughout the tube.
Immunofluorescence
Cells were fixed in 4% paraformaldehyde (or 4% paraformaldehyde/0.5% glutaraldehyde for visualization of tubulin) for 15 min, permeabilized in 0.2% Triton-100 (0.5% for Matrigel overlay cultures) for 5 min, and quenched with 0.5 mg/ml sodium borohydride in PBS for 10 min. Samples were incubated with primary antibody diluted in PBS for 1 h, washed extensively with PBS, and then incubated with secondary antibody (1:100) for 1 h in PBS. Finally, coverslips were washed in PBS and mounted with Mowiol.
RESULTS
Matrigel Overlay Assay of Capillary Tube Formation
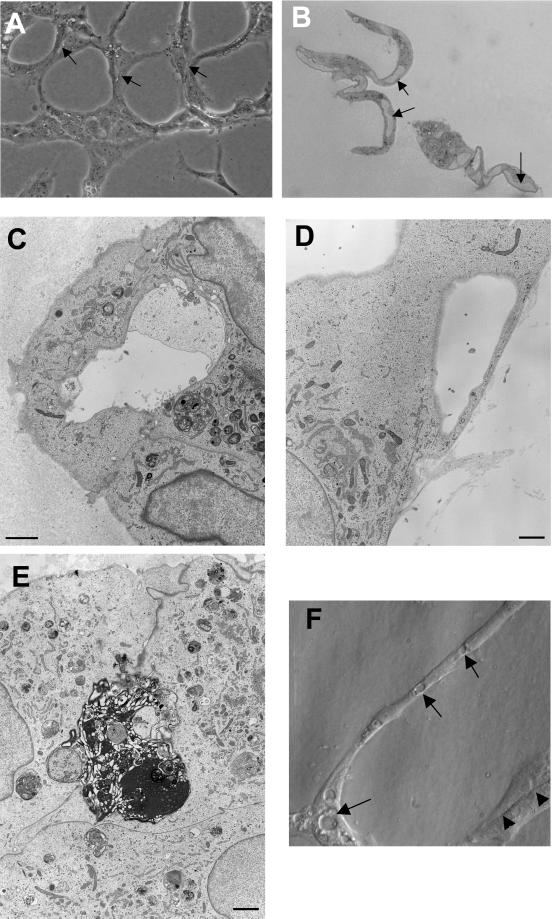
Many in vitro assays have been developed to study capillary tube formation and these usually involve the culture of endothelial cells on or within fibrin, collagen, or basement membrane-like Matrigel (Bischoff, 1995). A disadvantage with these assays is that the three-dimensional nature of the matrix renders the cells relatively inaccessible to further study. We have modified a Matrigel assay (Grant et al., 1989) by growing HUVECs on glass coverslips, then adding a thin overlay of 1:1 mixture of Matrigel and PBS. After the Matrigel has polymerized, 1–2 h later, endothelial growth media is added to the culture. After 24 h, a complex network of branching capillary tubes can be seen. The tubes can be one or several cells in diameter and by light microscopy have intercellular spaces resembling early lumens (Figure 1A, arrows). Lumens of at least one cell body in length were seen in 74 ± 10.5% (means ± SD) of interconnecting segments and 68 ± 12.1% of branch points of the network. Cells prepared for electron microscopy were also examined by light microscopy and lumens were readily visible in ∼90% of cases (Figure 1B). Serial 1- to 30-μm sections of these samples confirmed the presence of lumens throughout the cells. By electron microscopy, tubes are seen to have lumens formed by two or more cells (Figure 1C). Lumens are frequently seen to contain matrix material (Figure 1C) or apoptotic cells (Figure 1E). In some instances, single cells have lumens or large vacuoles (Figure 1D). Sections are also examined by light microscopy, and vacuoles and intercellular spaces were readily identified by modulation contrast optics (Figure 1 F). Vacuoles occurred in both junctional areas and interconnecting segments of tube structures.
Figure 1.
Phase contrast and electron micrographs of capillary tube formation. (A) Endothelial cells overlaid with Matrigel form a network of capillary tube-like structures composed of multiple cells with intercellular spaces or lumens. Cells are bipolar and are aligned in the axis of the tube. (B) High resolution phase contrast of a 1-μm transverse section through capillary-like tubes demonstrating lumen formation (arrows). (C) Electron micrograph demonstrating lumen in capillary tube. Lumens frequently contain debris or matrix-like material. (D) Some capillary tubes are composed of single endothelial cells containing a large vacuole or lumen. (E) Apoptotic cell surrounded by three cells and detached from matrix. (F) Modulation contrast image of capillary tube with vacuoles (arrows) and intercellular spaces (arrowheads). Bar, 100 μm in A, and 1 μm in C-E.
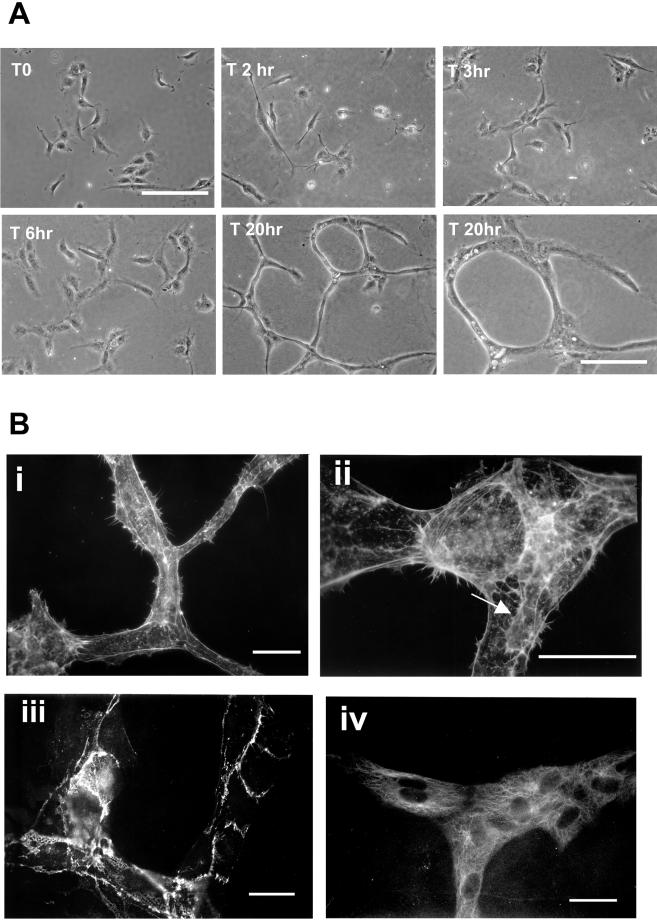
Endothelial cells growing on coverslips are well spread with lamellae and membrane ruffles (Figure 2A). By time-lapse videomicroscopy, they begin to send out multiple cellular protrusions within 30–60 min after Matrigel overlay, and these can be seen to extend and retract rapidly with marked membrane ruffling. Over the next 2–6 h, small aggregates of aligned cells making short cord-like structures are formed. The cellular cords do not have an identifiable lumen by light microscopy at this stage (Figure 2A, T6 h). Cell migration, often along neighboring cells, then leads to expansion of these shorter structures into a complex interconnecting network of capillary tubes with readily identifiable lumens (Figure 2A, T 20 h). A representative set of phase contrast micrographs demonstrates this time course in Figure 2A.
Figure 2.
Morphological changes during capillary tube formation. (A) Phase contrast micrographs showing cell morphology changes during capillary tube formation. Final panel is a higher resolution view of mature tubules. Bar, 100 μm. (B) Endothelial cells were fixed and labeled with rhodamine phalloidin (i and ii), anti-VE cadherin antibody (iii), and anti-tyr-tubulin antibody (iv). Capillary tube structures had little organized filamentous actin structures and formed branching networks, with some cells extending protrusions along one another (arrow in iii). Cells formed adherens junctions (iii), and microtubules aligned along the long axis of the tubule (iv). Bar, 50 μm.
Phalloidin staining of mature capillary structures demonstrates that they contain little or no organized filamentous actin; in fact, individual cells are not clearly distinguishable (Figure 2B, i). At junctional areas, cells can be seen to be arranged in a Y-shaped formation and extend lamellipodia along adjacent cells (Figure 2B, ii, arrow). This form of guided migration in capillary assembly has previously been described (Nehls et al., 1998). The endothelial specific VE-cadherin localizes to areas of cell-cell contact (Figure 2B, iii), and microtubules are aligned along the long axis of the tube (Figure 2B, iv).
Rho GTPases Are Required for Capillary Tube Formation
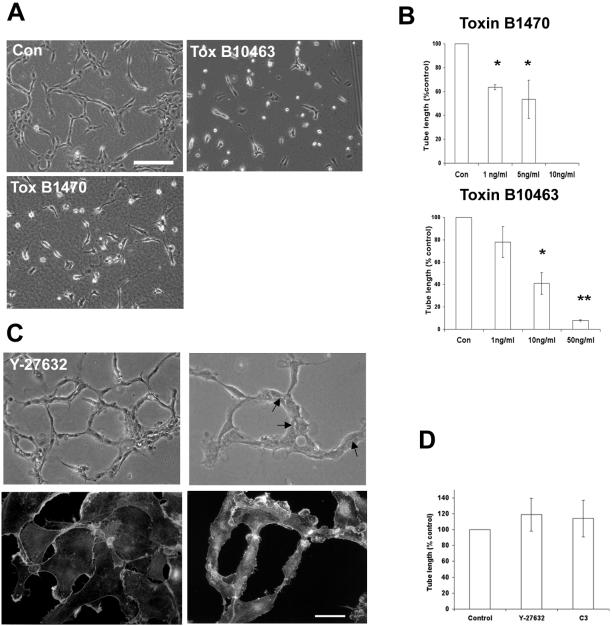
To investigate the role of Rho GTPases in capillary tube formation, we first made use of toxins from the bacterium Clostridium Difficile, which inhibit small GTPase function. Toxin B 10463 inhibits Rho, Rac, and Cdc42, whereas Toxin B 1470 inhibits R-ras, Ral, Rac, and Rap (Aktories et al., 2000). Cells were preincubated with toxin for 1 h before Matrigel overlay and continuously after Matrigel polymerization. Capillary formation was quantified by measuring total cord length. Both toxins gave similar results and inhibited capillary tube formation in a dose-dependent manner (Figure 3, A and B). These results suggest that small GTPases, and in particular Rac (a common substrate of both toxins), are likely to play an important role in capillary assembly.
Figure 3.
Rho is not required for capillary tube formation. (A) Cells were treated with the indicated concentration of toxin B 10463 or 1470 and were overlaid with Matrigel. (B) Capillary tube formation was inhibited in a dose-dependent manner. Results represent mean ± SD from at least three independent experiments performed in duplicate. *P < 0.01, **P < 0.001 in unpaired t test. Bar, 100 μm. (C) Cells were treated with the Rho kinase inhibitor Y-27632 (10 μM). Capillary tube formation was not affected, and intercellular spaces (arrows) resembling lumens were readily seen (upper panels). Cells were labeled with rhodamine phalloidin after overnight treatment with Y-27361and after tube formation in the presence of the drug. Lamellipodia formation was not affected, but actin stress fiber formation was inhibited (lower panels). Bar, 50 μm. (D) Cells were treated with C3 exoenzyme (3 μg/ml) or Y27632 (10 μM) and were overlaid with Matrigel. Tube formation was assessed after 24 h. Data represents mean ± SD from experiments performed in duplicate at least four times. No significant differences by unpaired t test.
Rho Is Not Required for Capillary Tube Formation
To examine the role of Rho in capillary assembly, we made use of C3 exoenzyme, which inactivates Rho by ADP ribosylation. Pretreatment of growing HUVECs with recombinant C3 at a concentration of 3 μg/ml for 18 h resulted in a loss of actin stress fibers and focal adhesions (our unpublished observations). Higher concentrations of C3 result in cell rounding and loss of attachment. Treatment of growing HUVECs with 10 μM Y27632, a specific inhibitor of the Rho effector protein Rho kinase, also resulted in a loss of actin stress fibers (Figure 3C, lower panels) and focal adhesions (not shown). In both cases, the formation of lamellipodia or filopodia was unaffected (Figure 3C, lower left panel).
Neither C3 nor Y27632 inhibited the assembly of capillary tubes (Figure 3D). Tube structure formed in the presence of Y27632 appeared less compacted or stable than control tubes, but still contained intercellular spaces resembling lumens (Figure 3C, upper panels). Similar results were obtained with C3 exoenzyme. Phalloidin staining of capillary structures showed that cells still contained many small lamellipodia and by time lapse microscopy, were highly motile, with extensive remodeling of the network (Figure 3C, lower right panel and our unpublished observations). We conclude from these experiments that Rho-dependent cell adhesion, stress fiber formation, and actin-myosin contractility are not required for assembly of capillary-like structures in this assay.
Rac Is Required for Capillary Tube Assembly
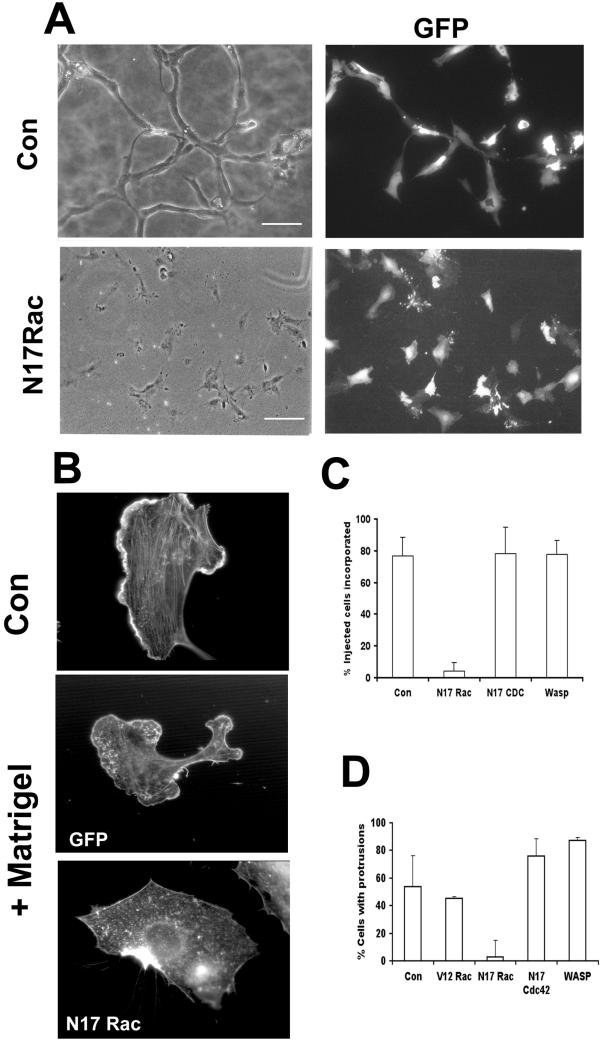
To examine the role of Rac and Cdc42, we microinjected dominant-negative (N17Rac and N17 Cdc42) mutants of each GTPase or a fragment of the effector WASp, which inhibits Cdc42 activity. Up to 150 cells in patches were coinjected with an expression vector for GFP and injected cells were identified by GFP expression. Expression of the GTPase construct was confirmed by immunofluorescence detection of myc-tagged constructs and could be seen ∼2 h after injection. Cells were overlaid with Matrigel and were photographed 20–24 h later. This experiment was quantified by counting the number of GFP-positive cells incorporated into capillary tubes. Cells injected with N17 Cdc42, WASp fragment, or control plasmid were incorporated into capillary tubes as normal (Figures 4, A and C and our unpublished observations), and it appears that Cdc42 activity is not required for capillary formation or cell migration. However, cells injected with N17 Rac were not incorporated into tube-like structures, but remained as single cells or groups of cells (Figure 4, A and C).
Figure 4.
Rac is required for capillary morphogenesis. (A and C) Cells were injected with the indicated constructs and GFP vector. Injected cells incorporated into capillary-like structures composed of at least three cells were scored as positive. Cells injected with N17 Rac were not incorporated into capillary tubes. Results (mean ± SD) from at least four independent experiments performed in duplicate are shown. **P < 0.001 in unpaired t test. Bar, 100 μM. (B and D) Cells were microinjected with N17 Rac and GFP or GFP expression vector alone, overlaid with Matrigel, and fixed and stained with rhodamine phalloidin after 3 h. A cell without Matrigel overlay is shown for comparison (upper panel). Bar, 10 μm. (D) Cells were injected with the indicated constructs and the percentage that formed protrusions after Matrigel overlay is shown. Results show mean ± SD from least four experiments performed in duplicate. *P < 0.05 in unpaired t test.
To investigate further the role of Rac function during capillary assembly, cells were fixed 3 h after Matrigel overlay and were visualized with phalloidin to examine cell morphology. Growing HUVECs show a wide array of ordered actin structures, including lamellipodia and actin stress fibers (Figure 4B, upper panel). After Matrigel overlay, the cells disassemble these ordered filamentous structures, and phalloidin staining reveals a generalized reduction in actin stress fibers and the presence of large cellular protrusions with actin-rich lamellipodia (Figure 4B, middle panel). Cells injected with GFP alone or with N17Cdc42 or WASp fragment still formed protrusive structures and lamellipodia (Figure 4D). In contrast, cells injected with N17 Rac did not form protrusions or lamellipodia, but displayed a characteristic “scalloped” appearance (Figure 4B, lower panel and Nobes and Hall, 1999). We conclude from these experiments that Rac activity is essential for capillary assembly and is required for the cell shape changes induced by Matrigel.
Actin Polymerization and Myosin-Dependent Contractility Are Required for Cell Motility But Not Early Morphological Change
It has been proposed that the formation of capillary structures in Matrigel and other matrices is influenced by cellular traction or mechanical forces generated by the cell's interaction with ECM (Ingber and Folkman, 1989a; Vernon et al., 1992). Therefore, it would be expected that the actin cytoskeleton would play a role in these events.
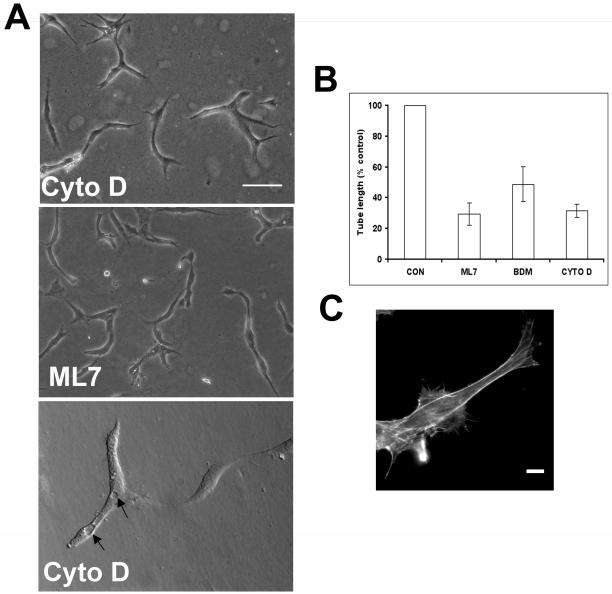
To analyze the role of actin polymerization, we have used cytochalasin D, an inhibitor of actin polymerization. At a concentration of 100 nM, cells were devoid of organized actin filaments (higher concentrations resulted in loss of cell attachment and an increase in apoptotic cells as assessed by Hoechst staining), but this had no effect on the initial morphological changes or on the formation of short capillary-like structures. These short segments still contained vacuoles (Figure 5A). However, it completely prevented the formation of an interconnecting network of tubes (Figure 5, A and B). Using phalloidin staining to detect filamentous actin, cells treated with cytochalasin D lacked lamellipodia but still formed elongated extensions after 3 h and assumed a bipolar aligned morphology after 24 h (Figure 5, A and C).
Figure 5.
The actin cytoskeleton is not required for the early morphological response to Matrigel. (A and B) Cells were treated with Cytochalasin D (100 nM), ML7 (10 nM), and BDM (10 mM). Capillary tube formation was quantified 24 h after Matrigel overlay and results represent the mean ± SD from at least four independent experiments performed in duplicate. **P < 0.001 by unpaired t test. Bar 100, μm. Lower panel in A shows vacuole formation (arrows) still present in cells treated with cytochalasin D. (C) Cells treated with cytochalasin D and labeled with rhodamine phalloidin and antitubulin antibody. Protrusion formation was not affected by treatment with cytochalasin D. Bar, 10 μm.
To determine whether actin-myosin-based contractility is required, BDM, an inhibitor of myosin II, or ML7, an inhibitor of myosin light chain kinase, were used. Both inhibitors blocked formation of an interconnecting capillary network, resulting in the formation of short segments of capillary-like structures (Figure 5, A and C and our unpublished observations). Cell shape change was not affected using these contractility inhibitors (our unpublished observations). We conclude from these experiments that actin polymerization and actin-myosin-based contractility are not required for the cell shape or morphogenetic changes induced by Matrigel. However, cell motility is inhibited and is required for the formation of an expanded interconnecting network of capillary tubes.
Microtubule Dynamics Are Required for Cell Shape Change and Motility
The microtubule cytoskeleton is required for migration of several cell types, including astrocytes and neurons (Tanaka at al, 1995; Etienne-Manneville and Hall, 2001). Growing HUVECs contain a typical radial array of microtubules (Figure 6B), but after Matrigel overlay, cells elaborate protrusive structures that contain organized microtubule filaments (Figure 6B).
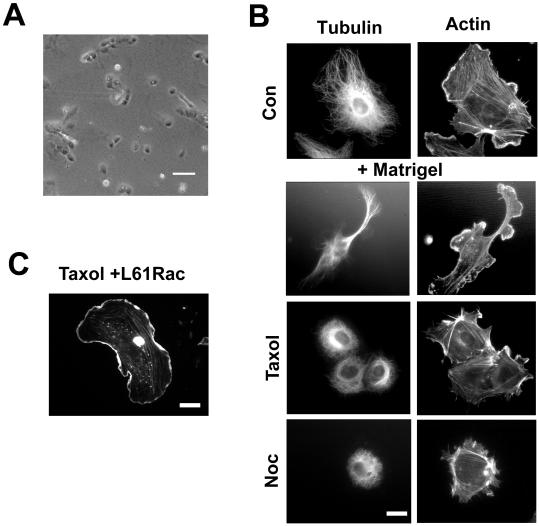
Figure 6.
The microtubule cytoskeleton is required for morphological response to Matrigel. (A) Cells treated with low-dose taxol (0.05 μM) do not form capillary-like structures. (B) Cells were labeled with antitubulin antibody and rhodamine phalloidin. Top panel shows growing HUVECs. Bottom panels show cells 3 h after Matrigel overlay either untreated or treated with taxol (0.05 μM) or Nocodazole (0.1 μM). Bar, 10 μM. (C) Taxol-treated cells injected with L61 Rac and overlaid with Matrigel. L61 Rac restored membrane ruffling but not protrusion formation. Bar, 10 μm in B and C.
To examine the role of microtubules in the endothelial cell shape changes seen during this assay, we inhibited microtubule dynamics using low doses of nocodazole (0.1 μM) or taxol (0.05 μM). At these concentrations, the microtubule network appeared intact (Figure 6B), but in both cases, capillary network formation was completely inhibited (Figure 6A and our unpublished observations). Closer inspection revealed that the cells no longer adopted an elongated morphology in response to Matrigel and that they remained round and nonmotile (Figure 6A and our unpublished observations).
To further examine the relationship between Rac and microtubule dynamics, we microinjected cells with L61 Rac and after treatment with low-dose taxol, overlaid cells with Matrigel. Three hours after overlay, injected cells had membrane ruffling but no protrusion formation (Figure 6C). We conclude that Rac regulates endothelial morphogenetic responses through a process that is dependent on microtubule dynamics.
Pak Is Required for Motility But Not Microtubule-Dependent Shape Change during Capillary Morphogenesis
The serine-threonine kinase Pak is a downstream target of Rac that has been shown to be important for endothelial cell migration (Kiosses et al., 1999). Pak can also regulate microtubule dynamics (Daub and Hall, 2001; Etienne-Manneville and Hall, 2001). To investigate the role of Pak kinase activity in capillary tube formation, we microinjected the kinase auto-inhibitory fragment of Pak into growing HUVECs before Matrigel overlay (Zhao et al., 1998). This construct had no effect on endothelial cell shape change or incorporation of cells into primitive capillary-like tubes (Figure 7, A and B). However, it did inhibit endothelial cell migration and prevented the formation of an extensive branched capillary network (Figure 7, A and B and our unpublished observations). This effect was similar to that seen with cytochalasin D or contractility inhibitors. A kinase dead mutant of Pak also had a similar effect (Figure 7A). We conclude from this that Pak is required for endothelial cell migration but is not essential for endothelial shape changes during capillary assembly. Rac, on the other hand, is required for both.
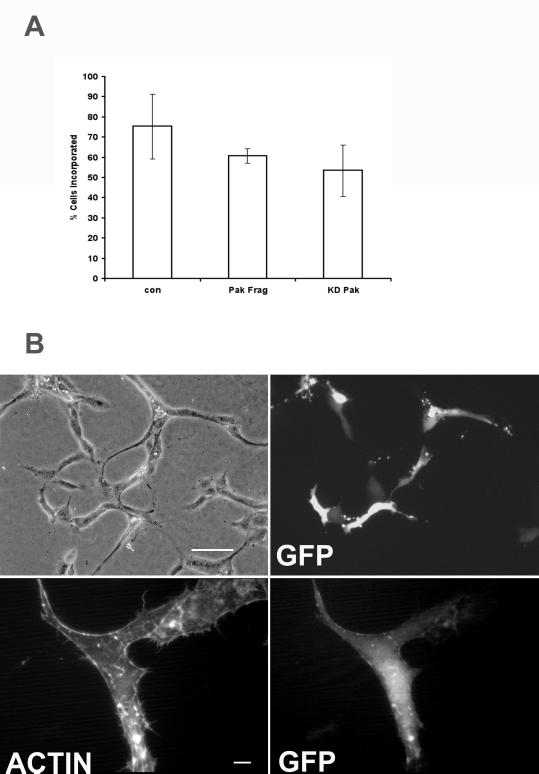
Figure 7.
Pak is required for endothelial motility but not the morphological response to Matrigel. (A) Cells were microinjected with the Pak inhibitory fragment (amino acids 83–149), kinase dead full-length Pak, or control vector and E-GFPC1. The number of injected cells incorporated into capillary structures is shown in and represents mean ± SD from at least three experiments performed in duplicate. No significant difference was detected (unpaired t test). (B) Cells were injected with Pak inhibitory fragment and photographed 24 h after Matrigel overlay (upper panels) or were fixed and stained with rhodamine phalloidin to demonstrate cell morphology (lower panels). Injected cells were incorporated into capillary-like structures and adopted an elongated bipolar morphology. Bar, 100 μm in upper panels and 10 μm in lower panels.
DISCUSSION
Branching morphogenesis is a complex developmental program that regulates the formation of the vascular system. A wide variety of extracellular cues, including soluble growth factors, matrix proteins, cell surface integrin receptors, and cell-cell adhesion molecules, influence assembly and patterning of the capillary plexus. These signals are integrated by the cell to regulate the complex changes in endothelial cell shape and motility required to form a network of endothelial tubes.
Study of the morphological and cytoskeletal change during capillary formation has been hampered by the nature of the in vitro assays previously used. Cells in three-dimensional matrix are relatively inaccessible to antibodies and other molecular tools. To address this issue, we have developed an assay in which endothelial cells are overlaid with a thin layer of Matrigel and in which an interconnecting network of endothelial capillary tubes is rapidly formed. These structures resemble capillaries in that they are multicellular and contain lumens. Tube formation occurs through an ordered sequence of events. Cells first elaborate large, highly dynamic cellular protrusions and then form small capillary-like aggregates or cords. These early cord structures do not have lumens. Cells then migrate to form a complex network of tube-like structures and eventually lumen-containing vessels. Lumens appear to arise through the formation of vacuoles by engulfment of dead cells or by simple alignment and structural contact between adjacent cells. Although the exact extracellular stimulus in Matrigel that is responsible for these complex changes is unknown, similar events have been described in other in vitro assays using defined extracellular matrix molecules (Folkman and Haudenschild, 1980; Montesano and Orci, 1985; Meyer et al., 1997). The advantages of the assay described here are the ability to directly visualize the changes in cell morphology and the cytoskeleton during capillary tube formation and the ability to microinject cells before Matrigel overlay. Assays of this nature, when interpreted with caution, provide important information into the mechanisms of capillary assembly.
Cells stimulated to form capillary tubes in the presence of Matrigel disassemble their organized actin stress fibers and form dynamic protrusive structures, leading to migration and capillary assembly. Many protrusions have membrane ruffles or lamellipodia at their leading edge. Surprisingly, however, inhibition of actin polymerization blocked lamellipodia formation but did not affect protrusion formation or the assembly of short primitive capillary-like structures. Inhibition of actin polymerization did block subsequent cell migration and expansion of the capillary network. Similarly, inhibitors of actin-myosin-based contractility also prevented cell migration but not the initial morphological changes. The loss of actin stress fibers and strong adhesion after Matrigel overlay may allow cells to alter their morphology and migrate freely. This likely promotes an increase in cell aggregation and facilitates the cell-cell adhesion needed for capillary assembly. Interestingly, the down-regulation of adhesive proteins such as vinculin has been reported during endothelial tube formation and differentiation (Deroanne et al., 1996). However, it is clear that adhesion to the ECM and integrin signaling are essential requirements for endothelial cell survival and angiogenesis (Brooks et al., 1994).
Inhibition of Rho or of its effector, Rho-kinase, did not block the morphogenetic response to Matrigel or capillary tube formation, although cells retained lamellipodia and capillary structures appeared less stable. Unfortunately, for technical reasons, we have been unable to directly measure Rho activity after Matrigel overlay, but the loss of actin stress fibers seen during morphogenesis suggests that down-regulation of Rho may in fact be required for capillary assembly. In support of this, overexpression of an activated mutant of Rho induces cell contraction and inhibits the morphological response to Matrigel (our unpublished observations). A similar situation exists in neurons where activation of Rho has been shown to inhibit neurite migration and induce growth cone retraction (Jalink et al., 1994). In this context, it is interesting that many ECM proteins with an antiadhesive function such as tenascin C, SPARC, or thrombospondin have all been shown to play a role in angiogenesis. Tenascin C, for example, induces an elongated morphology in endothelial cells and has been shown to down-regulate Rho activity and stress fibers in fibroblasts (Schenk et al. 1999; Wenk et al., 2000).
Although the Matrigel-induced changes in endothelial cell shape and the formation of primitive capillary-like structures are independent of actin polymerization, they are totally dependent on microtubule dynamics. Relatively little is known of the role of microtubules in endothelial function, although complete disruption of the microtubule network has been reported to inhibit endothelial migration in a wound healing assay (Ettenson and Gotlieb, 1992). Microtubules are required for migration and shape changes in other cells types such as neurons and astrocytes but not in fibroblasts (Tanaka et al. 1995; Nobes and Hall, 1999; Etienne-Manneville and Hall, 2001).
The protrusive structures we describe in endothelial cells, like those seen in astrocytes and neurons, are also dependent on Rac function. Because increased microtubule dynamics (for example, after nocodazole treatment) has been shown to result in Rac activation and lamellipodia formation (Waterman-Storer et al., 1999), we wanted to examine whether the role of microtubules in endothelial cells was simply to activate Rac. However, this was not the case because in cells treated with low-dose taxol, injection of L61 Rac did not restore protrusion formation. As Rac is essential for the earliest changes in cell morphology, we were unable to test directly whether it might also play a role in the later actin-dependent events during capillary morphogenesis. Nevertheless, a dual role for Rac is strongly suggested by the finding that the Rac effector, Pak, is required for endothelial cell motility but not for the formation of microtubule-dependent protrusions or primitive capillary-like structures.
The role of microtubules in capillary tube formation resembles that in neuronal outgrowth where microtubules are required for growth cone extension and guidance (Tanaka et al., 1995). Recent evidence has uncovered a link between neurogenesis and vascular development, with both processes sharing many of the same patterning cues (Shima and Mailhos, 2000). Our results suggest that as in neurons, Rho family GTPases integrate the signals from these cues to control endothelial morphogenesis. They control cell shape and movement and are essential for formation of new capillaries in the in vitro assay used here. Further study is needed to define their role in other aspects of angiogenesis such as the establishment of cell polarity, the control of transcription, and the maintenance of capillary architecture.
The potent chemotherapeutic agent taxol, and another antitumor drug, Combresatin A, target microtubules and may also inhibit tumor angiogenesis (Belotti et al., 1996; Grosios et al. 1999). It has been assumed that their antitumor effects may be through inhibition of mitosis and cell division. Our results outline a potential alternative mechanism for this effect and suggest that targeting microtubule dynamics may be a useful antiangiogenic strategy. The central role of Rac in the control of microtubule-dependent morphogenesis suggests that the downstream effectors of Rac in this pathway are deserving of further study and this may also be a potential avenue for novel therapeutic research.
ACKNOWLEDGMENTS
We thank Prof. Bruce Hendry for his encouragement at the outset of this project, Dr. Richard Lamb and Dr. C. Nobes for advice and stimulating discussions, and the members of the Hall laboratory for their continuing support. This work was generously supported by the Medical Research Council clinician scientist program (J.C. and N.S.) and by the Cancer Research Campaign (A.H.).
Abbreviations used:
- ECM
extracellular matrix
- PBS
phosphate-buffered saline
- GFP
green fluorescent protein
- BDM
2,3-butanedione monoxime
- HUVEC
human umbilical vein endothelial cell
- WAS
Wiskott-Aldrich syndrome
- Pak
p21-activated kinase
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–01–0006. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–01–0006.
REFERENCES
- Adams RH, Wilkinson GA, Weiss C, Diella F, Gale NW, Deutsh U, Risau W, Klein R. Roles of the ephrin B ligands and Eph receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev. 1999;12:667–678. doi: 10.1101/gad.13.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aktories K, Schmidt G, Just I. Rho GTPases as targets of bacterial protein toxins. Biol Chem. 2000;381:421–426. doi: 10.1515/BC.2000.054. [DOI] [PubMed] [Google Scholar]
- Ambler CA, Nowicki JL, Burke AC, Bautch VL. Assembly of trunk and limb blood vessels involves extensive migration and vasculogenesis of somite-derived angioblasts. Dev Biol. 2001;234:352–364. doi: 10.1006/dbio.2001.0267. [DOI] [PubMed] [Google Scholar]
- Bach TL, Barsigian C, Chalupowicz DG, Busler D, Yaen CH, Grant DS, Martinez J. VE-cadherin mediates endothelial cell capillary tube formation in fibrin and collagen gels. Exp Cell Res. 1998;238:324–334. doi: 10.1006/excr.1997.3844. [DOI] [PubMed] [Google Scholar]
- Bazzoni G, Dejana E, Lampugnani MG. Endothelial adhesion molecules in the development of the vascular tree: the garden of forking paths. Curr Opin Cell Biol. 1999;11:573–581. doi: 10.1016/s0955-0674(99)00023-x. [DOI] [PubMed] [Google Scholar]
- Belotti D, Vergani V, Drudis T, Borsotti P, Pitelli MR, Viale G, Giavazzi R, Taraboletti G. The microtubule-affecting drug paclitaxel has antiangiogenic activity. Clin Cancer Res. 1996;2:1843–1849. [PubMed] [Google Scholar]
- Bischoff J. Approaches to studying cell adhesion molecules in angiogenesis. Trends Cell Biol. 1995;5:69–74. doi: 10.1016/s0962-8924(00)88949-7. [DOI] [PubMed] [Google Scholar]
- Brooks PC, Clark RAF, Cheresh DA. Requirement of vascular integrin avB3 for angiogenesis. Science. 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, et al. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell. 1999;98:147–157. doi: 10.1016/s0092-8674(00)81010-7. [DOI] [PubMed] [Google Scholar]
- Daub H, Gevaert K, Vanderkerkhoue S, Sobel A, Hall A. Rac/Cdc42 and p65PAK regulate microtubule-destabilizing protein stathmin through phosphorylation at serine 16. J Biol Chem. 2001;276:1877–1880. doi: 10.1074/jbc.C000635200. [DOI] [PubMed] [Google Scholar]
- Deroanne CF, Colige AC, Nusgens BV, Lapiere CM. Modulation of expression and assembly of vinculin during in vitro fibrillar collagen-induced angiogenesis and its reversal. Exp Cell Res. 1996;224:215–223. doi: 10.1006/excr.1996.0131. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. Integrin-mediated activation of cdc42 controls cell polarity in migrating astrocytes through PKCl. Cell. 2001;106:489–498. doi: 10.1016/s0092-8674(01)00471-8. [DOI] [PubMed] [Google Scholar]
- Ettenson DS, Gotlieb AI. Centrosomes, microtubules, and microfilaments in the reendothelialization and remodeling of double-sided in vitro wounds. Lab Invest. 1992;66:722–733. [PubMed] [Google Scholar]
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- Folkman J, Haudenschild C. Angiogenesis in vitro. Nature. 1980;281:551–556. doi: 10.1038/288551a0. [DOI] [PubMed] [Google Scholar]
- Friedlander M, Brooks PC, Shaffer RW, Kincaid CM, Varner JA, Cheresh DA. Definition of two angiogenic pathways by distinct α v integrins. Science. 1995;270:1500–1502. doi: 10.1126/science.270.5241.1500. [DOI] [PubMed] [Google Scholar]
- Gale N, Yancopoulos GD. Growth factors acting via endothelial cell-specific receptor tyrosine kinases: VEGFs, angiopoietins and ephrins in vascular development. Genes Dev. 1999;13:1055–1066. doi: 10.1101/gad.13.9.1055. [DOI] [PubMed] [Google Scholar]
- Grant DS, Tashiro KI, Segui-Real B, Yamada Y, Martin GR, Kleinman HK. Two different laminin domains mediate the differentiation of human endothelial cells into capillary-like structures in vitro. Cell. 1989;58:933–943. doi: 10.1016/0092-8674(89)90945-8. [DOI] [PubMed] [Google Scholar]
- Grosios K, Holwell SE, McGown AT, Pettit GR, Bibby MC. In vivo and in vitro evaluation of combretastatin A-4 and its sodium phosphate pro-drug. Br J Cancer. 1999;81:1318–1327. doi: 10.1038/sj.bjc.6692174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Hogan BLM. Morphogenesis. Cell. 1999;96:225–233. doi: 10.1016/s0092-8674(00)80562-0. [DOI] [PubMed] [Google Scholar]
- Hopkins CR, Trowbridge IS. Internalization of transferrin and the transferrin receptor in epidermoid carcinoma cells. J Cell Biol. 1983;97:508–521. doi: 10.1083/jcb.97.2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber DE, Folkman J. Mechanochemical switching between growth and differentiation during fibroblast growth factor stimulated angiogenesis in vitro: role of the extracellular matrix. J Cell Biol. 1989a;109:317–330. doi: 10.1083/jcb.109.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber DE, Folkman J. How does the extracellular matrix control capillary morphogenesis? Cell. 1989b;58:803–805. doi: 10.1016/0092-8674(89)90928-8. [DOI] [PubMed] [Google Scholar]
- Jalink K, van Corven EJ, Hengeveld T, Morii N, Narumiya S, Moolenaar WH. Inhibition of lysophosphatidate- and thrombin-induced neurite retraction and neuronal cell rounding by ADP ribosylation of the small GTP-binding protein Rho. J Cell Biol. 1994;126:801–810. doi: 10.1083/jcb.126.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiosses WB, Daniels RH, Otey C, Bokoch GM, Schwartz MA. A role for p21-activated kinase in endothelial cell migration. J Cell Biol. 1999;147:831–843. doi: 10.1083/jcb.147.4.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozma RS, Sarner S, Ahmed S, Lim L. Rho family GTPases and neuronal growth cone remodeling: relationship between increased complexity induced by Cdc42Hs, Rac 1, and acetylcholine and collapse induced by Rho A and lysophosphatidic acid. Mol Cell Biol. 1995;17:1201–1211. doi: 10.1128/mcb.17.3.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger RJ, Krasnow MA. Genetic control of branching morphogenesis. Science. 1999;284:1635–1639. doi: 10.1126/science.284.5420.1635. [DOI] [PubMed] [Google Scholar]
- Meyer GT, Matthias LJ, Noack L, Vadas MA, Gamble JR. Lumen formation during angiogenesis in vitro involves phagocytic activity, formation and secretion of vacuoles, cell death, and capillary tube remodeling by different populations of endothelial cells. Anat Rec. 1997;249:327–340. doi: 10.1002/(SICI)1097-0185(199711)249:3<327::AID-AR3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Montesano R, Orci L. Tumor-promoting phorbol esters induce angiogenesis in vitro. Cell. 1985;42:469–477. doi: 10.1016/0092-8674(85)90104-7. [DOI] [PubMed] [Google Scholar]
- Nehls V, Herrmann R, Huhnken M. Guided migration as a novel mechanism of capillary network remodeling is regulated by basic fibroblast growth factor. Histochem Cell Biol. 1998;109:319–329. doi: 10.1007/s004180050232. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho, Rac and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho GTPases control polarity, protrusion and adhesion during cell movement. J Cell Biol. 1999;144:1235–1244. doi: 10.1083/jcb.144.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- Schenk S, Chiquet-Ehrismann R, Battegay EJ. The fibrinogen globe of tenascin-C promotes basic fibroblast growth factor-induced endothelial cell elongation. Mol Biol Cell. 1999;9:2933–2943. doi: 10.1091/mbc.10.9.2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima DT, Mailhos C. Vascular developmental biology: getting nervous. Curr Opin Genet Dev. 2000;10:536–542. doi: 10.1016/s0959-437x(00)00124-6. [DOI] [PubMed] [Google Scholar]
- Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin 1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92:735–745. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- Springer ML, Chen AS, Kraft PE, Bednarski M, Blau HM. VEGF gene delivery to muscle: potential role for vasculogenesis in adults. Mol Cell. 1998;2:549–558. doi: 10.1016/s1097-2765(00)80154-9. [DOI] [PubMed] [Google Scholar]
- Tanaka E, Ho T, Kirschner MW. The role of microtubule dynamics in growth cone motility and axonal growth. J Cell Biol. 1996;128:139–155. doi: 10.1083/jcb.128.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon RB, Angello M, Ireula-Arispe L, Lane TF, Sage EH. Reorganisation of basement membrane matrices by cellular traction promotes the formation of cellular networks in vitro. Lab Invest. 1992;66:536–547. [PubMed] [Google Scholar]
- Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin B2 and its receptor EphB4. Cell. 1998;93:741–753. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- Waterman-Storer CM, Worthylake RA, Liu BP, Burridge K, Salmon ED. Microtubule growth activates Rac1 to promote lamellipodial protrusion in fibroblasts. Nat Cell Biol. 1999;1:45–50. doi: 10.1038/9018. [DOI] [PubMed] [Google Scholar]
- Wenk MB, Midwood KS, Schwarzbauer JS. Tenascin-C suppresses Rho activation. J Cell Biol. 2000;150:913–919. doi: 10.1083/jcb.150.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZS, Manser E, Chen XQ, Chong C, Leung T, Lim L. A conserved negative regulatory region in alpha PAK: inhibition of PAK kinases reveals their morphological roles downstream of Cdc42 and Rac1. Mol Cell Biol. 1998;18:2153–2163. doi: 10.1128/mcb.18.4.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]