Abstract
Caveolae are vesicular invaginations of the plasma membrane. Caveolin-1 is the principal structural component of caveolae in vivo. Several lines of evidence are consistent with the idea that caveolin-1 functions as a “transformation suppressor” protein. In fact, caveolin-1 mRNA and protein expression are lost or reduced during cell transformation by activated oncogenes. Interestingly, the human caveolin-1 gene is localized to a suspected tumor suppressor locus (7q31.1). We have previously demonstrated that overexpression of caveolin-1 arrests mouse embryonic fibroblasts in the G0/G1 phase of the cell cycle through activation of a p53/p21-dependent pathway, indicating a role of caveolin-1 in mediating growth arrest. However, it remains unknown whether overexpression of caveolin-1 promotes cellular senescence in vivo. Here, we demonstrate that mouse embryonic fibroblasts transgenically overexpressing caveolin-1 show: 1) a reduced proliferative lifespan; 2) senescence-like cell morphology; and 3) a senescence-associated increase in β-galactosidase activity. These results indicate for the first time that the expression of caveolin-1 in vivo is sufficient to promote and maintain the senescent phenotype. Subcytotoxic oxidative stress is known to induce premature senescence in diploid fibroblasts. Interestingly, we show that subcytotoxic level of hydrogen peroxide induces premature senescence in NIH 3T3 cells and increases endogenous caveolin-1 expression. Importantly, quercetin and vitamin E, two antioxidant agents, successfully prevent the premature senescent phenotype and the up-regulation of caveolin-1 induced by hydrogen peroxide. Also, we demonstrate that hydrogen peroxide alone, but not in combination with quercetin, stimulates the caveolin-1 promoter activity. Interestingly, premature senescence induced by hydrogen peroxide is greatly reduced in NIH 3T3 cells harboring antisense caveolin-1. Importantly, induction of premature senescence is recovered when caveolin-1 levels are restored. Taken together, these results clearly indicate a central role for caveolin-1 in promoting cellular senescence and they suggest the hypothesis that premature senescence may represent a tumor suppressor function mediated by caveolin-1 in vivo.
INTRODUCTION
Caveolae are vesicular invaginations of the plasma membrane. Caveolins are the structural components of caveolae. It has been proposed that caveolins participate in vesicular trafficking events and signal transduction processes (Lisanti et al., 1994; Couet et al., 1997a; Okamoto et al., 1998). Several independent lines of evidence suggest that signaling molecules are sequestered, organized, and functionally regulated by caveolae microdomains. These caveolin-interacting proteins include Ha-Ras, Src-family tyrosine kinases, G protein α subunits, endothelial nitric oxide synthase, epidermal growth factor receptor, Neu, protein kinase A, and protein kinase C (Smart et al., 1993; Lisanti et al., 1994; Shenoy-Scaria et al., 1994; Li et al., 1995; Schnitzer et al., 1995; Feron et al., 1996; Li et al., 1996; Mineo et al., 1996; Song et al., 1996a; Couet et al., 1997b; Ju et al., 1997; Liu et al., 1997; Segal et al., 1999). In many cases, mutational activation of these molecules (e.g., G-proteins, H-Ras, and Src-family kinases) prevents regulated interaction with caveolin. These activating mutations include H-Ras and Gαs variants that are found in human cancers (Song et al., 1996a).
The mammalian caveolin gene family consists of caveolin-1, -2, and -3 (Scherer et al., 1996; Tang et al., 1996; Okamoto et al., 1998). Caveolin-1 and -2 are coexpressed and form a hetero-oligomeric complex (Scherer et al., 1997) in many cell types, with particularly high levels in adipocytes, whereas expression of caveolin-3 is muscle specific and is found in both cardiac and skeletal muscle, as well as smooth muscle cells (Song et al., 1996b; Minetti et al., 1998; Galbiati et al., 1999; Galbiati et al., 2000a,b; Galbiati et al., 2001a). These caveolin homo- and hetero-oligomers directly interact with cholesterol and represent the functional assembly units of caveolae (Sargiacomo et al., 1995). In addition, the caveolin gene family is structurally and functionally conserved from worms (C. elegans) to man (Tang et al., 1997), supporting the idea that caveolins play an essential role.
Most cells cannot divide indefinitely due to a process termed cellular senescence. Cellular senescence appears to be a fundamental feature of somatic cells, with the exception of most tumor cells and certain stem cells. Senescent cells enter into irreversible growth arrest, display a large and flat morphology, up-regulate several cell cycle inhibitory proteins, and show increased acid β-galactosidase activity (Dimri et al., 1995; Dumont et al., 2000; Frippiat et al., 2001). It has been suggested that cellular senescence may represent a powerful tumor suppressive mechanism (Kim et al., 1994; Dimri et al., 1995; Lee et al., 1998; Wynford-Thomas, 1999; Black et al., 2000; Lundberg et al., 2000; Sherr and DePinho, 2000).
Caveolin-1 is down-regulated in human tumors, in cell lines derived from human tumors, and in cell lines transformed by oncogenes (i.e., H-Ras and Abl; Sager et al., 1994; Koleske et al., 1995; Engelman et al., 1997; Engelman et al., 1998; Lee et al., 1998). On the other hand, up-regulation of caveolin-1 is sufficient to revert the transformed phenotype of oncogene-transformed cell lines (Engelman et al., 1997). These results suggest that caveolin-1 may have tumor suppressor activity. In support of this hypothesis, we previously overexpressed caveolin-1 in mice as a transgene (Galbiati et al., 2001b) and we demonstrated that overexpression of caveolin-1 in mouse embryonic fibroblasts is sufficient to block these cells in the G0/G1 phase of the cell cycle. We also demonstrated that caveolin-1-mediated cell cycle arrest occurs through a p53/p21-dependent pathway, as verified by the activation of the p53 responsive element and the up-regulation of p21WAF1/Cip1 protein expression in caveolin-1-overexpressing mouse embryonic fibroblasts (MEFs).
A block in cellular proliferation and inhibition of cell cycle progression are two fundamental steps in achieving cellular senescence. In fact, molecules that negatively regulate cell cycle progression are considered possible players in promoting and/or maintaining the senescent phenotype. Thus, caveolin-1 may represent a candidate protein in mediating cellular senescence. Interestingly, it has been previously reported that caveolin-1 expression is up-regulated in senescent human diploid fibroblasts (Park et al., 2000; Wheaton et al., 2001). Up-regulation of caveolin-1 in these cells was associated with lack of kinase activity in the caveolar-enriched fraction and unresponsiveness to EGF stimulation. However, whether caveolin-1 up-regulation is a “trigger factor” in promoting and/or maintaining cellular senescence remains unknown.
Here, we address this question directly by performing a series of biochemical analyses on MEFs that transgenically overexpress caveolin-1. We demonstrate that MEFs overexpressing caveolin-1 have a shorter proliferative lifespan when grown in culture as compared with MEFs expressing normal levels of endogenous caveolin-1. In addition, caveolin-1-overexpressing MEFs show a senescence-like cell morphology and express high levels of senescence-associated β-galactosidase activity.
One of the manifestations of aging is the accumulation of damage at both the cellular and organismal levels. This damage is initiated by endogenous and exogenous stimuli and may lead to cellular senescence. Cellular stress, for example, may induce premature senescence. Thus, cellular senescence represents a physiological endpoint for many diploid cells. However, stressful stimuli can harmfully accelerate the process. Stressful conditions include exposure to hydrogen peroxide (H2O2; Chen and Ames, 1994; Chen et al., 1998; Frippiat et al., 2001), UV light (Bayreuther et al., 1988; Rodemann et al., 1989), tert-butylhydroperoxide (Toussaint et al., 1992), hyperoxia (von Zglinicki et al., 1995), and radioactivity (Bumann et al., 1995). Cellular senescence may also be achieved when cells are passaged for a long period of time in culture (Thomas et al., 1997). Here, we demonstrate that subcytotoxic levels of H2O2 induce premature senescence in NIH 3T3 cells and up-regulate endogenous caveolin-1 expression. Interestingly, costimulation with H2O2 and quercetin or vitamin E, two well-known antioxidant agents, does not promote premature senescence and does not up-regulate caveolin-1 protein expression. We also demonstrate that H2O2-induced premature senescence is dramatically reduced in NIH 3T3 cells harboring antisense caveolin-1. Taken together, these results support the hypothesis that caveolin-1 overexpression is sufficient to induce cellular senescence and that up-regulation of caveolin-1 may represent a fundamental step in mediating stress-induced premature senescence (SIPS).
MATERIALS AND METHODS
Materials
Antibodies and their sources were as follows: anti-caveolin-1 immunoglobulin (Ig) G (monoclonal antibody [mAb] 2297; BD Transduction Laboratories, Lexington, KY); anti-caveolin-2 IgG (mAb 65; BD Transduction Laboratories); and anti-β-actin IgG (mAb AC-15; Sigma, St. Louis, MO). Other reagents were purchased commercially: DMEM (Cellgro, Herndon, VA) and donor bovine calf serum (DBCS; JRH Biosciences, Lenexa, KS). All other biochemicals used were of the highest purity available and were obtained from regular commercial sources.
Cell Culture
Normal NIH 3T3 cells and caveolin-1 antisense revertants (Rev-Cav-1-AS) were grown in DMEM supplemented with glutamine, antibiotics (penicillin and streptomycin) and 10% DBCS. NIH 3T3 caveolin-1 antisense cells (Cav-1-AS) were grown in DMEM supplemented with glutamine, antibiotics (penicillin and streptomycin), 10% DBCS, and hygromycin B (200 μg/ml). MEF cells were grown in DMEM supplemented with glutamine, antibiotics (penicillin and streptomycin), and 10% fetal bovine calf serum.
Immunoblotting
Cells were collected in boiling sample buffer and were homogenized using a 26-gauge needle. Cellular proteins were resolved by SDS-PAGE (12.5% acrylamide) and were transferred to BA83 nitrocellulose membranes (0.2 μm, Schleicher & Schuell, Keene, NH). Blots were incubated for 2 h in Tris-buffered saline/Tween 20 (TBST; 10 mM Tris-HCl, pH 8.0, 150 mM NaCl, and 0.2% Tween 20) containing 2% powdered skim milk and 1% bovine serum albumin. After three washes with TBST, membranes were incubated for 2 h with the primary antibody (∼1000-fold diluted in TBST) and for 1 h with horseradish peroxidase-conjugated goat anti-rabbit/mouse IgG (∼5000-fold diluted). Bound antibodies were detected using an ECL detection kit (Amersham Pharmacia Biotech, Piscataway, NJ).
Proliferative Lifespan Assay of MEFs
MEFs were passaged into a new 10-cm dish (1:3 division) every time they reached subconfluency until they showed irreversible growth arrest (as verified by counting the cell number and BrdU incorporation assays, our unpublished results). The number of passages MEFs underwent before showing irreversible growth arrest was recorded.
Acid β-Galactosidase Staining
Cells were subjected to acid β-galactosidase staining using the Senescence β-galactosidase Staining kit (Cell Signaling) according to the manufacture's recommendations. Briefly, cells were washed twice with phosphate-buffered saline (PBS) and were fixed with the fixative solution for 15 min. Cells were then washed twice with PBS and incubated overnight at 37°C with the staining solution. Cells were then examined for the development of blue color. Cells were photographed at low magnification (×10) using a BX50WI Optical light microscope (Olympus, Tokyo, Japan).
Induction of Premature Senescence in NIH 3T3 Cells
Cells were seeded in 60-mm dishes at 270,000 cells/dish. After 24 h, cells were exposed to subcytotoxic oxidative stress with different concentrations of H2O2 (1–150 μM) in the presence or absence of 300 μM quercetin or 300 μM vitamin E for 2 h. Cells were also treated with 300 μM quercetin or 300 μM vitamin E alone for 2 h. Cells were then washed twice and allowed to recover in complete medium for different periods of time (1–11 d). Although NIH 3T3 cells exposed to subcytotoxic oxidative stress alone did not proliferate during recovery, proliferation was not affected in control cells, cells exposed to subcytotoxic oxidative stress in the presence of quercetin or vitamin E, and cells treated with quercetin or vitamin C alone (as verified by counting the cell number, our unpublished results) and they were passaged as necessary. Cells at similar confluency were then subjected to immunoblot analysis and senescence acid β-galactosidase staining.
Luciferase Reporter Assay
Cells were seeded in 60-mm dishes at 270,000 cells/dish. The following day, cells were transiently transfected, using a modified calcium-phosphate precipitation method, with 2 μg of the caveolin-1 promoter luciferase reporter (3 Kb + Intron-1) or the luciferase reporter plasmid pTA-p53RE. Six hours post-transfection, cells were rinsed twice with PBS and were incubated in medium containing 150 μM H2O2 with or without 300 μM quercetin for 2 h. Cells were then washed twice and incubated in complete medium at 37°C for an additional 72 h. Cells were then lysed in 500 μl of extraction buffer; 200 μl was used to measure luciferase activity (Engelman et al., 1999). Three independent experiments were performed for each condition.
UV Radiation Treatment
Cells were seeded in 60-mm dishes at 270,000 cells/dish. After 24 h, cells were irradiated with a subLD of UV-C light (10 J/m2). During irradiation, cells were deprived of growth medium. Cells were allowed to recover in complete medium for different periods of time (2–11 d). Cells were then subjected to immunoblot analysis and senescence acid β-galactosidase staining.
4,6-Diamidino-2-Phenylindole (DAPI) Staining
Normal NIH 3T3, Cav-1-AS, and Rev-Cav-1-AS cells (untreated or treated with H2O2) were washed twice with PBS and fixed with 3.7% paraformaldehyde at room temperature for 20 min. Cells were then incubated with RNase A (10 μg/ml in PBS) for 10 min and with DAPI (1 μg/ml in PBS) for 10 min. Nuclear morphology was examined with a Provis fluorescent microscope (Olympus).
RESULTS
MEFs That Transgenically Overexpress Caveolin-1 Show Premature Irreversible Growth Arrest in Culture and Have a Large, Flat Morphology
We have previously achieved broad expression of caveolin-1 in mice as a transgene using the cDNA for caveolin-1 inserted into a vector (pCAGGS) driven by the cytomegalovirus enhancer and the chicken β-actin promoter, followed by the rabbit β-globin polyadenylation signal (Galbiati et al., 2001b). By Western-blot analysis, we demonstrated that high levels of caveolin-1 transgene expression are present in fat, kidney, liver, brain, lung, spleen, skeletal muscle, and the heart (Galbiati et al., 2001b). In addition, we generated MEFs overexpressing caveolin-1 and demonstrated that these cells show an increased tendency toward cell cycle arrest in the G0/G1 phase of the cell cycle; this G0/G1 arrest is mediated by activation of a p53/p21-dependent pathway (Galbiati et al., 2001b).
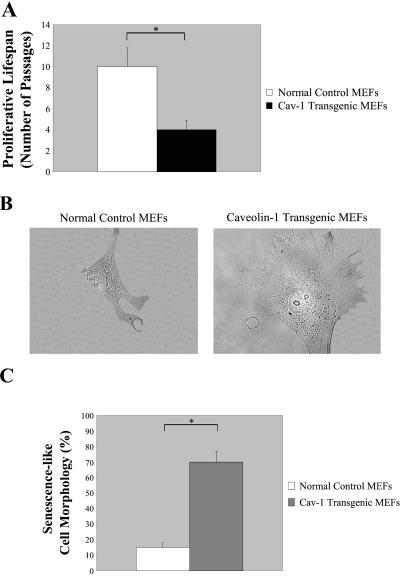
To test the hypothesis that caveolin-1 may also induce cellular senescence in these MEFs, we began to evaluate the proliferative lifespan of these cells in culture. Two independent MEF primary cell lines overexpressing caveolin-1 (#1 and #3) and two independent MEF primary cell lines expressing normal levels of caveolin-1 (#2 and #4) were used for these studies. The results reported in Figure 1 and Figure 2 were obtained with MEFs #1 and #2. Similar results were achieved with MEFs #3 and #4 (our unpublished results). MEFs (1 × 106 cells, passage 1) derived from normal control mice and from caveolin-1 transgenic mice were plated into a 10-cm dish and were allowed to expand. Every time the culture reached subconfluency, the cells were split 1:3 into a new 10-cm dish. This protocol was followed until more then 95% of the cells lost the ability to proliferate (as verified by counting the cell number and by BrdU incorporation assays, our unpublished results). We observed that MEFs expressing normal levels of caveolin-1 lost their ability to proliferate after 10 ± 1.8 (means ± SD) passages (n = 8; Figure 1A). In contrast, caveolin-1-overexpressing MEFs clearly showed premature irreversible growth arrest (4 ± 0.9 passages; n = 8; Figure 1A).
Figure 1.
Caveolin-1-overexpressing MEFs show premature irreversible growth arrest and senescence-like cell morphology. (A) MEFs derived from normal control mice and from caveolin-1 transgenic mice were passaged into a new 10-cm dish (1:3 division) every time they reached subconfluency. The number of passages MEFs were passaged before showing irreversible growth arrest was recorded. Note that overexpression of caveolin-1 induces premature growth arrest in MEFs. Values represent means ± SEM *P < 0.001. (B) Cells representative of the two MEF cell populations are shown (×60 magnification). (C) MEFs derived from normal control mice and from caveolin-1 transgenic mice were examined using a BX50WI Optical light microscope (Olympus) at a magnification of ×10. The percentage of cells showing senescence-like cell morphology was recorded. Note that 70 ± 5% of MEFs (passage 1) overexpressing caveolin-1 shows a large and flat morphology as compared with 15 ± 3% of MEFs derived from normal control mice. Values represent means ± SEM. *P < 0.0005.
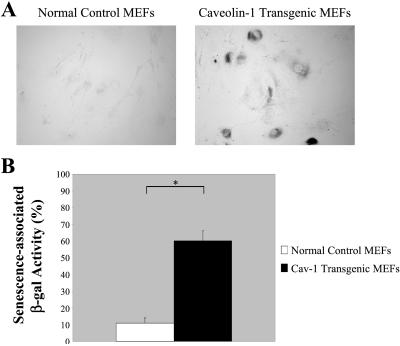
Figure 2.
Primary cultures of MEFs that transgenically express caveolin-1 show senescence-associated acid β-galactosidase activity. MEFs derived from normal control mice and from caveolin-1 transgenic mice were assayed for senescence-associated acid β-galactosidase activity (S-A β-gal). (A) Cells were examined using a BX50WI Optical light microscope (Olympus) at a magnification of ×10. A representative field is shown. (B) Quantitation of the senescence-associated acid β-galactosidase activity assay is shown. Note that 59 ± 6% of MEFs overexpressing caveolin-1 are positive for S-A β-gal as compared with only 12 ± 2% of MEFs expressing normal levels of endogenous caveolin-1. Values represent means ± SEM. *P < 0.0005.
It is well known that senescent cells display a typical large and flat morphology (Dimri et al., 1995; Dumont et al., 2000; Frippiat et al., 2001). Next, we evaluated the cell morphology of MEFs derived from caveolin-1 transgenic embryos as compared with MEFs derived from normal control littermate embryos. Representative cells for each of the two MEF populations are shown in Figure 1B. Light microscopy experiments indicated that 70 ± 5% of MEFs (passage 1; n = 20) overexpressing caveolin-1 show a large and flat morphology as compared with 15 ± 3% (n = 18) of MEFs derived from normal control mice (Figure 1C). These results indicate that overexpression of caveolin-1 in MEFs is responsible for a reduced proliferative lifespan in culture and for inducing a large and flat cell morphology, features that are typical of the senescent phenotype.
Acid β-Galactosidase Activity Is Elevated in MEFs Overexpressing Caveolin-1
Our results suggest that overexpression of caveolin-1 in MEFs may induce a senescent phenotype (Figure 1, A-C). Thus, we next assessed whether overexpression of caveolin-1 was associated with elevated acid β-galactosidase enzymatic activity. Acid β-galactosidase activity (at pH 6) is a well-established biochemical marker that is associated with the senescent cell phenotype (Dimri et al., 1995; Dumont et al., 2000; Frippiat et al., 2001). Figure 2A clearly indicates that a higher percentage of caveolin-1-overexpressing MEFs (passage 1) are positive for acid β-galactosidase enzymatic activity as compared with MEFs derived from normal control mice. Quantitation of this staining is shown in Figure 2B. Note that 59 ± 6% (n = 20) of MEFs overexpressing caveolin-1 were positive as compared with only 12 ± 2% (n = 20) of MEFs expressing normal levels of endogenous caveolin-1 (Figure 2B).
Up-Regulation of Caveolin-1 Occurs during SIPS in NIH 3T3 Fibroblasts
We have demonstrated that overexpression of caveolin-1 induces a G0/G1 arrest via a p53/p21WAF1/Cip1-dependent mechanism (Galbiati et al., 2001b) and promotes a premature senescence phenotype (this report) in MEFs in vivo. To further elaborate on these findings, we decided to focus our studies on the role of caveolin-1 in SIPS. Subcytotoxic oxidative stress, for example, has been shown to induce premature senescence in fibroblasts in culture (Chen and Ames, 1994; Chen et al., 1995; Dimri et al., 1995; von Zglinicki et al., 1995; Dumont et al., 2000; Frippiat et al., 2001). If caveolin-1 is directly involved in mediating cellular senescence, we would expect up-regulation of caveolin-1 in NIH 3T3 cells stimulated with subcytotoxic levels of H2O2.
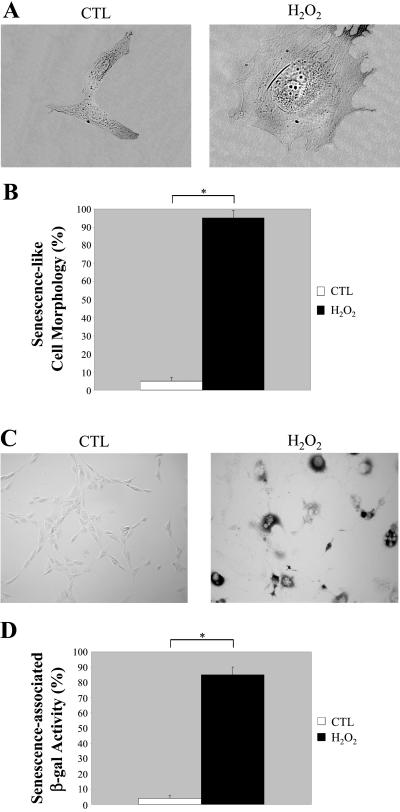
NIH 3T3 cells were treated with 150 μM H2O2 for 2 h in complete medium. Cells were then washed twice and allowed to recover in complete medium for 11 d. Figure 3 shows that we successfully induced premature senescence. The flat and large morphology typical of senescent NIH 3T3 cells is shown in Figure 3A. Figure 3B indicates that 95 ± 3% (n = 16) of NIH 3T3 cells treated with 150 μM H2O2 for 2 h showed a senescence-like cell morphology after 11 d as compared with 5 ± 2% (n = 18) of untreated cells. In addition, we demonstrated that NIH 3T3 cells subjected to oxidative stress (150 μM H2O2 for 2 h) displayed positive acid β-galactosidase staining after 11 d in culture (Figure 3C). Importantly, normal untreated cells were essentially negative in this assay system. Quantitation of the acid β-galactosidase staining is shown in Figure 3D. Approximately 84 ± 4% (n = 20) of H2O2-treated NIH 3T3 cells showed senescence-associated β-galactosidase activity. Conversely, only 3 ± 2% (n = 20) of normal untreated cells were positive. Similar results were also obtained with lower doses of H2O2 (15 and 50 μM, our unpublished results).
Figure 3.
Oxidative stress induces premature senescence in NIH 3T3 cells. NIH 3T3 cells were left untreated or treated with 150 μM H2O2 for 2 h and recovered for 11 d. (A) Cells representative of the two cell populations (untreated or H2O2-treated NIH 3T3 cells) were photographed at a magnification of ×60. (B) Cells were observed under a BX50WI Optical light microscope (Olympus) at a magnification of ×10. The percentage of cells showing a large and flat morphology was scored. Values represent means ± SEM. *P < 0.0005. (C) Untreated and H2O2-treated NIH 3T3 cells were subjected to senescence-associated β-galactosidase activity assay and were observed under a BX50WI Optical light microscope (Olympus) at a magnification of ×10. A representative field is shown. (D) Quantitation of the acid β-galactosidase activity assay shown in C. Note that the treatment with H2O2 successfully promotes premature senescence in NIH 3T3 cells. Values represent means ± SEM. *P < 0.0005.
Next, we evaluated whether subcytotoxic levels of H2O2 affect caveolin-1 expression. In Figure 4A, NIH 3T3 cells were treated with the indicated concentration of H2O2 for 2 h. Cells were washed twice and allowed to recover in complete medium for 11 d. The cells were then collected and subjected to immunoblot analysis with a caveolin-1-specific antibody probe.
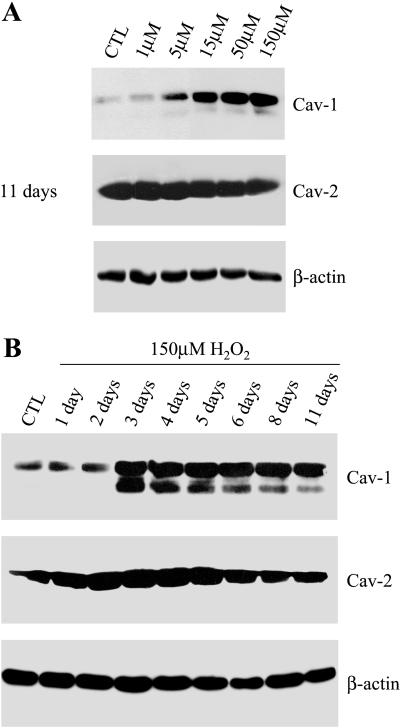
Figure 4.
SIPS is associated with up-regulation of caveolin-1. (A) NIH 3T3 cells were left untreated or treated for 2 h with the indicated concentration of H2O2 and were allowed to recover for 11 d. Cells were then subjected to immunoblot analysis with monospecific antibody probes that recognize only caveolin-1 (mAb 2297) or caveolin-2 (mAb 65). Note that the treatment with H2O2 (15–150 μM) induces up-regulation of endogenous caveolin-1, but not caveolin-2. Immunoblotting with anti-β-actin IgG served as a control for equal loading. (B) NIH 3T3 cells were left untreated or treated with 150 μM H2O2 for 2 h and were allowed to recover for the indicated period of time. Cells were then subjected to immunoblot analysis with monospecific antibody probes that recognize only caveolin-1 (mAb 2297) or caveolin-2 (mAb 65). Note that caveolin-1 protein expression is induced 3 d after the treatment with 150 μM H2O2 and remains elevated for up to 11 d. In contrast, caveolin-2 expression was not affected. Immunoblotting with anti-β-actin IgG served as a control for equal loading.
Figure 4A shows that caveolin-1 protein expression is significantly up-regulated in cells treated with at least 15 μM H2O2. In contrast, caveolin-2 protein expression was not affected by the H2O2 treatment. Immunoblotting with β-actin was performed as a control for equal protein loading. In addition, we treated NIH 3T3 cells with 150 μM H2O2 for 2 h and allowed them to recover for the indicated period of time. Figure 4B shows that 3 d after stimulation with subcytotoxic levels of H2O2, caveolin-1 expression is significantly increased and remained significantly higher over a period of 11 d. Caveolin-2 expression was not significantly affected by the H2O2 treatment. These results indicate that up-regulation of caveolin-1 occurs during SIPS in fibroblasts.
Antioxidants Prevent H2O2-Induced Premature Senescence and Block the Up-Regulation of Caveolin-1 in NIH 3T3 Cells
Dietary antioxidants are believed to prevent the accumulation of age-related oxidative damages (Halliwell, 1996; Palmer and Paulson, 1997). Next, we investigated the potential for the dietary antioxidant quercetin to reverse H2O2-induced premature senescence in NIH 3T3 cells and to modulate caveolin-1 protein expression.
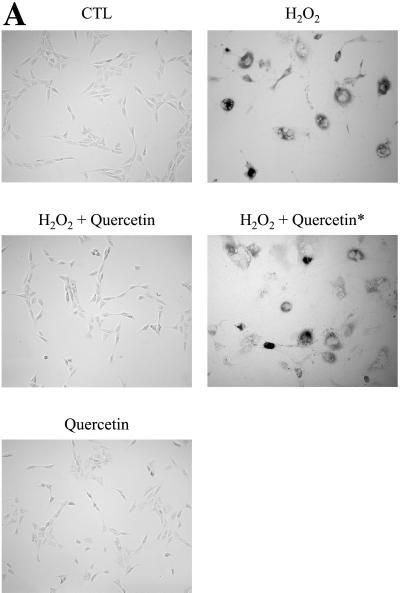
NIH 3T3 cells were stimulated for 2 h with 150 μM H2O2 alone or in combination with 300 μM quercetin. Cells were washed twice and allowed to recover in complete medium for 11 d. Cells were then subjected to acid β-galactosidase staining and Western-blot analysis. Figure 5A illustrates that H2O2 alone (not in combination with quercetin) promoted the expression of senescence-associated β-galactosidase activity in NIH 3T3 cells. However, quercetin successfully prevented this H2O2-induced premature senescence. As an important control, we ruled out the possibility that quercetin had a direct inhibitory effect on acid β-galactosidase activity. In fact, when NIH 3T3 cells were stimulated for 2 h with 150 μM H2O2 alone and recovered for 11 d, treatment with 300 μM quercetin for 2 h before assaying for β-galactosidase activity (H2O2 + quercetin*) did not prevent the expression of senescence-associated β-galactosidase activity (Figure 5A). As an additional control, quercetin alone did not stimulate acid β-galactosidase activity (Figure 5A).
Figure 5.
SIPS and caveolin-1 up-regulation are prevented by the treatment with the antioxidant quercetin. (A) β-galactosidase activity. NIH 3T3 cells were left untreated (CTL), treated with 150 μM H2O2 alone (H2O2) or in combination with 300 μM quercetin (H2O2 + quercetin), or treated with 300 μM quercetin alone (quercetin) for 2 h and the cells were allowed to recover for 11 d. Cells were then subjected to an acid β-galactosidase activity assay. In addition, NIH 3T3 cells were treated with 150 μM H2O2 alone for 2 h, recovered for 11 d, and then incubated with 300 μM quercetin for 2 h before assaying for acid β-galactosidase activity (H2O2 + quercetin*). Cells were photographed using a BX50WI Optical light microscope (Olympus) at a magnification of ×10. Note that treatment with quercetin completely prevented the induction of senescence-associated β-galactosidase activity in H2O2-treated cells. (B) Immunoblotting. Cells were treated as in A. Cell lysates were then prepared and subjected to SDS-PAGE/Western-blot analysis with anti-caveolin-1 and anti-caveolin-2 mAb probes. Interestingly, the antioxidant quercetin abolished the up-regulation of caveolin-1 protein expression induced by H2O2. Immunoblotting with anti-β-actin shows equal protein loading. (C) Immunoblotting. Cells were left untreated or treated with 300 μM quercetin for 2 h and were allowed to recover for the indicated period of time. Cells were then subjected to immunoblot analysis with monospecific antibody probes that recognize only caveolin-1 (mAb 2297) or caveolin-2 (mAb 65). Note that caveolin-1 protein expression was not affected after 3 d of recovery, but was down-regulated 11 d after the treatment. Interestingly, caveolin-2 protein expression was similarly affected. Immunoblotting with anti-β-actin was performed to show equal protein loading in all lanes.
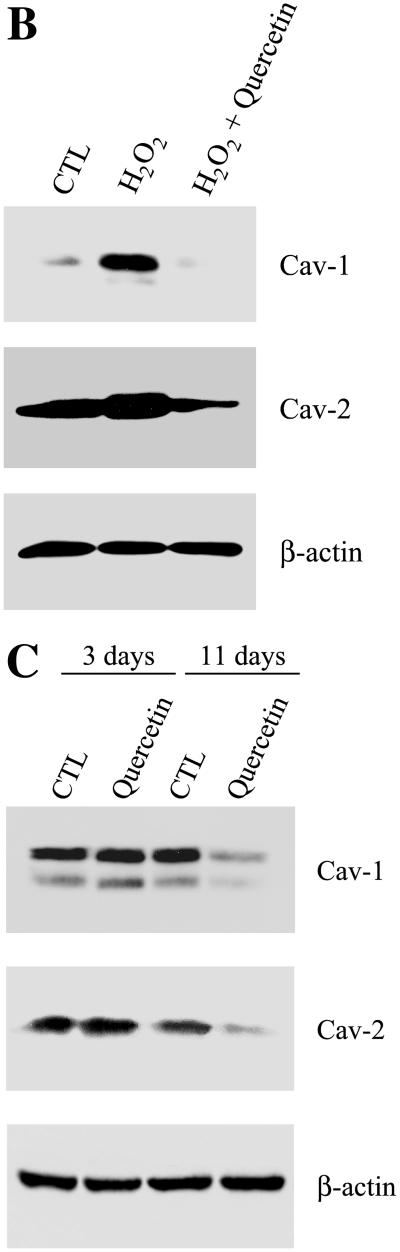
In Figure 5B, we next evaluated the effect of the antioxidant quercetin, in combination with H2O2, on caveolin-1 protein expression. Interestingly, quercetin totally prevented the up-regulation of caveolin-1 induced by the treatment with H2O2 alone after 11 d of recovery. Similar results were obtained after only 3 d of recovery (our unpublished results). In addition, caveolin-1 expression appeared slightly reduced by treatment with H2O2 in combination with quercetin. Caveolin-2 protein expression was not significantly affected by the treatment with H2O2 alone and was slightly reduced, similar to caveolin-1, by the treatment of H2O2 in combination with quercetin. Immunoblotting with β-actin was performed as a control for equal protein loading. Taken together, these results indicate that the antioxidant quercetin successfully prevented the H2O2-induced premature senescent phenotype and blocked the up-regulation of caveolin-1 protein expression in NIH 3T3 cells.
As we observed a slight reduction in caveolin-1 protein expression by the treatment with quercetin in combination with H2O2 (Figure 5B), we next investigated whether quercetin alone has any effect on caveolin-1 protein expression. NIH 3T3 cells were treated for 2 h with 300 μM quercetin. Cells were then washed twice and allowed to recover in complete medium for the indicated period of time. Figure 5C shows that quercetin significantly reduced caveolin-1 protein expression after 11 d of recovery. Importantly, caveolin-1 expression was not affected by quercetin treatment after 3 d of recovery. Interestingly, caveolin-2 protein expression was down-regulated, similarly to caveolin-1, only after 11 d of recovery. This result is consistent with the idea that caveolin-1 expression is required to stabilize the caveolin-2 protein product. In support of this hypothesis, caveolin-2 protein expression is reduced in MEFs derived from caveolin-1 null mice (Razani et al., 2001). Because quercetin prevented H2O2-induced caveolin-1 up-regulation after 3 d of recovery (our unpublished results), one possibility is that quercetin may act through two distinct mechanisms in affecting caveolin-1 expression. In the short term, quercetin indirectly prevents caveolin-1 up-regulation induced by H2O2, whereas in the long term, quercetin directly negatively regulates caveolin-1 protein expression. An alternative explanation may be that cells are simply more vulnerable to quercetin's effects when stressed by H2O2.
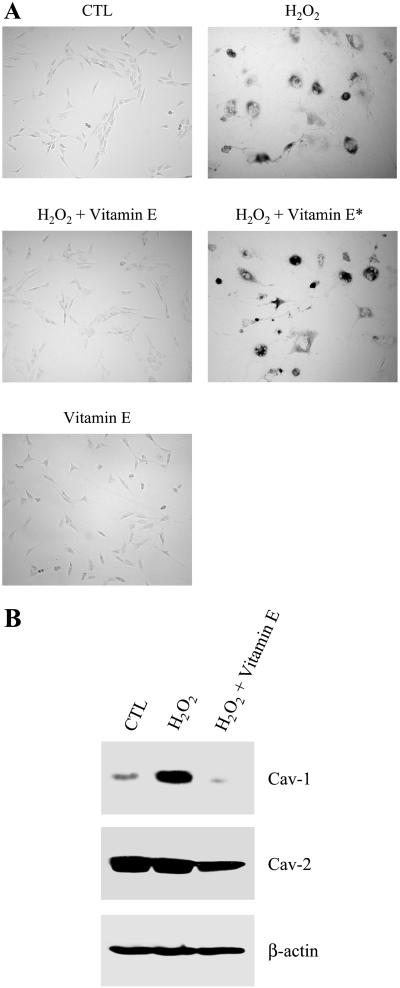
To assess whether other antioxidants are capable of preventing H2O2-induced premature senescence and up-regulation of caveolin-1, we next treated NIH 3T3 cells for 2 h with 150 μM H2O2 alone or in combination with 300 μM vitamin E. Cells were washed twice and allowed to recover in complete medium for 11 d. Cells were then subjected to acid β-galactosidase staining and Western-blot analysis. Figure 6A illustrates that treatment with vitamin E successfully prevented the H2O2-induced expression of acid β-galactosidase activity. Like quercetin, vitamin E did not have a direct inhibitory effect on acid β-galactosidase activity (H2O2 + vitamin E*). Importantly, Figure 6B shows that treatment with H2O2 in combination with vitamin E did not up-regulate caveolin-1 protein expression in NIH 3T3 after 11 d of recovery. These results indicate that quercetin's effects can be replicated by vitamin E.
Figure 6.
Quercetin's effects can be replicated by the antioxidant vitamin E. (A) β-galactosidase activity. NIH 3T3 cells were left untreated (CTL), treated with 150 μM H2O2 alone (H2O2) or in combination with 300 μM vitamin E (H2O2 + vitamin E), or treated with 300 μM vitamin E alone (vitamin E) for 2 h and the cells were allowed to recover for 11 d. Cells were then subjected to an acid β-galactosidase activity assay. In addition, NIH 3T3 cells were treated with 150 μM H2O2 alone for 2 h, recovered for 11 d, and then incubated with 300 μM vitamin E for 2 h before assaying for acid β-galactosidase activity (H2O2 + vitamin E*). Cells were photographed using a BX50WI Optical light microscope (Olympus) at a magnification of ×10. Interestingly, vitamin E completely prevented the induction of senescence-associated β-galactosidase activity in H2O2-treated cells. As a control, treatment with vitamin E alone did not stimulate acid β-galactosidase activity. (B) Immunoblotting. Cells were treated as in A. Cell lysates were then prepared and subjected to SDS-PAGE/Western-blot analysis with anti-caveolin-1 and anti-caveolin-2 mAb probes. Interestingly, the antioxidant vitamin E abolished the up-regulation of caveolin-1 protein expression induced by H2O2. Caveolin-2 protein expression was not significantly affected by the treatment with H2O2 alone and was slightly reduced, similar to caveolin-1, by the treatment of H2O2 in combination with vitamin E. Immunoblotting with anti-β-actin shows equal protein loading.
Oxidative Stress Promotes Caveolin-1 Transcriptional Activity and Activates the p53 Responsive Element
To evaluate whether the up-regulation of caveolin-1 protein expression induced by H2O2 was correlated with increased caveolin-1 transcriptional activity, we next examined caveolin-1 promoter activity in NIH 3T3 cells stimulated with H2O2 alone or in combination with quercetin. For these experiments, we used a previously characterized luciferase-based caveolin-1 promoter construct. This construct consists of ∼3-kb region upstream of the caveolin-1 ATG, plus caveolin-1/exon 1, caveolin-1/intron 1, and a portion of caveolin-1/exon 2 inserted upstream of the luciferase gene (Engelman et al., 1999).
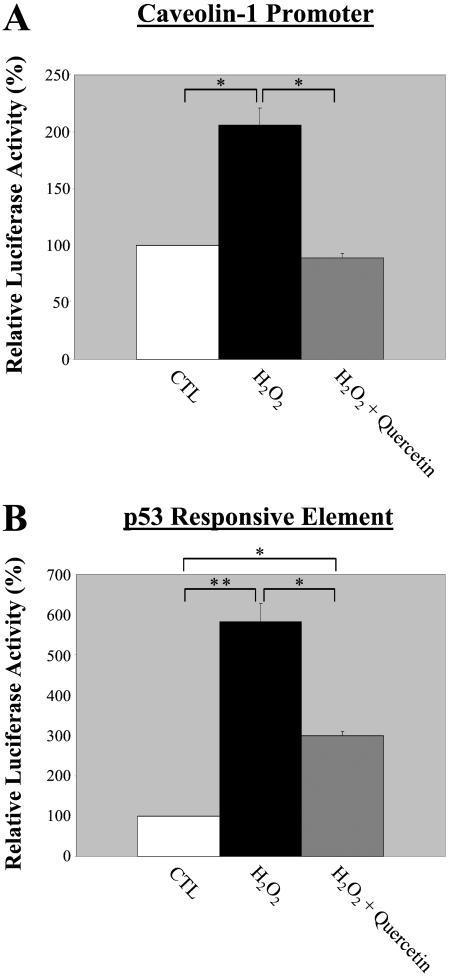
Figure 7A shows that 72 h after induction of oxidative stress with 150 μM H2O2, caveolin-1 promoter activity was increased by ∼twofold. Interestingly, when NIH 3T3 cells were treated with 150 μM H2O2 in combination with 300 μM quercetin, the expected increase in caveolin-1 promoter activity was completely abolished. These results suggest that up-regulation of caveolin-1 protein expression observed in senescent NIH 3T3 cells is due, at least in part, to increased transcriptional activity.
Figure 7.
The antioxidant quercetin totally prevents the stimulation of the caveolin-1 promoter activity, but only partially abrogates the activation of the p53-responsive element induced by H2O2. NIH 3T3 cells were transiently transfected with 2 μg of the caveolin-1 promoter luciferase reporter (3 Kb + Intron-1; A) or with the luciferase reporter plasmid pTA-p53RE (B). Six hours post-transfection, cells were rinsed with PBS and incubated in medium containing 150 μM H2O2 with or without 300 μM quercetin for 2 h. Cells were washed in complete medium and were incubated at 37°C for an additional 72 h. Cells were then lysed and the luciferase activity was measured. Note that H2O2 stimulates the caveolin-1 promoter activity (A) and the p53 responsive element (B). Importantly, quercetin completely abolished the stimulation of the caveolin-1 promoter activity induced by H2O2, but only partially inhibited the activation of the p53-responsive element. Values represent means ± SEM (n = 9 for each experimental point). *P < 0.001; **P < 0.0005.
We have previously reported that transient overexpression of caveolin-1 in NIH 3T3 cells and stable overexpression of caveolin-1 in MEFs activates the p53 responsive element (Galbiati et al., 2001b). Thus, we next decided to examine whether up-regulation of endogenous caveolin-1 induced by oxidative stress was associated with increased activation of the p53 responsive element.
Figure 7B shows that 3 d after stimulation with H2O2 (150 μM for 2 h), the p53 responsive element was activated by ∼five- to sixfold. However, when normal NIH 3T3 cells were stimulated with H2O2 in combination with quercetin, activation of the p53 responsive element was reduced by ∼50% (Figure 7B). Thus, although quercetin completely abolished H2O2-induced caveolin-1 transcriptional activity, it only partially abrogated the activation of the p53 responsive element. These results suggest that the p53-dependent pathway, when activated by oxidative stress, consists of two components: one that is caveolin-1 sensitive and the other is caveolin-1 insensitive. As such, treatment with the antioxidant quercetin prevented only the activation of the caveolin-1-sensitive p53-dependent pathway. Because in Figure 5A we demonstrated that treatment with quercetin totally abolished the acquisition of the senescent phenotype induced by H2O2, these data indicate that the activation of the caveolin-1-sensitive p53-dependent pathway is selectively responsible for promoting the senescent phenotype.
Loss of Caveolin-1 Protein Expression Prevents Premature Senescence Induced by Oxidative Stress
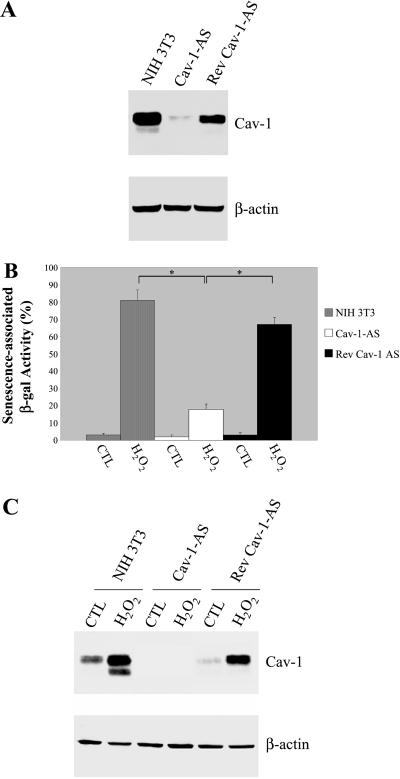
Because up-regulation of caveolin-1 occurs in SIPS (this report), we next investigated whether loss of caveolin-1 protein expression was sufficient to prevent the development of the senescent phenotype in H2O2-stimulated NIH 3T3 cells. We took advantage of three independent stable NIH 3T3 cell lines that express dramatically reduced levels of caveolin-1 (Cav-1-AS) that we have previously derived using an antisense approach (Galbiati et al., 1998). Caveolin-1 antisense cells stably express caveolin-1 antisense mRNA that constitutively reduces caveolin-1 protein expression (Galbiati et al., 1998). These cells are characterized by a transformed phenotype, as demonstrated by hyperactivation of the p42/44 mitogen-activated protein kinase pathway, anchorage-independence growth, and formation of tumors in immunodeficient mice (Galbiati et al., 1998). As we previously reported (Galbiati et al., 1998), NIH 3T3 cells harboring Cav-1-AS expressed substantially reduced levels of caveolin-1 (Figure 8A). After NIH 3T3 cells that harbor Cav-1-AS were cultured in the absence of selection media for four passages, caveolin-1 expression was restored (approaching ∼70% of normal levels), due to loss of the caveolin-1 antisense vector (Rev Cav-1-AS; Figure 8A; Galbiati et al., 1998). These results indicate that down-regulation of caveolin-1 in this system is reversible. The results illustrated in Figure 8 and Figure 9 were obtained using one Cav-1-AS cell line and the corresponding Rev-Cav-1-AS cell line. Importantly, similar results were obtained using three independent Cav-1-AS cell lines and the corresponding Rev-Cav-1-AS cell lines that we have previously generated (our unpublished results; Galbiati et al., 1998).
Figure 8.
Down-regulation of caveolin-1 protein expression confers resistance to SIPS. (A) Immunoblotting. Normal NIH 3T3 cells, Cav-1-AS cells, and Rev-Cav-1-AS cells were subjected to Western-blot analysis using a mAb probe specific for caveolin-1. Note that caveolin-1 expression is significantly reduced in Cav-1-AS cells. Immunoblotting with anti-β-actin antibody shows equal protein loading. (B) β-galactosidase activity. Normal NIH 3T3 cells, Cav-1-AS cells, and Rev-Cav-1-AS cells were left untreated or treated with 150 μM H2O2 for 2 h and were allowed to recover for 11 d. Cells were then observed under a BX50WI Optical light microscope (Olympus; ×10 magnification) and the percentage of cells positive for senescence-associated acid β-galactosidase activity was recorded. Note that cells expressing low levels of caveolin-1 display a dramatically reduced number of acid β-galactosidase activity-positive cells. Values represent means ± SEM. *P < 0.0005. (C) Immunoblotting. Cells were treated as in B. Cell lysates were then subjected to SDS-PAGE/Western-blot analysis with a mAb probe specific for caveolin-1. Interestingly, H2O2 promoted up-regulation of endogenous caveolin-1 only in normal NIH 3T3 cells and in Rev-Cav-1-AS cells. Immunoblotting with anti-β-actin IgG served as a control for equal protein loading.
Figure 9.
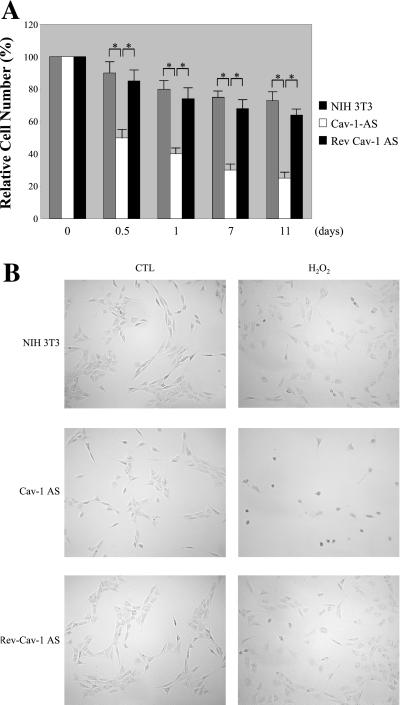
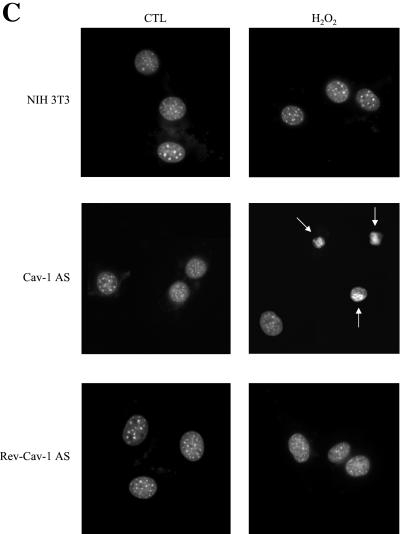
Oxidative stress promotes apoptosis in cells with low caveolin-1 protein expression. (A) Cell death. Normal NIH 3T3 cells, Cav-1-AS cells, and Rev-Cav-1-AS cells were treated with 150 μM H2O2 for 2 h and the cells were allowed to recover for the indicated period of time. The cells remaining in the dish were then collected and counted. Interestingly, H2O2 induces a significantly higher degree of cell death in the Cav-1-AS cells (i.e., that express low levels of caveolin-1). Values represent means ± SEM (n = 8 for each experimental point). *P < 0.001. (B) Cell morphology. Cells were left untreated or treated as in A and allowed to recover for 12 h. Cells were then observed under a BX50WI Optical light microscope (Olympus; ×10 magnification). Note that Cav-1-AS cells clearly show cell shrinkage when stimulated with H2O2. (C) Nuclear morphology. Cells were left untreated or treated as in B, stained with DAPI to visualize their nuclear morphology, and then observed under a Provis fluorescence microscope (Olympus). Note that nuclear condensation is observed only in Cav-1-AS cells.
Thus, we next evaluated the ability of subcytotoxic levels of H2O2 to induce premature senescence in NIH 3T3 cells harboring Cav-1-AS and as well in caveolin-1 antisense revertants. Cells were treated with 150 μM H2O2 for 2 h or were left untreated, washed twice, and allowed to recover in complete medium for the indicated period of time. Interestingly, 11 d after the treatment with H2O2, 81 ± 6% (n = 20) of normal NIH 3T3 cells and 67 ± 4% (n = 20) of caveolin-1 antisense revertants displayed a senescent phenotype, as verified by acid β-galactosidase staining (Figure 8B). In contrast, only 18 ± 3% (n = 20) of caveolin-1-AS cells showed senescence-associated β-galactosidase activity (Figure 8B). As expected, oxidative stress induced up-regulation of caveolin-1 expression in normal NIH 3T3 cells and in caveolin-1 antisense revertants without affecting caveolin-1 protein expression in caveolin-1 antisense cells (Figure 8C). These results indicate that up-regulation of caveolin-1 protein expression is necessary to fully achieve oxidative stress-induced cellular senescence.
Cells Expressing Low Levels of Endogenous Caveolin-1 Preferentially Respond to Oxidative Stress Undergoing Apoptosis
Interestingly, Cav-1-AS cells showed severe intolerance to treatment with subcytotoxic levels of H2O2. In fact, only 12 h after stimulation with H2O2, ∼50% of these cells died as compared with the ∼10–15% of cell death observed with normal NIH 3T3 cells and Rev-Cav-1-AS cells (Figure 9A). Importantly, treatment of Cav-1-AS cells with quercetin in combination with H2O2 completely prevented the H2O2-induced cell death (our unpublished results).
Because H2O2 has been shown to induce apoptosis in a variety of cell types (Hampton and Orrenius, 1997; Macho et al., 1997; Chen et al., 2000), we next investigated whether the cell death we observed was actually apoptosis. Apoptosis is characterized by morphological changes such as cell shrinkage and nuclear condensation. We first evaluated the cell morphology of normal NIH 3T3 cells, Cav-1-AS cells, and Rev- Cav-1-AS cells 12 h after the treatment with H2O2 (150 μM for 2 h) as compared with untreated controls.
Figure 9B shows that Cav-1-AS cells rounded-up and started detaching from the dish when treated with H2O2. In contrast, only a few cells displayed cell shrinkage in H2O2-treated normal NIH 3T3 cells and Cav-1-AS revertants. Untreated controls showed the regular fibroblast-like cell morphology.
Next, we evaluated whether the cell shrinkage observed in Cav-1-AS cells treated with H2O2 was associated with nuclear condensation. Twelve hours after stimulation with 150 μM H2O2 for 2 h, cells were stained with DAPI (1 μg/ml in PBS for 10 min) to visualize their nuclear morphology. Consistent with our above data, nuclear condensation was observed in H2O2-treated Cav-1 AS cells, but not in H2O2-treated normal NIH 3T3 cells and Rev-Cav-1-AS cells (Figure 9C). Regular nuclear morphology was observed in untreated controls (Figure 9C). Similar results were obtained 3 h after H2O2 stimulation (our unpublished results).
Taken together, these results suggest that oxidative stress may promote either premature senescence or apoptosis, depending on the expression levels of caveolin-1 in the cell. Upon H2O2 treatment, cells that responded to oxidative stress through up-regulation of caveolin-1 protein expression preferentially achieved premature senescence. In contrast, cells with low levels of endogenous caveolin-1 protein expression preferentially underwent apoptosis.
UV Irradiation Up-Regulates Caveolin-1 Protein Expression
Besides oxidative stress, other stressful conditions have been shown to promote premature senescence. Such stressful conditions include UV light (Bayreuther et al., 1988; Rodemann et al., 1989). To study the effect of UV light on caveolin-1 protein expression, NIH 3T3 cells were irradiated with subLDs of UV-C light (10 J/m2). Cells were then allowed to recover for the indicated period of time and were subjected to Western-blot analysis with a caveolin-1-specific antibody probe.
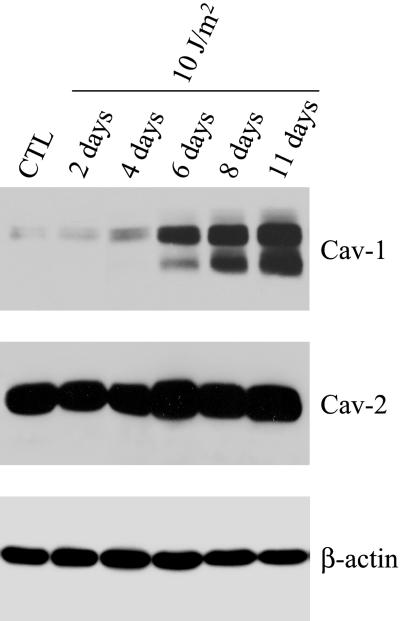
Figure 10 shows that 6 d after UV-C stimulation, caveolin-1 protein expression was significantly increased. Caveolin-1 protein expression remained elevated over a period of 11 d. Also, Figure 10 shows that caveolin-2 protein expression was not affected by UV-C light stimulation. Immunoblotting with β-actin was performed as a control for equal protein loading. In addition, NIH 3T3 cells stimulated with UV-C light (10 J/m2) showed an increased acid β-galactosidase activity after 11 d (our unpublished results). Taken together, these results suggest that up-regulation of caveolin-1 is associated with the premature senescence phenotype induced by different stressful conditions, including both oxidative stress and UV light.
Figure 10.
UV-C light treatment induces the up-regulation of caveolin-1 protein expression. NIH 3T3 cells were left untreated or treated with subLDs of UV-C light (10 J/m2) and were allowed to recover for the indicated period of time. Cells were then subjected to immunoblotting with monospecific antibody probes that recognize only caveolin-1 (mAb 2297) or caveolin-2 (mAb 65). Note that 6 d after stimulation with subLDs of UV-C light, caveolin-1 protein expression is up-regulated. Caveolin-1 expression remains elevated up to 11 d. In contrast, caveolin-2 protein expression was not affected by UV irradiation. Immunoblotting with anti-β-actin IgG shows equal protein loading.
DISCUSSION
Several lines of evidence indicate that caveolin-1 may function as a general negative regulator to inhibit the basal activity of many signaling proteins. One would predict that down-regulation of caveolin-1 leads to increased basal activity for a number of signaling pathways and subsequent cell transformation. Numerous independent but complementary data support this hypothesis. In fact, caveolin-1 mRNA and protein expression are lost or reduced during cell transformation by activated oncogenes such as v-Abl and H-ras (G12V); caveolae are absent from these cell lines. In addition, induction of caveolin-1 expression in v-Abl- and H-ras (G12V)-transformed NIH 3T3 cells abrogated the anchorage-independent growth of these cells in soft agar and resulted in the de novo formation of caveolae (Engelman et al., 1997). Moreover, antisense-mediated reduction of caveolin-1 protein expression in NIH 3T3 cells is sufficient to drive oncogenic transformation and constitutively activate the p42/44 mitogen-activated protein kinase cascade (Galbiati et al., 1998). Thus, down-regulation of caveolin-1 expression and caveolae organelles may be critical for maintaining the transformed phenotype.
Consistent with this idea, we have recently demonstrated that overexpression of caveolin-1 in MEFs induces cell cycle arrest in the G0/G1 phase of the cell cycle, a reduction in cell proliferation, and a reduction in the DNA replication rate (Galbiati et al., 2001b). We have also shown that a p53/p21-dependent pathway mediates this caveolin-1-induced cell cycle arrest (Galbiati et al., 2001b). Given the role of caveolin-1 in mediating cell cycle arrest, one might predict a possible role for caveolin-1 in promoting cellular senescence. In fact, cell cycle arrest is an essential step in achieving the senescent phenotype. In support of this hypothesis, up-regulation of caveolin-1 was recently observed during the serial passaging of normal human diploid fibroblasts (Park et al., 2000). However, whether up-regulation of caveolin-1 is a necessary step in promoting cellular senescence remains unknown.
In the present study, we directly investigated the importance of caveolin-1 protein in mediating cellular senescence in vivo. We demonstrated that MEFs overexpressing caveolin-1 show premature irreversible growth arrest, have a senescence-like cell morphology, and are enriched in senescence-associated acid β-galactosidase activity, which is typical of senescent cells. In addition, the senescent phenotype is characterized by induction of cell cycle inhibitory proteins (Dimri et al., 1995; Dumont et al., 2000; Frippiat et al., 2001). Importantly, we have previously demonstrated that overexpression of caveolin-1 activates a p53/p21-dependent pathway in MEFs (Galbiati et al., 2001b). Taken together, these results indicate that overexpression of caveolin-1 in vivo is sufficient to promote and maintain the senescent phenotype.
Tumor development is initiated by a multiplicity of genetic abnormalities. Moreover, tumor cells need to escape barriers that limit uncontrolled cell proliferation. One of these barriers is represented by cellular senescence. Cancer cells need to overcome this obstacle to produce a clinically relevant tumor mass. For these reasons, cellular senescence represents a natural tumor suppressor mechanism. In recent years, several independent lines of evidence have emerged that suggest that caveolin-1 functions as a “tumor suppressor protein” in mammalian cells. In fact, modification and/or inactivation of caveolin-1 expression appears to be a common feature of the transformed phenotype. For example, caveolin-1 protein expression has been demonstrated to be absent in several transformed cell lines derived from human mammary carcinomas, including MT-1, MCF-7, ZR-75–1, T47D, MDA-MB-361, and MDA-MB-474 (Sager et al., 1994). We show here that caveolin-1 expression is critical in achieving the senescent phenotype. These results suggest that cellular senescence may represent one of the molecular mechanisms through which caveolin-1 acts as a tumor suppressor protein.
Cellular senescence is spontaneously achieved by somatic cells. However, many external and internal cellular stimuli can accelerate the acquisition of the senescent phenotype. Oxidative stress, for example, has been widely demonstrated to be responsible for premature senescence (Chen and Ames, 1994; Chen et al., 1995; Dimri et al., 1995; von Zglinicki et al., 1995; Dumont et al., 2000; Frippiat et al., 2001). Understanding at the molecular level the intracellular pathways affected by cellular stresses will improve our knowledge of the more complicated aging process. In this report, we demonstrated that overexpression of caveolin-1 induces premature senescence. As a consequence, we next asked whether premature senescence induced by oxidative stress is associated with increased endogenous caveolin-1 expression. H2O2 has been previously demonstrated to induce premature senescence in human diploid fibroblasts (Chen and Ames, 1994; Chen et al., 1995; von Zglinicki et al., 1995). We demonstrate here that treatment of NIH 3T3 cells with subcytotoxic doses of H2O2 induces premature senescence and up-regulation of caveolin-1 at the transcriptional level and at the protein level. Interestingly, 3 d after subcytotoxic stimulation with H2O2, caveolin-1 expression was up-regulated and remained elevated up to 11 d, whereas senescence-associated β-galactosidase activity was first observed only after 7 d post-H2O2 stimulation (our unpublished results) and remained elevated up to 11 d. These results indicate that up-regulation of caveolin-1 precedes the onset of the senescent phenotype, suggesting that caveolin-1 expression may be necessary to initiate and maintain cellular senescence.
The maintenance of a “physiological redox tone” is essential to prevent the degenerative processes associated with aging. Dietary antioxidants are believed to prevent and/or contain oxidative damages induced by oxidative stress (Halliwell, 1996; Palmer and Paulson, 1997). We demonstrated that quercetin, a flavanoid found in foods of plant origin, and vitamin E prevented the premature senescence phenotype and the up-regulation of caveolin-1 induced by H2O2. We also found that quercetin directly negatively regulates caveolin-1 protein expression. These results support the idea of a tight correlation between the senescence phenotype and up-regulation of caveolin-1. This data is supported by studies showing that endogenous antioxidants such as reduced glutathione decrease with age (Hu et al., 2000). Interestingly, caveolin-1 protein expression has been demonstrated to increase with age (Park et al., 2000). Because we demonstrated in this report that oxidative stress up-regulates caveolin-1, whereas the dietary antioxidant quercetin down-regulates caveolin-1 protein expression, we may speculate that up-regulation of caveolin-1 occurring with the aging process may be due in part to the accumulation of oxidants and the reduction of endogenous antioxidants.
Using an antisense-based approach, we previously generated and characterized NIH 3T3 cells that express substantially reduced levels of caveolin-1 (Galbiati et al., 1998). These cells are characterized by a transformed phenotype. In fact, they form foci in petri dishes, exhibit anchorage-independent growth in soft agar, and form tumors in immunodeficient mice (Galbiati et al., 1998). If caveolin-1 expression is a key element in promoting cellular senescence, NIH 3T3 cells harboring caveolin-1 antisense should be protected against SIPS. We demonstrate in this report that Cav-1-AS cells express significantly lower levels of acid β-galactosidase activity when stimulated with subcytotoxic levels of H2O2 as compared with normal control NIH 3T3 cells. Interestingly, the ability of these cells to express high levels of senescence-associated β-galactosidase activity is recovered when caveolin-1 levels are restored. Taken together, these data indicate that caveolin-1 may be a fundamental player in the intracellular pathway that leads to premature senescence.
The role of caveolin-1 in mediating apoptosis remains contradictory. On one hand, caveolin-1 has been shown to promote ceramide-induced apoptosis in diploid fibroblasts (Zundel et al., 2000). Moreover, Lisanti and colleagues (Liu et al., 2001) have demonstrated that caveolin-1 sensitizes fibroblasts and epithelial cells to staurosporine-induced programmed cell death and that caveolin-1 antisense cells are resistant to staurosporine-induced apoptosis. In addition, we have shown that transgenic expression of caveolin-1 in MEFs sensitizes these cells to staurosporine-induced programmed cell death (Galbiati et al., 2001b). On the other hand, caveolin-1 has been shown to act as a suppressor of c-myc-induced apoptosis in LNCaP cells, a human epithelial prostate cancer-derived cell line (Timme et al., 2000). Thus, evidence has been presented that caveolin-1 is both a facilitator and a suppressor of programmed cell death in different contexts. In the present study, we show that cells expressing low levels of caveolin-1 (Cav-1-AS cells) preferentially undergo apoptosis when stimulated with subcytotoxic levels of H2O2 as compared with normal control NIH 3T3 cells and Rev-Cav-1-AS cells, as verified by cell and nuclear morphology. This result is not surprisingly because it has been previously reported that subcytotoxic concentrations of H2O2 can induce apoptosis in human fibroblasts (Hampton and Orrenius, 1997; Macho et al., 1997; Chen et al., 2000). In addition, this result is consistent with the idea that the role of caveolin-1 in mediating apoptosis may be different depending on the nature of the extracellular apoptotic stimulus. We can speculate that diploid fibroblasts react differently to oxidative stress depending on the level of endogenous caveolin-1. Cells with normal levels of caveolin-1 react to oxidative stress by choosing premature senescence through up-regulation of caveolin-1, whereas cells in which caveolin-1 expression is kept low (by the antisense vector in the case of Cav-1-AS cells) preferentially undergo programmed cell death.
It is interesting to point out that ionizing radiation and chemotherapeutic drugs, which induce apoptosis in cancer cells, represent sources of reactive oxygen species. We know that caveolin-1 protein expression is down-regulated during cell transformation. Also, caveolin-1 has been shown to be up-regulated in multidrug-resistant cancer cells (Lavie et al., 1998; Yang et al., 1998). We speculate that differences in caveolin-1 protein expression may facilitate or prevent the efficacy of specific anticancer treatments. In addition, the dual role of caveolin-1 in promoting senescence and apoptosis is not uncommon. In fact, the tumor suppressor protein p53 is directly involved in both cell cycle arrest/senescence and programmed cell death (Amundson et al., 1998; el-Deiry, 1998; Sionov and Haupt, 1998; Bates and Vousden, 1999). Lisanti and colleagues (Razani et al., 2000) have demonstrated that caveolin-1 gene transcription is induced by p53. We have recently shown that caveolin-1 expression can increase the activity of p53 (Galbiati et al., 2001b). Thus, caveolin-1 and p53 may act synergistically in their dual role of promoting senescence and apoptosis in different cellular contexts.
Because transgenic expression of caveolin-1 in MEFs induces cellular senescence and SIPS correlates with increased endogenous caveolin-1 expression, we speculate that caveolin-1 transgenic mice may represent an interesting mouse model for the study of the aging process and the characterization of the molecular mechanisms underlying degenerative diseases. However, additional experiments are necessary to directly test this hypothesis.
ACKNOWLEDGMENTS
We thank Dr. Ian J. Reynolds and Dr. Simon Watkins for help with light and fluorescence microscopy, respectively. This work was supported by grants from the American Heart Association and the American Cancer Society (IRG-60–002-40), and by start-up funds from the Department of Pharmacology (to F.G.). M.P.L. was supported by grants from the National Institutes of Health, the Muscular Dystrophy Association, the American Heart Association, and the Susan B. Komen Breast Cancer Foundation.
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.01–11–0529. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.01–11–0529.
REFERENCES
- Amundson SA, Myers TG, Fornace AJ., Jr Roles for p53 in growth arrest and apoptosis: putting on the brakes after genotoxic stress. Oncogene. 1998;17:3287–3299. doi: 10.1038/sj.onc.1202576. [DOI] [PubMed] [Google Scholar]
- Bates S, Vousden KH. Mechanisms of p53-mediated apoptosis. Cell Mol Life Sci. 1999;55:28–37. doi: 10.1007/s000180050267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayreuther K, Rodemann HP, Hommel R, Dittmann K, Albiez M, Francz PI. Human skin fibroblasts in vitro differentiate along a terminal cell lineage. Proc Natl Acad Sci USA. 1988;85:5112–5116. doi: 10.1073/pnas.85.14.5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black EJ, Clark W, Gillespie DA. Transient deactivation of ERK signaling is sufficient for stable entry into G0 in primary avian fibroblasts. Curr Biol. 2000;10:1119–1122. doi: 10.1016/s0960-9822(00)00699-0. [DOI] [PubMed] [Google Scholar]
- Bumann J, Santo-Holtje L, Loffler H, Bamberg M, Rodemann HP. Radiation-induced alterations of the proliferation dynamics of human skin fibroblasts after repeated irradiation in the subtherapeutic dose range. Strahlenther Onkol. 1995;171:35–41. [PubMed] [Google Scholar]
- Chen Q, Ames BN. Senescence-like growth arrest induced by hydrogen peroxide in human diploid fibroblast F65 cells. Proc Natl Acad Sci, USA. 1994;91:4130–4134. doi: 10.1073/pnas.91.10.4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Fischer A, Reagan JD, Yan LJ, Ames BN. Oxidative DNA damage and senescence of human diploid fibroblast cells. Proc Natl Acad Sci, USA. 1995;92:4337–4341. doi: 10.1073/pnas.92.10.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen QM, Bartholomew JC, Campisi J, Acosta M, Reagan JD, Ames BN. Molecular analysis of H2O2-induced senescent-like growth arrest in normal human fibroblasts: p53 and Rb control G1 arrest but not cell replication. Biochem J. 1998;332(Pt 1):43–50. doi: 10.1042/bj3320043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen QM, Liu J, Merrett JB. Apoptosis or senescence-like growth arrest: influence of cell-cycle position, p53, p21 and bax in H2O2 response of normal human fibroblasts. Biochem J. 2000;347:543–551. doi: 10.1042/0264-6021:3470543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couet J, Li S, Okamoto T, Scherer PS, Lisanti MP. Molecular and cellular biology of caveolae: paradoxes and plasticities. Trends Cardiovasc Med. 1997a;7:103–110. doi: 10.1016/S1050-1738(97)00001-7. [DOI] [PubMed] [Google Scholar]
- Couet J, Sargiacomo M, Lisanti MP. Interaction of a receptor tyrosine kinase, EGF-R, with caveolins. Caveolin binding negatively regulates tyrosine and serine/threonine kinase activities. J Biol Chem. 1997b;272:30429–30438. doi: 10.1074/jbc.272.48.30429. [DOI] [PubMed] [Google Scholar]
- Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont P, Burton M, Chen QM, Gonos ES, Frippiat C, Mazarati JB, Eliaers F, Remacle J, Toussaint O. Induction of replicative senescence biomarkers by sublethal oxidative stresses in normal human fibroblast. Free Radic Biol Med. 2000;28:361–373. doi: 10.1016/s0891-5849(99)00249-x. [DOI] [PubMed] [Google Scholar]
- el-Deiry WS. p21/p53, cellular growth control and genomic integrity. Curr Top Microbiol Immunol. 1998;227:121–137. doi: 10.1007/978-3-642-71941-7_6. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Lee RJ, Karnezis A, Bearss DJ, Webster M, Siegel P, Muller WJ, Windle JJ, Pestell RG, Lisanti MP. Reciprocal regulation of Neu tyrosine kinase activity and caveolin-1 protein expression in vitro and in vivo. Implications for mammary tumorigenesis. J Biol Chem. 1998;273:20448–20455. doi: 10.1074/jbc.273.32.20448. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Wycoff CC, Yasuhara S, Song KS, Okamoto T, Lisanti MP. Recombinant expression of caveolin-1 in oncogenically transformed cells abrogates anchorage-independent growth. J Biol Chem. 1997;272:16374–16381. doi: 10.1074/jbc.272.26.16374. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Zhang XL, Razani B, Pestell RG, Lisanti MP. p42/44 MAP kinase-dependent and -independent signaling pathways regulate caveolin-1 gene expression. Activation of Ras-MAP kinase and protein kinase a signaling cascades transcriptionally down-regulates caveolin-1 promoter activity. J Biol Chem. 1999;274:32333–32341. doi: 10.1074/jbc.274.45.32333. [DOI] [PubMed] [Google Scholar]
- Feron O, Belhassen L, Kobzik L, Smith TW, Kelly RA, Michel T. Endothelial nitric oxide synthase targeting to caveolae. Specific interactions with caveolin isoforms in cardiac myocytes and endothelial cells. J Biol Chem. 1996;271:22810–22814. doi: 10.1074/jbc.271.37.22810. [DOI] [PubMed] [Google Scholar]
- Frippiat C, Chen QM, Zdanov S, Magalhaes JP, Remacle J, Toussaint O. Subcytotoxic H2O2 stress triggers a release of transforming growth factor-β 1, which induces biomarkers of cellular senescence of human diploid fibroblasts. J Biol Chem. 2001;276:2531–2537. doi: 10.1074/jbc.M006809200. [DOI] [PubMed] [Google Scholar]
- Galbiati F, Engelman JA, Volonte D, Zhang XL, Minetti C, Li M, Hou H, Jr, Kneitz B, Edelmann W, Lisanti MP. Caveolin-3 null mice show a loss of caveolae, changes in the microdomain distribution of the dystrophin-glycoprotein complex, and t-tubule abnormalities. J Biol Chem. 2001a;276:21425–21433. doi: 10.1074/jbc.M100828200. [DOI] [PubMed] [Google Scholar]
- Galbiati F, Volonte D, Chu JB, Li M, Fine SW, Fu M, Bermudez J, Pedemonte M, Weidenheim KM, Pestell RG, Minetti C, Lisanti MP. Transgenic overexpression of caveolin-3 in skeletal muscle fibers induces a Duchenne-like muscular dystrophy phenotype. Proc Natl Acad Sci USA. 2000a;97:9689–9694. doi: 10.1073/pnas.160249097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbiati F, Volonte D, Engelman JA, Watanabe G, Burk R, Pestell RG, Lisanti MP. Targeted down-regulation of caveolin-1 is sufficient to drive cell transformation and hyperactivate the p42/44 MAP kinase cascade. EMBO J. 1998;17:6633–6648. doi: 10.1093/emboj/17.22.6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbiati F, Volonte D, Liu J, Capozza F, Frank PG, Zhu L, Pestell RG, Lisanti MP. Caveolin-1 expression negatively regulates cell cycle progression by inducing G(0)/G(1) arrest via a p53/p21(WAF1/Cip1)-dependent mechanism. Mol Biol Cell. 2001b;12:2229–2244. doi: 10.1091/mbc.12.8.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbiati F, Volonte D, Minetti C, Bregman DB, Lisanti MP. Limb-girdle muscular dystrophy (LGMD-1C) mutants of caveolin-3 undergo ubiquitination and proteasomal degradation. Treatment with proteasomal inhibitors blocks the dominant negative effect of LGMD-1C mutants and rescues wild-type caveolin-3. J Biol Chem. 2000b;275:37702–37711. doi: 10.1074/jbc.M006657200. [DOI] [PubMed] [Google Scholar]
- Galbiati F, Volonte D, Minetti C, Chu JB, Lisanti MP. Phenotypic behavior of caveolin-3 mutations that cause autosomal dominant limb girdle muscular dystrophy (LGMD-1C). Retention of LGMD-1C caveolin-3 mutants within the Golgi complex. J Biol Chem. 1999;274:25632–25641. doi: 10.1074/jbc.274.36.25632. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Oxidative stress, nutrition and health. Experimental strategies for optimization of nutritional antioxidant intake in humans. Free Radic Res. 1996;25:57–74. doi: 10.3109/10715769609145656. [DOI] [PubMed] [Google Scholar]
- Hampton MB, Orrenius S. Dual regulation of caspase activity by hydrogen peroxide: implications for apoptosis. FEBS Lett. 1997;414:552–556. doi: 10.1016/s0014-5793(97)01068-5. [DOI] [PubMed] [Google Scholar]
- Hu HL, Forsey RJ, Blades TJ, Barratt ME, Parmar P, Powell JR. Antioxidants may contribute in the fight against ageing: an in vitro model. Mech Ageing Dev. 2000;121:217–230. doi: 10.1016/s0047-6374(00)00212-8. [DOI] [PubMed] [Google Scholar]
- Ju H, Zou R, Venema VJ, Venema RC. Direct interaction of endothelial nitric-oxide synthase and caveolin-1 inhibits synthase activity. J Biol Chem. 1997;272:18522–18525. doi: 10.1074/jbc.272.30.18522. [DOI] [PubMed] [Google Scholar]
- Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- Koleske AJ, Baltimore D, Lisanti MP. Reduction of caveolin and caveolae in oncogenically transformed cells. Proc Natl Acad Sci USA. 1995;92:1381–1385. doi: 10.1073/pnas.92.5.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavie Y, Fiucci G, Liscovitch M. Up-regulation of caveolae and caveolar constituents in multidrug-resistant cancer cells. J Biol Chem. 1998;273:32380–32383. doi: 10.1074/jbc.273.49.32380. [DOI] [PubMed] [Google Scholar]
- Lee SW, Reimer CL, Oh P, Campbel LDB, Schnitzer JE. Tumor cell growth inhibition by caveolin re-expression in human breast cancer cells. Oncogene. 1998;16:1391–1397. doi: 10.1038/sj.onc.1201661. [DOI] [PubMed] [Google Scholar]
- Li S, Couet J, Lisanti MP. Src tyrosine kinases, G α subunits and H-Ras share a common membrane-anchored scaffolding protein, caveolin. Caveolin binding negatively regulates the auto-activation of Src tyrosine kinases. J Biol Chem. 1996;271:29182–29190. doi: 10.1074/jbc.271.46.29182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Okamoto T, Chun M, Sargiacomo M, Casanova JE, Hansen SH, Nishimoto I, Lisanti MP. Evidence for a regulated interaction of hetero-trimeric G proteins with caveolin. J Biol Chem. 1995;270:15693–15701. doi: 10.1074/jbc.270.26.15693. [DOI] [PubMed] [Google Scholar]
- Lisanti MP, Scherer P, Tang Z-L, Sargiacomo M. Caveolae, caveolin and caveolin-rich membrane domains: a signaling hypothesis. Trends Cell Biol. 1994;4:231–235. doi: 10.1016/0962-8924(94)90114-7. [DOI] [PubMed] [Google Scholar]
- Liu J, Lee P, Galbiati F, Kitsis RN, Lisanti MP. Caveolin-1 expression sensitizes fibroblastic and epithelial cells to apoptotic stimulation. Am J Physiol Cell Physiol. 2001;280:C823–C835. doi: 10.1152/ajpcell.2001.280.4.C823. [DOI] [PubMed] [Google Scholar]
- Liu P, Ying Y, Anderson RG. Platelet-derived growth factor activates mitogen-activated protein kinase in isolated caveolae. Proc Natl Acad Sci USA. 1997;94:13666–13670. doi: 10.1073/pnas.94.25.13666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg AS, Hahn WC, Gupta P, Weinberg RA. Genes involved in senescence and immortalization. Curr Opin Cell Biol. 2000;12:705–709. doi: 10.1016/s0955-0674(00)00155-1. [DOI] [PubMed] [Google Scholar]
- Macho A, Hirsch T, Marzo I, Marchetti P, Dallaporta B, Susin SA, Zamzami N, Kroemer G. Glutathione depletion is an early and calcium elevation is a late event of thymocyte apoptosis. J Immunol. 1997;158:4612–4619. [PubMed] [Google Scholar]
- Mineo C, James GL, Smart EJ, Anderson RGW. Localization of EGF-stimulated Ras/Raf-1 interaction to caveolae membrane. J Biol Chem. 1996;271:11930–11935. doi: 10.1074/jbc.271.20.11930. [DOI] [PubMed] [Google Scholar]
- Minetti C, Sotogia F, Bruno C, Scartezzini P, Broda P, Bado M, Masetti E, Mazzocco P, Egeo A, Donati MA, Volonte D, Galbiati F, Cordone G, Bricarelli FD, Lisanti MP, Zara F. Mutations in the caveolin-3 gene cause autosomal dominant limb-girdle muscular dystrophy. Nat Genet. 1998;18:365–368. doi: 10.1038/ng0498-365. [DOI] [PubMed] [Google Scholar]
- Okamoto T, Schlegel A, Scherer PE, Lisanti MP. Caveolins, a family of scaffolding proteins for organizing “pre-assembled signaling complexes” at the plasma membrane. J Biol Chem. 1998;273:5419–5422. doi: 10.1074/jbc.273.10.5419. [DOI] [PubMed] [Google Scholar]
- Palmer HJ, Paulson KE. Reactive oxygen species and antioxidants in signal transduction and gene expression. Nutr Rev. 1997;55:353–361. doi: 10.1111/j.1753-4887.1997.tb01561.x. [DOI] [PubMed] [Google Scholar]
- Park WY, Park JS, Cho KA, Kim DI, Ko YG, Seo JS, Park SC. Up-regulation of caveolin attenuates epidermal growth factor signaling in senescent cells. J Biol Chem. 2000;275:20847–20852. doi: 10.1074/jbc.M908162199. [DOI] [PubMed] [Google Scholar]
- Razani B, Altschuler Y, Zhu L, Pestell RG, Mostov KE, Lisanti MP. Caveolin-1 expression is down-regulated in cells transformed by the human papilloma virus in a p53-dependent manner. Replacement of caveolin-1 expression suppresses HPV-mediated cell transformation. Biochemistry. 2000;39:13916–13924. doi: 10.1021/bi001489b. [DOI] [PubMed] [Google Scholar]
- Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, Marks CB, Macaluso F, Russell RG, Li M, Pestell RG, Di Vizio D, Hou H, Jr, Knietz B, Lagaud G, Christ GJ, Edelmann W, Lisanti MP. Caveolin-1 null mice are viable, but show evidence of hyper-proliferative and vascular abnormalities. J Biol Chem. 2001;276:38121–38138. doi: 10.1074/jbc.M105408200. [DOI] [PubMed] [Google Scholar]
- Rodemann HP, Bayreuther K, Francz PI, Dittmann K, Albiez M. Selective enrichment and biochemical characterization of seven human skin fibroblasts cell types in vitro. Exp Cell Res. 1989;180:84–93. doi: 10.1016/0014-4827(89)90214-0. [DOI] [PubMed] [Google Scholar]
- Sager R, Sheng S, Anisowicz A, Sotiropoulou G, Zou Z, Stenman G, Swisshelm K, Chen Z, Hendrix MJC, Pemberton P, Rafidi K, Ryan K. RNA Genetics of breast cancer: maspin as a paradigm. Cold Spring Harbor Sym Quant Biol. 1994;LIX:537–546. doi: 10.1101/sqb.1994.059.01.060. [DOI] [PubMed] [Google Scholar]
- Sargiacomo M, Scherer PE, Tang Z-L, Kubler E, Song KS, Sanders MC, Lisanti MP. Oligomeric structure of caveolin: implications for caveolae membrane organization. Proc Natl Acad Sci USA. 1995;92:9407–9411. doi: 10.1073/pnas.92.20.9407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer PE, Lewis RY, Volonte D, Engelman JA, Galbiati F, Couet J, Kohtz DS, van Donselaar E, Peters P, Lisanti MP. Cell-type and tissue-specific expression of caveolin-2. Caveolins-1 and -2 co-localize and form a stable hetero-oligomeric complex in vivo. J Biol Chem. 1997;272:29337–29346. doi: 10.1074/jbc.272.46.29337. [DOI] [PubMed] [Google Scholar]
- Scherer PE, Okamoto T, Chun M, Nishimoto I, Lodish HF, Lisanti MP. Identification, sequence and expression of caveolin-2 defines a caveolin gene family. Proc Natl Acad Sci USA. 1996;93:131–135. doi: 10.1073/pnas.93.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer JE, Liu J, Oh P. Endothelial caveolae have the molecular transport machinery for vesicle budding, docking, and fusion including VAMP, NSF, SNAP, annexins, and GTPases. J Biol Chem. 1995;270:14399–14404. doi: 10.1074/jbc.270.24.14399. [DOI] [PubMed] [Google Scholar]
- Segal SS, Brett SE, Sessa WC. Codistribution of NOS and caveolin throughout peripheral vasculature and skeletal muscle of hamsters. Am J Physiol. 1999;277:H1167–H1177. doi: 10.1152/ajpheart.1999.277.3.H1167. [DOI] [PubMed] [Google Scholar]
- Shenoy-Scaria AM, Dietzen DJ, Kwong J, Link DC, Lublin DM. Cysteine-3 of Src family tyrosine kinases determines palmitoylation and localization in caveolae. J Cell Biol. 1994;126:353–363. doi: 10.1083/jcb.126.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr CJ, DePinho RA. Cellular senescence: mitotic clock or culture shock? Cell. 2000;102:407–410. doi: 10.1016/s0092-8674(00)00046-5. [DOI] [PubMed] [Google Scholar]
- Sionov RV, Haupt Y. Apoptosis by p53: mechanisms, regulation, and clinical implications. Springer Semin Immunopathol. 1998;19:345–362. doi: 10.1007/BF00787230. [DOI] [PubMed] [Google Scholar]
- Smart EJ, Foster D, Ying Y-S, Kamen BA, Anderson RGW. Protein kinase C activators inhibit receptor-mediated potocytosis by preventing internalization of caveolae. J Cell Biol. 1993;124:307–313. doi: 10.1083/jcb.124.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song KS, Li S, Okamoto T, Quilliam L, Sargiacomo M, Lisanti MP. Copurification and direct interaction of Ras with caveolin, an integral membrane protein of caveolae microdomains. Detergent-free purification of caveolae membranes. J Biol Chem. 1996a;271:9690–9697. doi: 10.1074/jbc.271.16.9690. [DOI] [PubMed] [Google Scholar]
- Song KS, Scherer PE, Tang Z-L, Okamoto T, Li S, Chafel M, Chu C, Kohtz DS, Lisanti MP. Expression of caveolin-3 in skeletal, cardiac, and smooth muscle cells. Caveolin-3 is a component of the sarcolemma and co-fractionates with dystrophin and dystrophin-associated glycoproteins. J Biol Chem. 1996b;271:15160–15165. doi: 10.1074/jbc.271.25.15160. [DOI] [PubMed] [Google Scholar]
- Tang Z, Okamoto T, Boontrakulpoontawee P, Katada T, Otsuka AJ, Lisanti MP. Identification, sequence, and expression of an invertebrate caveolin gene family from the nematode Caenorhabditis elegans: implications for the molecular evolution of mammalian caveolin genes. J Biol Chem. 1997;272:2437–2445. doi: 10.1074/jbc.272.4.2437. [DOI] [PubMed] [Google Scholar]
- Tang Z-L, Scherer PE, Okamoto T, Song K, Chu C, Kohtz DS, Nishimoto I, Lodish HF, Lisanti MP. Molecular cloning of caveolin-3, a novel member of the caveolin gene family expressed predominantly in muscle. J Biol Chem. 1996;271:2255–2261. doi: 10.1074/jbc.271.4.2255. [DOI] [PubMed] [Google Scholar]
- Thomas E, al-Baker E, Dropcova S, Denyer S, Ostad N, Lloyd A, Kill IR, Faragher RG. Different kinetics of senescence in human fibroblasts and peritoneal mesothelial cells. Exp Cell Res. 1997;236:355–358. doi: 10.1006/excr.1997.3760. [DOI] [PubMed] [Google Scholar]
- Timme TL, Goltsov A, Tahir S, Li L, Wang J, Ren C, Johnston RN, Thompson TC. Caveolin-1 is regulated by c-myc and suppresses c-myc-induced apoptosis. Oncogene. 2000;19:3256–3265. doi: 10.1038/sj.onc.1203654. [DOI] [PubMed] [Google Scholar]
- Toussaint O, Houbion A, Remacle J. Aging as a multi-step process characterized by a lowering of entropy production leading the cell to a sequence of defined stages. II. Testing some predictions on aging human fibroblasts in culture. Mech Ageing Dev. 1992;65:65–83. doi: 10.1016/0047-6374(92)90126-x. [DOI] [PubMed] [Google Scholar]
- von Zglinicki T, Saretzki G, Docke W, Lotze C. Mild hyperoxia shortens telomeres and inhibits proliferation of fibroblasts: a model for senescence? Exp Cell Res. 1995;220:186–193. doi: 10.1006/excr.1995.1305. [DOI] [PubMed] [Google Scholar]
- Wheaton K, Sampsel K, Boisvert FM, Davy A, Robbins S, Riabowol K. Loss of functional caveolae during senescence of human fibroblasts. J Cell Physiol. 2001;187:226–235. doi: 10.1002/jcp.1071. [DOI] [PubMed] [Google Scholar]
- Wynford-Thomas D. Cellular senescence and cancer. J Pathol. 1999;187:100–111. doi: 10.1002/(SICI)1096-9896(199901)187:1<100::AID-PATH236>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Yang C-P, Galbiati F, Volonte D, Horwitz SB, Lisanti MP. Upregulation of caveolin-1 and caveolae organelles in Taxol-resistant A549 cells. FEBS Lett. 1998;439:368–372. doi: 10.1016/s0014-5793(98)01354-4. [DOI] [PubMed] [Google Scholar]
- Zundel W, Swiersz LM, Giaccia A. Caveolin 1-mediated regulation of receptor tyrosine kinase-associated phosphatidylinositol 3-kinase activity by ceramide. Mol Cell Biol. 2000;20:1507–1514. doi: 10.1128/mcb.20.5.1507-1514.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]