Abstract
Dimerization and phosphorylation of the epidermal growth factor (EGF) receptor (EGFR) are the initial and essential events of EGF-induced signal transduction. However, the mechanism by which EGFR ligands induce dimerization and phosphorylation is not fully understood. Here, we demonstrate that EGFRs can form dimers on the cell surface independent of ligand binding. However, a chimeric receptor, comprising the extracellular and transmembrane domains of EGFR and the cytoplasmic domain of the erythropoietin receptor (EpoR), did not form a dimer in the absence of ligands, suggesting that the cytoplasmic domain of EGFR is important for predimer formation. Analysis of deletion mutants of EGFR showed that the region between 835Ala and 918Asp of the EGFR cytoplasmic domain is required for EGFR predimer formation. In contrast to wild-type EGFR ligands, a mutant form of heparin-binding EGF-like growth factor (HB2) did not induce dimerization of the EGFR-EpoR chimeric receptor and therefore failed to activate the chimeric receptor. However, when the dimerization was induced by a monoclonal antibody to EGFR, HB2 could activate the chimeric receptor. These results indicate that EGFR can form a ligand-independent inactive dimer and that receptor dimerization and activation are mechanistically distinct and separable events.
INTRODUCTION
Epidermal growth factor receptor (EGFR), a member of the ErbB family of receptor tyrosine kinases, was the earliest noted growth factor receptor. EGFR is an ∼180-kDa transmembrane glycoprotein consisting of an extracellular domain containing two cysteine-rich regions, a single transmembrane domain, and an intracellular domain. EGFR has several ligands with similar structures, including EGF, transforming growth factor α, heparin-binding EGF-like growth factor (HB-EGF), amphiregulin, betacellulin, and epiregulin (Marquart et al., 1984; Shoyab et al., 1989; Higashiyama et al., 1991; Shing et al., 1993; Toyoda et al., 1995). The first step in EGFR activation is receptor dimer formation. EGF family molecules activate an EGFR homodimer and an EGFR heterodimer with other ErbB receptors (Tzahar and Yarden, 1998). Dimerized EGFR induces autophosphorylation of tyrosine residues in the carboxyl terminal of EGFR by its own kinase domain. The resulting phosphorylated tyrosine residues serve as binding sites for molecules containing Src homology 2 domains and initiate intracellular signaling cascades linked to versatile cellular responses, including regulation of gene expression.
Although dimerization and autophosphorylation are the critical events in the activation of EGFR (Yarden and Schlessinger, 1987a,b), the precise mechanisms underlying these events have not been fully elucidated. EGF-induced dimerization of EGFR has been demonstrated in a number of studies, many of which made use of covalent cross-linking agents (Cochet et al., 1988; Lax et al., 1988; Lax et al., 1989; Tanner and Kyte, 1999). Circular dichroism analysis and steady-state fluorescence measurements show that EGF induces a conformational change in the EGFR ectodomain (Greenfield et al., 1989), which may be the basis of EGFR activation. Electron microscopy shows that the ligand not only induces a conformational change of the soluble form of EGFR but also stimulates its oligomerization (Lax et al., 1991). Small-angle x-ray scattering assays and isothermal titration calorimetry suggest that the stoichiometry of the ligand binding to soluble EGFR is 2:2 (Lemmon et al., 1997). Dimerization of EGFR and activation of its tyrosine kinase are coincidental events (Canal, 1992); thus, it has been thought that dimerization and activation of EGFR are mechanistically indistinguishable events.
Studies for determining fluorescence resonance energy transfer on fixed A431 cells (Gadella and Jovin, 1995) or single-molecule imaging of EGFR on the surface of living A431 cells (Sako et al., 2000) have implied that preformed dimers of EGFR may exist in intact cell membranes without ligand stimulation. Earlier studies of A431 cells by sucrose density gradient centrifugation or cross-linking also showed that EGFR could form dimers without exogenous ligand stimulation (Boni-Schnetzler and Pilch, 1987; Cochet et al., 1988). However, these studies did not exclude the possibility that EGFR dimers were induced by EGFR ligands provided from the A431 cells themselves (Van de Vijver et al., 1991). Thus, there is no direct evidence to prove the existence of ligand-independent EGFR dimer. We demonstrate here ligand-independent EGFR dimers using Ba/F3 cells and other cell lines. We found that such ligand-independent EGFR dimers were not activated. Comparison of EGFR with an EGFR-erythropoietin receptor (EpoR) chimeric receptor and the use of a HB-EGF mutant indicated that dimer formation and the activation of EGFR are distinguishable processes.
MATERIALS AND METHODS
Reagents and Antibodies
Human recombinant EGF was purchased from Boehringer-Mannheim Co. (Indianapolis, IN). Rabbit anti-EGFR antibody, mouse anti-Cbl monoclonal antibody (mAb), rabbit anti–hemagglutinin-tag antibody, rabbit anti-myc antibody, and rabbit anti–signal transducer and activator of transcription (STAT) 5 were all acquired from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Mouse anti-EGFR mAb (clone LA1) was purchased from Upstate Biotechnology Inc. (Charlottesville, VA), mouse anti-EGFR mAb (clone EGFR.1) from Neo Markers, horseradish peroxidase (HRP)-conjugated goat antimouse immunoglobulin G (IgG) from Zymed Laboratories Inc. (San Francisco, CA), HRP-conjugated goat anti-rabbit IgG from Chemicon International Inc. (Temecula, CA), mouse anti-phosphotyrosine mAb (6D12) from MBL Co (Nagoya, Japan), and mouse anti-Flag mAb (M2) from Sigma Chemical Co. (St. Louis, MO). Rabbit anti–mitogen-activated protein kinase (MAPK) and phospho-MAPK antibody were purchased from New England Biolabs Inc. (Beverly, MA).
Plasmid Construction
A plasmid encoding a glutathione S-transferase (GST) fusion protein containing the EGF-like domain of proHB-EGF, corresponding to amino acids 106–149 of human proHB-EGF, was constructed by insertion of the corresponding cDNA sequences of proHB-EGF into the EcoRI/BamHI sites of the pGEX-3X plasmid (Pharmacia). The inserted DNA fragment encoding proHB-EGF was prepared by polymerase chain reaction using plasmid pRTHG-1 (Mitamura et al., 1995) as a template. The resulting GST fusion protein, referred to as HB1, encompasses the entire EGF-like domain. Next, HB2, a GST fusion protein containing a mutated EGF-like domain of proHB-EGF, was produced: The coding sequence of proHB-EGF cDNA was mutated from 379CGGAAA to CTTTCA and from 388AAG to GAC. These substitutions resulted in amino acid alterations from 110Arg-111Lys to Leu-Ser and 113Lys to Asp. cDNA of the resulting mutant proHB-EGF, corresponding to amino acids 106–149 and containing the above substitutions, was inserted into the EcoRI/BamHI sites of the pGEX-3X plasmid. Truncated EGFR mutants were constructed: pRc/CMV-HA was constructed by the insertion of a DNA fragment encoding the HA-tag epitope into the XbaI site of pRc/CMV (Invitrogen, San Diego, CA). Deletion of EGFR was generated by polymerase chain reaction using pTJNEO-EGFR (Gotoh et al., 1992) as the template, and synthesized products were inserted between the HindIII and XbaI sites of pRc/CMV-HA. The sequence of each EGFR mutant was confirmed by sequence analysis.
Purification of GST Fusion Protein
The GST fusion proteins were purified with glutathione Sepharose 4B (Pharmacia, Piscataway, NJ) according to the manufacturer's instructions. GST-HB1 and GST-HB2, eluted from glutathione Sepharose, were dialyzed against HEPES-buffered saline (20 mM HEPES, 150 mM NaCl, pH 7.2) for use in the following experiments. Protein concentrations were determined by the Bradford method using BSA as a standard.
Cell Culture and Transfection
Ba/F3 cells were cultured in RPMI 1640 medium containing 10% fetal calf serum (FCS) and 5% WEHI-3 cell-conditioned medium as a source of interleukin 3 (IL-3). Stable transformants of Ba/F3 cells expressing EGFR or EGFR-EpoR were obtained by selection in medium containing G418 as previously described (Iwamoto et al., 1999). COS-7 cells were maintained in DMEM with 10% FCS. Chinese hamster ovary (CHO) cells were cultured in Ham's F12 medium with 10% FCS. Transfection was carried out by electroporation (Gene Pulser, Bio-Rad, Richmond, CA) according to the manufacturer's instructions.
Treatment with EGF Ligands
Before cross-linking and coimmunoprecipitation assays, cells indicated were incubated with 100 nM of EGF or the recombinant forms of HB-EGF for 3 min, washed with PBS, and then used for further analysis.
Chemical Cross-linking
Chemical cross-linking was carried out as described previously, with minor modifications (Iwamoto et al., 1994). Briefly, the cells were washed with PBS (137 mM NaCl, 0.67 mM KCl, 8 mM Na2HPO4, 1.4 mM KH2PO4) three times and incubated for 30 min at 4°C with 1 mM dithiobis-(sulfosuccinimdylpropionate) (DTSSP) (Pierce Chemical Co., Rockford, IL) in PBS, followed by washing three times with Tris-buffered saline (TBS) (20 mM Tris-HCl, 100 mM NaCl, pH 7.5) before use in the following studies.
Immunoprecipitation and Immunoblotting
Cells were lysed with 1% Triton X-100 in lysis buffer (0.15 M NaCl, 2 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 1 mM NaVO4, 50 mM Tris pH 7.5) and then centrifuged for 20 min at 15,000 × g. The supernatants were cleared with Sepharose 4B for 1 h and then incubated with primary antibody for 2 h, followed by addition of Sepharose 4B-conjugated secondary antibody. The Sepharose beads were washed three times with lysis buffer and once with deionized water and then were boiled for 5 min in SDS-PAGE sample buffer with or without 50 mM dithiothreitol. Samples were run on SDS-PAGE and electrotransferred to an Immobilon membrane. The membrane was blocked with 3% skim milk in TBS (20 mM Tris, 0.1 M NaCl, pH 7.5) at 37°C for 1 h, then incubated with primary antibody in TBS containing 1% skim milk at room temperature for 1 h. Next, the membrane was washed four times with TTBS (TBS containing 0.05% Tween 20), incubated with HRP-conjugated secondary antibody, and finally analyzed with an ECL-Western blotting kit (Amersham International plc, Buckinghamshire, England).
DNA Synthesis Assay
Cells were seeded into 24-well plates at a density of 5 × 104 cells/well with or without each EGF ligand in fresh RPMI 1640 medium containing 10% serum, then cultured at 37°C for 24 h and incubated with [3H]thymidine (37 kBq/ml) for 4 h. Cells were harvested, and radioactivity incorporated into DNA was determined as described previously (Iwamoto et al., 1999). The rate of DNA synthesis was expressed as a percentage of the average of the maximum value (40,000 cpm).
Preparation of Fab Fragment
Anti-EGFR mAb (EGFR.1), 1 mg/ml, was incubated with papain (0.1 mg/ml) in PBS at 37°C for 18 h, at which point iodoacetamide (30 mM) was added to stop the reaction. The mixture was dialyzed in PBS at 4°C for 12 h and then purified with goat antimouse IgG Fc antibody conjugated to Sepharose 4B. The size of the Fab fragment was checked by SDS-PAGE, and the Fab concentration was determined by measuring absorbance at 280 nm.
RESULTS
EGF Receptor Exists as an Inactive Dimer without Ligand
Ba/F3 cells are an IL-3–dependent preB-lymphocyte cell line and do not express endogenous murine EGFR, ErbB2, and ErbB4. Although the expression of the detectable amount of ErbB3 transcript was reported (Riese et al., 1995), we did not detect ErbB3 in this cell line at the protein level. BE cells (Ba/F3 cells expressing EGFR), which were generated by transfection of Ba/F3 cells with vectors encoding human EGFR, can respond to and proliferate with EGF and other EGFR ligands. In the absence of IL-3, BE cells can proliferate in an EGFR ligand-dependent manner. Therefore, this cell line allows EGFR activation to be monitored precisely by measuring cell proliferation.
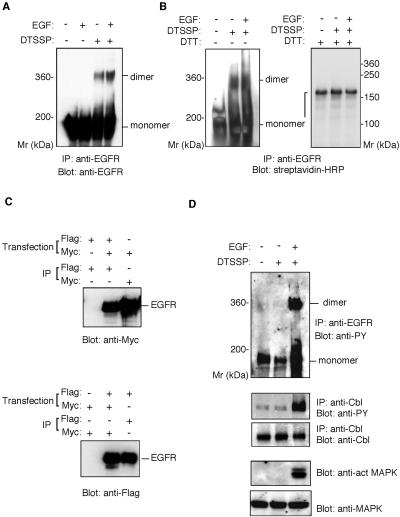
To examine the oligomeric state of EGFR, we undertook a cross-linking analysis with DTSSP, a membrane-impermeable homobifunctional reagent. BE cells cultured in serum-free medium were preincubated with or without EGF and then treated with DTSSP. The cell lysate was immunoprecipitated with anti-EGFR antibody, followed by SDS-PAGE and Western blotting using an anti-EGFR antibody. In the absence of DTSSP, EGF induces dimerization and activation of EGFR, but the dimers are separated into monomers upon SDS electrophoresis. Therefore, only the monomeric 180-kDa form of EGFR was detected by the anti-EGFR antibody (Figure 1A). When EGF-stimulated BE cells were treated with DTSSP, a 360-kDa polypeptide, corresponding to the size of the EGFR dimer, was detected by the anti-EGFR antibody in addition to the 180-kDa EGFR monomer. It should be noted that this 360-kDa molecule was observed in samples from unstimulated BE cells treated with DTSSP. Neither the 360-kDa nor 180-kDa bands were observed when Ba/F3 cells that did not express EGFR were treated with DTSSP (data not shown), thus excluding the possibility of a nonspecific cross-reaction of the anti-EGFR antibody with polypeptides unrelated to EGFR.
Figure 1.
Ligand-independent preformed dimer of EGFR in BE cells. (A) Cross-linking assay of the EGFR dimer. BE cells were preincubated with or without EGF, then treated with DTSSP. The cell lysates were precipitated with rabbit anti-EGFR antibody. Precipitated material was separated by 4% SDS-PAGE in the absence of reducing agents and detected by immunoblot with anti-EGFR mAb. (B) Cross-linking of surface-biotinylated EGFR. BE cells were biotinylated with sulfo-N-hydroxysulfosuccinimide-biotin, incubated with or without EGF, and then treated with DTSSP. The cell lysates were precipitated with anti-EGFR polyclonal antibody. Precipitated material was separated by SDS-PAGE in the presence or absence of 5 mM dithiothreitol and detected by immunoblot with streptavidin-HRP. (C) Coimmunoprecipitation assay of epitope-tagged EGFR. COS cells were cotransfected with plasmids encoding EGFR-myc and EGFR-Flag or transfected with either one of the plasmids alone. The transfected cells were lysed and the cell lysates immunoprecipitated by rabbit anti-myc antibody or anti-Flag mAb. The precipitated materials were separated by 7% SDS-PAGE, transferred to an Immobilon membrane, and blotted with rabbit anti- myc or anti-Flag mAb. (D) Phosphorylation of EGFR, Cbl, and MAP kinase. BE cell lysates were immunoprecipitated with anti-EGFR polyclonal antibody or anti-Cbl antibody. Precipitated materials were subjected by SDS-PAGE and Western blotting using anti-phosphotyrosine mAb or anti-Cb1 antibody. MAP kinase and phosphorylated MAP kinase were detected from the cell lysate by Western blotting using anti-MAP kinase or anti-phosphorylated MAP kinase antibody.
To obtain further evidence that the 360-kDa band represents EGFR homodimers, we performed a cross-linking analysis using cells whose surface was prelabeled by the biotinylation reagent sulfo–N-hydroxysulfosuccinimide–biotin. After cross-linking with DTSSP, the cell lysate was immunoprecipitated with anti-EGFR antibody, and the precipitated material was subjected to SDS-PAGE in the presence or absence of a reducing agent. Because DTSSP is cleaved in the presence of a reducing agent, cross-linked proteins would be split into their monomeric forms. In the absence of the reducing agent, the 360-kDa and 180-kDa polypeptides were detected by Western blotting with streptavidin-HRP, whereas only the 180-kDa polypeptide was detected in the presence of the reducing agent (Figure 1B), indicating that the 360-kDa cross-linked dimers had been reduced to 180-kDa EGFR monomers. BE cells do not express any other ErbB family proteins, excluding the possibility of heterodimer formation of EGFR with other ErbB family receptors.
To confirm the formation of EGFR homodimers, we performed coprecipitation assays using two kinds of epitope-tagged EGFR constructs. EGFR-Flag and EGFR-myc contain Flag and Myc tags, respectively, in the C-terminal region of EGFR. EGFR-Flag and EGFR-myc were cotransfected into COS-7 cells and incubated in serum-free media. The cell lysates were precipitated with anti-Flag antibody, and the precipitated material was analyzed by Western blotting using the anti-Myc antibody, or vice versa. Figure 1C shows that EGFR-myc coprecipitated with the anti-Flag antibody, and EGFR-Flag coprecipitated with anti-Myc antibody, from cell lysates prepared by culturing cells in the absence of EGF ligands. This confirms the ability of EGFR to form homodimers in the absence of ligand stimulation.
The above results indicate that EGFR, or at least some of the EGFR molecules on the cell surface, may exist as dimers in the absence of ligand stimulation. Although it appears that EGFR activation should not be able to occur without EGF binding, our use of BE cells preincubated with serum-free medium 2 h before the cross-linking study led us to explore the activation state of preformed EGFR dimers under serum-free conditions. When EGF-treated BE cells were analyzed by the cross-linking assay, the 360-kDa homodimer and the 180-kDa monomer of EGFR were highly phosphorylated, as demonstrated by an anti-phosphotyrosine antibody (Figure 1D). In EGF-untreated cells, however, both EGFR homodimers and monomers were found to be not highly phosphorylated. Although a weak band of 180 kDa appeared, it was the basal-level phosphorylation of unstimulated EGFR (Figure 1D). Cbl and MAPK, downstream substrates of the EGFR signal, were also unphosphorylated (Figure 1D), supporting the case for an inactive state of EGFR.
Ligand-independent Dimer Formation Requires the Cytoplasmic Domain of EGFR
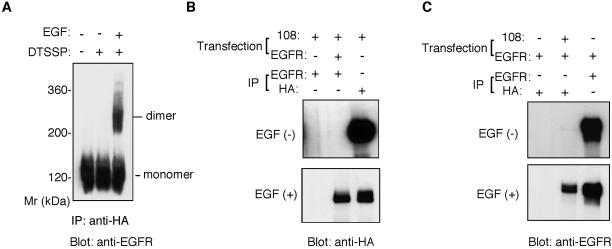
The EGFR-EpoR chimeric receptor, designated −108 (Iwatsuki et al., 1997), brings together the extracellular and the transmembrane domains of EGFR with part of the cytoplasmic domain of EpoR and is linked to an HA tag at its carboxy terminus. Cells expressing this chimeric receptor proliferate under the influence of EGFR ligands (Ohashi et al., 1994). The chimeric receptor was used to examine whether the cytoplasmic domain of EGFR is required for the predimer formation. Like BE cells, B108 cells, stable transformants of Ba/F3 cells expressing chimeric receptor, can respond and proliferate upon stimulation with EGF and other EGF ligands. B108 cells were preincubated with or without EGF, treated with DTSSP, lysed, and finally analyzed by SDS-PAGE and Western blotting. The monomeric form of the chimeric receptor gives a band of ∼120 kDa in SDS gels (Figure 2A). When B108 cells were stimulated with EGF and then treated with the cross-linker DTSSP, a dimer of the chimeric EGFR-EpoR molecule of ∼240 kDa was observed in addition to the monomer (Figure 2A). However, unlike wild-type EGFR, dimers of the chimeric receptor were not observed in the absence of EGF stimulation (Figure 2A).
Figure 2.
Failure of EGFR-EpoR chimeric receptors to form ligand-independent dimers. (A) Cross-linking analysis of chimeric receptor dimers. B108 cells were preincubated with or without EGF and then treated with DTSSP. The cell lysates were immunoprecipitated with rabbit anti–HA-tag antibody. Precipitated material was separated by 4.5% SDS-PAGE in the absence of reducing agents and detected by immunoblot with mouse anti-EGFR mAb. (B and C) Coimmunoprecipitation assay for the detection of preformed heterodimers of EGFR-EpoR chimeric receptors with EGFR. COS cells were cotransfected with plasmids encoding the EGFR-EpoR chimeric receptor (108) and EGFR or transfected with either of the plasmids alone. The transfected cells were incubated with or without EGF. The cell lysates were immunoprecipitated by rabbit anti-EGFR antibody or rabbit anti–HA-tag antibody. The precipitated materials were separated by SDS-PAGE, transferred to an Immobilon membrane, and blotted with rabbit anti–HA-tag antibody (B) or anti-EGFR antibody (C).
The EGFR-EpoR chimeric receptor was further studied by coimmunoprecipitation experiments. Both wild-type EGFR and the EGFR-EpoR chimeric receptor were transiently expressed in COS-7 cells, which were then treated with or without EGF. The cell lysates were immunoprecipitated for coprecipitation assay with an anti–HA-tag antibody, which recognizes the EGFR-EpoR chimeric receptor, or an anti-EGFR antibody, which specifically recognizes the cytoplasmic domain EGFR and does not bind to the EGFR-EpoR chimeric receptor. When cells were treated with EGF, the EGFR-EpoR chimeric receptor coprecipitated with EGFR and the anti-EGFR antibody, as confirmed by Western blotting using an anti–HA-tag antibody (Figure 2B). However, such coprecipitation of the EGFR-EpoR chimeric receptor was not observed without EGF stimulation (Figure 2B). Similar results were obtained by immunoprecipitation with the anti-HA antibody, in which the coprecipitation of wild-type EGFR was detected by the anti-EGFR antibody (Figure 2C). These results, together with the results of the cross-linking experiments, indicate that the EGFR-EpoR chimeric receptor does not homodimerize in the absence of ligand stimulation and that the cytoplasmic domain of EGFR is required for formation of such predimers.
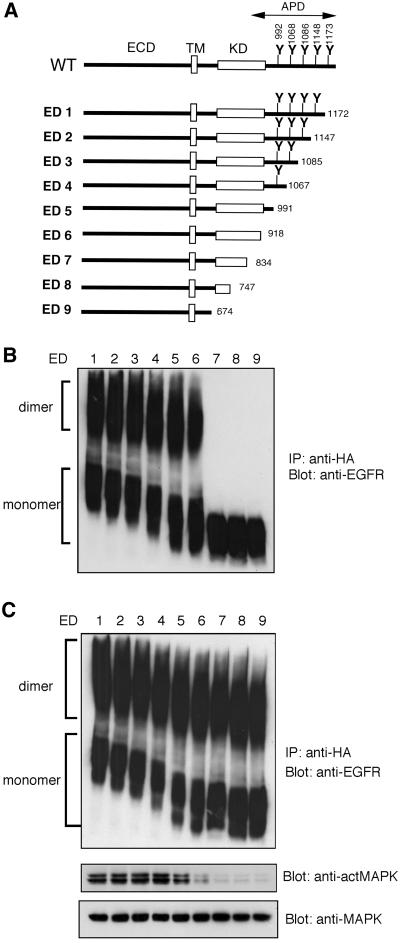
To confirm the role of the cytoplasmic domain of EGFR in its predimer formation and to narrow down the responsible region, we made a series of truncation mutants of EGFR. The mutants ED1–ED5 possess the protein kinase domain but are lacking parts of the autophosphorylation domain, whereas ED6–ED9 are lacking either part of or all of the kinase and the autophosphorylation domains (Figure 3A). All the EGFR mutants were transiently expressed in CHO cells, and the cross-linking assay was performed. Mutants from ED1 to ED6 formed homodimers without EGF stimulation, whereas ED7, ED8, and ED9 failed to form predimers (Figure 3B). However, in the presence of EGF stimulation, all the truncated EGFR mutants were induced to form homodimers (Figure 3C). Because ED7, ED8, and ED9 are lacking part or all of the kinase domain, these mutants could not generate signals to activate the MAPK pathway on EGF treatment (Figure 3C). These results indicate that the region between 835Ala and 918Asp is important for both EGFR predimer formation and EGFR activation.
Figure 3.
Predimer formation of EGFR mutants. (A) Schematic structure of EGFR mutants. Amino acids are shown as one-letter symbols, and numbers in the figure indicate the number of amino acids from the N-terminus of human EGFR. ECD, extracellular domain; TM, transmembrane domain; KD, kinase domain; APD, autophosphorylation domain; WT, wild-type. (B and C) Cross-linking analysis of EGFR mutants. CHO cells were transfected with plasmids encoding each EGFR mutant. Cells were incubated with (C) or without (B) EGF and then treated with DTSSP. The cell lysates were immunoprecipitated with anti-EGFR mAb. Precipitated material was separated by 4.5% SDS-PAGE in the absence of reducing agents and detected by immunoblot with rabbit anti–HA-tag antibody. MAP kinase and phosphorylated MAP kinase were detected from the cell lysates by Western blotting using anti-MAP kinase or anti–phosphorylated MAP kinase antibody (C).
EGFR-EpoR Chimeric Receptor and EGFR Show Different Mitogenic Responses
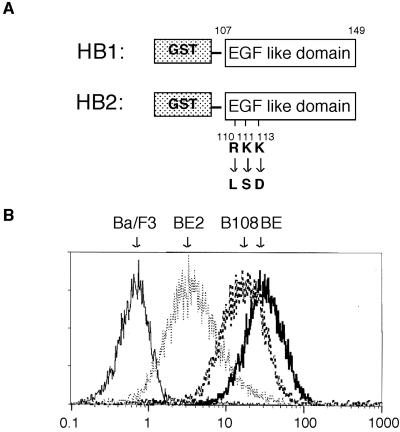
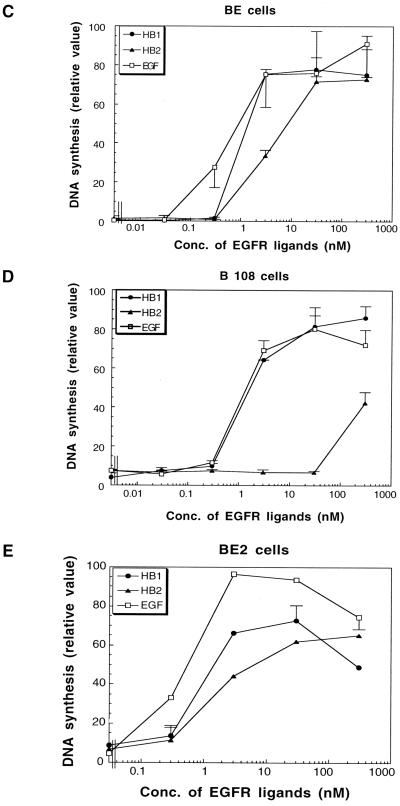
Because EGFR predimer formation and activation require a similar region, it is difficult to use EGFR truncation mutants to study the biological significance of EGFR predimer formation. To further explore the biological significance of preformed EGFR dimers, we studied wild-type EGFR and EGFR-EpoR chimera and compared their physiological reactions to treatment with EGFR ligands. First, we examined the mitogenic response, as measured by the level of DNA synthesis activity, of BE and B108 cells upon stimulation with EGF or related ligands. Figure 4, C and D, show that DNA synthesis in BE and B108 cells is stimulated equally by EGF. Mitogenic responses were also studied for two kinds of recombinant HB-EGF molecules. HB1 is a GST fusion protein of HB-EGF containing only the EGF-like domain of human HB-EGF, whereas HB2 is similar to HB1 but has substitutions of three amino acid residues in the EGF-like domain (Figure 4A). Like EGF, HB1 stimulated DNA synthesis equally in BE and B108 cells. However, HB2 was a less efficient mitogen for B108 cells. HB2 at 3 and 30 nM induced DNA synthesis in BE cells to ∼38 and 70% of maximum induction, respectively, but not at all in B108 cells. Fifty times more HB2 was necessary to achieve the same 40% of maximum induction in B108 compared with BE cells.
Figure 4.
Mitogenic activities of EGF and recombinant HB-EGF proteins in BE, B108, and BE2 cells. (A) Schematic structures of HB1 and HB2. HB1 is a GST fusion protein containing the EGF-like domain of human HB-EGF. HB2 is essentially similar to HB1 except for three basic amino acids within the EGF-like domain that are changed to the indicated amino acids. Amino acids are shown as one-letter symbols, and numbers in the figure indicate the number of amino acids from the N-terminus of human HB-EGF. (B) Flow cytometric analysis of cell surface EGFR and EGFR-EpoR chimeric receptor with anti-EGFR antibody. Ba/F3, BE, BE2, and B108 cells treated with anti-EGFR antibody recognizing the extracellular domain of EGFR were stained with FITC-conjugated secondary antibody. (C–E) The mitogenic activities of EGF, HB1, and HB2 toward BE cells (C), B108 cells (D), and BE2 cells (E) were determined by measuring DNA synthesis. BE cells, B108 cells, and BE2 cells were cultured with EGF, HB1, or HB2 for 24 h, followed by incubation with [3H]thymidine (37 kBq/ml) at 37°C for 4 h. Cells were harvested, and the amounts of [3H]thymidine incorporated into DNA were measured. Data are shown as means ± SD obtained from three independent experiments.
The number of the EGFR-EpoR chimeric receptors on B108 cells is 2 times less than that of EGFR on BE cells (Figure 4B). To examine whether or not the different mitogenic response of B108 cells to HB2 is a result of the lesser amount of receptor molecules on the cell surface, we isolated another clone of Ba/F3 cells that expresses a smaller amount of EGFR. As shown in Figure 4B, BE2 cells express EGFR, but the number of EGFRs on the cell surface is ∼9 times less than that of BE cells. BE2 cells showed a mitogenic response to HB2 similar to that of HB1 (Figure 4E), also as in BE cells, indicating that the different mitogenic response of B108 cells to HB2 is not caused by the smaller amounts of receptor molecules on the cell surface.
Defect of HB2 in the Dimerization of EGFR-EpoR Chimeric Receptor
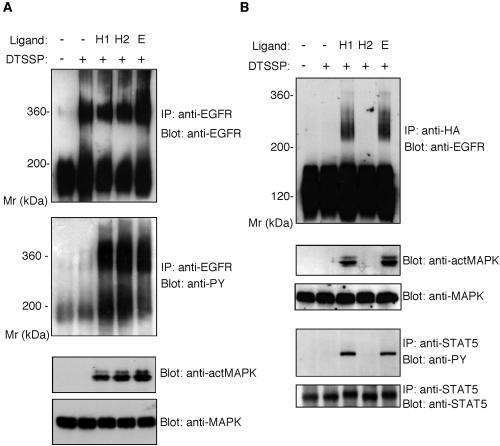
We have shown in this study that the wild-type EGFR, but not the EGFR-EpoR chimera, forms homodimers even in the absence of EGFR ligands. We explored whether the decreased mitogenic response of B108 cells to HB2, compared with that of BE cells, is related to their inability to form dimers in the absence of ligand. Cross-linking analysis by DTSSP indicated that, whereas homodimer formation in BE cells is EGFR ligand-independent, phosphorylation of the EGFR homodimer and the subsequent activation of downstream signaling molecules are ligand-dependent (Figure 1). Like EGF and HB1, HB2 stimulated phosphorylation of the EGFR homodimer and MAPK in BE cells (Figure 5A). In the case of the EGFR-EpoR chimeric receptor, treatment of B108 cells with EGF or HB1 resulted in dimer formation and the activation of MAPK and STAT5, the substrates downstream of EpoR (Figure 5B). However, neither chimeric receptor dimer formation nor MAPK/STAT5 activation was induced by treatment of B108 cells with HB2.
Figure 5.
Dimerization and activation of EGFR and the EGFR-EpoR chimeric receptor by EGF, HB1, or HB2. (A) BE cells were treated with HB1, HB2, or EGF, followed by incubation with or without DTSSP. Cell lysates were immunoprecipitated with an anti-EGFR polyclonal antibody, and the precipitated materials were separated by 4% SDS-PAGE in the absence of a reducing agent and transferred to an Immobilon membrane. The membrane was blotted with anti-EGFR mAb or anti-phosphotyrosine mAb. Total cell lysates were used for detection of MAPK and phosphorylated MAPK by anti-MAPK or anti–activated MAPK antibody. (B) B108 cells were treated with HB1, HB2, or EGF followed by treatment with or without DTSSP. Cell lysates were immunoprecipitated with anti-HA polyclonal antibody, and the precipitated materials were separated by 4.5% SDS-PAGE in the absence of reducing agents and transferred to an Immobilon membrane. The membrane was blotted with anti-EGFR mAb. Total cell lysates were used for detection of MAPK and phosphorylated MAPK by anti-MAPK or anti–activated MAPK antibody. For the detection of STAT5, cell lysates were immunoprecipitated with anti-STAT5 antibody, and the membrane was blotted with anti-STAT 5 antibody or anti-phosphotyrosine mAb.
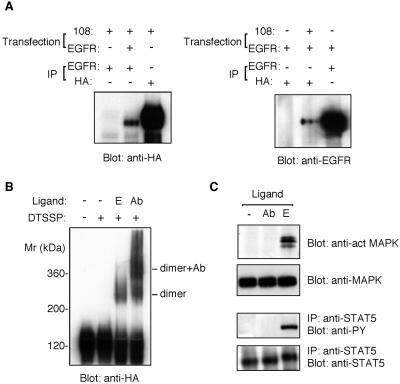
The above results, including the failure of HB2 to induce chimeric receptor dimer formation, lead to the hypothesis that HB2 activates EGFR only when it exists as a preformed dimer. To examine this possibility, we decided to induce chimeric receptor dimer formation artificially using an mAb directed to EGFR. The anti-EGFR antibody used in this experiment (EGFR.1) recognizes the extracellular domain of EGFR but neither activates EGFR nor inhibits ligand binding (Waterfield et al., 1982). The ability of the antibody to induce dimers was first monitored by coimmunoprecipitation assay. Plasmids encoding EGFR and EGFR-EpoR chimeric receptor were cotransfected into COS-7 cells. Cells were treated with the anti-EGFR mAb, and the cell lysates were subjected to coprecipitation assay. As shown in Figure 6A, EGFR and EGFR-EpoR chimeras coprecipitated with each other, indicating that the anti-EGFR antibody can induce ligand-independent dimer formation between the EGFR and EGFR-EpoR chimera. Figure 6B also demonstrates antibody-mediated formation of EGFR-EpoR chimeric receptor dimers in B108 cells, as demonstrated by the cross-linking assay. B108 cells were first incubated with the EGFR.1 antibody and then treated with DTSSP. Western blotting using an anti–HA-tag antibody detected the formation of high-molecular-weight bands corresponding to or higher than the size of the chimeric receptor homodimer. Such high-molecular-weight bands were not observed in control cells that were not treated with the EGFR.1 antibody. However, analysis of the phosphorylation states of MAPK and STAT5 revealed that the antibody-mediated dimer or oligomer formation did not activate the chimeric receptor, in contrast to EGF-induced homodimer formation (Figure 6C).
Figure 6.
Antibody-mediated dimer formation of EGFR. (A) Coprecipitation assay of antibody-mediated formation of EGFR/EGFR-EpoR chimeric receptor heterodimers. COS cells were transfected with plasmids encoding EGFR and EGFR-EpoR (108). The transfected cells were incubated with anti-EGFR mAb (EGFR.1) for 2 h. Cells were lysed, and the cell lysates were subjected to the coimmunoprecipitation assay as described in MATERIALS AND METHODS and Figure 2. (B) Cross-linking assay of antibody-mediated formation of EGFR-EpoR chimeric receptor dimers. B108 cells were incubated with anti-EGFR mAb (Ab) or EGF (E) and then treated with or without DTSSP. Cells were lysed, and cell lysates were immunoprecipitated by anti-HA antibody. Precipitated materials were analyzed by SDS-PAGE and immunoblot using an anti-HA antibody. (C) The activation state of the antibody-mediated chimeric receptor dimer. B108 cells were incubated with anti-EGFR mAb (EGFR.1) (Ab) or EGF (E) and then lysed. The lysates were separated by SDS-PAGE, followed by immunoblotting with anti–phosphorylated MAPK or anti-MAPK antibody. An equal amount of cell lysate was subjected to immunoprecipitation with rabbit anti-STAT5 polyclonal antibody and then immunoblotted with anti-phosphotyrosine mAb or anti-STAT5 antibody.
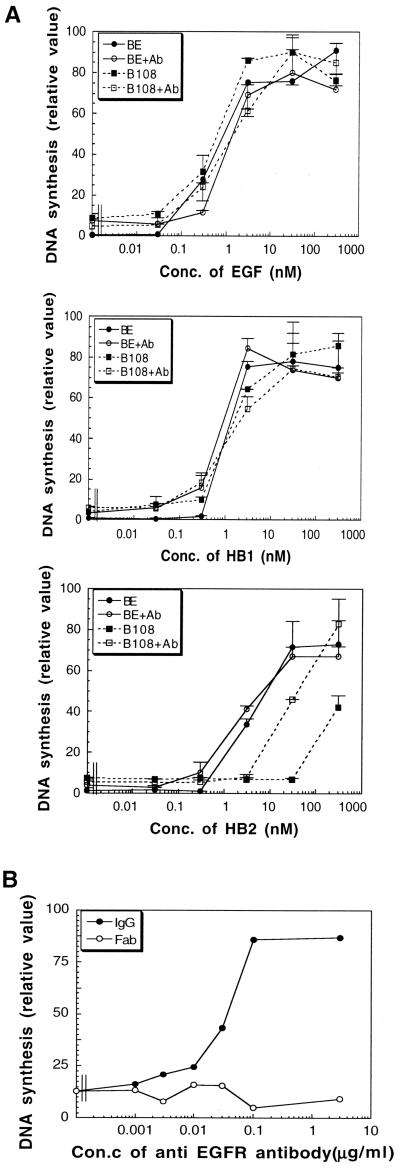
Next, we compared the mitogenic activities of EGF, HB1, and HB2 with both BE and B108 cells in the presence or absence of the EGFR.1 antibody. DNA synthesis induced by EGF, HB1, and HB2 in BE cells and by EGF and HB1 only in B108 cells was not affected by the presence of the EGFR.1 antibody (Figure 7A). However, the mitogenic activity of HB2 on B108 cells was increased by ∼10 times in the presence of 1 μg/ml of the EGFR.1 antibody. This effect of the EGFR.1 antibody on the mitogenic activity of HB2 was lost when only the Fab fragment of the EGFR.1 antibody was used (Figure 7B), indicating that bivalency of the antibody is required for its effect.
Figure 7.
Effect of antibody-mediated formation of the chimeric receptor dimers on the mitogenic activities of EGF, HB1, and HB2. (A) BE and B108 cells were cultured with EGF, HB1, or HB2 in the presence or absence of anti-EGFR mAb (EGFR.1, 1 μg/ml) for 24 h, followed by incubation with [3H]thymidine (37 kBq/ml) at 37°C for 4 h. Cells were harvested, and the amounts of [3H]thymidine incorporated into DNA were measured. Data are shown as means ± SD obtained from three independent experiments. (B) B108 cells were incubated with HB2 (100 nM) in the presence of the indicated amounts of anti-EGFR mAb (EGFR.1) or its Fab fragment for 24 h, followed by incubation with [3H]thymidine (37 kBq/ml) at 37°C for 4 h. Cells were harvested, and the amounts of [3H]thymidine incorporated into DNA were measured. Conc., concentration
From these results, we concluded that the mitogenic activity of HB2 is greatly affected by the presence or absence of preformed EGFR dimers, whereas EGF and HB1 are less affected by the oligomerization state.
DISCUSSION
Crosslinking studies using the chemical cross-linker DTSSP have provided biochemical evidence for the existence of a ligand-independent preformed dimer of EGFR in BE cells. Conversely, ligand-independent dimer formation was not observed with an EGFR-EpoR chimeric receptor, excluding the possibility that the bifunctional cross-linker itself artificially forms the dimer. By cross-linking studies and by single-molecule analysis (Sako, Yu, Mekada, and Yanagida, unpublished observations), we have also observed that EGFR expressing in another cell line (CHO cells) forms a ligand-independent dimer, whereas the EGFR-EpoR chimeric receptor expressed in the same cell line does not. Thus, the present results are not specific for Ba/F3 cells. Because DTSSP cannot penetrate the cell membrane, the preformed EGFR dimer must be on the plasma membrane. Phosphorylation analysis of EGFR and its downstream signaling molecules demonstrates that EGFR is not activated under serum-free conditions. In addition, using epitope-tagged EGFRs in coimmunoprecipitation assays, we confirmed the physical interaction between the two kinds of epitope-tagged EGFRs in COS-7 cells. Taking into account all earlier studies (Boni-Schnetzler and Pilch, 1987; Cochet et al., 1988; Gadella and Jovin, 1995; Sako et al., 2000; Moriki et al., 2001), we conclude that EGFR forms dimers or oligomers on the cell surface in the absence of ligand stimulation.
Our work also indicates that EGFR dimer formation is not sufficient for receptor activation and downstream signaling. The amount of cross-linked EGFR dimers on BE cells was almost the same in EGF-treated or -untreated cells. However, phosphorylation assays of EGFR and its downstream molecules Cbl and MAPK indicate that EGF-untreated dimers are not activated at all. Although weak phosphorylation of Cbl and EGF-untreated EGFR monomers was seen in some cases, it was basal-level phosphorylation probably caused by the insufficient serum starvation. In addition, the bivalent anti-EGFR mAb used here can induce dimer formation without EGFR activation. In contrast to the ligand-independent dimerization state, binding of EGFR ligands, such as EGF and HB-EGF, to EGFRs results in strong activation of the receptor. These results clearly show that dimer formation is not enough for receptor activation. A similar phenomenon has also been demonstrated in EpoR and other receptor systems (Burke et al., 1997; Constantinescu et al., 2001). Therefore, the present conclusion that dimerization is not sufficient for receptor activation should be applicable to other receptor systems (Jiang and Hunter, 1999).
In this study, we used two kinds of recombinant HB-EGF. HB1, which contains the EGF-like domain of human HB-EGF, showed mitogenic activity similar to that of EGF. HB2, which covers the EGF-like domain of human HB-EGF but has three basic amino acids, which are thought to contribute in part to the heparin-binding property of HB-EGF, was replaced with the corresponding amino acids from EGF. The initial purpose of making these substitutions was to inactivate the heparin-binding region of HB-EGF and to examine instead its diphtheria toxin-binding activity, because interaction of the heparin-binding domain with heparin or heparin-like molecules is important for diphtheria toxin binding (Shishido et al., 1995). However, we noticed in this study that HB2 differed from HB1 in its mitogenic activity; EGF and HB1 showed similar mitogenic activity in BE, BE2, and B108 cells, whereas HB2 was quite a weak mitogen for B108 cells compared with EGF and HB1. Cross-linking experiments indicated that HB2 is defective in its induction of EGFR-EpoR chimeric receptor dimer formation and therefore fails to activate MAPK and STAT5; in contrast, EGF and HB1 are able to induce chimeric receptor dimer formation under the same conditions. Although HB2 is defective in dimer formation, it seems to retain its ability to induce activation and phosphorylation of preformed EGFR dimers. The finding that pretreatment of B108 cells with a bivalent, but not monovalent, anti-EGFR mAb increases the mitogenic activity of HB2 indicates that the reduced mitogenic activity of HB2 on B108 cells is a result of its deficiency in inducing dimer formation. These results suggest that native EGFR ligands, including EGF and HB-EGF, have both receptor dimerization and receptor activation activities, whereas HB2 has little or no capacity to induce receptor dimerization.
What are the physiological implications of preformed EGFR dimers? As mentioned above, native EGFR ligands are generally able to induce EGFR dimer formation. However, without predimers, dimerization would take longer, because receptor molecules need to move around the cell surface looking for their partner molecules. We have recently observed that EGFR induced tyrosine phosphorylation within 1 minute after the addition of EGF, whereas the EGFR-EpoR chimeric receptor required much longer to induce significant tyrosine phosphorylation (Sako et al., unpublished observations). Therefore, it is likely that predimers are responsible for the accelerated signal transduction of EGFR. The amounts of EGFR on the in vivo cell surfaces are much smaller than those of the cell lines we used in experiments, so ligand-induced dimer formation would not be efficient. Preformed dimers may also circumvent such disadvantages as the low surface EGFR concentration. As shown in Figure 3, BE2 cells, which express much smaller amounts of EGFR than BE cells, respond to EGF in the same way as BE cells. It has been reported that in tissue cells, EGFR can still respond to EGF stimulation even when receptor numbers go down to 2000 per cell (Carpenter, 1987), perhaps suggesting the existence of ligand-independent dimers in vivo. Although cross-linking studies would be ineffective for detecting preformed dimers in cells expressing such low amounts of EGFR, HB2 may be a useful tool to monitor the dimerization states of EGFR.
The present study also provides information regarding the domain of EGFR required for predimer formation. The EGFR-EpoR chimeric receptor does not form a predimer. This chimeric molecule possesses the extracellular and transmembrane domains of EGFR, but the cytoplasmic domain of EGFR is substituted with the cytoplasmic domain of EpoR. Thus, the results indicate that the cytoplasmic domain of EGFR is necessary for ligand-independent dimer formation. The present results are supported by an earlier report that only the extracellular domain of EGFR does not form a dimer without a ligand (Lax et al., 1991). The extracellular domain of EGFR has a role in the prevention of EGFR autoactivation (Adelsman et al., 1996), as shown in the platelet-derived growth factor receptor (Uren et al., 1997). Further studies on truncated EGFR mutants indicate that the region from 835Ala and 918Asp of EGFR is required for EGFR predimer formation. This region is included in the kinase domain of EGFR, located close to the ATP binding site, and may regulate the kinase domain orientation (Groenen et al., 1997). Thus, it suggests the intimate relationship between EGFR predimer formation and its activation. The dual function of this region may also support the “twist model” of EGFR activation, i.e., EGF induces rotation of each molecule of the EGFR predimer, rather than one of simply dimerization (Gadella and Jovin, 1995; Burke and Stern, 1998; Bell et al., 2000).
The mechanism by which dimers form in a ligand-independent manner remains to be elucidated. It would be possible that another molecule is involved in ligand-independent predimer formation. Although there is no direct evidence identifying such a molecule, proteins such as ZPR1 (Galcheva-Gargova et al., 1996), which has two identical domains that bind to the cytoplasmic domain of EGFR, may contribute to predimer formation.
ACKNOWLEDGMENTS
We are grateful to A. Yoshimura for critical discussion and for providing the plasmid encoding the EGFR-EpoR chimeric receptor. E.M. was supported in part by a grant from the Research for the Future Program of the Japan Society for the Promotion of Science (Project No. 97L00303).
Abbreviations used:
- EGF
epidermal growth factor
- EGFR
EGF receptor
- FCS
fetal calf serum
- HB-EGF
heparin-binding EGF-like growth factor
- mAb
monoclonal antibody
- proHB-EGF
membrane-anchored form of HB-EGF
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.01–08–0411. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.01–08–0411.
REFERENCES
- Adelsman MA, Huntley BK, Maihle NJ. Ligand-independent dimerization of oncogenic v-erbB products involves covalent interactions. J Virol. 1996;70:2533–2544. doi: 10.1128/jvi.70.4.2533-2544.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell CA, Tynan JA, Hart KC, Meyer AN, Robertson SC, Donoghue DJ. Rotational coupling of transmembrane and kinase domains of the neu receptor tyrosine kinase. Mol Biol Cell. 2000;11:3589–3599. doi: 10.1091/mbc.11.10.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boni-Schnetzler M, Pilch PF. Mechanism of epidermal growth factor receptor autophosphorylation and high-affinity binding. Proc Natl Acad Sci USA. 1987;84:7832–7836. doi: 10.1073/pnas.84.22.7832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke CL, Lemmon MA, Coren BA, Engelman DM, Stern DF. Dimerization of the p185neu transmembrane domain is necessary but not sufficient for transformation. Oncogene. 1997;14:687–696. doi: 10.1038/sj.onc.1200873. [DOI] [PubMed] [Google Scholar]
- Burke CL, Stern DF. Activation of neu (ErbB-2) mediated by disulfide bond-induced dimerization reveals a receptor tyrosine kinase dimer interface. Mol Cell Biol. 1998;18:5371–5379. doi: 10.1128/mcb.18.9.5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canal F. Signal transmission by epidermal growth factor receptor: coincidence of activation and dimerization. Biochemistry. 1992;31:4493–4501. doi: 10.1021/bi00133a016. [DOI] [PubMed] [Google Scholar]
- Carpenter G. Receptors for epidermal growth factor and other polypeptide mitogens. Annu Rev Biochem. 1987;56:881–914. doi: 10.1146/annurev.bi.56.070187.004313. [DOI] [PubMed] [Google Scholar]
- Cochet C, Kashles O, Chambaz EM, Borrello I, King CR, Schlessinger J. Demonstration of epidermal growth factor-induced receptor dimerization in living cells using a chemical covalent cross-linking agent. J Biol Chem. 1988;263:3290–3295. [PubMed] [Google Scholar]
- Constantinescu SN, Keren T, Socolovsky M, Nam H, Henis YI, Lodish HF. Ligand-independent oligomerization of cell-surface erythropoietin receptor is mediated by the transmembrane domain. Proc Natl Acad Sci USA. 2001;98:3479–3484. doi: 10.1073/pnas.081069198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadella TW, Jr, Jovin TM. Oligomerization of epidermal growth factor receptors on A431 cells studied by time-resolved fluorescence imaging microscopy: a stereochemical model for tyrosine kinase receptor activation. J Cell Biol. 1995;129:1543–1558. doi: 10.1083/jcb.129.6.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galcheva-Gargova Z, Konstantinov KN, Wu I, Klier FG, Barrett T, Davis RJ. Binding of zinc finger protein ZPR1 to the epidermal growth factor receptor. Science. 1996;272:1797–1802. doi: 10.1126/science.272.5269.1797. [DOI] [PubMed] [Google Scholar]
- Gotoh N, Tojo A, Hino M, Yazaki Y, Shibuya M. A highly conserved tyrosine residue at codon 845 within the kinase domain is not required for the transforming activity of human epidermal growth factor receptor. Biochem Biophys Res Commun. 1992;186:768–774. doi: 10.1016/0006-291x(92)90812-y. [DOI] [PubMed] [Google Scholar]
- Greenfield C, Hiles I, Waterfield MD, Federwisch M, Wollmer A, Blundell TL, McDonald N. Epidermal growth factor binding induces a conformational change in the external domain of its receptor. EMBO J. 1989;8:4115–4123. doi: 10.1002/j.1460-2075.1989.tb08596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenen LC, Walker F, Burgess AW, Treutlein HR. A model for the activation of the epidermal growth factor receptor kinase: involvement of an asymmetric dimer? Biochemistry. 1997;36:3826–3836. doi: 10.1021/bi9614141. [DOI] [PubMed] [Google Scholar]
- Higashiyama S, Abraham JA, Miller J, Fiddes JC, Klagsbrun M. A heparin-binding growth factor secreted by macrophage-like cells that is related to EGF. Science. 1991;251:936–939. doi: 10.1126/science.1840698. [DOI] [PubMed] [Google Scholar]
- Iwamoto R, Handa K, Mekada E. Contact-dependent growth inhibition and apoptosis of epidermal growth factor (EGF) receptor-expressing cells by the membrane-anchored form of heparin-binding EGF-like growth factor. J Biol Chem. 1999;274:25906–25912. doi: 10.1074/jbc.274.36.25906. [DOI] [PubMed] [Google Scholar]
- Iwamoto R, Higashiyama S, Mitamura T, Taniguchi N, Klagsbrun M, Mekada E. Heparin-binding EGF-like growth factor, which acts as the diphtheria toxin receptor, forms a complex with membrane protein DRAP27/CD9, which up-regulates functional receptors and diphtheria toxin sensitivity. EMBO J. 1994;13:2322–2330. doi: 10.1002/j.1460-2075.1994.tb06516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwatsuki K, Endo T, Misawa H, Yokouchi M, Matsumoto A, Ohtsubo M, Mori KJ, Yoshimura A. STAT5 activation correlates with erythropoietin receptor-mediated erythroid differentiation of an erythroleukemia cell line. J Biol Chem. 1997;272:8149–8152. doi: 10.1074/jbc.272.13.8149. [DOI] [PubMed] [Google Scholar]
- Jiang G, Hunter T. Receptor signaling: when dimerization is not enough. Curr Biol. 1999;9:R568–R571. doi: 10.1016/s0960-9822(99)80357-1. [DOI] [PubMed] [Google Scholar]
- Lax I, Bellot F, Howk R, Ullrich A, Givol D, Schlessinger J. Functional analysis of the ligand binding site of EGF-receptor utilizing chimeric chicken/human receptor molecules. EMBO J. 1989;8:421–427. doi: 10.1002/j.1460-2075.1989.tb03393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lax I, Burgess WH, Bellot F, Ullrich A, Schlessinger J, Givol D. Localization of a major receptor-binding domain for epidermal growth factor by affinity labeling. Mol Cell Biol. 1988;8:1831–1834. doi: 10.1128/mcb.8.4.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lax I, Mitra AK, Ravera C, Hurwitz DR, Rubinstein M, Ullrich A, Stroud RM, Schlessinger J. Epidermal growth factor (EGF) induces oligomerization of soluble, extracellular, ligand-binding domain of EGF receptor. J Biol Chem. 1991;266:13828–13833. [PubMed] [Google Scholar]
- Lemmon MA, Bu Z, Ladbury JE, Zhou M, Pinchasi D, Lax I, Engelman D, Schlessinger J. Two EGF molecules contribute additively to stabilization of EGFR dimer. EMBO J. 1997;16:281–294. doi: 10.1093/emboj/16.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquart HM, Hunkapiller W, Hood LE, Todaro GJ. Rat transforming growth factor type 1: structure and relation to epidermal growth factor. Science. 1984;223:1079–1082. doi: 10.1126/science.6320373. [DOI] [PubMed] [Google Scholar]
- Mitamura T, Higashiyama S, Taniguchi N, Klagsbrun M, Mekada E. Diphtheria toxin binds to the epidermal growth factor (EGF)-like domain of human heparin-binding EGF-like growth factor/diphtheria toxin receptor and inhibits specially its mitogenic activity. J Biol Chem. 1995;270:1015–1019. doi: 10.1074/jbc.270.3.1015. [DOI] [PubMed] [Google Scholar]
- Moriki T, Maruyama H, Maruyama IN. Activation of preformed EGF receptor dimers by ligand-induced rotation of the transmembrane domain. J Mol Biol. 2001;311:1011–1026. doi: 10.1006/jmbi.2001.4923. [DOI] [PubMed] [Google Scholar]
- Ohashi H, Maruyama K, Lui Y-C, Yoshimura A. Ligand-induced activation of chimeric receptors between the erythropoietin receptor and receptor tyrosine kinases. Proc Natl Acad Sci USA. 1994;91:158–162. doi: 10.1073/pnas.91.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riese DJ, II, Van Raaij TM, Plowman GD, Andrews GC, Stern DF. The cellular response to neuregulins is governed by complex interactions of the erbB family. Mol Cell Biol. 1995;15:5770–5776. doi: 10.1128/mcb.15.10.5770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sako Y, Minoguchi S, Yanagida T. Single-molecule imaging of EGFR signaling on the surface of living cells. Nat Cell Biol. 2000;2:168–172. doi: 10.1038/35004044. [DOI] [PubMed] [Google Scholar]
- Shing Y, Christofori G, Hanahan D, Ono Y, Sasada R, Igarashi K, Folkman J. Betacellulin: a mitogen from pancreatic beta cell tumors. Science. 1993;259:1604–1607. doi: 10.1126/science.8456283. [DOI] [PubMed] [Google Scholar]
- Shishido Y, Sharma KD, Higashiyama S, Klagsbrun M, Mekada E. Heparin-like molecules on the cell surface potentiate binding of diphtheria toxin to the diphtheria toxin receptor/membrane-anchored heparin-binding epidermal growth factor-like growth factor. J Biol Chem. 1995;270:29578–29585. doi: 10.1074/jbc.270.49.29578. [DOI] [PubMed] [Google Scholar]
- Shoyab MG, Plowman G, McDonald VL, Bradley JG, Todaro GJ. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1989;243:1074–1076. doi: 10.1126/science.2466334. [DOI] [PubMed] [Google Scholar]
- Tanner KG, Kyte J. Dimerization of the extracellular domain of the receptor for epidermal growth factor containing the membrane-spanning segment in response to treatment with epidermal growth factor. J Biol Chem. 1999;274:35985–35990. doi: 10.1074/jbc.274.50.35985. [DOI] [PubMed] [Google Scholar]
- Toyoda H, Komuasaki T, Uchida D, Takayama Y, Isobe T, Okuyama T, Hanada K. Epiregulin, a novel epidermal growth factor with mitogenic activity for rat primary hepatocytes. J Biol Chem. 1995;270:7495–7500. doi: 10.1074/jbc.270.13.7495. [DOI] [PubMed] [Google Scholar]
- Tzahar E, Yarden Y. The ErbB-2/HER2 oncogenic receptor of adenocarcinomas: from orphanhood to multiple stromal ligands. Biochim Biophys Acta. 1998;1377:M25–M37. doi: 10.1016/s0304-419x(97)00032-2. [DOI] [PubMed] [Google Scholar]
- Uren A, Yu JC, Karcaaltincaba M, Pierce JH, Heidaran MA. Oncogenic activation of the aPDGFR defines a domain that negatively regulates receptor dimerization. Oncogene. 1997;14:157–162. doi: 10.1038/sj.onc.1200810. [DOI] [PubMed] [Google Scholar]
- Van de Vijver MJ, Kumar R, Mendelsohn J. Ligand-induced activation of A431 cell epidermal growth factor receptors occurs primarily by an autocrine pathway that acts upon receptors on the surface rather than intracellularly. J Biol Chem. 1991;266:7503–7508. [PubMed] [Google Scholar]
- Waterfield MD, Mayes ELV, Stroobant P, Bennet PLP, Young S, Goodfellow PN, Banting GS, Ozanne B. A monoclonal antibody to the human epidermal growth factor receptor. J Cell Biochem. 1982;20:149–161. doi: 10.1002/jcb.240200207. [DOI] [PubMed] [Google Scholar]
- Yarden Y, Schlessinger J. Self-phosphorylation of epidermal growth factor receptor: evidence for a model of intermolecular allosteric activation. Biochemistry. 1987a;26:1434–1442. doi: 10.1021/bi00379a034. [DOI] [PubMed] [Google Scholar]
- Yarden Y, Schlessinger J. Epidermal growth factor induces rapid, reversible aggregation of the purified epidermal growth factor receptor. Biochemistry. 1987b;26:1443–1451. doi: 10.1021/bi00379a035. [DOI] [PubMed] [Google Scholar]