Abstract
Purpose
Montelukast is used extensively in children and adolescents for allergic rhinitis and asthma. However, concerns have been raised regarding the increased risk of neuropsychiatric adverse events (NPAEs) associated with montelukast use. Therefore, our case-crossover study was conducted to observe whether there is an increased risk of NPAEs associated with montelukast use in children and adolescents.
Materials and methods
A population-based case-crossover study using the customised Health Insurance Review and Assessment (HIRA) dataset was conducted. Paediatric patients aged between 0 and 19 years diagnosed with allergic rhinitis and/or asthma with a history of at least one montelukast prescription between 1 January 2018 and 31 December 2021 were included. Exposure to montelukast was assessed during 3-, 7-, 14-, 28- and 56-day hazard periods prior to each patient’s NPAE. Stratified analyses according to age group, gender and season for the risk of NPAEs associated with montelukast use in the previous 7 days and 14 days were performed, respectively. Conditional logistic regression analysis was used to calculate adjusted ORs (aORs) with their corresponding 95% CIs, adjusting for concomitant medications.
Results
A total of 161 386 paediatric patients was identified. An increased risk of NPAEs associated with montelukast was found in all time window periods, including 3-day (aOR 1.28, 95% CI 1.24 to 1.32), 7-day (aOR 1.29, 95% CI 1.26 to 1.33), 14-day (aOR 1.34, 95% CI 1.31 to 1.37), 28-day (aOR 1.38, 95% CI 1.36 to 1.41) and 56-day (aOR 1.21, 95% CI 1.19 to 1.22) preceding hazard periods compared with use in the four control periods.
Conclusion
Children and adolescents with allergic rhinitis and/or asthma should be prescribed montelukast with caution considering clinical benefits.
Keywords: pharmacology, adolescent health, child psychiatry, infant
WHAT IS ALREADY KNOWN ON THIS TOPIC.
WHAT THIS STUDY ADDS
A case-crossover study was conducted to see if there was an increased risk of NPAEs associated with montelukast use in children and adolescents with allergic rhinitis and/or asthma. We observed an increased risk of NPAEs associated with montelukast use. Our findings highlight the need for careful prescribing of montelukast in children and adolescents.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
In the practice aspect, our study conveys an important message that more caution is needed when prescribing montelukast in children and adolescents. In terms of the research aspect, our study conducted the case-crossover study that examined the association between montelukast use and the risk of NPAEs in the paediatric population using the large, representative and most recent claims database. Our study provides the important evidence to establish a healthcare policy to reduce the risk of NPAEs, especially in children and adolescents, and to develop or improve future treatment options to avoid potential risks.
Introduction
Montelukast, a leukotriene receptor antagonist (LTRA), is widely prescribed for allergic rhinitis and asthma in children and adolescents.1 It is used as a preventative measure for patients with allergic rhinitis and asthma, and as an additional treatment for those whose asthma symptoms are uncontrolled by inhaled corticosteroids.1 2 Montelukast has been used extensively in children and adolescents because it can be easily administered orally. However, postmarketing case reports showed a possible risk of neuropsychiatric adverse events (NPAEs) associated with montelukast use.1 3 Therefore, in 2008, a warning about NPAEs was included under the ‘Precautions Section’ of the montelukast label as advised by the US Food and Drug Administration (FDA).3 The reported NPAEs were agitation, aggression, anxiety, depression, insomnia, irritability, suicidal ideation/attempts and suicide.1 3 The FDA further announced the strengthening of an existing boxed warning of montelukast in 2020.4 A possible explanation for montelukast-associated NPAEs is a montelukast-glutathione (GSH) conjugate leading to the possible progression of neuropsychiatric disorders such as depression, anxiety and stress.5
An increased risk of NPAEs associated with montelukast was not found in other observational studies, according to the FDA.4 However, these studies had shortcomings.4 There were possibilities of imprecise defining of exposures, outcomes, inclusion and exclusion criteria, and covariates, in which the Sentinel study recommended a careful interpretation of data.4 In addition, the data used in the study were from 2000 to 2015. To reflect on the current situation more effectively, the use of the most recent data available is needed. Other studies have shown a possible risk of NPAEs in association with montelukast. The overall risk of NPAEs did not increase in patients using LTRAs; an increase was noticed in a 4-day to 14-day window of initiation of LTRAs from a self-controlled case series (SCCS) study.1A nested case-control study showed children with asthma who experienced an NPAE had nearly twice the odds of having been prescribed montelukast.3 The association between the risk of NPAEs and montelukast use remains unclear.
This study was conducted to observe whether there is an increased risk of NPAEs associated with montelukast use in children and adolescents using a case-crossover study design that allows study subjects to act as their own controls, minimising intersubject variability.
Materials and methods
Data source
The customised claims data of the Health Insurance Review and Assessment Service (HIRA) in South Korea were used for the study. The claims data of HIRA cover 50 million patients per year, which account for 98% of the total population in Korea.6 The database contains comprehensive information regarding relevant healthcare services, including procedures, surgeries, examinations, treatments, prescriptions and sociodemographic characteristics of patients.6 7 In this study, the customised claims dataset of paediatric patients aged between 0 and 19 years with at least one diagnosis of allergic rhinitis or asthma (10th revision of the International Classification of Diseases (ICD-10) codes J30, J45 and J46) and a history of at least one montelukast prescription between 1 January 2018 and 31 December 2021 was obtained.
Case-crossover design
A case-crossover design was implemented to see the association between montelukast exposure and the risk of NPAEs, in which the study design allows study subjects to act as their own controls at previous time points.8 9 The study design is appropriate to use when observing potential causes of sudden events when the exposure is intermittent, the effect on risk is transient and the outcome is abrupt.8 9 The association between exposure times and outcome times within individuals can be observed.8 Confounding by time-invariant subject characteristics such as sex, socioeconomic status, genetics and underlying state of health that were difficult to measure can be minimised as study participants serve as their own controls.1 9 Therefore, the number of patients exposed to montelukast in the hazard period, which is the period immediately before the outcome of interest, can be compared with the number of those exposed to montelukast in control periods, which are periods prior to and of the same length as the hazard period.9 The Odds Ratio (OR) is calculated by comparing the odds of exposure between hazard and control periods.
Case definition
Children and adolescents aged between 0 and 19 years who had been prescribed montelukast at least once along with the primary or secondary NPAE diagnosis between 1 January 2018 and 31 December 2021 were identified. It is often more difficult to diagnose psychiatric conditions in infants and toddlers compared with children and adolescents; however, removing infants and toddlers who experienced NPAEs and were exposed to montelukast from the study could possibly introduce a potential bias.10 Therefore, our study decided to include all paediatric patients. Patients who were not diagnosed with NPAEs in 2017, a year before the initiation of the study period, were included in the study to assure incident cases of NPAEs and to account for both chronic and acute psychiatric conditions.
Cases were defined as paediatric patients with any NPAE primary or secondary diagnosis during hospitalisations or outpatient visits, including anxiety disorders, cognitive disorders, mood disorders, movement disorders, personality disorders, psychotic disorders, sleep-related disorders, self-harm and others. The NPAEs were defined based on previous studies, and ICD-10 codes were selected according to NPAE diagnoses1 9 11 (online supplemental table S1).
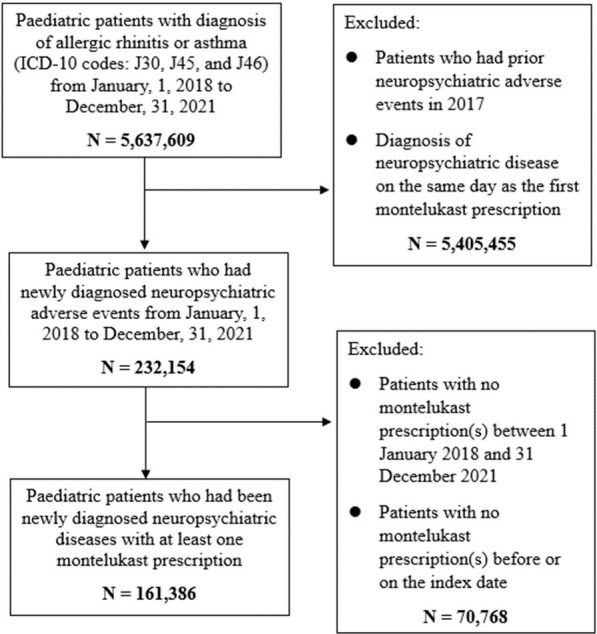
The exclusion criteria were as follows: (1) previous history of NPAE(s) in 2017 to assure incident cases of NPAE(s); (2) diagnosis of NPAE(s) on the same day as the first montelukast prescription since the time association cannot be confirmed in the claims database; (3) no history of montelukast prescription(s) between 1 January 2018 and 31 December 2021 and (4) no montelukast prescription(s) before or on the index date (figure 1).
Figure 1. Flow diagram of study subjects.
Exposure to montelukast
Exposure to montelukast was defined as a paediatric patient who had received at least one montelukast prescription (Anatomic Therapeutic Chemical code R03DC03). Paediatric patients who were given montelukast prescriptions from both inpatient and outpatient settings were included in the study (online supplemental table S2).
Different hazard time window periods were applied; 3-day, 7-day, 14-day, 28-day and 56-day time periods were chosen. The 7-day and 14-day time frames were selected as previous studies, which assessed the association between the risk of NPAEs and montelukast, reported the average onset of NPAEs after the montelukast exposure occurred between 7 and 14 days.1 12 A 3-day interval was also analysed to see if there was an abrupt onset of NPAEs. Additionally, 28-day and 56-day hazard periods were examined to see the change in the NPAE risk with a longer follow-up duration.
To see if there was a change in the risk among different age groups, genders and seasons, stratified analyses were performed. NPAEs associated with montelukast use usually occurred between 7 and 14 days after montelukast exposure; therefore, stratified analyses were conducted when montelukast use was within 7 days and 14 days of the hazard period, respectively.1 12
Four consecutive control periods were set with the same duration as the hazard period for each outcome. A washout period of 5 days between hazard and control periods was established to avert any carry-over effect (figure 2). The mean plasma half-life of montelukast ranged from 2.7 to 5.5 hours in healthy young adults.13 Therefore, it is anticipated that montelukast will be in the system for approximately 30.3 hours.13 Considering study subjects were paediatric patients, in whom the elimination rate could be different from that of adults, we decided setting a washout period of 5 days was plausible.14
Figure 2. Case-crossover study design of montelukast and NPAEs. The hazard period was defined as the 3-, 7-, 14-, 28- and 56-day time window before the first diagnosis of an NPAE. The washout period of 5 days was set between the hazard and the control periods. Four consecutive control periods were chosen. NPAE, neuropsychiatric adverse events.

Time-varying confounders
A case-crossover study design itself is controlled for time-invariant confounders including sex, genetic factors, socioeconomic status and underlying state of health.1 9 However, concomitant medications that study subjects take may change across hazard and control periods. Therefore, concomitant medications were measured as within-patient time-varying confounders and were adjusted in the analysis. Antidepressants, antiepileptics, antidepressants, antihistamines, antipsychotics, benzodiazepines, corticosteroids, histamine H2-receptor antagonists, neuraminidase inhibitors, non-steroidal anti-inflammatory drugs (NSAIDs), opioids and psychostimulants (methylphenidate and atomoxetine) were determined as medications associated with NPAEs based on previous studies1 9 11 (online supplemental table S3).
Statistical analyses
Baseline characteristics of the study subjects were presented by using descriptive statistics. Categorical variables were presented as numbers with percentages. The conditional logistic regression analysis was conducted to calculate crude and adjusted ORs (aORs) with their corresponding 95% CIs. The odds of montelukast exposure between the hazard and four control periods before the NPAEs were compared at different hazard time windows.
Subgroup analyses according to age groups, genders and seasons were conducted when the montelukast use was within the previous 7 days and 14 days of the hazard period. These subgroup analyses were performed to see if there was a difference in results in terms of different age groups, genders and seasons, as other previous studies found the differences.1 10 15 The age groups were divided into 0–2 years, 3–5 years, 6–9 years, 10–12 years and 13–19 years to observe the possible risk in infants, toddlers, children and adolescents distinctively. The aORs were estimated by adjusting concomitant medications. The joint test for exposure and strata variable interaction was also conducted to see whether montelukast affects NPAEs differently depending on age groups, genders and seasons.
To observe the risk of NPAEs associated with the use of montelukast over the preceding 7 days of the hazard period using three additional control periods, which were 3, 6 and 9 months before the index date, an additional analysis was performed. This was conducted to see if our study appropriately controlled for seasonality. The first control period used in each analysis started from 3, 6 and 9 months before the index date correspondingly and included the preceding 7 days.
Additional sensitivity analyses were performed for pranlukast, another LTRA, to see if pranlukast was associated with the increased risk of NPAEs at different hazard time periods (3, 7, 14, 28 and 56 days). Subgroup analyses were also conducted according to age groups, genders and seasons when the pranlukast exposure was within previous 7 days of the hazard period. In both analyses, a conditional logistic regression analysis was used, and the joint test for exposure and strata variable interaction was also performed (tables 6 and 7).
All analyses were performed using SAS V.9.4 (SAS Institute) and a p value of <0.05 was considered statistically significant in this study.
Patient and public involvement
The patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Results
In total, 161 386 children and adolescents were dispensed montelukast prescriptions and diagnosed with NPAEs between 1 January 2018 and 31 December 2021 (figure 1). The baseline characteristics of children and adolescents with montelukast use preceding NPAEs are shown in table 1. 58.3% of study patients were male, and the age distribution was as follows: 3.30% aged 0–2 years, 13.2% aged 3–5 years, 30.4% aged 6–9 years, 13% aged 10–12 years and 40.1% aged 13–19 years. In terms of season, the greatest number of patients were diagnosed in autumn (29.2%) followed by summer, spring and winter. Concomitant medications during the study period were observed in the following proportions: antihistamines (72.6%), NSAIDs (55.5%), corticosteroids (47.5%) and histamine H2-receptor antagonists (19%). NPAEs diagnosed during the study period were identified in the following order: anxiety disorders (29.7%), mood disorders (28.6%), personality disorders (22.9%), sleep-related disorders (6.60%) and others (6.30%) (table 1).
Table 1. Baseline characteristics.
| Variables | Patients (n=161 386)n (%) | |
| Age group (years) | ||
| 0–2 | 5332 | (3.30) |
| 3–5 | 21 312 | (13.21) |
| 6–9 | 49 079 | (30.41) |
| 10–12 | 21 026 | (13.03) |
| 13–19 | 64 637 | (40.05) |
| Gender | ||
| Male | 94 049 | (58.28) |
| Female | 67 337 | (41.72) |
| Season | ||
| Spring | 35 912 | (22.25) |
| Summer | 43 872 | (27.18) |
| Autumn | 47 157 | (29.22) |
| Winter | 34 445 | (21.34) |
| Concomitant medications* | ||
| Antihistamines | 117 113 | (72.57) |
| Non-steroidal anti-inflammatory drugs | 89 518 | (55.47) |
| Corticosteroids | 76 684 | (47.52) |
| Histamine H2-receptor antagonists | 30 673 | (19.01) |
| Opioids | 18 773 | (11.63) |
| Benzodiazepines | 14 317 | (8.87) |
| Antipsychotics | 7865 | (4.87) |
| Psychostimulants | 7497 | (4.65) |
| Neuraminidase inhibitors | 6663 | (4.13) |
| Antiepileptics | 2732 | (1.69) |
| Antidepressants | 2222 | (1.38) |
| Neuropsychiatric adverse events | ||
| Anxiety disorder | 47 911 | (29.69) |
| Mood disorder | 46 114 | (28.57) |
| Personality disorder | 36 899 | (22.86) |
| Sleep-related disorder | 10 616 | (6.58) |
| Others | 10 194 | (6.32) |
| Movement disorder | 7076 | (4.38) |
| Psychotic disorder | 1469 | (0.91) |
| Cognitive disorder | 1087 | (0.67) |
| Self-harm | 20 | (0.01) |
Overalpped.
The use of montelukast in the previous 3 days was associated with a 1.28-fold higher risk of NPAEs after the adjustment for concomitant medications (95% CI 1.24 to 1.32; p≤0.0001). The risk remained elevated in 7 days (aOR 1.29; 95% CI 1.26 to 1.33; p≤0.0001), in 14 days (aOR 1.34; 95% CI 1.31 to 1.37; p≤0.0001), in 28 days (aOR 1.38; 95% CI 1.36 to 1.41; p≤0.0001) and 1.21-fold higher in the 56-day time window (95% CI 1.19 to 1.22; p<0.0001) (table 2).
Table 2. Risk of neuropsychiatric adverse events associated with the use of montelukast at different time window periods (3, 7, 14, 28 and 56 days).
| Time window period (days) | No. of exposed in hazard period (n=161 386)N (%) | No. of exposed in control period (n=645 544)N (%) | Crude | Adjusted | ||||||
| OR (95% CI)* | P value | OR (95% CI)† | P value | |||||||
| 3 | 9773 | (6.06) | 38 276 | (5.93) | 1.04 | (1.01 to 1.08) | 0.0090 | 1.28 | (1.24 to 1.32) | <0.0001 |
| 7 | 12 673 | (7.85) | 48 882 | (7.57) | 1.07 | (1.04 to 1.09) | <0.0001 | 1.29 | (1.26 to 1.33) | <0.0001 |
| 14 | 17 080 | (10.58) | 63 578 | (9.85) | 1.13 | (1.10 to 1.15) | <0.0001 | 1.34 | (1.31 to 1.37) | <0.0001 |
| 28 | 24 568 | (15.22) | 87 991 | (13.63) | 1.20 | (1.18 to 1.22) | <0.0001 | 1.38 | (1.36 to 1.41) | <0.0001 |
| 56 | 35 969 | (22.29) | 135 123 | (20.93) | 1.11 | (1.10 to 1.13) | <0.0001 | 1.21 | (1.19 to 1.22) | <0.0001 |
Calculated by conditional logistic regression.
Calculated by conditional logistic regression adjusted for concomitant medications.
The highest NPAE risk associated with the use of montelukast in the previous 7 days was observed among adolescents aged 13–19 years, with an aOR of 1.44 (95% CI 1.37 to 1.52). The risk was also noted in other age groups except for the risk in children aged 0–2 years, in which the risk was statistically not significant. The risk of NPAEs was higher in female children and adolescents (aOR 1.35; 95% CI 1.29 to 1.41). However, the difference was not significant (p value for interaction=0.1195) (table 3).
Table 3. Risk of NPAEs associated with the use of montelukast in the previous 7 days, stratified analysis according to age group, gender and season.
| Variables | Exposed in hazard period | Exposed in control period | Crude | Adjusted | P value for interaction* | ||||
| N/N | (%) | N/N | (%) | OR (95% CI)† | OR (95% CI)‡ | ||||
| Total | 12 673/161 386 | (7.85) | 48 882/645 544 | (0.08) | 1.07 | (1.04 to 1.09) | 1.23 | (1.20 to 1.27) | |
| Age group (years) | 0.0063 | ||||||||
| 0–2 | 864/5332 | (16.20) | 3518/21 328 | (16.49) | 0.97 | (0.88 to 1.07) | 1.10 | (0.99 to 1.22) | |
| 3–5 | 2530/21 312 | (11.87) | 10 199/85 248 | (11.96) | 0.99 | (0.93 to 1.05) | 1.14 | (1.08 to 1.22) | |
| 6–9 | 4648/49 079 | (9.47) | 17 996/196 316 | (9.17) | 1.06 | (1.02 to 1.11) | 1.23 | (1.18 to 1.29) | |
| 10–12 | 1413/21 026 | (6.72) | 5450/84 104 | (6.48) | 1.07 | (0.99 to 1.15) | 1.36 | (1.25 to 1.47) | |
| 13–19 | 3218/64 637 | (4.98) | 11 719/258 548 | (4.53) | 1.16 | (1.11 to 1.22) | 1.44 | (1.37 to 1.52) | |
| Gender | 0.1195 | ||||||||
| Male | 8137/94 049 | (8.65) | 31 812/376 196 | (8.46) | 1.04 | (1.01 to 1.08) | 1.26 | (1.22 to 1.30) | |
| Female | 4536/67 337 | (6.74) | 17 070/269 348 | (6.34) | 1.11 | (1.06 to 1.16) | 1.35 | (1.29 to 1.41) | |
| Season | <0.0001 | ||||||||
| Spring | 2973/35 912 | (8.28) | 10 680/143 648 | (7.43) | 1.21 | (1.15 to 1.28) | 1.40 | (1.32 to 1.48) | |
| Summer | 2344/43 872 | (5.34) | 10 841/175 488 | (6.18) | 0.78 | (0.73 to 0.82) | 0.97 | (0.91 to 1.03) | |
| Autumn | 4259/47 157 | (9.03) | 14 048/188 628 | (7.45) | 1.40 | (1.34 to 1.47) | 1.71 | (1.63 to 1.79) | |
| Winter | 3097/34 445 | (8.99) | 13 313/137 780 | (9.66) | 0.88 | (0.84 to 0.93) | 1.08 | (1.02 to 1.14) | |
Joint test for exposure and strata variable interaction, indicating whether montelukast affects NPAEs differently depending on age group, gender, and season.
Calculated by conditional logistic regression.
Calculated by conditional logistic regression adjusted for concomitant medication.
NPAEsneuropsychiatric adverse events
The highest NPAE risk associated with montelukast in the previous 14 days was observed among adolescents aged 13–19 years, with an aOR of 1.43 (95% CI 1.37 to 1.50). The risk was found in all other age groups, in which the lowest risk was seen in paediatric patients aged 0–2 years (aOR 1.16; 95% CI 1.07 to 1.27). The difference in risk according to age groups was not significant. The risk was higher in females (aOR 1.36; 95% CI 1.31 to 1.41). However, the difference in risk according to gender was not significant (p value for interaction=0.8972) (table 4).
Table 4. Risk of NPAEs associated with the use of montelukast in the previous 14 days, stratified analysis according to age group, gender and season.
| Variables | Exposed in hazard period | Exposed in control period | Crude | Adjusted | P value for interaction* | ||||
| N/N | (%) | N/N | (%) | OR (95% CI)† | OR (95% CI)‡ | ||||
| Total | 17,080/161 386 | (10.58) | 63,578/645 544 | (9.985) | 1.13 | (1.10 to 1.15) | 1.28 | (1.25 to 1.31) | |
| Age group (years) | 0.1821 | ||||||||
| 0–2 | 1173/5332 | (22.00) | 4586/21 328 | (21.50) | 1.04 | (0.96 to 1.13) | 1.16 | (1.07 to 1.27) | |
| 3–5 | 3433/21 312 | (16.11) | 13 165/85 248 | (15.44) | 1.08 | (1.03 to 1.14) | 1.23 | (1.17 to 1.30) | |
| 6–9 | 6293/49 079 | (12.82) | 23 526/196 316 | (11.98) | 1.12 | (1.08 to 1.16) | 1.29 | (1.24 to 1.34) | |
| 10–12 | 1896/21 026 | (9.02) | 7103/84 104 | (8.45) | 1.11 | (1.04 to 1.19) | 1.37 | (1.28 to 1.47) | |
| 13–19 | 4285/64 637 | (6.63) | 15 198/258 548 | (5.88) | 1.19 | (1.15 to 1.24) | 1.43 | (1.37 to 1.50) | |
| Gender | 0.8972 | ||||||||
| Male | 10 965/94 049 | (11.66) | 41 073/376 196 | (10.92) | 1.12 | (1.09 to 1.15) | 1.32 | (1.28 to 1.36) | |
| Female | 6115/67 337 | (9.08) | 22 205/269 348 | (8.36) | 1.14 | (1.10 to 1.18) | 1.36 | (1.31 to 1.41) | |
| Season | <0.0001 | ||||||||
| Spring | 3989/35 912 | (11.11) | 13 309/143 648 | (9.27) | 1.35 | (1.29 to 1.42) | 1.53 | (1.46 to 1.60) | |
| Summer | 3231/43 872 | (7.36) | 15 729/175 488 | (8.96) | 0.73 | (0.69 to 0.76) | 0.89 | (0.85 to 0.93) | |
| Autumn | 5679/47 157 | (12.04) | 16 089/188 628 | (8.53) | 1.80 | (1.69 to 1.83) | 2.08 | (1.99 to 2.16) | |
| Winter | 4181/34 445 | (12.14) | 18 451/137 780 | (13.39) | 0.85 | (0.81 to 0.88) | 1.02 | (0.97 to 1.06) | |
Joint test for exposure and strata variable interaction, indicating whether montelukast affects NPAEs differently depending on age group, gender, and season.
Calculated by conditional logistic regression.
Calculated by conditional logistic regression adjusted for concomitant medication.
NPAEsneuropsychiatric adverse events
An increased risk of NPAEs associated with the use of montelukast over the preceding 7 days was identified using control periods 3, 6 and 9 months before the index date. The highest risk was found 3 months before the index date (aOR 1.66; 95% CI 1.62 to 1.71) (table 5).
Table 5. Risk of neuropsychiatric adverse events associated with the use of montelukast over the preceding 7 days using control periods (3, 6 and 9 months before the index date).
| First control period | Exposed in hazard period | Exposed in control period | Crude | P value | Adjusted | P value | ||||
| N/N | (%) | N/N | (%) | OR (95% CI)* | OR (95% CI)† | |||||
| 3 months before the index date | 12 673/161 386 | (7.85) | 43 917/484 158 | (9.07) | 1.26 | (1.23 to 1.30) | <0.0001 | 1.66 | (1.62 to 1.71) | <0.0001 |
| 6 months before the index date | 12 673/161 386 | (7.85) | 47 497/484 158 | (9.81) | 1.11 | (1.08 to 1.14) | <0.0001 | 1.46 | (1.42 to 1.49) | <0.0001 |
| 9 months before the index date | 12 673/161 386 | (7.85) | 47 079/484 158 | (9.72) | 1.12 | (1.10 to 1.15) | <0.0001 | 1.30 | (1.27 to 1.34) | <0.0001 |
Calculated by conditional logistic regression.
Calculated by conditional logistic regression adjusted for concomitant medications.
Discussion
Principal findings
Our study showed an increased risk of NPAEs associated with montelukast use in children and adolescents. An increased risk of NPAEs was noted in all time window periods (3, 7, 14, 28 and 56 days). The risk was higher when we adjusted for concomitant medications as time-varying confounders. A possible explanation for this finding is that for those who take concomitant medications, which could possibly contribute to an increased risk of NPAEs, an additional administration of montelukast could further increase the risk. According to our results, antihistamines were the most frequently prescribed concomitant medications (72.6%), followed by NSAIDs (55.5%) and corticosteroids (47.5%). Antihistamines and corticosteroids are classes of medications that are commonly prescribed for allergic rhinitis and asthma in conjunction with montelukast. A study by Bian et al found an increased risk of neuropsychiatric events related to montelukast, antihistamines and inhaled corticosteroids.12 34.1% of neuropsychiatric events were associated with antihistamines, and 11.1% of events were related to inhaled corticosteroids.12 The study advised more attention to be paid to specific patients treated with LTRAs and antihistamines.12 In addition, another study identified a strong association between the use of corticosteroids and the development of psychiatric and neurological side effects.16 NSAIDs, another widely prescribed medication among children and adolescents, were also known to be associated with an increased risk of psychiatric symptoms.17 Therefore, concomitant administration of the aforementioned medications along with montelukast could possibly further increase the risk of NPAEs.
Our study included antidepressants and antipsychotics as time-varying confounders, as these two drug classes were frequently associated with neuropsychiatric disorders.9 However, including these two drug classes as concomitant medications could possibly introduce confounding indications as antidepressants and antipsychotics are prescribed for treatments of neuropsychiatric conditions. Therefore, we performed additional analyses observing the risk of NPAEs at different time window periods and the risk of NPAEs in the previous 7 days and 14 days of the hazard period, excluding antidepressants and antipsychotics from concomitant medications (online supplemental tables S4–S6). The results were indifferent to those of the main analyses, which included antidepressants and antipsychotics as time-varying confounders, confirming that our study was not affected by the inclusion of antidepressants and antipsychotics.
In addition, in regard to stratified analyses for risk of NPAEs in the previous 7 days and 14 days of the hazard period, the highest increased risk was found in older paediatric patients aged between 13 and 19 years. A possible explanation for this finding is that in children and adolescents aged over 10 years, an increase in the diagnosis of depression, anxiety disorders and/or adjustment disorders was noticed, and especially more depressive symptoms were shown in adolescents than younger children.18,22 This pattern was more conspicuous during the COVID-19 pandemic.18,21 Alike results were noted in our study, in which 28.6% of children and adolescents with NPAEs were diagnosed with mood disorders and 29.7% with anxiety disorders. Furthermore, it is often more difficult to diagnose psychiatric conditions in infants and young children compared with adolescents, leading to more NPAE cases in this particular age group, resulting in a higher risk.10 In our study, a higher risk was seen in females, which could be due to the fact that female children and adolescents tend to be diagnosed with psychiatric conditions more than males.19,21
Comparison with other studies
Our findings on the increased risk of NPAEs in relation to monteluksat use within 3, 7 and 14 days of the hazard period were consistent with other studies.23 24 The SCCS study showed increased risks in the 4–7 and 8–14 days after initiation of LTRAs, and a study by Bian et al12 found most reported cases of NPAEs occurred within the first 10 days since the drug initiation.12 In addition, the SCCS study identified a notable risk, particularly in adolescents (incidence rate ratio 1.28; 95% CI 1.05 to 1.55), while the risk decreased in children aged between 3 and 11 years. A similar finding was seen in our study in which the risk of NPAEs was the highest in children and adolescents aged 13–19 years, and the lower risk was shown in patients aged 0–9 years. However, in our study, the risk of NPAEs remained elevated in 28 days and 56 days of the hazard period, whereas the risk was not found in 15–30, 31–90 and >90 days since LTRA initiation in the SCCS study. This difference could be due to a different study design and methodology. A case-crossover study is outcome-indexed, whereas the SCCS study is exposure-indexed, resulting in lower risk in the SCCS study design compared with a case-crossover study design.25 Additionally, an increased risk of NPAEs associated with montelukast use was also shown in a nested case-control study where children with asthma who experienced a new onset of neuropsychiatric events had nearly twice the odds of having been prescribed montelukast in the year before the event.3 A study by Paljarvi et al11 also found an increased risk in patients with asthma who were exposed to montelukast (OR 1.11; 95% CI 1.04 to 1.19), and the highest OR was for anxiety disorders.11 Similarly, in our study, the largest number of patients were diagnosed with anxiety disorders (29.7%). Interestingly, in a study by Paljarvi et al,11 the risk was higher in patients with asthma compared with patients with allergic rhinitis. One of the studies suggested that chronic montelukast treatment was not associated with depression-like behaviours but confirmed the association between depression and asthma in mice.26 27 Thus, it is possible that the asthma condition itself could be one of the possible reasons for the increased risk of psychiatric conditions like depression. To confirm whether the increased risk of NPAE is associated with montelukast or with the asthma condition itself, more studies need to be conducted. In contrast, some other studies found no association between the increased risk of NPAE and montelukast use.28,31 One of the matched nested case-control studies did not detect an increased risk of NPAEs associated with montelukast use in children with asthma.32 Patients exposed to montelukast during the prior year had an aOR of 1.01 (95% CI 0.88 to 1.14) for having NPAEs, indicating no significant positive association between montelukast and NPD in children with asthma.32 It is possible that the OR that was measured in patients exposed to montelukast during the prior year of NPAE diagnosis could have been attributed to this result. Several previous studies suggested the usual onset of NPAEs after montelukast exposure was between 7 and 14 days.1 18 Thus, since the OR was measured in the prior year of the neuropsychiatric condition diagnosis, not immediately before, the results of this nested case-control study were different to ours.
Pranlukast and the risk of NPAEs
Our findings on the increased risk of NPAEs associated with pranlukast use were consistent with those of montelukast. The risk of NPAEs in relation to the use of pranlukast at various time window periods (3, 7, 14, 28 and 56 days) was similar to that of montelukast (table 6). When stratified analysis of the risk of NPAEs in relation to pranlukast use in the previous 7 days was conducted, the overall aOR was 1.26 (95% CI 1.21 to 1.32), the highest risk was found in 13–19 years (aOR 1.44; 95% CI 1.32 to 1.58), and the risk was higher in females (aOR 1.28; 95% CI 1.19 to 1.38) (table 7). These results were all comparable to those of montelukast. It is not surprising that pranlukast was also related to the increased risk of NPAEs like montelukast, as these two medications belong to the same class, LTRAs, indicating that the biological mechanism of action would be similar. Studies conducted by Park et al1 and Kang et al,17 which observed the association between LTRAs and the risk of NPAEs, found both LTRAs were related to a higher risk of NPAEs.1 17 However, an interesting finding was noted from a recent study by Kim et al.33 Kim et al33 observed no association between the increased risk of the composite outcome of all measured NPAEs in study subjects diagnosed with asthma who were prescribed montelukast or pranlukast in comparison to those who were not.33 Nevertheless, the administration of montelukast was related to a higher risk of hallucinations (inverse probability treatment weighting HR 1.45; 95% CI 1.07 to 1.96) and attention difficulties (inverse probability weighting HR 1.24; 95% CI 1.01 to 1.52).33 Moreover, substantial negative hazards for disorientation, anxiety, stress reactions and somatic symptoms were seen in patients exposed to montelukast.33 Until our study, studies have been conducted to see the association between the risk of NPAEs and LTRA use and the risk of NPAEs and montelukast use; however, no studies have been performed to observe the association between the risk of NPAEs and pranlukast, respectively. Therefore, to confirm the possible NPAE risk associated with pranlukst use found in our study, a future study is needed to explore the association between the risk of NPAEs and the use of pranlukast thoroughly.
Table 6. Risk of neuropsychiatric adverse events associated with the use of pranlukast at different time window periods (3, 7, 14, 28 and 56 days).
| Time window period (days) | No. of exposed in hazard period (n=64 800) | No. of exposed in control period (n=2 59 200) | Crude | Adjusted | ||||||
| N (%) | N (%) | OR (95% CI)* | P value | OR (95% CI)† | P value | |||||
| 3 | 2525 | (3.90) | 9999 | (3.86) | 1.02 | (0.96 to 1.07) | 0.5747 | 1.21 | (1.15 to 1.28) | <0.0001 |
| 7 | 3568 | (5.51) | 13 633 | (5.26) | 1.07 | (1.02 to 1.12) | 0.0035 | 1.26 | (1.21 to 1.32) | <0.0001 |
| 14 | 5133 | (7.92) | 18 716 | (7.22) | 1.14 | (1.10 to 1.18) | <0.0001 | 1.32 | (1.27 to 1.37) | <0.0001 |
| 28 | 7908 | (12.20) | 27 441 | (10.59) | 1.22 | (1.19 to 1.26) | <0.0001 | 1.38 | (1.34 to 1.42) | <0.0001 |
| 56 | 12 241 | (18.89) | 43 481 | (16.78) | 1.19 | (1.16 to 1.22) | <0.0001 | 1.28 | (1.25 to 1.31) | <0.0001 |
Calculated by conditional logistic regression.
Calculated by conditional logistic regression adjusted for concomitant medications.
Table 7. Risk of NPAEs associated with the use of pranlukast in the previous 7 days, stratified analysis according to age group, gender and season.
| Variables | Exposed in hazard period | Exposed in control period | Crude | Adjusted | P value for interaction* | ||||
| N/N | (%) | N/N | (%) | OR (95% CI)† | OR (95% CI)‡ | ||||
| Total | 3 568/64 800 | (5.51) | 13 633/259 200 | (0.05) | 1.07 | (1.02 to 1.12) | 1.26 | (1.21 to 1.32) | |
| Age group (years) | 0.5798 | ||||||||
| 0–2 | 422/3285 | (12.85) | 1655/13 140 | (12.60) | 1.03 | (0.90 to 1.17) | 1.18 | (1.03 to 1.34) | |
| 3–5 | 940/12 976 | (7.24) | 3644/51 904 | (7.02) | 1.05 | (0.96 to 1.14) | 1.21 | (1.11 to 1.33) | |
| 6–9 | 1076/22 671 | (4.75) | 4253/90 684 | (4.69) | 1.02 | (0.94 to 1.10) | 1.17 | (1.08 to 1.28) | |
| 10–12 | 226/5948 | (3.80) | 889/23 792 | (3.74) | 1.02 | (0.86 to 1.22) | 1.25 | (1.05 to 1.50) | |
| 13–19 | 904/19 920 | (4.54) | 3192/79 680 | (4.01) | 1.19 | (1.09 to 1.30) | 1.44 | (1.32 to 1.58) | |
| Gender | 0.9691 | ||||||||
| Male | 2294/39 257 | (5.84) | 8782/157 028 | (5.59) | 1.07 | (1.01 to 1.13) | 1.25 | (1.18 to 1.32) | |
| Female | 1274/25 543 | (4.99) | 4851/102 172 | (4.75) | 1.07 | (1.00 to 1.15) | 1.28 | (1.19 to 1.38) | |
| Season | <0.0001 | ||||||||
| Spring | 903/14 256 | (6.33) | 3038/57 024 | (5.33) | 1.29 | (1.18 to 1.42) | 1.47 | (1.34 to 1.61) | |
| Summer | 733/17 573 | (4.17) | 3233/70 292 | (4.60) | 0.87 | (0.79 to 0.96) | 1.08 | (0.98 to 1.19) | |
| Autumn | 1206/19 111 | (6.31) | 3996/76 444 | (5.23) | 1.31 | (1.21 to 1.42) | 1.53 | (1.41 to 1.65) | |
| Winter | 726/13 860 | (5.24) | 3366/55 440 | (6.07) | 0.81 | (0.74 to 0.89) | 0.96 | (0.88 to 1.06) | |
Joint test for exposure and strata variable interaction, indicating whether montelukast affects NPAEDs differently depending on age group, gender, and season.
Calculated by conditional logistic regression.
Calculated by conditional logistic regression adjusted for concomitant medications.
NPAEsneuropsychiatric adverse events
Biological mechanism
Biological mechanisms for montelukast-associated NPAEs are not clearly understood. However, one study noted a montelukast-GSH conjugate where GSH has a vital role in protecting cells from oxidative stress, principally at the brain level.5 A decrease in GSH may compromise brain detoxification pathways and could also impact the l-glutamate levels.5 As a result, the progression of neuropsychiatric disorders such as depression, anxiety and stress could be affected.5 Another possible explanation is human genes that interact with montelukast.34 A functional enrichment analysis of human genes that interact with montelukast was conducted, and several genes were notably enriched in the biological processes of ‘neuroactive ligand–receptor interaction’.34 These genes were involved in mood disorders and major depressive disorders.34 However, until we find the definitive mechanism, further studies exploring the mechanisms for montelukast-related NPAEs are still needed.
Strengths and limitations
Our study has several strengths. We conducted the case-crossover study observing the risk of NPAEs associated with montelukast use in children and adolescents using the most recent population-level claims database. The case-crossover study design itself effectively controls both known and unknown confounders, such as gender, socioeconomic factors and genetic factors. In addition, the study design allows study subjects to act as their own controls, minimising intersubject variability. Furthermore, our study used the most recent database available at a population level from 2018 to 2021, which would have reflected reality more effectively.
There are some potential limitations that need to be considered. First, the use of claims databases can possibly result in inaccuracies in coding. However, given the fact that patients diagnosed with NPAEs recorded in the claims dataset would be more severe than those with no recorded NPAEs, there is no evidence that the distribution of montelukast exposure in the hazard and control periods would differ in these patients. With the use of a case-crossover study design, bias due to an unequal distribution of the outcome misclassification would not have occurred. Thus, it is not expected that the main outcome would be different. In addition, our study is based on montelukast prescriptions that were actually dispensed, meaning that we did not have information on patient compliance or the precise timing of medication administration. Lastly, it is possible that residual confounding may not have been removed due to the lack of information in the claims database. For example, there could be other reasons apart from the NPAE onset that could stop patients from taking montelukast, which could have been the cause of possible confounding and the information on these cannot be found in the claims database. Montelukast treatment could have been ineffective for asthma and/or allergic rhinitis, montelukast could have caused other side effects, or patients did not prefer to take montelukast.
In conclusion, children and adolescents with allergic rhinitis should be prescribed montelukast with caution considering its clinical benefits.
supplementary material
Footnotes
Funding: This research was supported by the SungKyunKwan University and the BK21 FOUR (Graduate School Innovation) funded by the Ministry of Education (MOE, Korea) and National Research Foundation of Korea (NRF).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Ethics approval: This research was approved by the Institutional Review Board of Sungkyunkwan University (IRB no. SKKU-2022-11-042). The board waived the requirement for obtaining informed consent as this study used anonymised administrative data.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Contributor Information
Jae Won Kim, Email: kjwmansai@naver.com.
Mideum Kim, Email: stdark@naver.com.
Min Sook Seo, Email: minsookseo@gmail.com.
Ju-Young Shin, Email: shin.jy@skku.edu.
Data availability statement
Data are available upon reasonable request.
References
- 1.Park JS, Cho YJ, Yun J-Y, et al. Leukotriene receptor antagonists and risk of neuropsychiatric events in children, adolescents and young adults: a self-controlled case series. Eur Respir J. 2022;60:2102467. doi: 10.1183/13993003.02467-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dixon EG, Rugg-Gunn CE, Sellick V, et al. Adverse drug reactions of leukotriene receptor antagonists in children with asthma: a systematic review. BMJ Paediatr Open . 2021;5:e001206. doi: 10.1136/bmjpo-2021-001206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glockler-Lauf SD, Finkelstein Y, Zhu J, et al. Montelukast and neuropsychiatric events in children with asthma: A nested case-control study. J Pediatr. 2019;209:176–82. doi: 10.1016/j.jpeds.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Drug Safety Communications FDA requires Boxed Warning about serious mental health side effects for asthma and allergy drug montelukast (Singulair) FDA (US) 2020 [Google Scholar]
- 5.Marques CF, Marques MM, Justino GC. The mechanisms underlying montelukast’s neuropsychiatric effects - new insights from a combined metabolic and multiomics approach. Life Sci. 2022;310:S0024-3205(22)00756-1. doi: 10.1016/j.lfs.2022.121056. [DOI] [PubMed] [Google Scholar]
- 6.Kim JA, Yoon S, Kim LY, et al. Towards actualizing the value potential of Korea health insurance review and assessment (HIRA) data as a resource for health research: Strengths, limitations, applications, and strategies for optimal use of HIRA data. J Korean Med Sci. 2017;32:718–28. doi: 10.3346/jkms.2017.32.5.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kyoung D-S, Kim H-S. Understanding and utilizing claim data from the Korean National Health Insurance Service (NHIS) and Health Insurance Review & Assessment (HIRA) database for research. J Lipid Atheroscler. 2022;11:103–10. doi: 10.12997/jla.2022.11.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewer D, Petersen I, Maclure M. The case-crossover design for studying sudden events. BMJ Med . 2022;1:e000214. doi: 10.1136/bmjmed-2022-000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang H-R, Lee E-K, Kim WJ, et al. Risk of neuropsychiatric adverse events associated with the use of oseltamivir: a nationwide population-based case-crossover study. J Antimicrob Chemother. 2019;74:453–61. doi: 10.1093/jac/dky445. [DOI] [PubMed] [Google Scholar]
- 10.Szaniecki E, Barnes J. Measurement issues: Measures of infant mental health. Child Adolesc Ment Health. 2016;21:64–74. doi: 10.1111/camh.12105. [DOI] [PubMed] [Google Scholar]
- 11.Paljarvi T, Forton J, Luciano S, et al. Analysis of neuropsychiatric diagnoses after montelukast initiation. JAMA Netw Open. 2022;5:e2213643. doi: 10.1001/jamanetworkopen.2022.13643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bian S, Li L, Wang Z, et al. Neuropsychiatric side reactions of leukotriene receptor antagonist, antihistamine, and inhaled corticosteroid: A real-world analysis of the Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS) World Allergy Organ J. 2021;14:100594. doi: 10.1016/j.waojou.2021.100594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singulair . Package insert. Merck&Co Inc; 2021. [Google Scholar]
- 14.Eidelman C, Abdel-Rahman SM. Pharmacokinetic considerations when prescribing in children. Int J Pharmacokinet. 2016;1:69–80. doi: 10.4155/ipk-2016-0001. [DOI] [Google Scholar]
- 15.Zhang R, Volkow ND. Seasonality of brain function: role in psychiatric disorders. Transl Psychiatry. 2023;13:65. doi: 10.1038/s41398-023-02365-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ciriaco M, Ventrice P, Russo G, et al. Corticosteroid-related central nervous system side effects. J Pharmacol Pharmacother. 2013;4:S94–8. doi: 10.4103/0976-500X.120975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Onder G, Pellicciotti F, Gambassi G, et al. NSAID-related psychiatric adverse events: who is at risk? Drugs (Abingdon Engl) 2004;64:2619–27. doi: 10.2165/00003495-200464230-00001. [DOI] [PubMed] [Google Scholar]
- 18.Kim SJ, Lee J. Introduction of child and adolescent mental health services in Korea and their role during the COVID-19 pandemic: focusing on the ministry of education policy. Soa Chongsonyon Chongsin Uihak. 2023;34:4–14. doi: 10.5765/jkacap.220034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin B, Lee S, Chung US. Jeopardized mental health of children and adolescents in coronavirus disease 2019 pandemic. Clin Exp Pediatr . 2022;65:322–9. doi: 10.3345/cep.2021.01753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Racine N, McArthur BA, Cooke JE, et al. Global prevalence of depressive and anxiety symptoms in children and adolescents during COVID-19: a meta-analysis. JAMA Pediatr. 2021;175:1142–50. doi: 10.1001/jamapediatrics.2021.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee J, Hong SW, Kim K. Mental health of adolescents and subjective economic deterioration caused by COVID-19 in Korea. J Korean Med Sci. 2022;37:e268. doi: 10.3346/jkms.2022.37.e268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aldea Perona A, García-Sáiz M, Sanz Álvarez E. Psychiatric disorders and montelukast in children: A disproportionality analysis of the VigiBase. Drug Saf. 2016;39:69–78. doi: 10.1007/s40264-015-0360-2. [DOI] [PubMed] [Google Scholar]
- 23.Jordan A, Toennesen LL, Eklöf J, et al. Psychiatric adverse effects of montelukast-a nationwide cohort study. J Allergy Clin Immunol Pract. 2023;11:2096–103. doi: 10.1016/j.jaip.2023.03.010. [DOI] [PubMed] [Google Scholar]
- 24.Jordan A, Toennesen LL, Eklöf J, et al. Psychiatric adverse effects of montelukast—A nationwide cohort study. J Allergy Clin Immunol Pract. 2023;11:2096–103. doi: 10.1016/j.jaip.2023.03.010. [DOI] [PubMed] [Google Scholar]
- 25.Bykov K, Franklin JM, Li H, et al. Comparison of self-controlled designs for evaluating outcomes of drug–drug interactions. Epidemiol (Sunnyvale) 2019;30:861–6. doi: 10.1097/EDE.0000000000001087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tel BC, Telli G, Onder S, et al. Investigation of the relationship between chronic montelukast treatment, asthma and depression-like behavior in mice. Exp Ther Med. 2021;21:27. doi: 10.3892/etm.2020.9459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gonca E. The effects of montelukast on depression and anxiety behaviors in rats. Psychiatr Clin Pharmacol. 2015;25 [Google Scholar]
- 28.Liu MJ, Lei WT. Montelukast does not increase the risk of neuropsychiatric disease in children with asthma: a nationwide population-based cohort study. J Allergy Clin Immunol. 2019;143:AB220. doi: 10.1016/j.jaci.2018.12.672. [DOI] [Google Scholar]
- 29.Schumock GT, Stayner LT, Valuck RJ, et al. Risk of suicide attempt in asthmatic children and young adults prescribed leukotriene-modifying agents: a nested case-control study. J Allergy Clin Immunol. 2012;130:368–75. doi: 10.1016/j.jaci.2012.04.035. [DOI] [PubMed] [Google Scholar]
- 30.Huang P-Y, Yang Y-H, Huang Y-H, et al. Montelukast does not increase the risk of attention-deficit/hyperactivity disorder in pediatric asthma patients: A nationwide population-based matched cohort study. J Formos Med Assoc. 2021;120:1369–76. doi: 10.1016/j.jfma.2020.10.018. [DOI] [PubMed] [Google Scholar]
- 31.Fox CW, Khaw CL, Gerke AK, et al. Montelukast and neuropsychiatric events - a sequence symmetry analysis. J Asthma. 2022;59:2360–6. doi: 10.1080/02770903.2021.2018705. [DOI] [PubMed] [Google Scholar]
- 32.Ali MM, O’Brien CE, Cleves MA, et al. Exploring the possible association between montelukast and neuropsychiatric events among children with asthma: a matched nested case-control study. Pharmacoepidemiol Drug Saf. 2015;24:435–45. doi: 10.1002/pds.3758. [DOI] [PubMed] [Google Scholar]
- 33.Kim J-H, Lee H, Jeong D, et al. The risk of neuropsychiatric adverse events with use of leukotriene receptor antagonists in patients with asthma: analysis of Korea’s national health insurance sharing service database. J Allergy Clin Immunol Pract. 2023;11:3690–9. doi: 10.1016/j.jaip.2023.08.037. [DOI] [PubMed] [Google Scholar]
- 34.Umetsu R, Tanaka M, Nakayama Y, et al. Neuropsychiatric adverse events of montelukast: An analysis of real-world datasets and drug-gene interaction network. Front Pharmacol. 2021;12:764279. doi: 10.3389/fphar.2021.764279. [DOI] [PMC free article] [PubMed] [Google Scholar]


