Abstract
Background
Cardiovascular diseases are the primary cause of nonobstetric morbidity and mortality in pregnant women worldwide. Pakistan's high maternal and neonatal mortality rates underscore the need for effective screening protocols to detect cardiovascular diseases during pregnancy.
Objectives
The objective of this study was to assess the prevalence and factors associated with structural heart disease among pregnant women without active cardiorespiratory symptoms (no symptoms or symptoms attributed to pregnancy) attending routine antenatal appointments.
Methods
Obstetric patients without known heart disease attending routine antenatal visits were enrolled between February 2023 and March 2024. All patients had a standardized limited transthoracic echocardiogram. Left ventricular ejection fraction was quantified using biplane Simpson's method. Multivariable binary logistic regression analysis was performed to identify determinants of left ventricular systolic dysfunction (LVSD).
Results
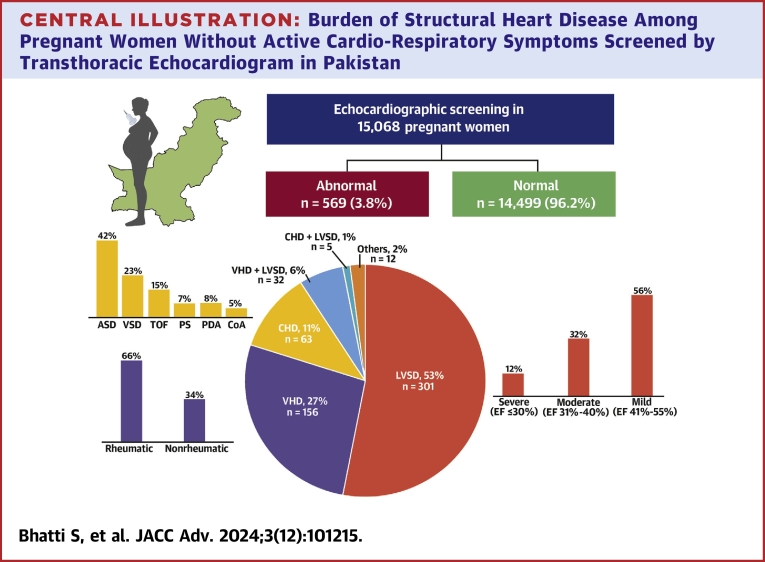
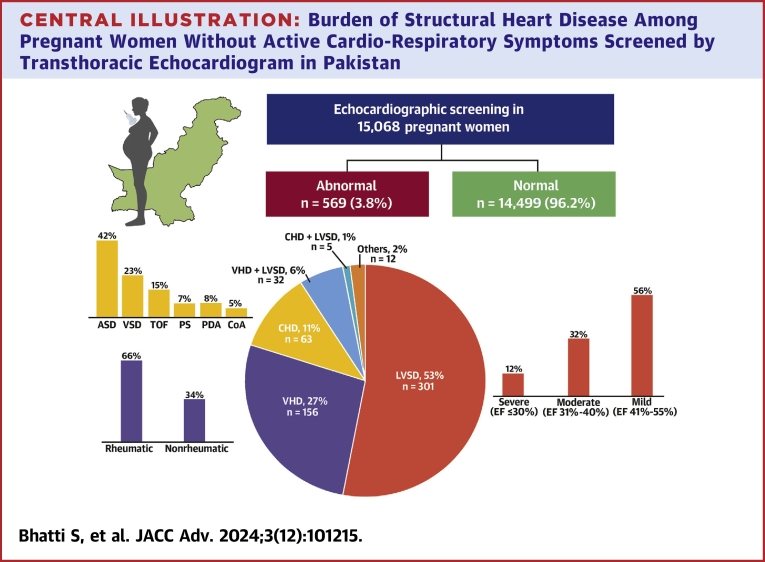
We enrolled 15,068 pregnant women (mean age 26.2 ± 5.1 years) of which 32.4% were primiparous and 0.9% had multifetal gestations. Screening occurred in the first trimester in 1.6% of patients and in the third trimester in 69.4%. structural heart disease was observed in 569 patients (3.8%): 338 (2.2%) with LVSD, 188 (1.2%) with valvular heart disease and 68 (0.5%) with congenital heart disease. Logistic regression analysis identified pregnancy-induced hypertension, tachycardia, and valvular heart disease as factors associated with LVSD.
Conclusions
In a large cohort of pregnant women in Pakistan undergoing routine antenatal echocardiographic screening, almost 4% had structural heart disease. Notably, 2.2% of women had LVSD without active cardiorespiratory symptoms. In select populations, screening can help facilitate early cardiac diagnosis and referral to cardio-obstetrics care. (Prospective Pakistan Registry of Echocardiographic Screening in Asymptomatic Pregnant Women [PRESAP]; NCT06004544)
Key words: cardioobstetric, left ventricular dysfunction, peripartum cardiomyopathy, PRESAP
Central Illustration
Cardiovascular diseases are a major contributor to maternofetal morbidity and mortality and may be due to structural heart disease (SHD). SHD in pregnant patients can be due to valvular heart disease (VHD), cardiomyopathies, and congenital heart disease. Furthermore, SHD may be first detected during pregnancy, especially in populations where access to cardiovascular care is limited. In Pakistan, the burden of poor maternal-fetal outcomes ranks among the highest worldwide and results from multifactorial factors, including inadequate nutrition, low income, early and multiple pregnancies, and limited access to medical care, both obstetric and cardiac.1, 2, 3
One of the challenges with detecting SHD during pregnancy lies in its clinical presentation, which may overlap with normal pregnancy symptoms or, in some cases, remain entirely asymptomatic. This asymptomatic state leaves unidentified SHD untreated that may adversely impact maternal and fetal outcomes.4 A crucial opportunity for early detection and improved outcomes lies in cardiac screening during routine obstetric appointments. However, the lack of current screening guidelines for SHD often leads to referrals for an echocardiogram only when symptoms raise concerns.5 Therefore, establishing effective screening protocols for SHD, early intervention, appropriate management, and follow-up echocardiograms are essential in addressing maternal morbidity and mortality.
The PRESAP (Pakistan Registry of Echocardiographic Screening in Asymptomatic Pregnant Women) registry is a prospective initiative that aims to address these critical aspects. This registry focuses on echocardiographic screening pregnant women attending the largest antenatal program in the country. The objective of our current study is to assess the prevalence and factors associated with SHD, especially focusing on left ventricular systolic dysfunction (LVSD), among pregnant women attending routine antenatal appointments without any active cardiorespiratory symptoms (defined as no symptoms at all or symptoms attributed to normal pregnancy). Additionally, we aim to investigate the maternofetal outcomes associated with SHD and establish a registry for long-term follow-up of patients diagnosed with SHD.
Methods
Study Design and Participant Enrollment
The present study is a prospective registry, known as the Pakistan Registry of Echocardiographic Screening in Asymptomatic Pregnant Women registry. We enrolled pregnant women without known heart disease attending routine antenatal visits at the largest obstetrics hospital in the Sindh Province, Pakistan, over a 14-month period from February 2023 to March 2024. Women were only screened once during the pregnancy. We excluded women who were already screened at prior antenatal visits.
Clinical assessment
Patient clinical data were obtained from medical records and the screening interview. The clinical data collected included obstetric history, comorbid conditions, and vitals. These data were entered into the registry using REDCAP.
Echocardiographic Screening Protocol
A standardized limited 2-dimensional transthoracic echocardiogram protocol was performed in all enrolled participants. The echocardiograms were performed by trained sonographers using General Electric ultrasound systems and a butterfly point-of-care ultrasound device. Data from butterfly point-of-care device was recorded on an online cloud-based server and it was accessible to the dedicated faculty through secured credentials. The following views were obtained during the echocardiographic assessment: parasternal long-axis view, parasternal short-axis view, apical 4-chamber view, and apical 2-chamber view. Additionally, color Doppler across mitral and aortic valves as well as continuous wave and pulse wave Doppler across the left ventricular (LV) outflow tract were performed to assess any gradients.
Evaluation of Left Ventricular Systolic Function
LV systolic function was evaluated using visual estimation by 2 readers and LV ejection fraction (EF) was calculated using Biplane Simpson's method. If there was a discrepancy between readers or between methods, a third reader was asked to adjudicate. In accordance with the recent ASE guidelines, severe LVSD was categorized as EF ≤30%, moderate LSVD as EF between 30% and 40%, mild LVSD as EF between 41% and 55%, and EF >55% was categorized as normal.6 Patients with EF <55% were categorized as significant LVSD.
Patients with Valve Disease or Congenital Heart Disease
Patients identified with significant (more than mild) valve disease or suspected congenital heart disease during the initial screening underwent a complete transthoracic echocardiogram to provide a more comprehensive assessment.
Management and Follow-Up
Patients diagnosed with SHD during the echocardiographic screening were referred to a cardio-obstetrics clinic, located adjacent to the screening facility, for further management and treatment. The management approach followed standard-of-care guidelines. These patients were then longitudinally followed up in the cardio-obstetrics registry.
Ethical Considerations
This study was approved by the institution review board of the National Institute of Cardiovascular Diseases (NICVD), Karachi, Pakistan (approval number: IRB-32/2023). Verbal informed consent was obtained from all the patients regarding their participation in the study and publication of data while maintaining confidentiality and anonymity. Due to observational nature of the study, IRB waived the written consent and verbal consent were approved by the IRB.
Statistical Analysis
Study data were entered and analyzed using IBM SPSS, version 21. Categorical response variables are summarized as frequency and percentages, while continuous variables with nonskewed distribution were presented as mean ± SD. Skewed distribution variables are summarized as median (IQR). Patients were categorized into 2 groups based on the presence of LVSD, and chi-squared tests or independent sample t-tests/Mann-Whitney U tests were applied to assess the association of various clinical and demographic factors with LVSD. Uni/multivariable binary logistic regression analyses were performed to identify clinical and demographic factors associated with LVSD. All the statistically significant variables in bivariate analysis and those with plausible clinical association with LVSD based on the literature were considered for the logistic regression analysis and variables with P value <0.20 in the univariable logistic regression analysis were considered for the multivariable logistic regression analysis. Statistical significance was determined at a 5% level.
Results
Approximately 22,295 new patients were enrolled in the antenatal clinics during the study period of 14 months. Of these, 15,608 (70%) were screened. There was a very small number of refusals to participate (<0.5%). Due to the large number of patients seen daily in the obstetrics clinics, not all the patients could be screened. Patients were screened while waiting to see their obstetricians.
A total of 15,068 pregnant women underwent echocardiographic screening, with a mean age of 26.2 ± 5.1 years. During the screening, 30.6% of the women were in the first 2 trimesters and 69.4% were in the third trimester of pregnancy. Among the participants, 32.4% were primiparous and 135 (0.9%) had a multifetal pregnancy. A total percentage of 20.2% patients had a history of spontaneous abortion. The echocardiographic screening revealed abnormal findings in 569 patients (3.8%) (Table 1).
Table 1.
Baseline Demographic and Clinical Characteristics of the Study Cohort Stratified by Left Ventricular Systolic Dysfunction Status
| Total (N = 15,068) | LV Dysfunction |
P Value | ||
|---|---|---|---|---|
| No (n = 14,730, 97.8%) | Yes (n = 338, 2.2%) | |||
| Mean age (y) | 26.2 ± 5.1 | 26.1 ± 5.1 | 26.6 ± 5.3 | 0.082 |
| Parity | ||||
| Primiparous | 4,888 (32.4%) | 4,795 (32.6%) | 93 (27.5%) | 0.050 |
| Multiparous | 10,180 (67.6%) | 9,935 (67.4%) | 245 (72.5%) | |
| History of neonatal death | 886 (5.9%) | 866 (5.9%) | 20 (5.9%) | 0.977 |
| Number of gestation | ||||
| 1 | 14,933 (99.1%) | 14,601 (99.1%) | 332 (98.2%) | 0.218 |
| 2 | 115 (0.8%) | 110 (0.7%) | 5 (1.5%) | |
| 3 | 20 (0.1%) | 19 (0.1%) | 1 (0.3%) | |
| Trimester | ||||
| 1st trimester | 247 (1.6%) | 234 (1.6%) | 13 (3.8%) | <0.001 |
| 2nd trimester | 4,360 (28.9%) | 4,293 (29.1%) | 67 (19.8%) | |
| 3rd trimester | 10,461 (69.4%) | 10,203 (69.3%) | 258 (76.3%) | |
| Mean systolic blood pressure (mm Hg) | 109.6 ± 11 | 109.6 ± 11 | 109.8 ± 12.1 | 0.781 |
| Mean diastolic blood pressure (mm Hg) | 71.4 ± 9.3 | 71.4 ± 9.3 | 72.4 ± 9.7 | 0.062 |
| Mean pulse rate (bpm) | 89.2 ± 8.4 | 89.1 ± 8.2 | 93.5 ± 12.2 | <0.001 |
| Diabetes mellitus | ||||
| Nondiabetic | 14,893 (98.8%) | 14,562 (98.9%) | 331 (97.9%) | 0.114 |
| Diabetic | 175 (1.2%) | 168 (1.1%) | 7 (2.1%) | |
| Hypertension | ||||
| Nonhypertensive | 14,813 (98.3%) | 14,489 (98.4%) | 324 (95.9%) | <0.001 |
| Hypertensive | 255 (1.7%) | 241 (1.6%) | 14 (4.1%) | |
| History of spontaneous abortion | 3,037 (20.2%) | 2,965 (20.1%) | 72 (21.3%) | 0.595 |
| Pregnancy induced hypertension | ||||
| No | 14,897 (98.9%) | 14,571 (98.9%) | 326 (96.4%) | <0.001 |
| Yes | 171 (1.1%) | 159 (1.1%) | 12 (3.6%) | |
| Anemia | ||||
| Normal | 5,238 (34.8%) | 5,110 (34.7%) | 128 (37.9%) | <0.001 |
| Anemia | 7,914 (52.5%) | 7,706 (52.3%) | 208 (61.5%) | |
| NA | 1,916 (12.7%) | 1,914 (13%) | 2 (0.6%) | |
| Mean hemoglobin (g/dL) | 10.1 ± 1.5 | 10.1 ± 1.5 | 9.9 ± 1.7 | 0.006 |
Values are mean ± SD or n (%).
Left Ventricular Systolic Dysfunction
LVSD was detected in 338 (2.2%) of the women. Among these patients, 191 (56%) had mildly reduced LV systolic function (LVEF 41%-55%), 107 (32%) had moderately reduced function (LVEF 31%-40%), and 40 (12%) had severely reduced function with an LVEF ≤30%. Anemia was more common in patients with LVSD (61.5%) compared to patients without LVSD (52.3%) P < 0.001.
Valvular and Congenital Heart Disease
Out of the participants, 188 patients (1.2%) were diagnosed with valvular heart disease, predominantly of rheumatic origin (66.5%). Among these, 73 patients had moderate to severe valvular lesions, with the majority being rheumatic mitral stenosis. Additionally, 68 patients (0.5%) had congenital heart disease (Table 2). An additional 2% of the patients had abnormal transthoracic echocardiogram findings such as pericardial effusion.
Table 2.
Echocardiographic Findings of the Study Cohort Stratified by Left Ventricular Systolic Dysfunction Status
| Total (N = 15,068) | LV Dysfunction |
P Value | ||
|---|---|---|---|---|
| No (n = 14,730, 97.8%) | Yes (n = 338, 2.2%) | |||
| Abnormal echocardiographic findings | 569 (3.8%) | 231 (1.6%) | 338 (100%) | <0.001 |
| Valvular lesions | 188 (1.2%) | 156 (1.1%) | 32 (9.5%) | <0.001 |
| Nonrheumatic | 63 (33.5%) | 48 (30.8%) | 15 (46.9%) | <0.001 |
| Rheumatic | 125 (66.5%) | 108 (69.2%) | 17 (53.1%) | |
| Mitral | 103 (82.4%) | 92 (85.2%) | 11 (64.7%) | <0.001 |
| Aortic | 1 (0.8%) | 1 (0.9%) | 0 (0%) | |
| Multivalve | 21 (16.8%) | 15 (13.9%) | 6 (35.3%) | |
| Congenital heart disease | 68 (0.5%) | 63 (0.4%) | 5 (1.5%) | 0.004 |
| Atrial septal defect | 29 (42.6%) | 28 (44.4%) | 1 (20%) | 0.287 |
| Ventricular septal defect | 16 (23.5%) | 15 (23.8%) | 1 (20%) | 0.847 |
| Patent ductus arteriosus | 6 (8.8%) | 5 (7.9%) | 1 (20%) | 0.360 |
| Tetralogy of Fallot | 10 (14.7%) | 9 (14.3%) | 1 (20%) | 0.728 |
| Pulmonary valve stenosis | 5 (7.4%) | 5 (7.9%) | 0 (0%) | 0.513 |
| Coarctation of aorta | 4 (5.9%) | 3 (4.8%) | 1 (20%) | 0.163 |
Values are n (%).
Factors Associated with LVSD
Logistic regression analysis revealed several factors associated with significant LV dysfunction, including pregnancy-induced hypertension, elevated pulse (>100 bpm), and valvular heart disease as factors associated with LVSD (Table 3).
Table 3.
Univariable and Multivariable Binary Logistic Regression: Factors Associated With Left Ventricular Systolic Dysfunction
| Univariable |
Multivariable |
|||
|---|---|---|---|---|
| OR [95% CI] | P Value | OR [95% CI] | P Value | |
| Age (y) | 1.02 (1.00-1.04) | 0.082 | 1.00 (0.98-1.02) | 0.950 |
| Multiparous | 1.27 (1.00-1.62) | 0.051 | 1.20 (0.92-1.56) | 0.172 |
| History of neonatal death | 1.01 (0.64-1.59) | 0.977 | - | - |
| Number of gestations | 1.75 (0.91-3.36) | 0.095 | 1.63 (0.82-3.23) | 0.160 |
| Systolic blood pressure (mm Hg) | 1.00 (0.99-1.01) | 0.781 | - | - |
| Diastolic blood pressure (mm Hg) | 1.01 (1.00-1.02) | 0.051 | 1.00 (0.99-1.02) | 0.450 |
| Pulse rate (beats/min) | 1.05 (1.04-1.06) | <0.001 | 1.05 (1.04-1.06) | <0.001 |
| Diabetes | 1.83 (0.85-3.93) | 0.120 | 1.22 (0.52-2.84) | 0.650 |
| Hypertension | 2.60 (1.50-4.50) | <0.001 | 1.85 (0.99-3.48) | 0.055 |
| History of spontaneous abortion | 1.07 (0.83-1.40) | 0.595 | - | - |
| Pregnancy-induced hypertension | 3.37 (1.86-6.13) | <0.001 | 2.79 (1.47-5.30) | 0.002 |
| Anemia | 1.08 (0.86-1.35) | 0.511 | - | - |
| Valvular lesions | 9.77 (6.57-14.53) | <0.001 | 8.47 (5.59-12.83) | <0.001 |
| Congenital heart disease | 3.50 (1.40-8.75) | 0.007 | 2.57 (0.98-6.72) | 0.054 |
Discussion
Cardiovascular diseases and in particular SHD such as peripartum cardiomyopathy (PPCM) and valvular heart disease remain a significant concern worldwide, contributing to maternal morbidity and mortality.7,8 In this study, we screened over 15000 pregnant women using a standardized echocardiographic protocol. Our study aimed to assess the prevalence of SHD in pregnant women and evaluate associated factors for LVSD. To our knowledge, this is the largest study of echocardiographic screening of pregnant women. Overall, the prevalence of SHD was found to be 3.8% with LVSD in 2.2% of the patients. Furthermore, we identified VHD in 1.2% and congenital heart disease in 0.5% of the patients (Central Illustration). Pregnancy-induced hypertension (PIH), elevated pulse, and VHD were found to be the factors associated with LVSD. An association between multiparity and one form of cardiomyopathy, PPCM, has been reported in prior studies.5 Anemia was more common in the LVSD group compared to the overall group. Previous studies have shown anemia to be a risk factor for asymptomatic LVSD.9 Our findings highlight the value of echocardiographic screening during pregnancy to identify high-risk individuals who may benefit from cardiac evaluation and referral to cardio-obstetric care.
Central Illustration.
Burden of Structural Heart Disease Among Pregnant Women Without Active Cardio-Respiratory Symptoms Screened by Transthoracic Echocardiogram in Pakistan
ASD = atrial septal defect; CHD = congenital heart disease; CoA = coarctation of aorta; EF = ejection fraction; LVSD = left ventricular systolic dysfunction; PDA = patent ductus arteriosus; PS = pulmonary valve stenosis; TOF = Tetralogy of Fallot; VHD = valvular heart disease; VSD = ventricular septal defect.
The detection of valvular heart disease, predominantly of rheumatic origin, in 1.2% of our study population emphasizes the importance of evaluating for valvular abnormalities during routine antenatal care. Rheumatic heart disease remains a significant health burden in certain regions, and early identification allows for timely referral and appropriate management including starting penicillin for secondary prophylaxis, medical therapy to control heart rate and symptoms if any and referral for intervention if needed.
LVSD in this study may have been due to a number of conditions. LVSD can be due to PPCM, hypertensive heart disease, gestational diabetes, myocarditis, VHD, congenital heart disease or previous cardiomyopathy of any cause. Anemia and nutritional deficiencies are also predisposing factors for LVSD in pregnancy. PPCM, in particular, is a significant contributor to maternal morbidity and mortality on a global scale.4 While epidemiologic studies from Africa, Haiti, European nations, and the United States of America have shed some light on PPCM, data on this condition remain relatively scarce in Pakistan.4,10,11 Notably, the incidence of PPCM varies considerably based on ethnic and regional backgrounds, with African and African-American individuals displaying a higher susceptibility. Incidence rates of 1:100 in Nigeria and 1:299 in Haiti have been reported.4,5,10,11 Early detection of cardiomyopathies, including PPCM, and other cardiovascular conditions provides an opportunity for timely initiation of guideline-directed medical therapy, with potential benefits for both maternal and fetal health.12,13 In the Investigators of Pregnancy-Associated Cardiomyopathy cohort,7 13% of women with PPCM experienced a major event (death, transplantation, or LVAD placement) or had persistent severe cardiomyopathy with an LVEF<35%. According to a position statement from the Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy, screening for peripartum cardiomyopathy should be considered in women with a history of peripartum cardiomyopathy, those with a family history of cardiomyopathy or sudden cardiac death, and those with multiple gestations.7
The findings from this registry align with the results reported in a large screening study conducted in India, reinforcing the significance of echocardiographic screening in asymptomatic pregnant women for early detection of structural heart abnormalities.14 Of 14,275 consecutive women screened in India, 353 (2.5%) were found to have a structural abnormality on a screening echocardiogram. A total of 93 women had mitral valve disease (two-thirds rheumatic). Of those with mitral stenosis, 90% had mild to moderate disease, and 67% had a Wilkins score <8. The prevalence of LVSD was 0.46% (66 women). These results underscore the substantial burden of structural heart abnormalities in pregnant women, regardless of symptoms potentially warranting the implementation of routine echocardiographic screening during antenatal visits. Our findings, along with the evidence from other studies, advocate for the incorporation of echocardiographic screening as a vital component of antenatal care, contributing to enhanced maternal cardiovascular health and overall perinatal well-being.15, 16, 17
While our study provides valuable insights into the prevalence of SHD and factors associated with LVSD in pregnant women without active cardiac symptoms, it is essential to acknowledge some limitations. The study was conducted at a single center in a specific region, which may limit the generalizability of the results. The definition of “asymptomatic” for the purposes of this registry was patient denial of any active cardiorespiratory symptoms; however, some patients could have denied having complaints because they thought that those were normal symptoms of pregnancy. Future multicenter studies with larger cohorts and diverse populations are warranted to validate these findings on a broader scale. While this is a screening registry, we do have a prospective follow-up arm to look at outcomes. More complete follow-up data will be informative in underscoring the importance of such screening and will be published in a follow-up paper.
Conclusions
Our prospective registry of echocardiographic screening in pregnant women revealed a substantial burden of SHD including LVSD and VHD. Early detection of cardiac abnormalities during routine antenatal visits provides an opportunity for early referral to the cardio-obstetrics team and timely management of cardiac abnormalities. The follow up data that is being collected postpartum will provide valuable insights into maternofetal outcomes. These findings support the need for further research and the development of screening guidelines specific to local populations. Implementing such protocols may contribute to reducing maternal morbidity and mortality in regions with a high burden of poor maternal-fetal outcomes.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: The prospective registry of echocardiographic screening in pregnant women demonstrated a significant prevalence of structural cardiac abnormalities. Identifying cardiac abnormalities during routine antenatal visits allows for early identification and management, which may improve maternal and fetal outcomes.
TRANSLATIONAL OUTLOOK: These findings underscore the importance of additional research and the establishment of tailored screening guidelines for the local population. By implementing such protocols, regions with high rates of poor maternal-fetal outcomes may reduce maternal morbidity and mortality.
Funding support and author disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgment
The authors acknowledge Dr Nasrien E. Ibrahim, Assistant Professor of Medicine at Harvard Medical School, for her valuable feedback and review of the manuscript.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Bhutta Z.A., Hafeez A., Rizvi A., et al. Reproductive, maternal, newborn, and child health in Pakistan: challenges and opportunities. Lancet. 2013;381(9884):2207–2218. doi: 10.1016/S0140-6736(12)61999-0. [DOI] [PubMed] [Google Scholar]
- 2.National Institute of Population Studies-NIPS . NIPS/ICF; Islamabad/Pakistan: 2020. https://www.dhsprogram.com/pubs/pdf/FR366/FR366.pdf (Pakistan Maternal Mortality Survey [Internet]). Available from. [Google Scholar]
- 3.Trends in maternal mortality 2000 to 2020: estimates by WHO, UNICEF, UNFPA, World Bank Group and UNDESA/Population Division. World Health Organization; Geneva: 2023. [Google Scholar]
- 4.Davis M.B., Arany Z., McNamara D.M., Goland S., Elkayam U. Peripartum cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75(2):207–221. doi: 10.1016/j.jacc.2019.11.014. [DOI] [PubMed] [Google Scholar]
- 5.Grodzinsky A., Florio K., Spertus J.A., et al. Importance of the cardio-obstetrics team. Curr Treat Options Cardiovasc Med. 2019;21(12):84. doi: 10.1007/s11936-019-0789-1. [DOI] [PubMed] [Google Scholar]
- 6.Lang R.M., Badano L.P., Mor-Avi V., et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16(3):233–271. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 7.Sliwa K., Hilfiker-Kleiner D., Petrie M.C., et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy. Eur J Heart Fail. 2010;12(8):767–778. doi: 10.1093/eurjhf/hfq120. [DOI] [PubMed] [Google Scholar]
- 8.Elkayam U., Akhter M.W., Singh H., et al. Pregnancy-associated cardiomyopathy: clinical characteristics and a comparison between early and late presentation. Circulation. 2005;111(16):2050–2055. doi: 10.1161/01.cir.0000162478.36652.7e. [DOI] [PubMed] [Google Scholar]
- 9.Bhatti S., Hakeem A., Dillie K.S., Cook J.R., Chang S.M. Prevalence, prognosis, and therapeutic implications of unrecognized left ventricular systolic dysfunction in patients with anemia and chronic kidney disease. Congest Heart Fail. 2010;16(6):271–277. doi: 10.1111/j.1751-7133.2010.00181.x. [DOI] [PubMed] [Google Scholar]
- 10.Karaye K.M., Ishaq N.A., Sa'idu H., et al. Incidence, clinical characteristics, and risk factors of peripartum cardiomyopathy in Nigeria: results from the PEACE Registry. ESC Heart Fail. 2020;7(1):236–244. doi: 10.1002/ehf2.12562. [DOI] [Google Scholar]
- 11.Mogos M.F., Piano M.R., McFarlin B.L., Salemi J.L., Liese K.L., Briller J.E. Heart failure in pregnant women: a concern across the pregnancy continuum. Circ Heart Fail. 2018;11(1) doi: 10.1161/circheartfailure.117.004005. [DOI] [Google Scholar]
- 12.DeFilippis E.M., Haythe J.H., Walsh M.N., Kittleson M.M. Intersection of heart failure and pregnancy: beyond peripartum cardiomyopathy. Circ Heart Fail. 2021;14(5) doi: 10.1161/circheartfailure.120.008223. [DOI] [Google Scholar]
- 13.McNamara D.M., Elkayam U., Alharethi R., et al. Clinical outcomes for peripartum cardiomyopathy in north America: results of the IPAC study (Investigations of pregnancy-associated cardiomyopathy) J Am Coll Cardiol. 2015;66(8):905–914. doi: 10.1016/j.jacc.2015.06.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel A., Ranard L.S., Aranoff N., et al. Use of routine echocardiographic screening for structural heart disease in at-risk pregnant women in India. JACC Cardiovasc Imaging. 2021;14(3):692–693. doi: 10.1016/j.jcmg.2020.08.032. [DOI] [PubMed] [Google Scholar]
- 15.Choi E.Y., Kim E.S., Kim J.Y., Song M.K., Kim S.H., Noh C.I. Pregnancy outcomes in patients with structural heart disease: a single center experience. Cardiovasc Diagn Ther. 2021;11(1):81–90. doi: 10.21037/cdt-20-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Regitz-Zagrosek V., Roos-Hesselink J.W., Bauersachs J., et al. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J. 2018;39(34):3165–3241. doi: 10.1093/eurheartj/ehy340. [DOI] [PubMed] [Google Scholar]
- 17.Bozkaya V.Ö., Oskovi-Kaplan Z.A., Erel O., Keskin L.H. Anemia in pregnancy: it’s effect on oxidative stress and cardiac parameters. J Matern Fetal Neonatal Med. 2021;34(1):105–111. doi: 10.1080/14767058.2020.181370. [DOI] [PubMed] [Google Scholar]