Abstract
Introduction
Systemic inflammatory and nutritional markers are associated with the prognosis of various cancers. However, their association with sinonasal squamous cell carcinoma (SNSCC) prognosis remains unclear. This study aimed to identify systemic inflammatory and nutritional markers associated with the postoperative prognosis of patients with SNSCC and to clarify the clinical value of these markers.
Materials and methods
Data from 129 patients with SNSCC were included. The optimal prognostic systemic inflammatory and nutritional markers were identified using the area under the curve. The prognostic value was evaluated using COX regression and subgroup analyses; a nomogram was built based on these data.
Results
The advanced lung cancer inflammation index (ALI) and systemic immune‐inflammatory index (SII) had higher prognostic values than the other indices; their cut‐off values were 27.80 and 791.35, respectively. The nomogram included tumor stage, ALI, and tumor primary site; the calibration and decision curves indicated that the model had good clinical value.
Conclusion
The ALI and SII have potential prognostic value for postoperative patients with SNSCC. The nomogram constructed in this study could be used as a tool to assist physicians in making clinical decisions.
Level of evidence
4.
Keywords: advanced lung cancer inflammation index, nomogram, overall survival, sinonasal cancer, squamous cell carcinoma
This study aimed to identify the systemic inflammatory and nutritional markers associated with the prognosis of patients with SNSCC and clarify their clinical value. Our results indicated that the advanced lung cancer inflammation index (ALI) and systemic immune‐inflammatory index (SII) had potential prognostic value for this patient population. In addition, the ALI was superior to the SII for predicting the prognosis of these patients.
1. INTRODUCTION
Sinonasal squamous cell carcinoma (SNSCC) is the most common type of malignant tumor of the nasal cavity and sinuses, occurring in the mucosal epithelium of the nasal cavity and sinuses and is usually highly invasive. 1 Currently, the primary treatment for SNSCC is surgical resection combined with radiochemotherapy or induction chemotherapy. 2 According to data from several studies, the 5‐year survival rate of patients with SNSCC ranges from 40% to 80%. 3 , 4 Local and regional tumor recurrence are the main causes of treatment failure, which mostly occur within 5 years after initial treatment. 5 , 6
An increasing number of researchers believe that inflammation plays a key role in the tumor microenvironment. A tumor‐induced prolonged inflammatory response enhances the proliferation and invasive ability of tumor cells, resulting in increased metabolism and energy expenditure in patients, and the promotion of tumorigenesis, progression, and metastasis. 7 , 8 However, nutritional status significantly affects patient tolerance and outcomes after receiving anticancer therapy. 9 The immune system of patients with high nutritional risk is weakened, and their resistance to tumors and infections is reduced. Only one‐third of patients with cancer who are at nutritional risk receive nutritional support before surgery. 10
Recently, many studies have calculated systemic inflammatory and nutritional indices from blood markers to reflect the inflammatory and nutritional statuses of patients. These indicators include the advanced lung cancer inflammation index (ALI), 11 neutrophil‐to‐lymphocyte ratio (NLR), 12 prognostic nutritional index (PNI), 13 systemic immune‐inflammation index (SII), 14 and platelet‐to‐lymphocyte ratio (PLR). 15 They not only provide a basis for decision‐making regarding whether a patient needs to receive additional supportive therapy before surgery but are also used for prognostic assessments. Specifically, ALI and SII have been linked to the prognosis in head and neck tumors. 16 , 17 ALI, comprising albumin and body mass index (BMI) as the numerator and neutrophils and lymphocytes as the denominator, is a composite indicator associated with better prognosis due to an improved preoperative nutritional status and a stable inflammatory endo‐environment. The opposite is true for SII, which primarily reflects the state of inflammation in the body. A high SII, indicating low plasma lymphocytes with elevated neutrophils and platelets, suggests a poor prognosis due to an adverse inflammatory tumor microenvironment.
However, SNSCC is often not treated as a distinct entity in research, but rather grouped together with other HNSCC types like salivary gland tumors. This general grouping leads to an underestimation of heterogeneity of SNSCC, leaving the prognostic impact of these indices for SNSCC unclear. Furthermore, the treatment regimen in these studies was less focused on surgery. In addition, the impact of these indicators in clinical work is relatively scarce because there is a lack of robust, application‐based tools available to transfer results to clinical settings. This study aimed to identify systemic inflammatory and nutritional indicators associated with the prognoses of postoperative patients with SNSCC using a survival analysis and to clarify the clinical value of these indicators. Additionally, we sought to construct a nomogram based on these for clinical use, providing a valuable reference for clinicians.
2. MATERIALS AND METHODS
2.1. Study population
We retrospectively collected data from patients who underwent first‐time resection of squamous carcinoma of the nasal cavity and sinuses between December 2013 and June 2023 at the Affiliated Hospital of Qingdao University. The inclusion criteria were: (1) age ≥ 18 years; (2) first treatment; and (3) a postoperative pathologic diagnosis of squamous carcinoma. The exclusion criteria were: (1) absence of important clinical data; (2) presence of other malignant tumors or distant metastases on admission; (3) preoperative radiotherapy or admission to the intensive care unit; (4) immunosuppression or use of immunomodulators, including HIV carriers, organ transplant recipients, and those with rheumatoid diseases.
This study was approved by the Ethics Committee of our hospital.
2.2. Calculation of systemic inflammation and nutritional indices
All laboratory indices were obtained 1 week prior to surgery, and these calculation formulas were used as in previous reports in the literature 11 , 12 , 13 , 14 , 15 : ; ; lymphocyte‐to‐monocyte ratio ; albumin‐to‐globulin ratio ; ; ; .
2.3. Other covariates
We also collected patient data included sex, age, smoking history, alcohol consumption history, BMI, tumor stage, primary site, tumor differentiation, N classification, and treatment regimen. Smoking status was defined as more than one cigarette per day for more than 1 year, regardless of age at the time of cessation and duration of cessation; alcohol consumption was defined as more than two drinks per week for more than 6 months; tumor staging was determined according to the eighth edition of the American Joint Committee on Cancer 18 ; and the primary site was classified as the nasal cavity, maxillary sinus, or other sinuses. The patients in our study were treated in four types: surgery, surgery and radiotherapy, surgery and chemoradiotherapy, and patients who received postoperative adjuvant therapy, but the specific treatment was not known. Surgical treatments included simple nasal endoscopic surgery, open surgery assisted by endoscopy, and simple open surgery. Because of the severity of missing data, patients' surgical margin status was excluded from this study, and other missing data were labeled as unknown.
2.4. Access to follow‐up data
Patients were followed up through telephone or re‐examinations at regular intervals. The date of the last follow‐up visit was February 22, 2024. Overall survival was defined as the time from the malignancy diagnosis until death from any cause. If death did not occur by the follow‐up cutoff time, the last follow‐up date was considered the end of follow‐up, and for patients who were lost to follow‐up, the date of their last contact was used.
2.5. Truncation value definition
Time‐dependent receiver operating characteristic (ROC) curves incorporated the time factor into the analyses, allowing the ROC curves to be constructed at multiple time points, and allowing the predictive power of each factor to be compared while considering the time nodes. The time‐dependent ROC curves were used to identify the best predictors in the study, followed by maximally selected rank statistics to determine the best cutoff values for the indicators. Restricted cubic spline (RCS) curves, a common method for analyzing nonlinear relationships, were used to visually assess the relationship between the selected metrics and patient hazards ratio (HR).
2.6. Statistical analyses
SPSS version 27.00 (IBM Corp., Armonk, NY, USA) and R software (version 4.3.3) were used for statistical analyses. Descriptive statistics for categorical variables are expressed as frequencies and percentages (%), and Pearson's chi‐square and Fisher's exact tests were used. Continuous variables were analyzed using Student's t‐ or Mann–Whitney U tests based on normal distributions. Survival curves were plotted using the Kaplan–Meier method, and the log‐rank test was used to compare survival differences. HR and 95% confidence intervals (CIs) were calculated using the Cox proportional risk regression model. All tests were two‐sided, and p < .05 was considered statistically significant. In the subgroup analysis, p < .1 was considered statistically significant.
3. RESULTS
3.1. Patient baseline data
A total of 129 patients with squamous carcinoma were included; 85 men and 44 women with a mean age of 59 years, 83 of whom were aged <65 years. The average follow‐up was 39 months, with a maximum of 122 months and a minimum of 1 month. Most patients had tumors originating in the maxillary sinus, and 95 patients received adjuvant therapy after surgical treatment. Among them, 42 had surgery combined with radiotherapy, 36 had surgery combined with radiochemotherapy, and 17 were unsure of their adjuvant therapy received after surgery. No lymph node metastases were detected in 67 patients, while lymph node status was unavailable for 24 patients. Detailed patient information is presented in Table 1. Survival curves of the patients are shown in Figure 1A. The 1‐, 3‐, and 5‐year survival rates were 82.4%, 57.4%, and 36.9%, respectively. In this study, there was no significant difference in the effect of different treatment regimens on patient prognosis (Figure S1).
TABLE 1.
Baseline characteristics of included patients.
| Characteristics | Overall (N = 129) | High ALI (N = 63) | Low ALI (N = 66) | p‐value | High SII (N = 61) | Low SII (N = 68) | p‐value |
|---|---|---|---|---|---|---|---|
| Gender, n (%) | .458 | 1.000 | |||||
| Male | 85 (65.89) | 44 (69.84) | 41 (62.12) | 45 (73.77) | 40 (58.82) | ||
| Female | 44 (34.11) | 19 (30.16) | 25 (37.88) | 23 (37.70) | 21 (30.88) | ||
| Age, years, mean ± SD | 59.98 ± 10.78 | 56.62 ± 10.52 | 63.2 ± 10.09 | <.001 | 61.41 ± 11.29 | 58.71 ± 10.22 | .156 |
| Age stratification, n (%) | .002 | .142 | |||||
| ≥65 | 46 (35.66) | 14 (22.22) | 32 (48.48) | 20 (32.79) | 26 (38.24) | ||
| <65 | 83 (64.34) | 49 (77.78) | 34 (51.52) | 48 (78.69) | 35 (51.47) | ||
| Smoking, n (%) | .014 | .008 | |||||
| Yes | 68 (52.71) | 26 (41.27) | 42 (63.64) | 40 (65.57) | 28 (41.18) | ||
| No | 61 (47.29) | 37 (58.73) | 24 (36.36) | 21 (34.43) | 40 (58.82) | ||
| Alcohol, n (%) | .480 | .861 | |||||
| Yes | 58 (44.96) | 26 (41.27) | 32 (48.48) | 28 (45.90) | 30 (44.12) | ||
| No | 71 (55.04) | 37 (58.73) | 34 (51.52) | 33 (54.10) | 38 (55.88) | ||
| Treatment regimen, n (%) | .023 | .109 | |||||
| Surgery | 34 (26.36) | 13 (20.63) | 21 (31.82) | 14 (22.95) | 20 (29.41) | ||
| Surgery + radiotherapy | 42 (32.56) | 28 (44.44) | 14 (21.21) | 16 (26.23) | 26 (38.24) | ||
| Surgery + chemoradiotherapy | 36 (27.91) | 17 (26.98) | 19 (28.79) | 19 (31.15) | 17 (25.00) | ||
| Surgery + unknown therapy | 17 (13.18) | 5 (7.94) | 12 (18.19) | 12 (19.67) | 5 (7.35) | ||
| Primary site, n (%) | .913 | .737 | |||||
| Nasal cavity | 25 (19.38) | 13 (20.63) | 12 (18.18) | 10 (16.39) | 15 (22.06) | ||
| Maxillary sinus | 75 (58.14) | 37 (58.73) | 38 (57.58) | 37 (60.66) | 38 (55.88) | ||
| Other sinuses | 29 (22.48) | 13 (20.63) | 16 (24.24) | 14 (22.95) | 15 (22.06) | ||
| Tumor stage, n (%) | .061 | .022 | |||||
| 1 | 16 (12.40) | 12 (19.05) | 4 (6.06) | 3 (4.92) | 13 (19.12) | ||
| 2 | 18 (13.95) | 11 (17.46) | 7 (10.61) | 7 (11.48) | 11 (16.18) | ||
| 3 | 31 (24.03) | 14 (22.22) | 17 (25.76) | 20 (32.79) | 11 (16.18) | ||
| 4 | 64 (49.61) | 26 (41.27) | 38 (57.58) | 31 (50.82) | 33 (48.53) | ||
| Tumor differentiation, n (%) | .817 | .720 | |||||
| Poorly | 47 (36.43) | 23 (36.51) | 24 (36.36) | 21 (34.43) | 26 (38.24) | ||
| Moderately | 42 (32.56) | 19 (30.16) | 23 (34.85) | 22 (36.07) | 20 (29.41) | ||
| Well | 40 (31.01) | 21 (33.33) | 19 (28.79) | 18 (29.51) | 22 (32.35) | ||
| N classification, n (%) | .142 | .071 | |||||
| N0 | 67 (51.94) | 34 (53.97) | 33 (50.00) | 35 (57.38) | 32 (52.46) | ||
| N1 | 27 (20.93) | 16 (25.40) | 11 (16.67) | 11 (18.03) | 16 (26.23) | ||
| N2 | 11 (8.53) | 2 (3.17) | 9 (13.64) | 8 (13.11) | 3 (4.92) | ||
| Unknown | 24 (18.60) | 11 (17.46) | 13 (19.70) | 7 (11.48) | 17 (27.87) | ||
| BMI, kg/m2, mean ± SD | 23.75 ± 3.99 | 25.62 ± 4.39 | 21.95 ± 2.52 | <.001 | 23.76 ± 4.63 | 23.74 ± 3.35 | .976 |
| NLR, M (Q1–Q3) | 3.06 (2.58–3.68) | 2.80 (2.35–3.36) | 3.41 (2.78–4.03) | <.001 | 3.41 (2.93–4.23) | 2.75 (2.28–3.35) | <.001 |
| PLR, M (Q1–Q3) | 138.38 (111.88–167.57) | 129.44 (110.75–162.76) | 142.15 (113.53–172.18) | .412 | 153.29 (123.18–188.85) | 125.52 (108.67–149.84) | .001 |
| LMR, M (Q1–Q3) | 3.60 (2.45–5.43) | 3.90 (2.70–5.52) | 3.01 (2.21–4.77) | .099 | 3.37 (2.27–4.92) | 3.71 (2.68–5.60) | .439 |
| ALI, M (Q1–Q3) | 27.71 (22.97–34.08) | — | — | — | 25.59 (19.79–30.23) | 29.55 (25.35–37.47) | <.001 |
| SII, M (Q1–Q3) | 779.38 (601.53–921.93) | 679.56 (586.11–842.18) | 869.53 (715.08–942.34) | .003 | — | — | — |
| AGR, M (Q1–Q3) | 1.32 (1.10–1.58) | 1.45 (1.24–1.63) | 1.18 (1.00–1.43) | <.001 | 1.27 (1.00–1.57) | 1.33 (1.14–1.59) | .345 |
| PNI, M (Q1–Q3) | 37.56 (31.86–42.05) | 40.52 (37.11–43.70) | 33.13 (27.39–40.27) | <.001 | 37.03 (31.37–42.95) | 38.27 (37.97–41.85) | .996 |
Abbreviations: AGR, albumin to globulin ratio; ALI, advanced lung cancer inflammation index; BMI, body mass index; LMR, lymphocyte‐to‐monocyte ratio; NLR, neutrophil‐to‐lymphocyte ratio; PLR, platelet‐to‐lymphocyte ratio; PNI, prognostic nutritional index; Q, quartile; SD, standard deviation; SII, systemic immune‐inflammation index.
FIGURE 1.
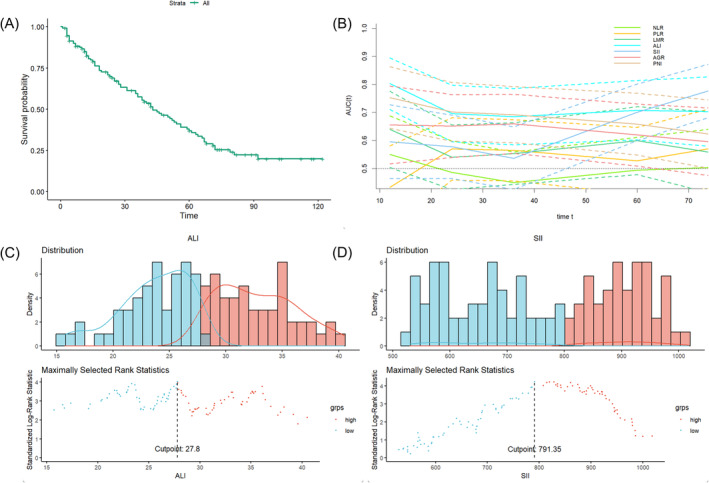
Survival curves and selection of cut‐off values. (A) Survival curves of the overall patients; (B) time‐dependent receiver operating characteristic curves; (C) maximum selection rank statistic to compute the optimal cutoff value of ALI; (D) maximum selection rank statistic to compute the optimal cutoff value of SII.
3.2. Optimal prognostic nutritional and inflammatory indicators and cut‐off values
The time‐dependent ROC results are shown in Figure 1B, and the area under the curve (AUC) values for the 5‐year survival prediction for the different indicators are displayed in Table 2. Among them, the 5‐year AUC values of 0.708 (0.602–0.814) and 0.700 (0.599–0.801) for the ALI and SII, respectively, were superior to the other variables; thus, we selected the ALI and SII for subsequent analyses. We calculated the optimal cutoff values using the rank statistic of maximum selection, which showed cutoff values of 27.80 and 791.35 for the ALI and SII, respectively (Figure 1C,D). Using the cutoff values, patients were categorized into high‐ and low‐value groups to compare their baseline information (Table 1). In the ALI group, statistically significant differences were observed between age, smoking history, BMI, NLR, SII, AGR, and PNI. In the SII group, there were statistically significant differences between tumor stage, smoking history, NLR, PLR, and ALI.
TABLE 2.
AUC values for the 5‐year survival prediction of the different indicators.
| Indicators | AUC | 95% CI |
|---|---|---|
| NLR | 0.493 | 0.375–0.611 |
| PLR | 0.527 | 0.408–0.647 |
| LMR | 0.599 | 0.478–0.720 |
| ALI | 0.708 | 0.602–0.814 |
| SII | 0.700 | 0.599–0.801 |
| AGR | 0.620 | 0.509–0.730 |
| PNI | 0.658 | 0.548–0.769 |
Abbreviations: AGR, albumin to globulin ratio; ALI, advanced lung cancer inflammation index; AUC, area under the curve; LMR, lymphocyte‐to‐monocyte ratio; NLR, neutrophil‐to‐lymphocyte ratio; PLR, platelet‐to‐lymphocyte ratio; PNI, prognostic nutritional index; SII, systemic immune‐inflammation index.
3.3. Nomogram construction
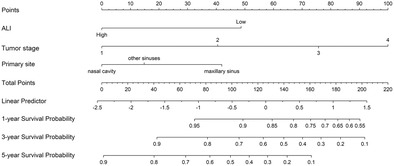
The results of the univariate Cox analysis for all variables are presented in Table 3; age, primary site, tumor stage, ALI, and SII had a remarkable effect on patient survival. Subsequently, we performed a multivariate COX analysis; primary site, tumor stage, and ALI were independent risk factors affecting patient prognosis. Therefore, we constructed a nomogram (Figure 2). The largest percentage in the nomogram was T stage, followed by ALI and primary site. The 1‐, 3‐, and 5‐year AUCs calculated using time‐dependent ROC curves were 0.870, 0.847, and 0.867, respectively; the calibration curves showed that the model had excellent predictive accuracy; the decision curves indicated that patients could benefit from the model (Figure 3). After excluding patients for which updated survivals could not be determined because of loss to follow‐up, the results of the sensitivity analyses showed similar survival outcomes (Table S1).
TABLE 3.
The univariate and multivariate cox analysis.
| Variables | Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | SE | p‐value | HR | 95% CI | SE | p‐value | |
| Gender (male) | 1.73 | 0.99–2.99 | 0.28 | .051 | ||||
| Age | 1.03 | 1.00–1.05 | 0.01 | .023 | 1.02 | 0.99–1.04 | 0.01 | .156 |
| Age stratification (≥65) | 1.46 | 0.93–2.27 | 0.23 | .097 | ||||
| Smoking (yes) | 1.48 | 0.95–2.29 | 0.22 | .081 | ||||
| Alcohol (yes) | 0.92 | 0.59–1.42 | 0.22 | .707 | ||||
| Treatment regimen | ||||||||
| Surgery | .219 | |||||||
| Surgery + radiotherapy | 0.684 | 0.380–1.231 | 0.300 | .205 | ||||
| Surgery + chemoradiotherapy | 1.078 | 0.608–1.910 | 0.293 | .797 | ||||
| Surgery + unknown therapy | 1.387 | 0.650–2.959 | 0.387 | .397 | ||||
| Primary site | ||||||||
| Nasal cavity | .009 | .024 | ||||||
| Maxillary sinus | 2.41 | 1.28–4.55 | 0.33 | .007 | 2.22 | 1.15–4.28 | 0.34 | .017 |
| Other sinuses | 1.38 | 0.66–2.86 | 0.37 | .392 | 1.31 | 0.61–2.81 | 0.39 | .488 |
| Tumor stage | ||||||||
| 1 | <.001 | <.001 | ||||||
| 2 | 2.58 | 0.91–7.33 | 0.53 | .075 | 2.18 | 0.76–6.22 | 0.54 | .146 |
| 3 | 5.78 | 2.15–15.55 | 0.51 | <.001 | 3.99 | 1.46–10.91 | 0.51 | .007 |
| 4 | 9.16 | 3.53–23.76 | 0.49 | <.001 | 6.73 | 2.56–17.69 | 0.49 | <.001 |
| Tumor differentiation | ||||||||
| Poorly | .347 | |||||||
| Moderately | 1.29 | 0.77–2.16 | 0.26 | .330 | ||||
| Well | 0.86 | 0.50–1.48 | 0.28 | .590 | ||||
| N classification | ||||||||
| N0 | .476 | |||||||
| N1 | 0.715 | 0.408–1.254 | 0.287 | .242 | ||||
| N2 | 0.957 | 0.408–2.249 | 0.436 | .921 | ||||
| Unknown | 0.195 | 0.358–1.233 | 0.316 | .195 | ||||
| ALI | 0.95 | 0.92–0.97 | 0.01 | <.001 | 0.47 | 0.29–0.77 | 0.25 | .002 |
| SII | 1.00 | 1.00–1.00 | 0.00 | .002 | 1.55 | 0.94–2.54 | 0.25 | .086 |
Abbreviations: ALI, advanced lung cancer inflammation index; CI, confidence interval; HR, hazards ratio; SE, standard error; SII, systemic immune‐inflammation index.
FIGURE 2.
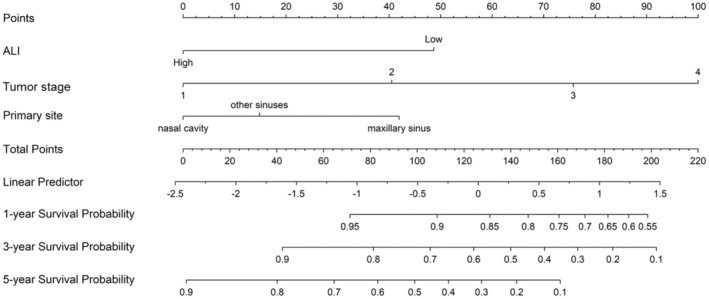
Nomogram for predicting the 1‐, 3‐, and 5‐year prognosis. ALI, advanced lung cancer inflammation index.
FIGURE 3.
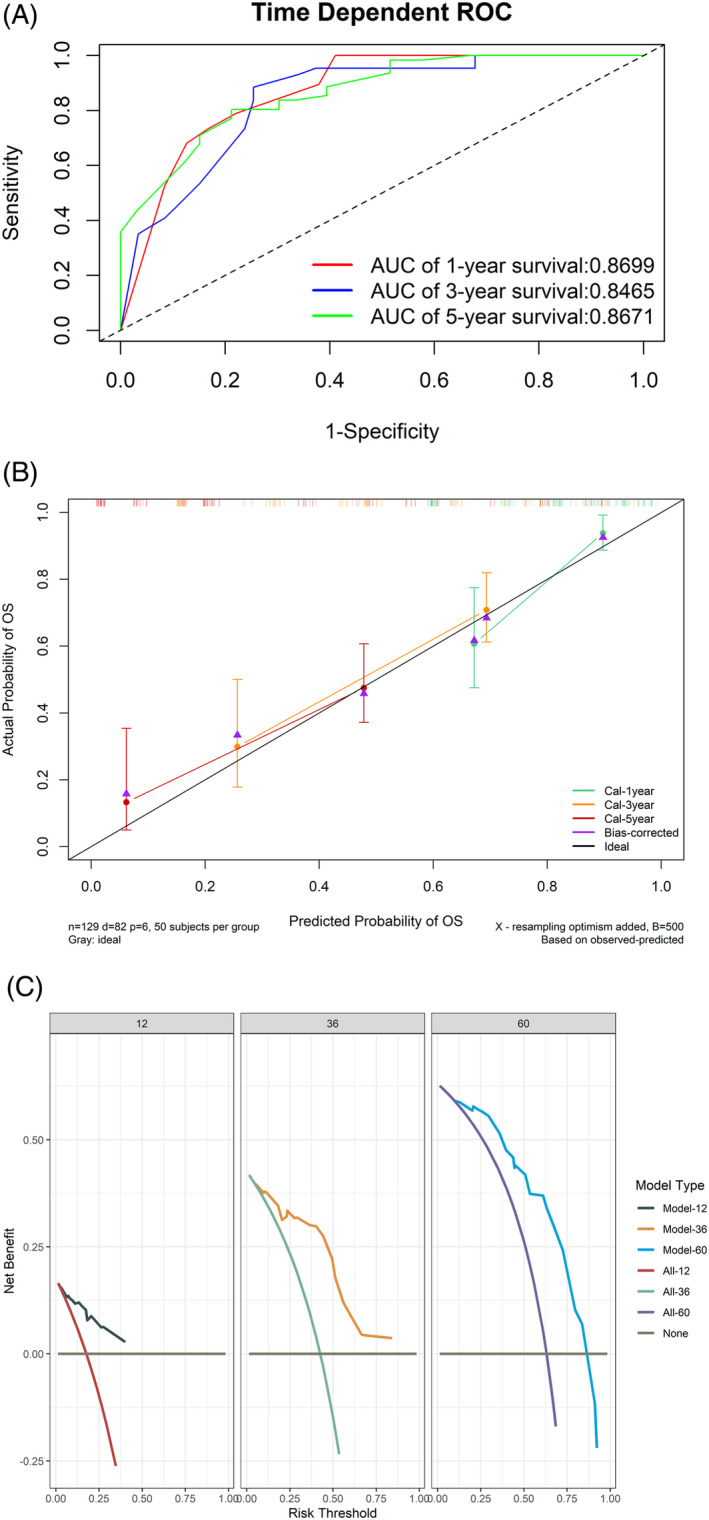
(A) The receiver operating characteristic curve for predicting the prognosis. (B) The calibration curve. (C) The decision curve for 12, 36, and 60 months. AUC, area under the curve.
3.4. Correlation between the ALI, SII, and indicators affecting prognosis
Although age, BMI, tumor stage, and primary site were correlated with patient prognosis, the relationships between the SII and ALI and these indicators were not clear; therefore, we analyzed the correlation between these variables and the SII and ALI. Box and line plot results indicated that the higher the tumor stage, the lower the patient ALI value, and patients with tumor stage 4 had lower ALI values than those with stage 1 (Figure S2A). SII values were not statistically different, although they tended to increase with a higher tumor stage (Figure S2B). In contrast, the primary site did not show a significant upward or downward trend for either variable (Figure S2C,D). The linear analysis revealed a marked negative correlation between the ALI, age, and SII; a positive correlation between the ALI and BMI; and a positive correlation between the SII and BMI (Figure S3).
3.5. Relationship between ALI, SII, and patient HRs
Survival curve analysis after grouping showed that patients with low ALI and high SII values had worse prognoses (Figure S4A,B). The relationship between the ALI, SII, and patient HRs were determined using RCS curves. The results indicated that as the ALI increased, the HR considerably decreased (Figure S4C). We achieved more consistent results in the analysis for the sex and age subgroups (Figure S4D,E). For the SII, the RCS curves showed increasing HRs with increasing SII values, although the increase in the HRs became insignificant as the SII values reached higher values (Figure S4F). This result also concurred with the results analyzed in the sex subgroups; however, it was poorly represented in the age subgroups (Figure S4G,H).
3.6. Subgroup analysis based on the ALI and SII
Subgroup analyses were performed for all patient categorical variables after grouping using the cutoff values. The results showed that the prognostic value for the ALI was remarkable in all sex groups of patients aged <65 years, with a primary site in the sinuses, who received comprehensive treatment (Figure 4). Conversely, the SII had a prognostic value in all age and sex groups of patients. However, following grouping by the remaining indicators, the overall performance of the SII was worse than that of the ALI (Table S2).
FIGURE 4.
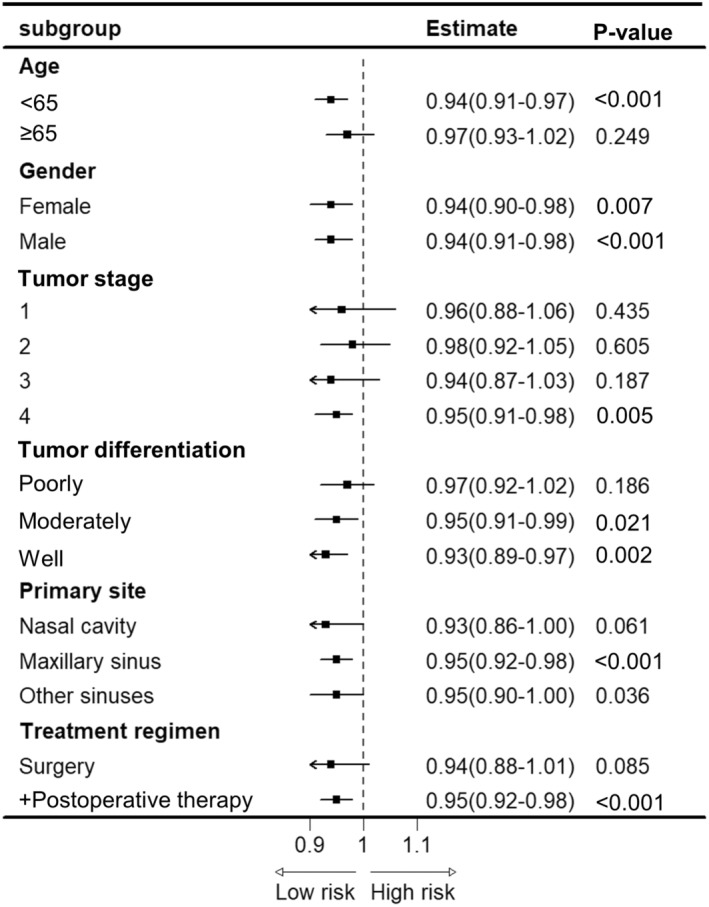
Stratification analysis of ALI.
4. DISCUSSION
This study identified the ALI and SII as potential prognostic indicators for postoperative patients with SNSCC. Many previous studies have indicated that inflammatory cells secrete various cytokines and chemokines, which not only promote tumor cell proliferation and survival but also enhance tumor invasiveness and metastasis by promoting angiogenesis and stromal remodeling. 19 , 20 Simultaneously, the inflammatory response may interfere with the body's immune surveillance of the tumor and anti‐tumor immune response. 21 Nutritional intake is critical for maintaining normal physiological functions and a healthy immune system. Malnutrition weakens the immune system, reduces resistance to tumors and infections, slows wound healing, and increases the risk of postoperative complications. 22 The inflammatory and nutritional status of the body can be determined by a variety of systemic inflammatory and nutritional indicators that are closely related to the occurrence, development, and prognosis of many tumors.
The ALI was initially proposed by Jafri et al. 11 to assess the degree of systemic inflammation and nutritional status of patients with advanced lung cancer. Follow‐up studies have found that the ALI has prognostic value for patients with B‐cell lymphoma, esophageal, gastric cancers, head and neck squamous carcinoma. 23 , 24 , 25 , 26 In patients with head and neck squamous carcinoma, Jank et al. 16 found that a low ALI value was considerably associated with lower overall survival. Brkic et al. 27 found a relationship between the ALI and overall survival in patients with SNSCC, which is consistent with our results; they determined a cutoff value of 29.5, which is slightly higher than that determined in this study. The ALI consists of BMI, albumin, and NLR results, all three of which are associated with prognosis in head and neck cancers. 28 , 29 BMI and serum albumin are traditional nutritional indices, whereas NLR consists mainly of neutrophils and lymphocytes.
Thus, a low ALI level implies low albumin levels, lymphopenia, and neutrophilia. Most albumin reductions in patients with cancer are due to increased degradation, weight loss, and inflammation, and this nutritional imbalance is an important cause of malignant diseases. 30 , 31 Additionally, studies have shown that albumin levels also reflect muscle mass 32 Although skeletal muscle mass was not evaluated in this study, prior research has found that sarcopenia affects overall survival in patients with sinonasal cancer. 33 Lymphocytes play a crucial role in the immune system because they induce cytotoxic cell death, which inhibits tumor cell survival and proliferation. 34 This indicates that the fewer the lymphocytes, the poorer the immune function of the body. Neutrophils support the growth of tumor cells and resist apoptosis by releasing large amounts of tumor necrosis factor locally, and the angiogenic factors they produce promote angiogenesis, providing a pathway for tumor cells to metastasize to distant sites. 35 , 36 Additionally, neutrophils inhibit the functions of lymphocytes, effector T cells, and natural killer cells, thereby reducing the immune ability of the body. 34 Our study also found that the higher the tumor stage, the lower the ALI value. This trend clearly illustrated the relationship between tumor progression and the ALI.
The SII, an important immunoinflammatory indicator, was developed and used for the prognostic evaluation of patients with hepatocellular carcinoma by Hu et al. 14 In a recent study, Akkas et al. 17 found that a high SII value was an independent adverse prognostic factor affecting overall survival and disease‐free survival in patients with head and neck tumors. In addition to lymphocytes and neutrophils, the SII is mainly composed of platelets. Physiologically, platelets play various important roles in hemostasis, wound healing, inflammatory responses, angiogenesis, and immunomodulation. Pathologically, similar to neutrophils, platelets interact with circulating tumor cells to form a complex that covers the tumor surface, thereby protecting tumor cells from removal by the immune system, and helps them adhere to the vessel wall. 37 , 38 Thus, a high SII value may imply elevated levels of circulating tumor cells in the blood, as well as decreased immune response capacity. Additionally, our study found a negative correlation between the SII and ALI, although the SII performed poorly in the subgroup analysis and nomogram. We defined a higher cutoff value for the SII compared with other similar studies. This performance of the SII might have been caused by the small sample size, which might have led to an overestimation of the effect of platelets as well as lymphocytes. Taken together, these results suggest that SII may have a lower potential value than the ALI.
Previous studies have found that 42%–77% of patients with advanced cancer do not receive the necessary nutritional support, despite being at high risk of malnutrition. 39 The current philosophy of enhanced recovery after surgery suggests that by addressing nutritional risks during the perioperative period, patients with cancer can enhance their recovery rates post‐surgery and reduce the likelihood of adverse prognostic events. 40 Our study supports this hypothesis. Based on our results, the preoperative identification of inflammatory and nutritional status in patients with SNSCC was of potential clinical significance. This emphasizes that the concept of accelerated rehabilitative surgery is particularly relevant for advanced‐stage populations. This also implies that some patients require more comprehensive treatment strategies, such as preoperative anti‐inflammatory and nutritional supportive therapies. In addition to the ALI, the nomogram also included tumor stage as well as primary site, with tumor stage accounting for the largest proportion. These results are consistent with those of several previous studies. 41 , 42
This study has some limitations. First, due to the retrospective design, it is inevitably subject to potential selection bias and confounding factors; For example, the nomogram lacked information on the patients' surgical margin status due to retrospective data loss. Assessing the surgical margins for SNSCC is challenging because the nasal anatomy lacks a well‐defined “safe border” (1 cm) for surgical treatment. Additionally, the resected pathological tissue is often fragmented and irregular, making positive margins common in clinical practice. This status considerably impacts the prognosis of patients with SNSCC, limiting the reliability of the conclusions of this study. Although the nomogram is easy to calculate, the clinical utility should not be overestimated, and the results should only serve as a therapeutic reference for clinicians. Second, the study was conducted at a single center with a small sample size, resulting in a limited level of evidence that restricts the generalizability of the findings. Finally, while acceptable results were obtained, there is a lack of validation from multicenter prospective studies. We hope that future research will address these issues and provide more reliable and widely applicable conclusions.
5. CONCLUSION
This study investigated the role of systemic inflammation and nutritional indicators in the postoperative prognosis of patients with SNSCC and found that the ALI had prognostic potential. We constructed a nomogram incorporating ALI, tumor stage, and primary site as survival risk factors, which may serve as a helpful reference for clinicians in treatment planning.
FUNDING INFORMATION
National Natural Science Foundation of China, grant/award number: 81770978.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflicts of interest.
Supporting information
TABLE S1. Stratification analysis of SII. HR, hazards ratio; LCI, lower confidence interval; SII, systemic immune‐inflammation index; UCI, upper confidence interval.
TABLE S2. Sensitivity analysis. ALI, advanced lung cancer inflammation index; CI, confidence interval; HR, hazards ratio; SE, standard error; SII, systemic immune‐inflammation index.
FIGURE S1. Kaplan–Meier survival curve for different treatment regimen. CRT, chemoradiotherapy; RT, radiotherapy.
FIGURE S2. Distribution of ALI and SII in different tumor stages and primary sites. (A) ALI in different tumor stages; (B) SII in different tumor stages; (C) ALI in different primary sites; (D) SII in different primary sites *p < .05. ALI, advanced lung cancer inflammation index; SII, systemic immune‐inflammation index.
FIGURE S3. The linear analysis demonstrated the relationship of ALI and SII to other indicators. ALI, advanced lung cancer inflammation index; BMI, body mass index; SII, systemic immune‐inflammation index.
FIGURE S4. Kaplan–Meier survival curve and restricted cubic spline curves based on optimal cutoff values. (A) Kaplan–Meier survival curve for ALI; (B) Kaplan–Meier survival curve for SII; (C) Restricted cubic spline curves for ALI in all patients; (D) ALI in different sex; (E) ALI in age stratification; (F) restricted cubic spline curves for SII in all patients; (G) SII in different sex; (H) SII in age stratification. ALI, advanced lung cancer inflammation index; SII, systemic immune‐inflammation index.
ACKNOWLEDGMENTS
We would like to thank Editage (www.editage.cn) for English language editing. This work was supported by grants from the Program for National Natural Science Foundation of China (81770978).
Wu C, Qi Z, Chen J, et al. Prognostic value of inflammation and nutritional indicators for sinonasal squamous cell carcinoma: A single‐center retrospective study. Laryngoscope Investigative Otolaryngology. 2025;10(1):e70046. doi: 10.1002/lio2.70046
Contributor Information
Longgang Yu, Email: yulonggang@qdu.edu.cn.
Yan Jiang, Email: jiangyanoto@qdu.edu.cn.
REFERENCES
- 1. El‐Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ. WHO Classification of Head and Neck Tumours. WHO; 2017. [Google Scholar]
- 2. Farrell NF, Mace JC, Detwiller KY, et al. Predictors of survival outcomes in sinonasal squamous cell carcinoma: an analysis of the National Cancer Database. Int Forum Allergy Rhinol. 2021;11(6):1001‐1011. doi: 10.1002/alr.22737 [DOI] [PubMed] [Google Scholar]
- 3. Sanghvi S, Khan MN, Patel NR, Yeldandi S, Baredes S, Eloy JA. Epidemiology of sinonasal squamous cell carcinoma: a comprehensive analysis of 4994 patients. Laryngoscope. 2014;124(1):76‐83. doi: 10.1002/lary.24264 [DOI] [PubMed] [Google Scholar]
- 4. Kuo P, Manes RP, Schwam ZG, Judson BL. Survival outcomes for combined modality therapy for Sinonasal undifferentiated carcinoma. Otolaryngol Head Neck Surg. 2017;156(1):132‐136. doi: 10.1177/0194599816670146 [DOI] [PubMed] [Google Scholar]
- 5. Birkenbeuel JL, Goshtasbi K, Adappa ND, Palmer JN, Tong CCL, Kuan EC. Recurrence rates of de‐novo versus inverted papilloma‐transformed sinonasal squamous cell carcinoma: a meta‐analysis. Rhinology. 2022;60(6):402‐410. doi: 10.4193/Rhin22.187 [DOI] [PubMed] [Google Scholar]
- 6. Orlandi E, Iacovelli NA, Ingargiola R, et al. Treatment options for recurrent anterior Skull Base tumors. Adv Otorhinolaryngol. 2020;84:231‐245. doi: 10.1159/000457942 [DOI] [PubMed] [Google Scholar]
- 7. Mantovani A. Cancer: inflaming metastasis. Nature. 2009;457(7225):36‐37. doi: 10.1038/457036b [DOI] [PubMed] [Google Scholar]
- 8. Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 2019;51(1):27‐41. doi: 10.1016/j.immuni.2019.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hamaker ME, Oosterlaan F, van Huis LH, Thielen N, Vondeling A, van den Bos F. Nutritional status and interventions for patients with cancer – a systematic review. J Geriatr Oncol. 2021;12(1):6‐21. doi: 10.1016/j.jgo.2020.06.020 [DOI] [PubMed] [Google Scholar]
- 10. Planas M, Álvarez‐Hernández J, León‐Sanz M, Celaya‐Pérez S, Araujo K, García de Lorenzo A. Prevalence of hospital malnutrition in cancer patients: a sub‐analysis of the PREDyCES® study. Support Care Cancer. 2016;24(1):429‐435. doi: 10.1007/s00520-015-2813-7 [DOI] [PubMed] [Google Scholar]
- 11. Jafri SH, Shi R, Mills G. Advance lung cancer inflammation index (ALI) at diagnosis is a prognostic marker in patients with metastatic non‐small cell lung cancer (NSCLC): a retrospective review. BMC Cancer. 2013;13:158. doi: 10.1186/1471-2407-13-158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ruan GT, Yang M, Zhang XW, et al. Association of systemic inflammation and overall survival in elderly patients with cancer cachexia ‐ results from a multicenter study. J Inflamm Res. 2021;14:5527‐5540. doi: 10.2147/jir.S332408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi. 1984;85(9):1001‐1005. [PubMed] [Google Scholar]
- 14. Hu B, Yang XR, Xu Y, et al. Systemic immune‐inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20(23):6212‐6222. doi: 10.1158/1078-0432.Ccr-14-0442 [DOI] [PubMed] [Google Scholar]
- 15. Jomrich G, Paireder M, Kristo I, et al. High systemic immune‐inflammation index is an adverse prognostic factor for patients with gastroesophageal adenocarcinoma. Ann Surg. 2021;273(3):532‐541. doi: 10.1097/sla.0000000000003370 [DOI] [PubMed] [Google Scholar]
- 16. Jank BJ, Kadletz L, Schnöll J, Selzer E, Perisanidis C, Heiduschka G. Prognostic value of advanced lung cancer inflammation index in head and neck squamous cell carcinoma. Eur Arch Otorhinolaryngol. 2019;276(5):1487‐1492. doi: 10.1007/s00405-019-05381-0 [DOI] [PubMed] [Google Scholar]
- 17. Atasever Akkas E, Erdis E, Yucel B. Prognostic value of the systemic immune‐inflammation index, systemic inflammation response index, and prognostic nutritional index in head and neck cancer. Eur Arch Otorhinolaryngol. 2023;280(8):3821‐3830. doi: 10.1007/s00405-023-07954-6 [DOI] [PubMed] [Google Scholar]
- 18. American Joint Committee on Cancer (AJCC) . Staging Manual. 8th ed. Springer; 2017. [Google Scholar]
- 19. Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124(2):263‐266. doi: 10.1016/j.cell.2006.01.007 [DOI] [PubMed] [Google Scholar]
- 20. Karin M. Nuclear factor‐kappaB in cancer development and progression. Nature. 2006;441(7092):431‐436. doi: 10.1038/nature04870 [DOI] [PubMed] [Google Scholar]
- 21. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883‐899. doi: 10.1016/j.cell.2010.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wu C, Yan X, Xie F, Lai X, Wang L, Jiang Y. Development of a nomogram for predicting pharyngocutaneous fistula based on skeletal muscle mass and systemic inflammation indices. Head Neck. 2024;46(3):571‐580. doi: 10.1002/hed.27614 [DOI] [PubMed] [Google Scholar]
- 23. Park YH, Yi HG, Lee MH, Kim CS, Lim JH. Prognostic value of the pretreatment advanced lung cancer inflammation index (ALI) in diffuse large B cell lymphoma patients treated with R‐CHOP chemotherapy. Acta Haematol. 2017;137(2):76‐85. doi: 10.1159/000452991 [DOI] [PubMed] [Google Scholar]
- 24. Feng JF, Huang Y, Chen QX. A new inflammation index is useful for patients with esophageal squamous cell carcinoma. Onco Targets Ther. 2014;7:1811‐1815. doi: 10.2147/ott.S68084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yin C, Toiyama Y, Okugawa Y, et al. Clinical significance of advanced lung cancer inflammation index, a nutritional and inflammation index, in gastric cancer patients after surgical resection: a propensity score matching analysis. Clin Nutr. 2021;40(3):1130‐1136. doi: 10.1016/j.clnu.2020.07.018 [DOI] [PubMed] [Google Scholar]
- 26. Gaudioso P, Borsetto D, Tirelli G, et al. Advanced lung cancer inflammation index and its prognostic value in HPV‐negative head and neck squamous cell carcinoma: a multicentre study. Support Care Cancer. 2021;29(8):4683‐4691. doi: 10.1007/s00520-020-05979-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brkic FF, Kadletz L, Jank B, Mayer C, Heiduschka G, Brunner M. Impact of pretherapeutic neutrophil‐to‐lymphocyte ratio, serum albumin, body‐mass index, and advanced lung cancer inflammation index on clinical outcome in sinonasal squamous cell carcinoma. J Craniomaxillofac Surg. 2020;48(1):33‐37. doi: 10.1016/j.jcms.2019.11.010 [DOI] [PubMed] [Google Scholar]
- 28. Gama RR, Song Y, Zhang Q, et al. Body mass index and prognosis in patients with head and neck cancer. Head Neck. 2017;39(6):1226‐1233. doi: 10.1002/hed.24760 [DOI] [PubMed] [Google Scholar]
- 29. Lim WS, Roh JL, Kim SB, Choi SH, Nam SY, Kim SY. Pretreatment albumin level predicts survival in head and neck squamous cell carcinoma. Laryngoscope. 2017;127(12):E437‐e442. doi: 10.1002/lary.26691 [DOI] [PubMed] [Google Scholar]
- 30. Hébuterne X, Lemarié E, Michallet M, de Montreuil CB, Schneider SM, Goldwasser F. Prevalence of malnutrition and current use of nutrition support in patients with cancer. JPEN J Parenter Enteral Nutr. 2014;38(2):196‐204. doi: 10.1177/0148607113502674 [DOI] [PubMed] [Google Scholar]
- 31. Argilés JM. The 2015 ESPEN sir David Cuthbertson lecture: inflammation as the driving force of muscle wasting in cancer. Clin Nutr. 2017;36(3):798‐803. doi: 10.1016/j.clnu.2016.05.010 [DOI] [PubMed] [Google Scholar]
- 32. Malietzis G, Johns N, Al‐Hassi HO, et al. Low muscularity and myosteatosis is related to the host systemic inflammatory response in patients undergoing surgery for colorectal cancer. Ann Surg. 2016;263(2):320‐325. doi: 10.1097/sla.0000000000001113 [DOI] [PubMed] [Google Scholar]
- 33. Kim JH, Mualla R, Mace JC, et al. Effect of sarcopenia on survival outcomes in patients with nasopharyngeal and sinonasal cancer. Int Forum Allergy Rhinol. 2023;13(8):1554‐1557. doi: 10.1002/alr.23112 [DOI] [PubMed] [Google Scholar]
- 34. Kataru RP, Ly CL, Shin J, et al. Tumor lymphatic function regulates tumor inflammatory and immunosuppressive microenvironments. Cancer Immunol Res. 2019;7(8):1345‐1358. doi: 10.1158/2326-6066.Cir-18-0337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Atretkhany KN, Gogoleva VS, Drutskaya MS, Nedospasov SA. Distinct modes of TNF signaling through its two receptors in health and disease. J Leukoc Biol. 2020;107(6):893‐905. doi: 10.1002/jlb.2mr0120-510r [DOI] [PubMed] [Google Scholar]
- 36. Donskov F. Immunomonitoring and prognostic relevance of neutrophils in clinical trials. Semin Cancer Biol. 2013;23(3):200‐207. doi: 10.1016/j.semcancer.2013.02.001 [DOI] [PubMed] [Google Scholar]
- 37. Tieken C, Versteeg HH. Anticoagulants versus cancer. Thromb Res. 2016;140(Suppl 1):S148‐S153. doi: 10.1016/s0049-3848(16)30114-1 [DOI] [PubMed] [Google Scholar]
- 38. Franchini M, Montagnana M, Favaloro EJ, Lippi G. The bidirectional relationship of cancer and hemostasis and the potential role of anticoagulant therapy in moderating thrombosis and cancer spread. Semin Thromb Hemost. 2009;35(7):644‐653. doi: 10.1055/s-0029-1242718 [DOI] [PubMed] [Google Scholar]
- 39. Arribas L, Hurtós L, Sendrós MJ, et al. NUTRISCORE: a new nutritional screening tool for oncological outpatients. Nutrition. 2017;33:297‐303. doi: 10.1016/j.nut.2016.07.015 [DOI] [PubMed] [Google Scholar]
- 40. Ljungqvist O. ERAS—enhanced recovery after surgery: moving evidence‐based perioperative care to practice. J Parenter Enteral Nutr. 2014;38(5):559‐566. doi: 10.1177/0148607114523451 [DOI] [Google Scholar]
- 41. Li W, You J, Xue H, Chao C. Factors contributing to diagnosis and prognosis in sinonasal malignancies. J Immunol Res. 2022;2022:4406838. doi: 10.1155/2022/4406838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Michel J, Fakhry N, Mancini J, et al. Sinonasal squamous cell carcinomas: clinical outcomes and predictive factors. Int J Oral Maxillofac Surg. 2014;43(1):1‐6. doi: 10.1016/j.ijom.2013.07.741 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1. Stratification analysis of SII. HR, hazards ratio; LCI, lower confidence interval; SII, systemic immune‐inflammation index; UCI, upper confidence interval.
TABLE S2. Sensitivity analysis. ALI, advanced lung cancer inflammation index; CI, confidence interval; HR, hazards ratio; SE, standard error; SII, systemic immune‐inflammation index.
FIGURE S1. Kaplan–Meier survival curve for different treatment regimen. CRT, chemoradiotherapy; RT, radiotherapy.
FIGURE S2. Distribution of ALI and SII in different tumor stages and primary sites. (A) ALI in different tumor stages; (B) SII in different tumor stages; (C) ALI in different primary sites; (D) SII in different primary sites *p < .05. ALI, advanced lung cancer inflammation index; SII, systemic immune‐inflammation index.
FIGURE S3. The linear analysis demonstrated the relationship of ALI and SII to other indicators. ALI, advanced lung cancer inflammation index; BMI, body mass index; SII, systemic immune‐inflammation index.
FIGURE S4. Kaplan–Meier survival curve and restricted cubic spline curves based on optimal cutoff values. (A) Kaplan–Meier survival curve for ALI; (B) Kaplan–Meier survival curve for SII; (C) Restricted cubic spline curves for ALI in all patients; (D) ALI in different sex; (E) ALI in age stratification; (F) restricted cubic spline curves for SII in all patients; (G) SII in different sex; (H) SII in age stratification. ALI, advanced lung cancer inflammation index; SII, systemic immune‐inflammation index.


