Abstract
Background
The prevalence of type 2 diabetes (T2D) and asthma is rising, yet evidence regarding the relationship between T2D and asthma, particularly in the context of genetic predispositions, remains limited.
Methods
This study utilized data from the UK Biobank longitudinal cohort, involving 388,775 participants. A polygenic risk score (PRS) for asthma was derived from genome-wide association studies summary. Cox regression models were used to assess the association between T2D and asthma, incorporating the asthma PRS.
Results
Over a median follow-up of 13.62 years, 10,211 asthma cases were documented. After adjusting for age, sex, current smoking status, and other confounding variables, T2D was significantly associated with an increased risk of developing asthma (Hazard Ratios [HR] 1.16, 95% confidence interval [CI] 1.06–1.26). This association remained significant after further adjustments for genetic susceptibility to asthma. Furthermore, T2D increased the risk of developing asthma across both high and low genetic risk groups.
Conclusions
T2D is associated with an increased risk of developing asthma, irrespective of genetic susceptibility. These findings underscore the importance of incorporating glucose regulation strategies into asthma prevention efforts.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12889-024-21266-2.
Keywords: Type 2 diabetes, Asthma, Polygenetic risk score
Background
Type 2 diabetes (T2D) is a major global public health issue, with its prevalence increasing rapidly worldwide [1]. According to the International Diabetes Federation Diabetes Atlas Tenth Edition, the global diabetes population has quadrupled from 1980 to 2021, reaching approximately 537 million people [2], with T2D accounting for nearly 95% of these cases. T2D, a multisystemic disease affecting numerous organs, has been hypothesized to be a potential risk factor for development of asthma. This proposed link may be due to the association between chronic hyperglycemia or insulin resistance and reduced pulmonary reserve [3], as well as impaired pulmonary function [4, 5].
While longitudinal studies have shown a significant association between T2D and an increased risk of asthma [6, 7], the nature of this relationship remains debated. Some studies have reported no significant relationship between T2D and asthma risk [8–10], highlighting the complexity of the relationship between T2D and asthma. This inconsistency underscores the need for more nuanced research, especially regarding potential confounding factors such as genetic predisposition to asthma.
To address these gaps, our study aimed to exam the association between T2D and asthma in an observational analysis while accounting for genetic predisposition to asthma using a polygenic risk score (PRS).
Methods
Study design and participants
We conducted an observational analysis to examine the association between T2D and asthma risk and to assess whether this association persisted when accounting for genetic risk factors for asthma. The UK Biobank served as our primary data source for this study, providing a comprehensive biomedical database for population health and genetic research. From 2006 to 2010, over 500,000 participants aged 40–69 years were recruited from 22 centers across the UK [11]. These participants completed questionnaires, underwent physical measurements, and provided blood samples. The UK Biobank study was approved by the Northwest Multicenter Research Ethics Committee (REC reference for UK Biobank 11/NW/0382), and all participants provided written informed consent.
From an initial cohort of 502,366 participants, we excluded those with a prior diagnosis of asthma (n = 61,023) and those with other forms of diabetes (n = 2,030) at baseline. We further excluded participants with new-onset T2D (n = 18,905) during follow-up, those missing glycosylated hemoglobin (HbA1c) data at baseline (n = 28,046), and those missing PRS data for asthma (n = 3,587), resulting in a final sample of 388,775 participants for analyses (Supplementary Figure S1).
Definition of T2D
T2D was defined based on the following criteria: (1) diagnoses recorded in hospital inpatients (International Classification of Diseases-Tenth Revision [ICD10 codes] [E11-E14]), death registry (ICD-10 codes [E11-E14]), or self-report; (2) HbA1c level ≥ 48 mmol/mol; or (3) used antidiabetic drugs at baseline. The field IDs of the exposures are presented in Supplementary Table S1.
Definition of asthma
The primary outcome, asthma, was diagnosed based on hospital admissions and cause of death registry records across England, Scotland, and Wales. Asthma diagnoses were confirmed using hospital records or as listed in cause of death registries, using ICD-10 code J45-J46. Detailed field IDs of asthma are presented in Supplementary Table S1. The follow-up period was from recruitment to the earliest occurrence of asthma, death, or loss of follow-up.
Assessment of covariates
Covariates for this study were collected at baseline. These covariates included age, sex, ethnicity, education levels, Townsend Deprivation Index (TDI), body mass index (BMI), smoking status, alcohol intake, physical activity, systolic blood pressure (SBP), diastolic blood pressure (DBP), nitrogen dioxide air pollution (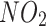 ), particulates matter with an aerodynamic diameter ≤ 2.5 μm (
), particulates matter with an aerodynamic diameter ≤ 2.5 μm (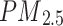 ), family history of bronchitis/emphysema, the hypertension status, and dyslipidaemia status. Ethnicity was categorised as White or other. Education level was categorised as college or university degree versus other. Smoking status was categorised as current smokers and others. Alcohol intake was categorized as current drinkers and others. Socioeconomic status was measured using the TDI, a composite score based on the participants’ residential postcode at recruitment and categorised by quartiles. Lower TDI values indicate higher socioeconomic levels. Physical activity was categorized as low or high. Participants who met the 2017 UK physical activity guidelines of 150 min of walking or moderate activity or 75 min of vigorous activity per week were assigned to the high physical activity group. Long-term exposure to
), family history of bronchitis/emphysema, the hypertension status, and dyslipidaemia status. Ethnicity was categorised as White or other. Education level was categorised as college or university degree versus other. Smoking status was categorised as current smokers and others. Alcohol intake was categorized as current drinkers and others. Socioeconomic status was measured using the TDI, a composite score based on the participants’ residential postcode at recruitment and categorised by quartiles. Lower TDI values indicate higher socioeconomic levels. Physical activity was categorized as low or high. Participants who met the 2017 UK physical activity guidelines of 150 min of walking or moderate activity or 75 min of vigorous activity per week were assigned to the high physical activity group. Long-term exposure to 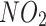 and PM2.5 were measured using a land use regression model developed for the European Study of Cohort for Air Pollution Effects (ESCAPE) [12]. A family history of bronchitis/emphysema was assessed through self-reported data. Specifically, participants completed a touchscreen questionnaire that asked, ‘Has/did your father ever suffer from? (You can select more than one answer)’ which allowed participants to select whether their father had a list of disorders, including chronic bronchitis/emphysema. A similar question was posed regarding the health condition of their mothers and siblings. Dyslipidaemia was defined as triglycerides ≥ 1.69 mmol/L or high-density lipoprotein cholesterol < 1 mmol/L for males and < 1.3 mmol/L for females.
and PM2.5 were measured using a land use regression model developed for the European Study of Cohort for Air Pollution Effects (ESCAPE) [12]. A family history of bronchitis/emphysema was assessed through self-reported data. Specifically, participants completed a touchscreen questionnaire that asked, ‘Has/did your father ever suffer from? (You can select more than one answer)’ which allowed participants to select whether their father had a list of disorders, including chronic bronchitis/emphysema. A similar question was posed regarding the health condition of their mothers and siblings. Dyslipidaemia was defined as triglycerides ≥ 1.69 mmol/L or high-density lipoprotein cholesterol < 1 mmol/L for males and < 1.3 mmol/L for females.
PRS for asthma
We implemented a standard quality control pipeline, excluding SNPs with a genotyping call rate below 0.99, those that deviated from Hardy-Weinberg Equilibrium (P < 10− 5), and those with a minor allele frequency (MAF) below 0.01. Additionally, individuals with a low call rate (< 0.98) were excluded. The PRS were derived with PRSice [13], a software tool that aggregates trait-associated alleles across multiple genetic loci, and typically weighted by effect sizes estimated from a genome-wide association study. To calculate the asthma PRS, we utilized GWAS summary comprising 2.83 million SNPs, derived from a meta-analysis that included 10,074 asthma cases and 103,164 controls from ethnically diverse populations [14]. We then divided the population into two groups, low genetic risk and high genetic risk, based on whether their PRS was below or above the median PRS value of asthma. The field IDs of the covariates are presented in Supplementary Table S1. Detailed information on the calculation of PRS is presented in Appendix 1.
Statistical analyses
Baseline variates were presented as means (standard deviation, [SD]) for continuous variables and frequency (percentages) for categorical variables. Continuous variables were assessed for statistical differences using Student’s t-test, and categorical variables were evaluated for differences using the 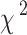 test. We used the Cox proportional hazards model to estimate hazard ratios and 95% confidence intervals (CI) of asthma incidence. Three models were generated for analysis: Model 1 adjusted for age and sex; Model 2 further adjusted for ethnicity, education levels, TDI, BMI (continuous), current smoking, current alcohol drinking, physical activity, family history of bronchitis/emphysema, hypertension status, dyslipidemia status,
test. We used the Cox proportional hazards model to estimate hazard ratios and 95% confidence intervals (CI) of asthma incidence. Three models were generated for analysis: Model 1 adjusted for age and sex; Model 2 further adjusted for ethnicity, education levels, TDI, BMI (continuous), current smoking, current alcohol drinking, physical activity, family history of bronchitis/emphysema, hypertension status, dyslipidemia status, 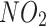 and PM2.5; Model 3 further adjusted for the PRS for asthma. The covariates included in the analysis were selected based on the relevant literature [7, 8, 15] and data availability. In addition, we further explored the association between T2D and asthma incidence in participants with different levels of genetic susceptibility to asthma (low and high genetic risk).
and PM2.5; Model 3 further adjusted for the PRS for asthma. The covariates included in the analysis were selected based on the relevant literature [7, 8, 15] and data availability. In addition, we further explored the association between T2D and asthma incidence in participants with different levels of genetic susceptibility to asthma (low and high genetic risk).
To enhance the comprehensiveness of our study, we conducted several sensitivity analyses. We excluded participants diagnosed with asthma within the first year of follow-up to mitigate potential reverse causality, as well as those with a family history of chronic bronchitis/emphysema. Additionally, we used the Fine-Gray sub-distribution hazard model to account for mortality as a competing event [16]. We also calculate the cumulative risk of asthma incidence among different glycemic status in Kaplan-Meier curves, which were assessed using the log-rank test. What’s more, we used logistic regression to examine the relationship between the PRS for asthma and T2D. All analyses were performed using R software (version 4.2.0). All statistical tests were two-tailed, and statistical significance was set at a P-value of less than 0.05.
Results
This study included 388,775 participants, among whom 21,512 (5.53%) were diagnosed with T2D, with a mean age of 56.52 years (standard deviation [SD], 8.07). Participants with T2D were more likely to be male, older, had a higher BMI, and have a family history of chronic bronchitis/emphysema, as well as history of hypertension, dyslipidemia (Table 1). And the distribution of the PRS for asthma is shown in Figure S2.
Table 1.
Baseline characteristics of participants stratified by T2D status at baseline
| Variables | Overall | Non-T2D | T2D | P |
|---|---|---|---|---|
| Number of participants | 388,775 | 367,263 | 21,512 | |
| Age at baseline (years) | 56.52 (8.07) | 56.34 (8.09) | 59.66 (7.15) | < 0.001 |
| Male, n (%) | 177,759 (45.72) | 164,220 (44.71) | 13,539 (62.94) | < 0.001 |
| Ethnicity, white, n (%) | 370,556 (95.75) | 351,882 (96.24) | 18,674 (87.51) | < 0.001 |
| College/university degree, n (%) | 127,294 (33.12) | 122,204 (33.64) | 5,090 (24.22) | < 0.001 |
| Townsend Deprivation Index, n (%) | < 0.001 | |||
| Q1 (least deprived) | 97,295 (25.03) | 93,508 (25.46) | 3,787 (17.60) | |
| Q2 | 97,225 (25.01) | 92,792 (25.27) | 4,433 (20.61) | |
| Q3 | 97,159 (24.99) | 91,603 (24.94) | 5,556 (25.83) | |
| Q4 (most deprived) | 97,096 (24.97) | 89,360 (24.33) | 7,736 (35.96) | |
BMI (kg/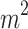 ) ) |
27.15 (4.58) | 26.92 (4.40) | 31.15 (5.68) | < 0.001 |
| Current smoker, n (%) | 40,384 (10.44) | 38,009 (10.40) | 2,375 (11.15) | < 0.001 |
| Current drinker, n (%) | 273,238 (70.43) | 261,562 (71.36) | 11,676 (54.55) | < 0.001 |
| Regularly physical activity, n (%) | 258,984 (82.08) | 246,448 (82.49) | 12,536 (74.93) | < 0.001 |
| Family history of chronic bronchitis/emphysema, n (%) | 57,536 (14.80) | 54,047 (14.72) | 3,489 (16.22) | < 0.001 |
| Glucose (mmol/L) | 5.08 (1.13) | 4.95 (0.64) | 7.37 (3.24) | < 0.001 |
| HbA1c (mmol/mol) | 35.71 (6.14) | 34.81 (3.61) | 51.83 (14.39) | < 0.001 |
| Systolic blood pressure (mmHg) | 139.54 (19.69) | 139.29 (19.73) | 143.67 (18.68) | < 0.001 |
| Diastolic blood pressure (mmHg) | 82.08 (10.67) | 82.09 (10.69) | 82.04 (10.36) | 0.565 |
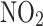 (μg⁄ (μg⁄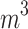 ) ) |
26.55 (7.55) | 26.48 (7.54) | 27.68 (7.75) | < 0.001 |
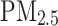 (μg⁄ (μg⁄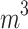 ) ) |
9.97 (1.05) | 9.96 (1.05) | 10.11 (1.06) | < 0.001 |
| Hypertension status at baseline, n (%) | 106,715 (27.45) | 92,098 (25.08) | 14,617 (67.95) | < 0.001 |
| Dyslipidemia status at baseline, n (%) | 170,314 (43.81) | 156,287 (42.55) | 14,027 (65.21) | < 0.001 |
| Forced expiratory volume in 1 s (Litres) | 2.90 (0.77) | 2.90 (0.78) | 2.72 (0.73) | < 0.001 |
| Forced vital capacity (Litres) | 3.81 (0.98) | 3.83 (0.98) | 3.59 (0.91) | < 0.001 |
Data are presented as mean (SD) for continuous variables and n (%) for categorical variables. 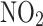 , nitrogen dioxide air pollution;
, nitrogen dioxide air pollution; 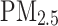 , particulates matter with an aerodynamic diameter ≤ 2.5 μm
, particulates matter with an aerodynamic diameter ≤ 2.5 μm
During a median follow-up period of 13.62 years, 10,211 participants were diagnosed with asthma, with 9,277 (90.85%) cases occurring in the non-T2D group and 934 (9.15%) cases in the T2D group. Participants with T2D exhibited a higher risk of asthma (Hazard Ratios [HR] 1.74, 95% Confidence Interval [CI] 1.63–1.87) in Model 1 (Table 2). The association remained significant after additional adjustments in Model 3, with a HR of 1.15 (95%CI 1.06–1.26). The higher cumulative incidence of asthma among individuals with T2D, compared to those without, persisted during follow-up, as illustrated in the Kaplan-Meier plot (Supplementary Figure S3).
Table 2.
Association between glycemic status at baseline and risk of asthma
| Category | N | Cases/Person-years | Model 1 h (95% CI) | Model 2 h (95% CI) | Model 3 h (95% CI) |
|---|---|---|---|---|---|
| Non-T2D | 367,263 | 9,277/ 4,853,636 | Reference | Reference | Reference |
| T2D | 21,512 | 934/ 267,477 | 1.74 (1.63–1.87) | 1.16 (1.06–1.26) | 1.15 (1.06–1.26) |
HR, hazard ratios. Model 1: adjusted for age and sex. Model 2: further adjusted for ethnicity, education levels, Townsend Deprivation Index, BMI, currently smoking, currently drinking, physical activity, family history of chronic bronchitis/emphysema, hypertension status, dyslipidemia status, 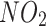 ,
, 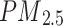 . Model 3: further adjusted for polygenic risk score of asthma
. Model 3: further adjusted for polygenic risk score of asthma
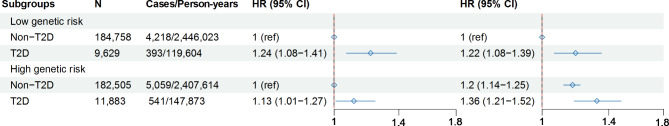
Regardless of whether individuals were in high-risk or low-risk group for asthma, we found an association between T2D and asthma. In the low genetic risk group, T2D was associated with an increased risk of developing asthma (HR 1.24, 95%CI 1.08–1.41). Similarly, in the high genetic risk group, T2D was also associated with an elevated risk of asthma (HR 1.13, 95%CI 1.01–1.27). Across the entire cohort, using non-T2D individuals with low genetic risk as the reference group, the risk of asthma was higher in several subgroups: T2D individuals with low genetic risk (HR 1.22, 95% CI 1.08–1.39), non-T2D individuals with high genetic risk (HR 1.2, 95% CI 1.14–1.25), and T2D individuals with high genetic risk (HR 1.36, 95%CI 1.21–1.52) (Fig. 1).
Fig. 1.
Association between T2D and long-term risk of asthma among individuals with different levels of PRS for asthma. The analysis was performed in Model 2 (adjusted for sex, age, ethnicity, education levels, Townsend Deprivation Index, BMI, currently smoking, currently drinking, physical activity, family history of chronic bronchitis/emphysema, hypertension status, dyslipidemia status, 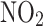 ,
, 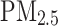 .). In the left forest plot, non-T2D individuals with low genetic risk severe as the reference group, the association between T2D and the risk of asthma was explored in low genetic risk participants. A similar association was observed in high genetic risk participants. In the right forest plot, using non-T2D and low genetic risk as the reference group, the association between non-T2D with high genetic risk, T2D with low genetic risk, T2D with high genetic risk and the risk asthma was explored in all participants
.). In the left forest plot, non-T2D individuals with low genetic risk severe as the reference group, the association between T2D and the risk of asthma was explored in low genetic risk participants. A similar association was observed in high genetic risk participants. In the right forest plot, using non-T2D and low genetic risk as the reference group, the association between non-T2D with high genetic risk, T2D with low genetic risk, T2D with high genetic risk and the risk asthma was explored in all participants
In the sensitivity analyses, after excluding participants with a family history of chronic/emphysema, T2D remained independently associated with an increased risk of asthma (Supplementary Table S2). Additionally, when excluding participants diagnosed with asthma within the first year of follow-up, the association between T2D and asthma risk persisted (Supplementary Table S3), reducing concerns of reverse causation. The Fine-Gray model analysis, which accounted for mortality as a competing risk, also confirmed these results (Supplementary Table S4). Moreover, the association between PRS for asthma and T2D was significantly with odds ratio (OR) of 1.26 (95%CI 1.25–1.28). However, this association became non-significant (OR 1.01, 95%CI 0.99–1.03) after further adjusting for confounding variables (Supplementary Table S5).
Discussion
Our prospective cohort study revealed several key findings: T2D is associated with an increased risk of developing asthma in the long term, and this association remains significant regardless of genetic predisposition to asthma. These findings suggest that the influence of T2D on asthma development operates independently of genetic factors. Sensitivity analyses further confirmed the robustness of this association, consistently showing that T2D is linked to a heightened risk of asthma.
Previous investigations, such as the cohort study by Thomsen et al., highlighted an association between T2D and asthma [6] but did not consider genetic predispositions [17]. By incorporating a PRS for asthma, our study addresses this limitation and enhances the understanding of the genetic factors involved in this association. Moreover, the use of the UK Biobank cohort, with its extensive sample size (n > 400,000) and prolonged follow-up period (median duration of 13.62 years), substantially strengthens the validity of our findings.
We investigated the relationship between T2D and asthma from two perspectives: electronic healthcare records and genetic evidence. After adjusting for the PRS of asthma and other confounding factors, we found that T2D remains an independent risk factor for asthma. These findings underscore the importance of addressing glucose regulation as part of asthma prevention strategies.
The mechanisms underlying the association between T2D and asthma are not fully elucidated [18]. Insulin resistance, a hallmark of T2D [19–21], has been associated with increased asthma-like symptoms, suggesting a potential pathway [22]. Additionally, advanced glycation end-products (AGEs) and their receptor for AGE, which promote chronic inflammation in the airways and vasculature, offer another mechanism linking T2D and asthma [23]. Obesity, which is frequently associated with T2D, is known to decrease adiponectin and increases in leptin levels, both of which are related to worsened systemic inflammation and airway hyper-reactivity [24]. Increased systemic inflammation, manifested by increased serum interleukin-6 (IL-6), has also been linked to a higher prevalence of asthma [25, 26].
Type 2 diabetes is a multi-systemic disease that affects many organs of the body. Diabetic retinopathy, nephropathy leading to end-stage renal disease, neuropathy, and macroangiopathy leading to cardiovascular disease and non-traumatic limb amputations are well-established complications of diabetes [27–29]. The lung as a target organ for diabetic injury is however a relatively less explored niche [30, 31]. In this study, we found that T2D was associated with an increased risk of asthma. Furthermore, T2D was not correlated with the PRS for asthma, indicating that T2D has an independent association with asthma. The association between T2D and asthma risk persisted regardless of genetic susceptibility to asthma, emphasizing the value of accounting for genetic predisposition in our study.
Despite these important insights, our study also has limitations. Firstly, although we controlled for several potential covariates in the analysis, residual confounding may still be present. Secondly, the absence of randomized interventions limits the strength of evidence for drawing causal conclusions. Future research should incorporate randomized controlled trials to explicitly investigate the causal relationship between T2D and asthma. Such an approach would provide more definitive answers regarding the causality of this relationship. Thirdly, it is important to note that the UK Biobank primarily includes Caucasians, which may limit the generalizability of the study findings to populations with different genetic backgrounds. Finally, asthma diagnoses were based solely on ICD code, which may introduce some bias.
Conclusions
Our study delineates a significant association between T2D and an increased risk of asthma, regardless of considering genetic susceptibility to asthma. These findings have profound implication for public health strategies and clinical management of individuals at risk of or living with T2D, suggesting a need for heightened vigilance for asthma symptoms in this population.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to express our appreciation to Xianbo Zuo, Chaozeng Si, and Jingkai Xu from the Clinical Big Data Center, China-Japan Friendship Hospital, Beijing 100029, China, for their invaluable assistance in the storage of UK Biobank data. Furthermore, our gratitude extends to Zhaoxu Geng from Institute of Biophysics, Chinese Academy of Sciences, Beijing, China, for the revision of the manuscript. This study was performed under UK Biobank application number 98577 (https://www.ukbiobank.ac.uk/enable-your-research/manage-your-project).
Abbreviations
- BMI
Body mass index
- CI
Confidence interval
- DBP
Diastolic blood pressure
- HbA1c
Glycosylated hemoglobin
- HR
Hazard ratios
- IL-6
Interleukin-6
- NF-
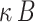
Nuclear factor-B
- NO2
Nitrogen dioxide air pollution
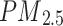
Particulates matter with an aerodynamic diameter ≤ 2.5 μm
- PRS
Polygenic risk score
- SBP
Systolic blood pressure
- SD
Standard deviation
- T2D
Type 2 diabetes
- TDI
Townsend Deprivation Index
- TNF
Tumor necrosis factor
Author contributions
Fei Chen and Bo Zhang design the study and contributed to the interpretation of the results. Fei Chen conducted the statistical analyses and drafted the first manuscript. Fei Chen, Ying Yang, Liping Yu, Lulu Song, Cong Zhang, Yifan He, Lili Wu, Wanlu Ma, and Bo Zhang contributed to the critical revision of the manuscript. Bo Zhang attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. Fei Chen and Bo Zhang have accessed and verified the underlying data. All authors had accepted responsibility for the decision to submit for publication.
Funding
This study was supported by National High Level Hospital Clinical Research Funding (2022-NHLHCRF-YS-01), National Key Research and Development Program of China (2018YFC1313902), and Young Elite Scientist Sponsorship Program By Bast (BYESS2023173).
Data availability
This research has been conducted using data from UK Biobank, a major biomedical database, https://www.ukbiobank.ac.uk/enable-your-research/manage-your-project. The UK Biobank study received ethical approval from the UK Northwest Multicenter Research Ethics Committee (Approval: 11/NW/0382). This study was performed under UK Biobank application number 98577. Participants of UK Biobank gave written informed consent before taking part.
Declarations
Ethics approval and consent to participate
This study was performed under UK Biobank application number 98577. Participants of UK Biobank gave written informed consent before taking part.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14:88–98. [DOI] [PubMed] [Google Scholar]
- 2.Zhou B, Lu Y, Hajifathalian K, Bentham J, Di Cesare M, Danaei G, et al. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4·4 million participants. Lancet. 2016;387:1513–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker EH, Archer JRH, Srivastava SA. Hyperglycemia, lung infection, and inflammation. Clin Pulm Med. 2009;16:258–64. [Google Scholar]
- 4.McKeever TM, Weston PJ, Hubbard R, Fogarty A. Lung function and glucose metabolism: an analysis of data from the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2005;161:546–56. [DOI] [PubMed] [Google Scholar]
- 5.Litonjua AA, Lazarus R, Sparrow D, DeMolles D, Weiss ST. Lung function in type 2 diabetes: the normative aging study. Respir Med. 2005;99:1583–90. [DOI] [PubMed] [Google Scholar]
- 6.Thomsen SF, Duffy DL, Kyvik KO, Skytthe A, Backer V. Risk of asthma in adult twins with type 2 diabetes and increased body mass index. Allergy. 2011;66:562–8. [DOI] [PubMed] [Google Scholar]
- 7.Ehrlich SF, Quesenberry CP, Van Den Eeden SK, Shan J, Ferrara A. Patients diagnosed with diabetes are at increased risk for asthma, chronic obstructive pulmonary disease, pulmonary fibrosis, and pneumonia but not lung cancer. Diabetes Care. 2010;33:55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baek JY, Lee SE, Han K, Koh EH. Association between diabetes and asthma. Ann Allergy Asthma Immunol. 2018;121:699–703. [DOI] [PubMed] [Google Scholar]
- 9.Rana JS, Mittleman MA, Sheikh J, Hu FB, Manson JE, Colditz GA, et al. Chronic obstructive pulmonary disease, asthma, and risk of type 2 diabetes in women. Diabetes Care. 2004;27:2478–84. [DOI] [PubMed] [Google Scholar]
- 10.Mueller NT, Koh W-P, Odegaard AO, Gross MD, Yuan J-M, Pereira MA. Asthma and the risk of type 2 diabetes in the Singapore Chinese health study. Diabetes Res Clin Pract. 2013;99:192–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562:203–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eeftens M, Beelen R, De Hoogh K, Bellander T, Cesaroni G, Cirach M, et al. Development of land use regression models for PM 2.5, PM 2.5 absorbance, PM 10 and PM coarse in 20 European study areas; results of the ESCAPE Project. Environ Sci Technol. 2012;46:11195–205. [DOI] [PubMed] [Google Scholar]
- 13.Euesden J, Lewis CM, O’Reilly PF. PRSice: polygenic risk score software. Bioinformatics. 2015;31:1466–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Day F, Karaderi T, Jones MR, Meun C, He C, Drong A, et al. Large-scale genome-wide meta-analysis of polycystic ovary syndrome suggests shared genetic architecture for different diagnosis criteria. PLOS Genet. 2018;14:e1007813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu TD, Brigham EP, Keet CA, Brown TT, Hansel NN, McCormack MC. Association between Pre-Diabetes/Diabetes and Asthma Exacerbations in a Claims-based obese asthma cohort. J Allergy Clin Immunol Pract. 2019;7:1868–e18735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Austin PC, Putter H, Lee DS, Steyerberg EW. Estimation of the Absolute Risk of Cardiovascular Disease and other events: issues with the use of multiple fine-Gray Subdistribution Hazard models. Circ Cardiovasc Qual Outcomes. 2022;15:e008368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han Y, Jia Q, Jahani PS, Hurrell BP, Pan C, Huang P, et al. Genome-wide analysis highlights contribution of immune system pathways to the genetic architecture of asthma. Nat Commun. 2020;11:1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torres RM, Souza MDS, Coelho ACC, De Mello LM, Souza-Machado C. Association between Asthma and type 2 diabetes mellitus: mechanisms and impact on asthma control—a literature review. Can Respir J. 2021;2021:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galicia-Garcia U, Benito-Vicente A, Jebari S, Larrea-Sebal A, Siddiqi H, Uribe KB, et al. Pathophysiology of type 2 diabetes mellitus. Int J Mol Sci. 2020;21:6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cantley J, Ashcroft FM. Q&A: insulin secretion and type 2 diabetes: why do β-cells fail? BMC Biol. 2015;13:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mezza T, Cinti F, Cefalo CMA, Pontecorvi A, Kulkarni RN, Giaccari A. β-Cell fate in human insulin resistance and type 2 diabetes: a perspective on islet plasticity. Diabetes. 2019;68:1121–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thuesen BH, Husemoen LLN, Hersoug L-G, Pisinger C, Linneberg A. Insulin resistance as a predictor of incident asthma-like symptoms in adults. Clin Exp Allergy J Br Soc Allergy Clin Immunol. 2009;39:700–7. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe T, Asai K, Fujimoto H, Tanaka H, Kanazawa H, Hirata K. Increased levels of HMGB-1 and endogenous secretory RAGE in induced sputum from asthmatic patients. Respir Med. 2011;105:519–25. [DOI] [PubMed] [Google Scholar]
- 24.Sood A, Shore SA. Adiponectin, Leptin, and Resistin in Asthma: Basic mechanisms through Population studies. J Allergy. 2013;2013:785835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan R, Kuai S, Li Q, Zhu X, Wang T, Cui Y. Diagnostic value of IL-6 for patients with asthma: a meta-analysis. Clin Immunol. 2023;12:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scott HA, Ng SH, McLoughlin RF, Valkenborghs SR, Nair P, Brown AC, et al. Effect of obesity on airway and systemic inflammation in adults with asthma: a systematic review and meta-analysis. Thorax. 2023;78:957–65. [DOI] [PubMed] [Google Scholar]
- 27.Jawa A, Kcomt J, Fonseca VA. Diabetic nephropathy and retinopathy. Med Clin North Am. 2004;88:1001–36. [DOI] [PubMed] [Google Scholar]
- 28.Valencia WM, Florez H. How to prevent the microvascular complications of type 2 diabetes beyond glucose control. BMJ. 2017;365:i6505. [DOI] [PubMed] [Google Scholar]
- 29.Tomic D, Shaw JE, Magliano DJ. The burden and risks of emerging complications of diabetes mellitus. Nat Rev Endocrinol. 2022;18:525–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khateeb J, Fuchs E, Khamaisi M. Diabetes and lung disease: an underestimated relationship. Rev Diabet Stud RDS. 2019;15:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mameli C, Ghezzi M, Mari A, Cammi G, Macedoni M, Redaelli FC, et al. The diabetic lung: insights into pulmonary changes in children and adolescents with type 1 diabetes. Metabolites. 2021;11:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This research has been conducted using data from UK Biobank, a major biomedical database, https://www.ukbiobank.ac.uk/enable-your-research/manage-your-project. The UK Biobank study received ethical approval from the UK Northwest Multicenter Research Ethics Committee (Approval: 11/NW/0382). This study was performed under UK Biobank application number 98577. Participants of UK Biobank gave written informed consent before taking part.