Abstract
Background
This article aims to use high-throughput sequencing to identify miRNAs associated with ferroptosis in myocardial ischemia–reperfusion injury, select a target miRNA, and investigate its role in H9C2 cells hypoxia-reoxygenation injury.
Methods
SD rats and H9C2 cells were used as subjects. ELISA kits quantified MDA, SOD, GSH, LDH, and ferritin levels. TTC staining evaluated infarction size. HE staining observed histopathological changes. DCFH-DA fluorescent probe detected ROS. CCK-8 kit measured cell viability. HiSeq 2000 sequencing performed differential expression analysis of miRNAs. qRT-PCR and Western blots assessed the expression levels of GPX-4, ACSL-4, HO-1, TFR-1 and TFR-2. SPSS 21.0 software conducted statistical analysis.
Results
Myocardial ischemia–reperfusion injury resulted in decreased levels of SOD and GSH, increased levels of LDH and MDA, up-regulation of ferritin, ACSL-4, HO-1, and TFR-2, down-regulation of GPX-4, increased tissue damage, and accumulation of ROS. However, DFO treatment reversed these changes. Subsequently, the target gene miRNA-541-5p was obtained by miRNA sequencing screening, and further validation revealed that miRNA-541-5p expression was increased in the myocardial tissues of rats in the I/R injury model group compared with those of rats in the NC group, P < 0.05. Subsequently, by constructing H9C2 cell lines with miRNA-541-5p overexpression and miRNA-541-5p expression inhibition, miRNA-541-5p expression was inversely correlated with the survival of H9C2 cells after hypoxia-reoxygenation injury. miRNA-541-5p up-regulation led to a decrease in SOD and GSH, an increase in ferritin and MDA, and an accumulation of ROS. wb and qRT-PCT demonstrated that high miRNA-541-5p expression up-regulated the expression of protein/mRNA expression of ACSL-4, HO-1, ferritin, and TFR-1, but down-regulated protein/mRNA expression of GPX-4. In addition, ADAM 7, FNIP 2, HOXD 10, HCCS and STK 3 were preliminarily identified as potential candidate target genes for miRNA-541-5p by bioinformatics analysis. Among them, ADAM7 emerges as the most suitable potential target gene based on the selection criteria.
Conclusion
In summary, miRNA-541-5p may be a biomarker of myocardial I/R damage diseases and can regulate oxidative stress and iron death by inhibiting the expression of miRNA-541-5p, thereby reducing mechanisms of I/R injury.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13019-024-03260-2.
Keywords: Myocardial ischemia–reperfusion injury, Ferroptosis, MiRNA-541-5p, MiRNA sequencing
Introduction
One prevalent cardiovascular condition that significantly contributes to fatalities related to non-communicable diseases is myocardial ischemia [1]. As the population ages and expands, the burden of these diseases escalates [2]. Myocardial reperfusion currently serves as the treatment for patients with myocardial ischemia, aiming to limit infarct size and cardiac remodeling by restoring blood flow to the ischemic myocardial tissue [2]. However, reperfusion can lead to a phenomenon known as cardiac reperfusion injury, resulting in cardiomyocyte death [3]. This irreversible damage subsequently leads to adverse events such as heart failure, cardiomyocyte death, and unfavorable cardiac remodeling [3, 4]. Therefore, preserving heart function post-reperfusion relies on preventing cardiomyocyte death.
Lipid peroxidation-induced ferroptosis is an iron-dependent form of cell death that impairs cardiomyocytes and exacerbates ischemia–reperfusion injury by disrupting cellular redox balance, inflammatory cascades, and calcium homeostasis [5, 6]. Endogenous glutathione (GSH), glutathione peroxidase 4 (Gpx-4), and the cystine/glutamate antiporter play pivotal roles in regulating ferroptosis. Strategies such as iron chelation or inhibition of lipid peroxidation can be employed to counteract ferroptosis in experimental models of cardiac ischemia–reperfusion injury [7, 8].
Non-coding RNAs (ncRNAs), such as microRNA (miRNA), are extensively distributed within eukaryotic cells and possess the capacity to regulate post-transcriptional gene expression [9, 10]. According to recent studies, miRNAs have been implicated in the pathogenesis and progression of myocardial ischemia reperfusion (I/R) injury. Dysregulation of miRNAs expression (whether upregulated or downregulated) are closely associated with myocardial contractility, relaxation function, inflammatory response, myocardial fibrosis, cell apoptosis, endoplasmic reticulum stress response, lysosomal function, intracellular iron homeostasis maintenance as well as mitochondrial structure and functional integrity [10]. Therefore, miRNAs hold great promise as an effective targeted therapy against myocardial I/R injury given the increasing understanding of their underlying biological mechanisms through further research.
The dysregulated expression of specific sets of miRNAs, including miR-1, miR-15, miR-144, miR-101, miR-21, miR-24, miR-29, miR-94a, miR-126, miR-214, miR-132, miR-451 and miR-494, has been reported in the context of myocardial I/R injury. These findings suggest that these abnormally expressed microRNAs have potential as valuable markers for diagnosing this disease [11]. The expression of miR-132 is significantly upregulated in the myocardial ischemia model and it exerts a cardioprotective effect by negatively regulating SIT1 and activating the oxidative stress signaling pathway PGC-1α/Nrf2 [12]. The activation of the AKT/mTOR signaling pathway can be facilitated by miR-486 through its targeting of PTEN and FoxO1, resulting in the inhibition of cardiomyocyte apoptosis and mitigation of myocardial (I/R) injury [13]. By activating the PTGS2/MAPK pathway mediated by miR-26b-5p, berberine exerts a cardioprotective effect against myocardial I/R injury by attenuating levels of IL-1β, TNF-α, IL-6, and malondialdehyde (MDA). Additionally, it enhances the antioxidant defense system through elevation of GSH, GSH-Px, and superoxide dismutase (SOD), thereby effectively suppressing oxidative stress and mitigating inflammatory response [14]. Consequently, miRNAs hold promising potential as candidates for regulating the molecular and cellular processes implicated in myocardial I/R injury, while also serving as valuable biomarkers for identifying and assessing risk factors associated with susceptible myocardial I/R injury.
The objective of this study is to investigate the potential amelioration of myocardial I/R injury through intervention in ferroptosis. Subsequently, utilizing high-throughput sequencing technology, we will identify miRNAs associated with ferroptosis during myocardial I/R injury and preliminarily predict the target genes of selected miRNA. Finally, cellular experiments will be conducted to further validate the regulatory effects and mechanisms of selected miRNA on ferroptosis. This article aims to provide a theoretical foundation for discovering effective biomarkers and therapeutic targets for myocardial I/R injury while establishing a basis for subsequent clinical research on miRNAs.
Methods
Experimental animals
Healthy male Sprague–Dawley (SD) rats aged 6–8 weeks and weighing 180–220 g were obtained from the Lanzhou Veterinary Research Institute of the Chinese Academy of Agricultural Sciences. The rats were housed at a room temperature of 25 ± 2 °C with a relative humidity of 45%, under a 12-h light–dark cycle, and provided with ad libitum access to food and water. Ethical approval for the use of experimental animals was obtained from the Ethics Committee of the First Hospital of Lanzhou University (No. LDYYLL2021-327). Prior to the experiment, the SD rats underwent a one-week acclimatization period. A fasting period of 12 h was observed before initiating the experiment.
Mouse myocardial I/R model
The SD rats were randomly divided into 4 groups: control, sham, ischemia–reperfusion injury model (I/R), and I/R + DFO (Deferoxamine) group (100 mg/kg, intraperitoneal injection). Experimental procedure: Rats were anesthetized with sodium pentobarbital (30 mg/kg) via intraperitoneal injection. After anesthesia, tracheal intubation was performed to connect them to a small animal ventilator (respiratory rate: 60/min, tidal volume: 6 mL/100 g, respiratory ratio: 3:2). Subsequently, the thoracic cavity was opened between the 3 and 4 left sternum to expose the heart. The left anterior descending (LAD) branch of the coronary artery was ligated using silk thread. After undergoing ischemic treatment for 30 min, the silk thread was loosened to allow reperfusion for 120 min. It is important to note that the control group received intraperitoneal injection of pentobarbital anesthesia without opening the chest, while the sham group only had silk thread placed on the LAD branch without ligation. Finally, the rats were euthanized by cervical dislocation for sample collection.
Measurement of MDA, SOD, GSH, LDH and Ferritin
According to the reagent manufacturer's instructions, Elisa kits were respectively used to detect malondialdehyde (MDA) (Maisha industries, Co., LTD, Jiangsu, China; Ruixin Biotech, Co., LTD, Quanzhou, China), superoxide dismutase (SOD) (Maisha industries, Co., LTD, Jiangsu, China; Ruixin Biotech, Co., LTD, Quanzhou, China), glutathione (GSH) (Maisha industries, Co., LTD, Jiangsu, China; Ruixin Biotech, Co., LTD, Quanzhou, China), lactate dehydrogenase (LDH) (Maisha industries, Co., LTD, Jiangsu, China; Ruixin Biotech, Co., LTD, Quanzhou, China) and ferritin (Maisha industries, Co., LTD, Jiangsu, China; Ruixin Biotech, Co., LTD, Quanzhou, China) in rat myocardial tissue and H9C2 cells.
TTC staining
The rat heart was flash-frozen in liquid nitrogen for 20 s. The myocardial tissue slices, approximately 3 mm thick, were obtained from the ligature point and midpoint of the apex. These slices were then incubated in a pH 7.4 triphenyltetrazolium chloride (TTC) (Solarbio life sciences, Co., Ltd., Beijing, China) solution (2%) at 37 °C for 30 min. After slicing, the myocardial tissue was fixed in a formalin solution (4%) for an hour before imaging with a digital camera. The results showed that non-infarction myocardial tissue appeared brick red, while infarcted myocardial tissue appeared gray-white. The infarcted and non-infarcted sections of each sliced sample were isolated and measured. Analysis revealed that the percentage of myocardial infarction size (%) was calculated as follows: (total weight of infarcted tissues/weight of entire heart) × 100%.
Hematoxylin and eosin staining
The heart tissue of rats was fixed in a 4% paraformaldehyde solution, followed by paraffin embedding and slicing into approximately 5–8 um thick sections after rinsing with cold saline water. The slices were then deparaffinized and hydrated. The tissue was stained with hematoxylin (Solarbio life sciences, Co., LTD, Beijing, China) for 5 min, followed by eosin staining (Solarbio life sciences, Co., LTD, Beijing, China) for another 5 min. After air-drying, the slices were mounted. Finally, an optical microscope (RCX41, sunny optical technology (group), Co., LTD, Ningbo, China) was used to observe and study pathological changes in the heart tissue sections.
ROS detection
SD rats myocardial tissue: After mincing the heart tissue, an appropriate amount of pancreatic enzyme was added to facilitate digestion for a duration of 20 min. The resulting cell suspension was obtained through filtration. In the suspension, 10µM of DCFH-DA (2, 7-dichlorofluorescin diacetate) (Applygen Technologies Inc., Beijing, China) was added. The negative control was treated with 0.01M PBS (Biosharp life sciences Co., Ltd., Hefei, China), while the positive control received treatment with 20 μM of a reactive oxygen species (Applygen Technologies Inc., Beijing, China) in combination with DCFH-DA; incubation took place at 37 °C for 30 min followed by centrifugation at 1000 g for 5 min to collect the cell precipitate which was then washed twice with PBS solution. Fluorescence microscopy (Leica DM2500, Germany) at a magnification of 200 × was employed to capture images; Image pro plus 7.0 software was utilized to measure green fluorescence intensity using an excitation wavelength of 500 nm and an emission wavelength of 525 nm.
H9C2 cells: The cells were cultured until the designated time point, and then the culture plates were removed. The positive control for reactive oxygen species (Solarbio life sciences Co., Ltd., Beijing, China) was added to each well at a ratio of 1:1000 and gently mixed before being incubated in a 37 °C incubator for 20 min. Subsequently, the supernatant was discarded, and each well was washed with 1mL of PBS (Biosharp life sciences Co., Ltd., Hefei, China). Next, 1 mL of DCFH-DA (Solarbio life sciences Co., Ltd., Beijing, China), diluted to a ratio of 1:6000 with FBS (Asia Biomaterials Co., Ltd., Wuhan, China), was added to each well and incubated at 37 °C in the cell culture incubator for 10 min. After washing the cells 3 times with FBS solution, they were observed under a fluorescence microscope (Leica DM2500, Germany) at a magnification of 200 × and photographed. Finally, the intensity of fluorescence was measured using Image pro plus software version 7.0 with an excitation wavelength of 488 nm and an emission wavelength of 525 nm.
qRT-PCR
The total RNA was extracted using the TRIZOL method (15596026, Invitrogen, Shanghai, China). Reverse transcription of the RNA was performed following the reagent instructions (SYBR Green Pro Taq HSpremixed qPCR kit; EvoM-MLV reverse transcription premix kit) (Yesen Biotechnology, Co., LTD, Shanghai, China) to synthesize cDNA. Quantitative real-time PCR analysis was conducted on an MA-6000 real-time fluorescent quantitative PCR instrument to validate the reliability of RT-PCR amplification curve and melting curve data. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as an internal control for calculating 2-ΔΔCt values of the target gene. The primer sequence is shown in the following Table 1.
Table 1.
Primers sequence
| Gene | Primer sequence | Product size (bp) |
|---|---|---|
| GAPDH |
AATGGTGAAGGTCGGTGTGAAC AGGTCAATGAAGGGGTCGTTG |
114 |
| Gpx4 |
AAGTACAGGGGTTGCGTGTG GGGCATCGTCCCCATTTACA |
250 |
| Tfr2 |
CGGGTGAGATGGATAGCGTC TATGGACAACAGAGGTTACCAGG |
110 |
| HO-1 |
AGCGAAACAAGCAGAACCCA ACCTCGTGGAGACGCTTTAC |
160 |
| ACSL4 |
TGCTACTGAGCAGATCCCAG AGCTAGTGAGTCGAAGTGCG |
153 |
| Tfr1 |
TGGAGACTACTTCCGTGCTAC TCCACTAAAGCTGAGAGGGTG |
186 |
High-throughput sequencing
According to the mentioned I/R modeling method, 9 samples were randomly divided into 3 groups: NC, Model, and DFO group, with 3 samples in each group. Briefly, approximately 20 mg of myocardial tissue was flash-frozen in liquid nitrogen and total miRNA was extracted using a commercially available miRNA extraction kit (Invitrogen, Carlsbad, CA, USA). The RNA concentration was quantified using a NanoDrop one microspectrophotometer (Themo Fisher Scientific, USA), with purity assessed by the OD260/OD280 ratio. The reverse transcription followed the standard protocol of the manufacturer (TruseqTM Small RNA sample prep Kit, Illumina, San Diego, CA, USA), while the construction of miRNA libraries adhered to their established procedure (TruseqTM Small RNA sample prep Kit, Illumina, San Diego,CA,USA). For each miRNA library,total RNA deep sequencing was performed on a HiSeq 2000 sequencing system(Illumina) following the manufacturer's instructions. The product yield was determined using TBS380 software (QuantiTTM Picogreen, Invitrogen), and miRNA differential expression analysis among groups utilized the DESeq package. GO enrichment analysis and KEGG pathway analysis were used to identify diseases and signaling pathways potentially associated with target genes.
Cell culture
The H9C2 (Procell Life Technology Co., LTD, Wuhan, China) cells were thawed and cultured in Dulbecco's modified Eagle medium (DMEM) (Cytiva, Shanghai, China) supplemented with 10% fetal bovine serum (FBS, Cellmax, Beijing, China), 2 nmol glutamine (Gibco, Shanghai, China), and 250 µg/mL penicillin/streptomycin (Gibco, Shanghai, China) at 37 °C and 5% CO2. Subsequently, the H9C2 cells were subjected to hypoxia environment (95% N2 and 5% CO2) for a duration of 6 h. Following this, the cells were transferred to normal culture conditions (5% CO2 and 95% air) for an additional 4 h to establish a model of hypoxia-reoxygenation (H/R) injury. The experimental groups are shown in Table 2.
Table 2.
Experimental groups
| Group | Deal with |
|---|---|
| Control | DMEM |
| I/R | Hypoxia for 6 h + Reoxygenation for 4 h |
| I/R + miR-541-5p mimics-NC | Transfection of miRNA-541-5p null set only |
| I/R + miR-541-5p mimics | Transfection of miRNA-541-5p group only |
| I/R + miR-541-5pinhibitor-NC | Transfection of miRNA-541-5p inhibitor null group only |
| I/R + miR-541-5p inhibitor | Transfection of miRNA-541-5p inhibitor group only |
| I/R + miR-541-5p + pcDNA3.1 | Transfection of miRNA-541-5p + pcDNA3.1 group only |
Target gene adenovirus vector construction
The miRNA-541-5p mimics, miRNA-541-5p inhibitor, and negative controls (NC) were synthesized by GenePharma (GenePharma Co., LTD, Shanghai, China). To investigate the loss and gain of functions of miRNA-541-5p, H9C2 cells (Procell Life Technology Co., LTD, Wuhan, China) were transfected with 200 nM concentration of miRNA-541-5p mimics or mimics negative control (mimics-NC), as well as miRNA-541-5p inhibitor or inhibitor negative control (inhibitor-NC), using Lipofectamine 3000 reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. The transfection efficiency was validated through qRT-PCR analysis.
The sequences were as follows: miRNA-541-5p mimics Sense sequence 5’-AAGGGAUUCUGAUGUUGGUCACACU-3’, miRNA-541-5p mimics Antisense sequence 5’-UGUGACCAACAUCAGAAUCCCUUUU-3’, miRNA-541-5p mimics-NC Sense sequence 5’-UUCUCCGAACGUGUCACGUTT-3’, miRNA-541-5p mimics-NC Antisense sequence 5’-ACGUGACACGUUCGGAGAATT-3’, miRNA-541-5p inhibitor, 5’-AGUGUGACCAACAUCAGAAUCCCUU-3’, miRNA-541-5p inhibitor-NC, 5’-CAGUACUUUUGUGUAGUACAA-3’.
CCK-8 for cell viability detection
After trypsin digestion, H9C2 cells (Procell Life Technology Co., LTD, Wuhan, China) were diluted to a density of 5 × 105 cells/mL in complete DMEM medium. Subsequently, 100 uL of cell suspension was added to each well of a 96-well plate, including the blank control group. Following the provided instructions, the culture plate was placed in a sealed container and transferred into an anaerobic bag before being incubated at 37 °C for 6 h under hypoxic conditions. The reoxygenation treatment was then administered for 4 h. Next, each well received an addition of 10uL CCK-8 reagent (Procell Life Technology Co., LTD, Wuhan, China) followed by incubation at 37 °C in darkness for 1 h. Finally, absorbance at a wavelength of 450 nm was measured using an ELISA reader.
Western blotting (WB) analysis
H9C2 cells were lysed using RIPA lysis buffer (Servicebio, Co., LTD, Wuhan, China), and the total protein content was measured with the BCA protein assay kit (Solarbio, Co., LTD, Wuhan, China). Subsequently, samples were separated by 10% SDS-PAGE and transferred onto PVDF membranes (Millipore, Co., LTD, Germany). The membranes were then blocked in TBST buffer with 5% skim milk at room temperature for 1 h, followed by overnight incubation at 4 °C with primary antibodies against β-actin (Servicebio, Co., LTD, Wuhan, China), HO-1 (Affinity, Co., LTD, Jiangsu, China), Acsl-4 (Affinity, Co., LTD, Jiangsu, China), Gpx-4 (BIOSS, Co., LTD Beijing, China), and ferritin (BIOSS,Co.,LTD Beijing, China). Secondary antibodies (Servicebio, Co., LTD, Wuhan, China) were diluted in TBST with 5% skim milk. Band visualization was performed using ECL detection reagent (Servicebio, Co., LTD, Wuhan, China) and Image J software was utilized to quantify the intensity of protein bands.
Statistical analysis
SPSS 21.0 software was used to make statistical analysis of the data, and one-way analysis of variance was used to compare the data among groups. Graphpad Prism 8 was used to plot, and the results were expressed as mean ± standard deviation (SD). p < 0.05 indicated that the difference was statistically significant.
Results
Exploring the role of ferroptosis in myocardial I/R injury based on animal experiments
TTC staining to observe myocardial infarction area
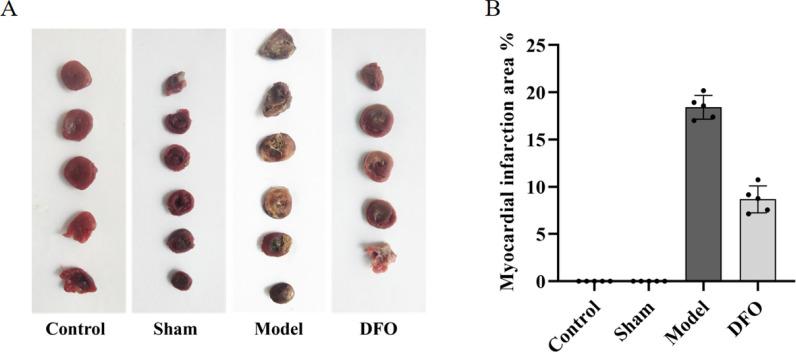
By staining with TTC, the myocardial infarction areas of each group of rats were visualized (Fig. 1A) and analyzed (Fig. 1B). The results revealed that the myocardium in the Control and Sham groups exhibited a healthy red color without any signs of infarction, indicating a myocardial infarction area percentage of 0%. In contrast, the Model group displayed a significant expansion in the extent of myocardial infarction (p < 0.01), reaching a percentage of (18.18 ± 1.72)%. Notably, treatment with DFO resulted in a remarkable reduction in myocardial infarction area compared to the Model group (p < 0.01), demonstrating a percentage decrease to (8.87 ± 1.81)%.
Fig. 1.
TTC staining and statistics of myocardial infarction area in each group of rats. A TTC staining of myocardial infarction area, the white part is the infarction area, the red part is the non-infarction area, B the percentage of myocardial infarction area in each group of rats. n = 5. ##p < 0.01 compared with the control group and the sham operation group; **p < 0.01 compared with the model group
HE staining to observe the pathological changes of myocardial tissue
The histological sections of myocardial tissues from each experimental group are presented in Fig. 2. In the Control group, myocardial fibers exhibited organized alignment with regular interstitial spaces, devoid of cellular edema, inflammatory cell infiltration, or hemorrhage. Compared to the Control group, the Sham group displayed no significant alterations in myocardial fiber arrangement; however, a slight increase in interstitial space width was observed without evident bleeding or inflammatory cell infiltration. In contrast to both the Control and Sham groups, the Model group demonstrated disrupted organization and even rupture of myocardial fibers accompanied by significantly widened interstitial spaces along with pronounced hemorrhage and inflammatory cell infiltration. Nevertheless, in comparison to the Model group, the DFO treatment exhibited partial restoration towards normalcy regarding myocardial fiber arrangement while still showing progressive widening of interstitial spaces and minimal bleeding alongside noticeable reduction in inflammatory cell infiltration.
Fig. 2.
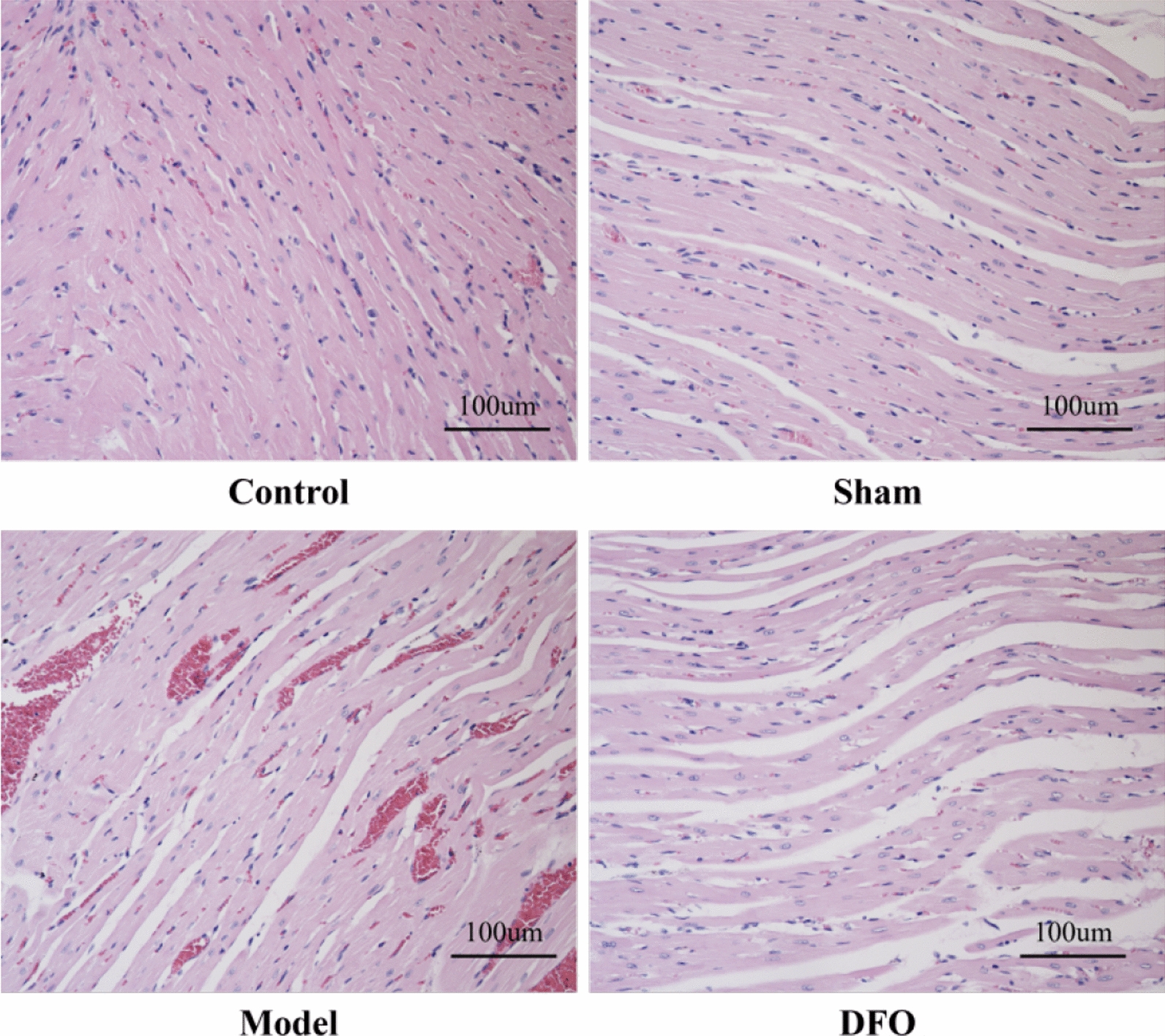
Pathological changes of myocardial tissue in rats in each group (200 ×)
Determination of serum LDH, MDA, SOD and GSH levels in rats
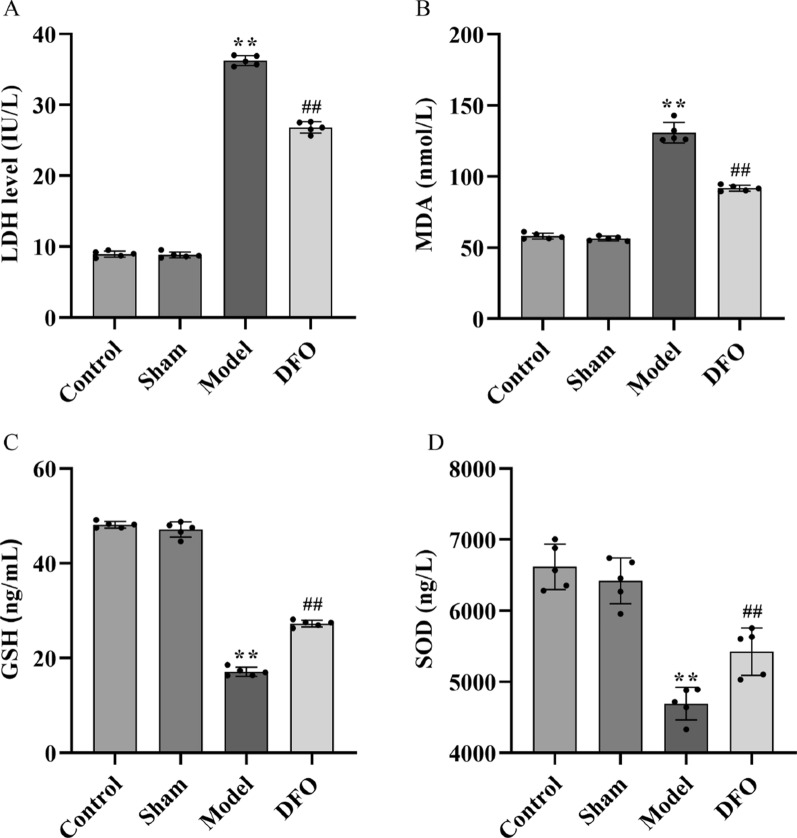
Using Elisa kits, we detected myocardial injury factor LDH (Fig. 3A), oxidative damage factor MDA (Fig. 3B), and antioxidant damage factors SOD (Fig. 3C) and GSH (Fig. 3D). The results revealed significantly elevated levels of LDH and MDA in the Model group compared to the Control group and Sham group (p < 0.01), accompanied by a significant decrease in GSH and SOD levels (p < 0.01). Conversely, the DFO group exhibited significantly reduced levels of LDH and MDA (p < 0.01) along with a substantial increase in GSH and SOD levels (p < 0.01) when compared to the Model group.
Fig. 3.
Determination of serum LDH, MDA, SOD, and GSH levels in rats of each group. A Serum LDH content; B serum MDA content; C serum GSH content; D serum SOD content. n = 5. ##p < 0.01 compared with the control group and sham operation group; **p < 0.01 compared with the model group
Determination of ferritin content in rat serum and myocardial tissue
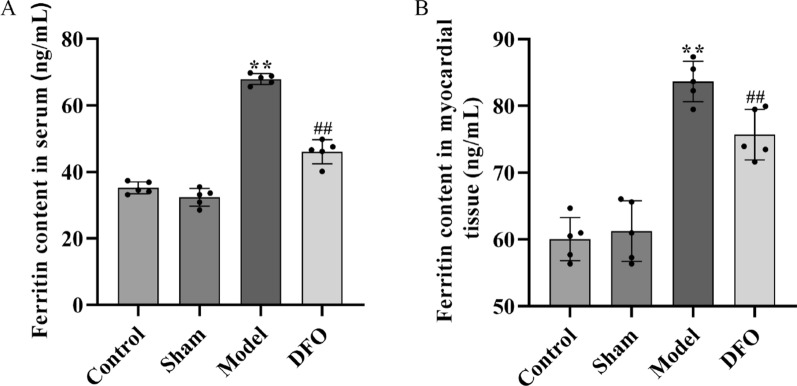
Utilizing Elisa test kits, the continuous assessment of ferritin content in serum (Fig. 4A) and myocardium (Fig. 4B) revealed a significant elevation in levels of ferritin in both serum and myocardium for the Model group compared to the Control group and Sham group (p < 0.01). Furthermore, treatment with iron chelator DFO demonstrated a notable reduction in ferritin content in both serum and myocardium when compared to the Model group (p < 0.01).
Fig. 4.
Determination of ferritin content in rat serum and myocardial tissue. A Determination of ferritin content in serum, B Determination of ferritin content in myocardial tissue. n = 5. ## Compared with the control group and sham operation group, p < 0.01; ** Compared with the model group, p < 0.01
Detection of ROS content in myocardial tissue
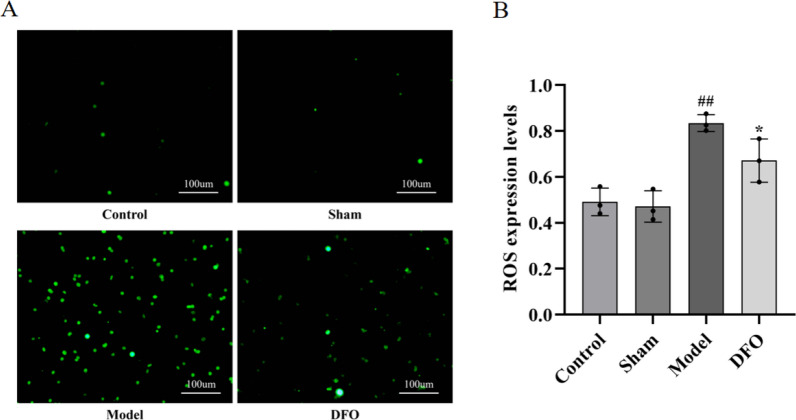
The results of ROS content detection in myocardial tissue (Fig. 5A) and the corresponding statistical analysis (Fig. 5B) are presented herein. The ROS content in the Control group was measured as 0.49 ± 0.06, while it was recorded as 0.47 ± 0.07 in the Sham group, with no statistically significant difference observed between them. In contrast, the Model group exhibited a substantial increase in ROS content (p < 0.01), measuring at 0.83 ± 0.04 compared to the Control and Sham groups. Furthermore, when compared to the Model group, the DFO group demonstrated a significant reduction in myocardial tissue ROS content (p < 0.05), which measured at 0.67 ± 0.09.
Fig. 5.
Detection of ROS content and statistics in myocardial tissue of rats in each group. A ROS expression levels in myocardial tissue of each group (200 ×); B Statistics of ROS expression levels in myocardial tissue of each group. ##p < 0.01 compared with the control group and sham operation group; *p < 0.05 compared with the model group
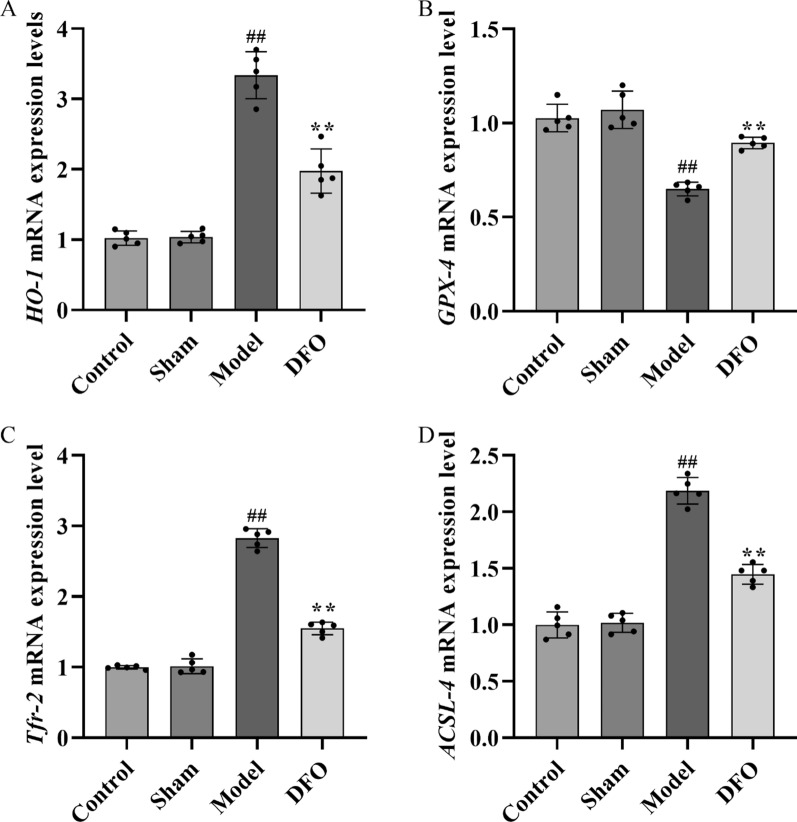
mRNA expression of ferroptosis related factors
By quantifying the mRNA expression levels of ferroptosis-related factors in myocardial tissue using qRT-PCR, it was observed that, compared to the Control and Sham groups, the Model group exhibited significantly elevated expression levels of HO-1 (Fig. 6A), TFR-2 (Fig. 6C), and ACSL-4 (Fig. 6D) (p < 0.01). Conversely, when compared to the Model group, the DFO group displayed significantly reduced expression levels of HO-1, TFR-2, and ACSL-4 (p < 0.01). Notably, relative to the Control and Sham groups, a significant decrease in GPX-4 (Fig. 6B) expression level was observed in the Model group (p < 0.01); however, this reduction was reversed in the DFO group where GPX-4's expression level showed a significant increase (p < 0.01).
Fig. 6.
mRNA expression levels of ferroptosis related factors were detected by qRT-PCR. A HO-1 mRNA expression level in myocardial tissue, B GPX-4 mRNA expression level in myocardial tissue, C TFR-2 mRNA expression level in myocardial tissue, D Acsl-4 mRNA expression level in myocardial tissue. n = 5 ## p < 0.01, as compared to Control and Sham group; ** p < 0.01, as compared to Model group
Screening of ferroptosis related miRNAs based on high-throughput sequencing technology
Based on the findings from previous animal experiments, it becomes evident that ferroptosis plays a crucial role in myocardial I/R injury. Furthermore, administration of the iron chelator DFO exhibits significant amelioration and improvement of myocardial I/R injury by antagonizing ferroptosis. Hence, high-throughput sequencing technology will be employed to screen for miRNAs associated with ferroptosis in myocardial I/R injury.
The experiment was divided into three groups, namely the NC group, Model group, and DFO group, each consisting of three samples. Firstly, the results of Principal Component Analysis (PCA) analysis (Fig. 7A) demonstrated a high degree of correlation among the samples within each group while revealing significant differences between the groups. These findings suggest the presence of differentially expressed miRNAs across the groups. Furthermore, volcano plots (Fig. 7B) revealed significant differential expression of miRNAs: 7 downregulated and 6 upregulated miRNAs between the NC group and Model group at a significance level of p < 0.05; 11 downregulated and 4 upregulated miRNAs between the Model group and DFO group; and 9 downregulated and 10 upregulated miRNAs between the NC group and DFO group. Subsequently, employing an online Venn diagram (Fig. 7C) facilitated the observation of varying numbers of differentially expressed miRNAs across the groups. Notably, a total of 369 miRNAs exhibited differential expression in the overlapping region among all three groups. Consequently, we conducted a comprehensive analysis to determine the number of differentially expressed miRNAs at various levels of significance between different groups (Fig. 7D). It is noteworthy that when p < 0.01, only one miRNA was found to be upregulated in the Model group compared to the NC group, while four miRNAs were downregulated in the DFO group compared to the Model group. When p < 0.1, a total of 27 miRNAs exhibited differential expression between the NC and Model groups, comprising 12 downregulated and 15 upregulated ones; similarly, there were a total of 30 differentially expressed miRNAs between the Model and DFO groups when p < 0.1, with 20 being downregulated and 10 being upregulated. For detailed information regarding statistical significance at p < 0.05 level, please refer to Fig. 7B.
Fig. 7.
Myocardial I/R injury results in changes of miRNA spectrum in myocardial tissue. A PCA was employed to evaluate the intra-group correlation and inter-group differences; B When p < 0.05, volcano plots were used to visually represent the differential expression of miRNAs across different groups; C The Venn diagram was utilized for the visualization of differentially expressed miRNAs across various groups; D The number of miRNAs exhibiting differential expression between groups was assessed at various levels of statistical significance
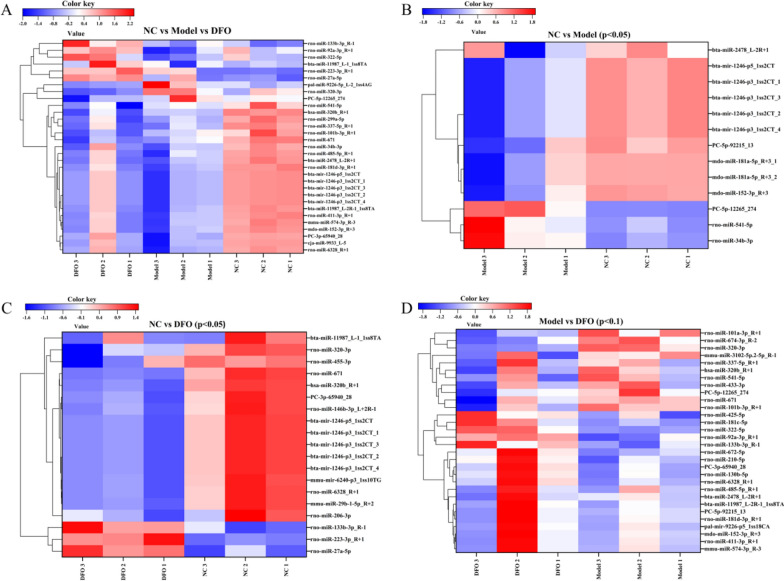
The heatmaps presented below depict the differential expression of miRNAs among and between each pair of groups. A total of 31 miRNAs were found to be differentially expressed (p < 0.05) in the NC group, Model group, and DFO group (Fig. 8A) (Table 3). Among them, miRNA-541-5p exhibited moderate expression levels with values of 6 in the NC group, 33 in the Model group, and 12 in the DFO group. Comparing the NC group with the Model group revealed a set of 13 differentially expressed miRNAs (p < 0.05), including miRNA-541-5p (Fig. 8B). Similarly, comparing the NC group with the DFO group identified a set of 19 differentially expressed miRNAs (p < 0.05), excluding interestingly miRNA-541-5p from this list (Fig. 8C). Lastly, when considering a significance level of p < 0.1 for comparisons between the Model group and DFO group (Fig. 8D), we observed a total of 30 differentially expressed miRNAs that included miRNA-541-5p.
Fig. 8.
The heatmap illustrates the differential expression patterns of miRNAs across distinct groups. A A total of 31 miRNAs exhibited significant differential expression (p < 0.05) among the NC group, Model group, and DFO group; B 13 miRNAs showed significant differential expression (p < 0.05) between the NC group and the Model group; C 19 miRNAs displayed significant differential expression (p < 0.05) between the NC and DFO groups; D 30 miRNAs demonstrated differential expression (p < 0.1) between the Model and DFO
Table 3.
A total of 31 miRNAs exhibited significant differential expression (p < 0.05) among the NC, Model and DFO groups
| miRNA name | NC (mean expression) | Model (mean expression) | DFO (mean expression) | p value |
|---|---|---|---|---|
| rno-miR-6328_R + 1 | 1 | 0 | 6 | < 0.01 |
| mdo-miR-152-3p_R + 3 | 1 | 6 | 0 | < 0.01 |
| rno-miR-671 | 45 | 46 | 21 | < 0.01 |
| rno-miR-27a-5p | 47 | 115 | 194 | < 0.01 |
| rno-miR-223-3p_R + 1 | 45 | 237 | 444 | < 0.01 |
| bta-miR-2478_L-2R + 1 | 2 | 10 | 0 | < 0.01 |
| PC-5p-12265_274 | 1 | 55 | 11 | < 0.01 |
| rno-miR-181d-3p_R + 1 | 9 | 6 | 0 | < 0.01 |
| PC-3p-65940_28 | 0 | 2 | 9 | < 0.05 |
| mmu-miR-574-3p_R-3 | 3 | 10 | 4 | < 0.05 |
| bta-miR-11987_L-2R-1_1ss8TA | 1 | 3 | 0 | < 0.05 |
| rno-miR-411-3p_R + 1 | 3 | 6 | 0 | < 0.05 |
| bta-mir-1246-p3_1ss2CT_4 | 5 | 3 | 3 | < 0.05 |
| bta-mir-1246-p3_1ss2CT_2 | 5 | 3 | 3 | < 0.05 |
| bta-mir-1246-p3_1ss2CT_3 | 5 | 3 | 3 | < 0.05 |
| bta-mir-1246-p3_1ss2CT_1 | 5 | 3 | 3 | < 0.05 |
| bta-mir-1246-p5_1ss2CT | 5 | 3 | 3 | < 0.05 |
| rno-miR-34b-3p | 10 | 24 | 19 | < 0.05 |
| rno-miR-337-5p_R + 1 | 5 | 15 | 0 | < 0.05 |
| rno-miR-485-5p_R + 1 | 1 | 9 | 0 | < 0.05 |
| hsa-miR-320b_R + 1 | 35 | 38 | 17 | < 0.05 |
| rno-miR-320-3p | 7880 | 7551 | 5373 | < 0.05 |
| bta-miR-11987_L-1_1ss8TA | 17 | 53 | 79 | < 0.05 |
| cja-miR-9933_L-5 | 0 | 2 | 6 | < 0.05 |
| rno-miR-101b-3p_R + 1 | 38 | 49 | 23 | < 0.05 |
| rno-miR-322-5p | 80 | 86 | 140 | < 0.05 |
| rno-miR-133b-3p_R-1 | 2783 | 2637 | 4260 | < 0.05 |
| rno-miR-541-5p | 6 | 33 | 12 | < 0.05 |
| rno-miR-299a-5p | 2 | 21 | 5 | < 0.05 |
| pal-miR-9226-5p_L-2_1ss4AG | 109 | 353 | 51 | < 0.05 |
| rno-miR-92a-3p_R + 1 | 10,614 | 7643 | 10,738 | < 0.05 |
After conducting further statistical analysis on the expression levels of miRNA-541-5p in Fig. 8A, it was observed that miRNA-541-5p exhibited significant upregulation in the Model group compared to the NC group (p < 0.05). However, there were no notable differences in expression levels between the DFO group and the NC group. Moreover, a significant downregulation of miRNA-541-5p was observed in the DFO group when compared to the Model group (p < 0.05) (Fig. 9). These findings suggest that miRNA-541-5p may play a role in the pathological process of myocardial I/R injury by influencing iron-mediated cell death. Interestingly, our results indicate that treatment with the iron chelator DFO can effectively restore miRNA-541-5p expression levels towards normalcy, which is consistent with the outcomes depicted in Fig. 8C.
Fig. 9.
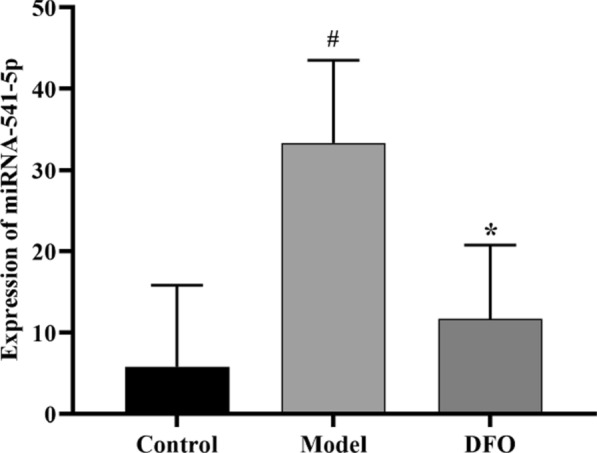
Relative expression of miRNA-541-5p. # p < 0.05, as compared to NC group; * p < 0.05, as compared to Model group.
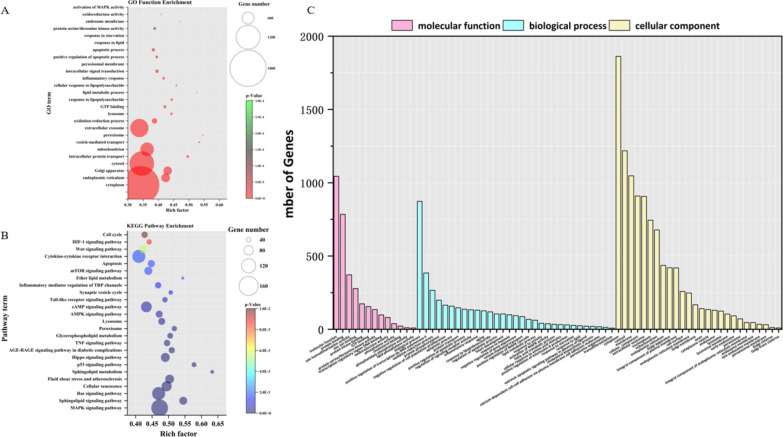
Finally, the miRNA action patterns and functional implications of these differentially expressed miRNAs were investigated through GO functional enrichment analysis and KEGG pathway enrichment analysis. The results of the GO functional enrichment analysis revealed that these differentially expressed miRNAs were primarily associated with cellular membrane function and integrity, endoplasmic reticulum stress, peroxisome function, mitochondrial function integrity, lysosome function, lipid metabolism, oxidation reduction reaction, inflammatory response, vesicle transport etc. (Fig. 10A). Additionally, the results of the KEGG pathway enrichment analysis indicated that these differentially expressed miRNAs were mainly enriched in MAPK signaling pathway, phospholipid metabolism pathway, p53 signaling pathway, peroxisome and lysosome-related signaling pathways AMPK signaling pathway,lipid metabolism signaling pathway,HIF-1 signaling pathway etc. (Fig. 10B). All of the aforementioned molecular functions and signal pathways are closely associated with the process of ferroptosis. The number of differentially expressed miRNAs in terms of molecular function, biological processes, and cellular components is summarized in Fig. 10C. Regarding molecular function, these differences primarily involve protein binding, protein dimer activity, serine/threonine kinase activity, and oxidoreductase activity. In terms of biological processes, they are mainly characterized by positive regulation of RNA polymerase II transcription, oxidation reduction processes, negative regulation of cell proliferation, inflammatory response, heart development, response to hypoxia and lipid metabolism. Concerning cellular components, the variations are predominantly observed in cytoplasmic membrane and organelle membrane compartments such as exosomes, mitochondria, endoplasmic reticulum membranes, lysosomes, peroxisomes etc.
Fig. 10.
GO functional enrichment analysis and KEGG pathway enrichment analysis. A GO functional enrichment analysis is employed to elucidate the biological functions associated with differentially expressed miRNAs; B KEGG pathway enrichment analysis is employed to elucidate the involvement of differentially expressed miRNAs in biological signal transduction pathways; C The number of differentially expressed miRNAs varies in terms of molecular function, biological processes, and cellular components
Study on miRNA-541-5p targeting iron death during myocardial ischemia reperfusion injury
qRT-PCR verified the successful construction of miRNA-541-5p vector
The expression levels of miRNA-541-5p in different vectors were detected and validated using qRT-PCR, along with the assessment of the effects of hypoxia-reoxygenation injury on miRNA-541-5p expression in H9C2 cells. As depicted in Fig. 11, compared to the Control group, a significant upregulation was observed in the I/R group for miRNA-541-5p expression (p < 0.01), which is consistent with the findings from high-throughput sequencing analysis. When compared to the I/R group, the expression level of miRNA-541-5p was significantly upregulated in the I/R + miRNA-541-5p mimics group (p < 0.01), while it was markedly downregulated in the I/R + miRNA-541-5p inhibitor group (p < 0.01). Furthermore, compared to the I/R + miRNA-541-5p mimics group, there was a significant decrease in miRNA-541-5p expression observed in the I/R + miRNA-541-5p inhibitor group (p < 0.01). These findings not only demonstrate successful construction of overexpressing/underexpressing vectors for miRNA-541-5p but also provide further validation that hypoxia-reoxygenation injury can induce upregulation of miRNA-541-5p expression.
Fig. 11.
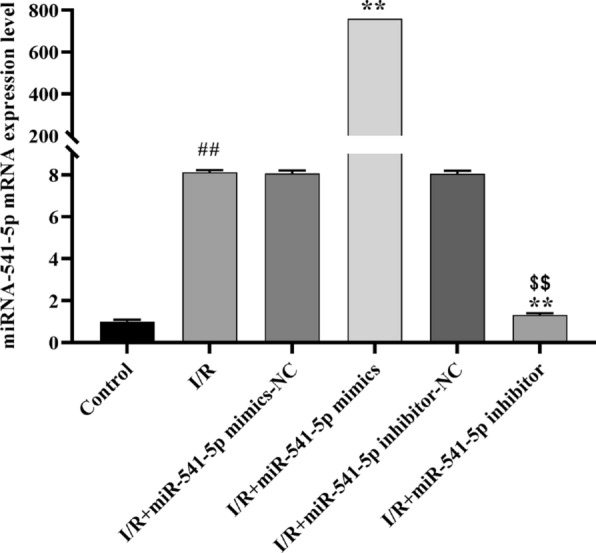
qRT-PCR was employed for the detection and validation of vectors harboring distinct expression levels of miRNA-541-5p. ##p < 0.01, as compared to Control group; **p < 0.01, as compared to I/R group; $$p < 0.01, as compared to I/R + miRNA-541-5p mimics group
Effect of miRNA-541-5p on myocardial cell viability
The CCK-8 kit was utilized to assess the impact of miRNA-541-5p with varying expression levels on H9C2 cell viability. As depicted in Fig. 12, compared to the Control group, there was a significant reduction in cell viability observed in the I/R group (p < 0.01), which is consistent with findings from animal experiments. Furthermore, cell survival was significantly decreased in the I/R + miRNA-541-5p mimics group compared to the I/R group (p < 0.05), whereas an improvement in cell viability was observed in the I/R + miRNA-541-5p inhibitor group (p < 0.05). Importantly, the cell survival rate was significantly increased in the I/R + miRNA-541-5p inhibitor group compared to the I/R + miRNA-541-5p mimics group (p < 0.01), indicating a significant inverse correlation between miRNA-541-5p expression level and cell survival rate.
Fig. 12.

The impact of miRNA-541-5p on the viability of myocardial cells as assessed by CCK-8 assay. ##p < 0.01, as compared to Control group; *p < 0.05, as compared to I/R group; $$p < 0.01, as compared to I/R + miRNA-541-5p mimics group
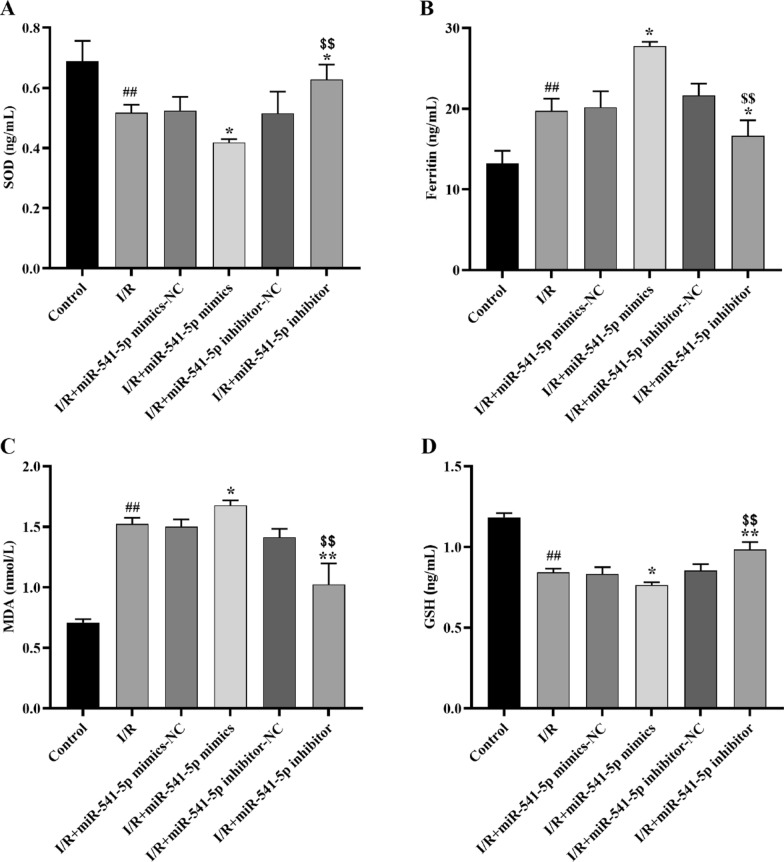
Effects of miRNA-541-5p on SOD, GSH, MDA and Ferritin content in cardiomyocytes
The Elisa kits were utilized to quantify the levels of markers for antioxidant damage, including SOD (Fig. 13A) and GSH (Fig. 13D), ferroptosis marker ferritin (Fig. 13B), and oxidative damage marker MDA (Fig. 13C) in H9C2 cells. The results demonstrated a significant decrease in SOD and GSH content (p < 0.01) and a significant increase in ferritin and MDA content (p < 0.01) in the I/R group compared to the Control group. Furthermore, when comparing with the I/R group, there was a further significant reduction in SOD and GSH content (p < 0.05) and an additional increase in ferritin (p < 0.01) and MDA (p < 0.05) content observed in the I/R + miRNA-541-5p mimics group; Compared with the I/R + miRNA-541-5p mock group, the I/R + miRNA-541-5p inhibitor group showed a significant increase in SOD and GSH content (p < 0.01) and a substantial decrease in MDA and ferritin content (p < 0.01).
Fig. 13.
The Elisa kits were utilized for the quantification of SOD, ferritin, MDA and GSH in H9C2 cells. A Antioxidant damage marker SOD content assay; B ferroptosis-related factor ferritin content assay; C oxidative damage marker MDA content assay; D antioxidant damage marker GSH content assay. ##p < 0.01, as compared to Control group; **p < 0.01, *p < 0.05, as compared to I/R group; $$p < 0.01, as compared to I/R + miRNA-541-5p mimics group
Effect of miRNA-541-5p on the expression of GPX-4, ACSL-4, HO-1 and ferritin in cardiomyocytes
Next, the protein expression levels of pro-ferroptotic factors, including HO-1 (Fig. 14A), Acsl-4 (Fig. 14C), and ferritin (Fig. 14D), as well as the anti-ferroptotic factor Gpx-4 (Fig. 14B), were assessed using Western blot (WB) analysis (Fig. 14E). The results demonstrated a significant upregulation (p < 0.01) of HO-1, Acsl-4, and ferritin levels in the I/R group compared to the Control group, while Gpx-4 expression level was significantly downregulated (p < 0.01). Furthermore, compared to the I/R group, the levels of HO-1, Acsl-4, and ferritin were further augmented in the I/R + miRNA-541-5p mimics group (p < 0.05), while Gpx-4 expression was further diminished (p < 0.05). Conversely, in the I/R + miRNA-541-5p inhibitor group, a significant reversal was observed in the results of HO-1, Acsl-4, ferritin, and Gpx-4 (p < 0.05). Interestingly, when comparing with the I/R + miRNA-541-5p mimics group, a significant decrease (p < 0.01) in HO-1, Acsl-4 and ferritin expression levels was observed in the I/R + miRNA-541-5p inhibitor group; conversely, there was a significant increase (p < 0.01) in Gpx-4 expression level. These findings suggest a positive correlation between miRNA-541-5p expression level and ferroptosis severity in H9C2 cells.
Fig. 14.
Western blot detection of GPX-4, ACSL-4, HO-1, ferritin expression levels. A GPX-4 protein expression level, B ACSL-4 protein expression, C HO-1 protein expression, D ferritin expression, E WB bands. ##p < 0.01, as compared to Control group; *p < 0.05, as compared to I/R group; $$p < 0.01, as compared to I/R + miRNA-541-5p mimics group
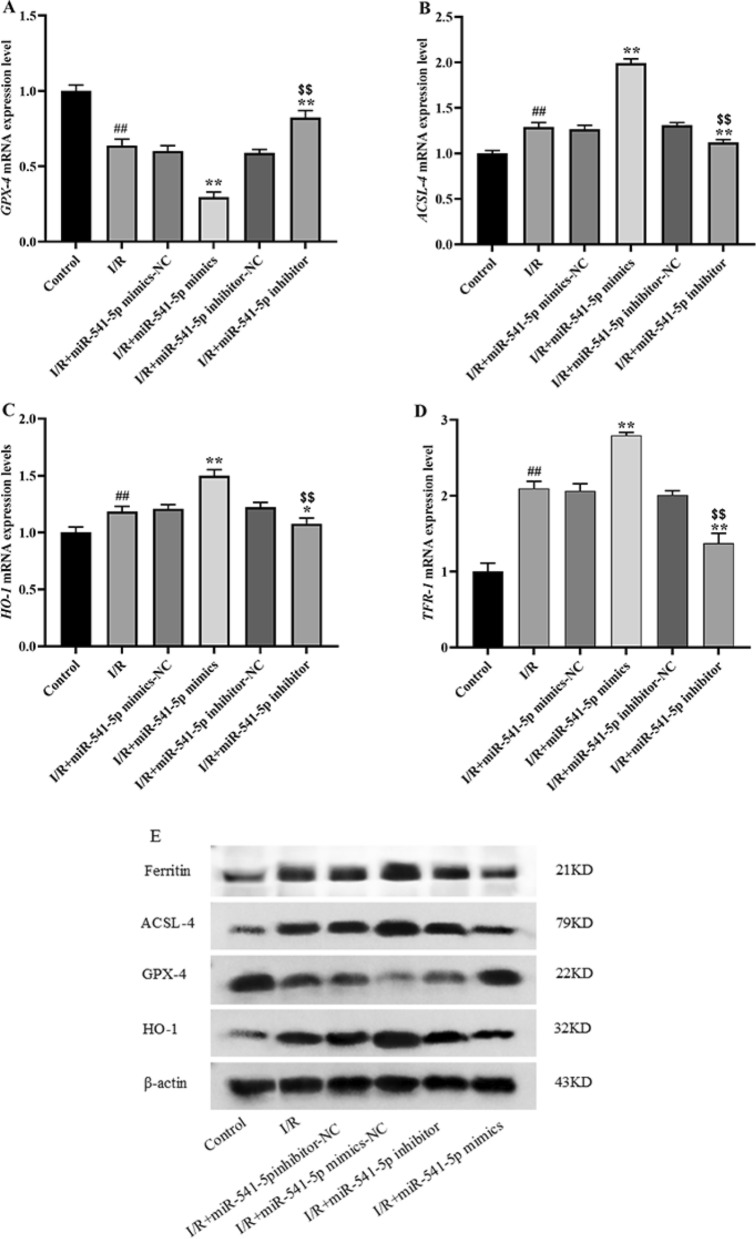
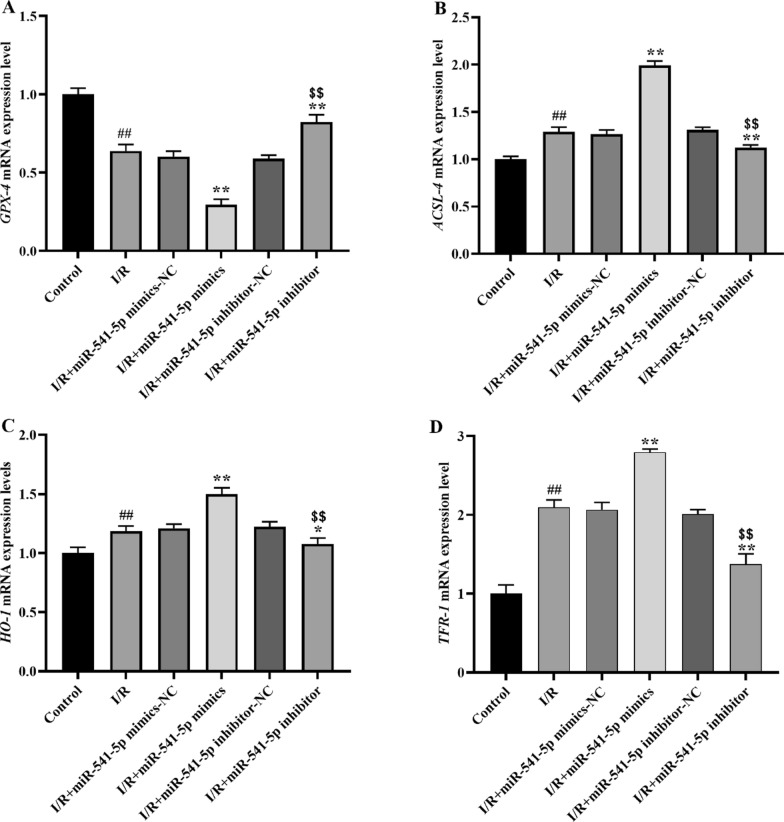
Effect of miRNA-541-5p on GPX-4, ACSL-4, and HO-1 mRNA expression in cardiomyocytes
Subsequently, the mRNA expression levels of the anti-ferroptotic factor GPX-4 (Fig. 15A) and the pro-ferroptotic factors ACSL-4 (Fig. 15B), HO-1 (Fig. 15C), and TFR-1 (Fig. 15D) were determined using the qRT-PCR method. We observed a significant downregulation of GPX-4 expression in the I/R group compared to the Control group (p < 0.01), while ACSL-4, HO-l and TFR-1 exhibited substantial upregulation (p < 0.01). In the I/R + miRNA541-5p mimics group, there was a more pronounced decrease in GPX-4 expression compared to the I/R group (p < 0.01), whereas ACSL-4, HO-l and TFR-1 showed a more prominent increase in expression levels (p < 0.01). Conversely, in the I/R + miRNA-541-5p inhibitor group, we noticed a noticeable upregulation of GPX-4 expression (p < 0.01) along with significant downregulation of ACSL-4 (p < 0.01) and HO-l (p < 0.05) expressions. Importantly, when comparing with the I/R + miRNA-541-5p mimics group, we observed a clear reversal in expressions of GPX-4, ACSL-4, and HO-l in the I/R + miRNA-541-5p inhibitor group (p < 0.01). These findings were consistent with those obtained from WB analysis.
Fig. 15.
mRNA expression levels of ferroptosis-related factors GPX-4, ACSL-4, HO-1 and TFR-1 were detected by qRT-PCR. A mRNA expression level of GPX-4; B mRNA expression level of ACSL-4; C mRNA expression level of HO-1; D mRNA expression level of TFR-1. ##p < 0.01, as compared to Control group; **p < 0.01, *p < 0.05, as compared to I/R group; $$p < 0.01, as compared to I/R + miRNA-541-5p mimics group
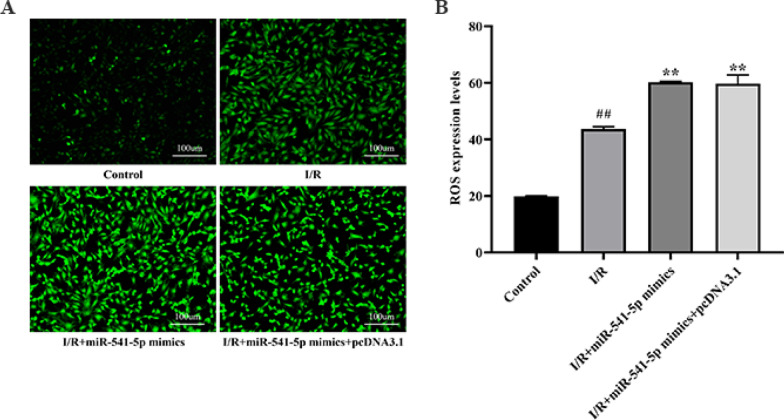
Effect of miRNA-541-5p on ROS content in cardiomyocytes
The effect of miRNA-541-5p on intracellular ROS levels in H9C2 cells is depicted in Fig. 16. It was observed that the Control group exhibited a ROS level of 19.81 ± 0.26. In comparison, the I/R group displayed a significantly higher ROS level of 43.62 ± 0.86 when compared to the Control group (p < 0.01). Furthermore, both the I/R + miRNA-541-5p mimics group and I/R + miRNA-541-5p mimics + pcDNA3.1 group demonstrated significantly elevated ROS levels at 60.20 ± 0.37 and 59.72 ± 3,10, respectively, as compared to the I/R group (p < 0.01). These findings provide further evidence supporting the ability of miRNA-541-5p to promote oxidative stress damage.
Fig. 16.
Effects of miRNA-541-5p on ROS content in cardiomyocytes (200 ×). A ROS fluorescence intensity; B ROS expression level. ##p < 0.01 vs. Control group; **p < 0.01 vs. I/R group, $$p < 0.01 vs. I/R + miRNA-541-5p group
Prediction of downstream target genes for miRNA-541-5p
According to the aforementioned results, we have discovered that miRNA-541-5p plays a role in exacerbating myocardial cell I/R injury by promoting ferroptosis. In this section, our objective is to provide preliminary predictions on the downstream target genes of miRNA-541-5p. By conducting thorough searches in miRNA-related databases, a total of 1918 potential target genes were identified from the Targetscan database, 14,924 potential target genes from the miRWalk database, and 97 potential target genes from the miRDB database. The intersection of these three databases resulted in 58 common potential target genes (Fig. 17). Table 4 (Supplementary materia) summarizes comprehensive information including functional descriptions, binding site lengths, miRDB scores, TargetScan cumulative weights, and TargetScan total weights for these 58 potential target genes. The URLs for accessing these databases are provided below.
Fig. 17.
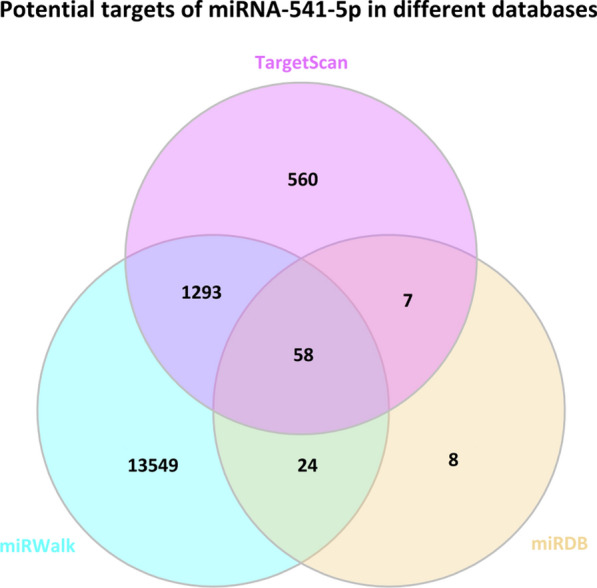
Screening potential target genes of miRNA-541-5p from TargetScan, miRWalk, and miRDB databases
miRWalk (http://mirwalk.umm.uni-heidelberg.de/rat/mirna).
miRDB (http://mirdb.org/cgi-bin/search.cgi).
Through a comprehensive analysis of these 58 potential target genes, we have identified 10 target genes with binding region lengths exceeding 40, namely ADAM7, RAG1, KAT2A, FNIP2, RPGRIP1L, VAPA, KLHL29, VAMP4, NPC1 and DNAJB1. Furthermore, we have discovered nine target genes with miRDB scores surpassing 80 points: STK3, ADAM7, HOXD10, PCBP2, HCCS, ADRA2B, FNIP2, PXDN, and USP15. Additionally, there were nine target genes exhibiting Targetscan total weights below −0.45: LEFTY1, CMPK1, SORCS1, LAPT, M4B, STK3, HCCS, ADAM7, and HOXD10 (Fig. 18). Based on a comprehensive analysis of region length, miRDB score, and Targetscan total weight, we have preliminarily identified ADAM7, FNIP2, HOXD10, HCCS, and STK3 as potential candidate target genes for miRNA-541-5p. Among them, ADAM7 emerges as the most suitable potential target gene based on the selection criteria.
Fig. 18.
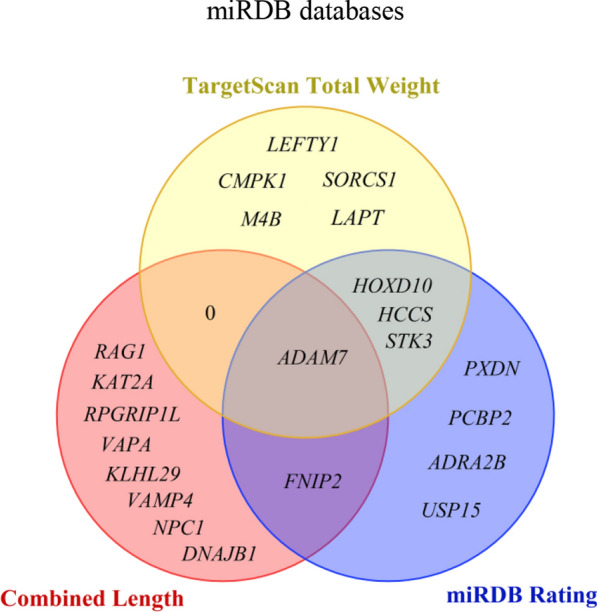
Based on three fundamental criteria, including a cumulative region length exceeding 40, a Targetscan total weight below − 0.45, and an miRDB score above 80 points, further screening was conducted for 58 potential target genes
Discussion
Ischemic heart disease is characterized by the narrowing of coronary arteries, leading to myocardial ischemia and hypoxia. As a prevalent form of coronary artery disease, it poses a significant threat to human health. Early diagnosis, treatment, and preservation of the compromised myocardium have consistently been focal points in clinical practice [15]. When coronary artery blood flow is interrupted, myocardial cells undergo enhanced anaerobic metabolism, disruption of the mitochondrial energy supply chain, an imbalance in AMP/ATP ratio, accumulation of toxic metabolic byproducts such as lactate and free radicals, and impaired cellular functions. Failure to promptly restore oxygen supply to the cells can ultimately result in irreversible damage or even myocardial cell death [16]. The expeditious restoration of coronary artery blood flow has always been the primary treatment approach for ischemic heart disease, encompassing percutaneous coronary intervention (PCI) and coronary artery bypass surgery (CABG) [17]. However, one cannot overlook the issue of I/R injury; upon reestablishment of blood flow to ischemic myocardial tissue, it can lead to more severe detrimental effects than the original ischemia and even an expansion of the infarcted area [18].
Myocardial I/R injury encompasses a multitude of intricate molecular mechanisms and signaling pathways, wherein diverse factors synergistically contribute to the exacerbation of myocardial cell damage. Current research predominantly focuses on elucidating the involvement of apoptosis, autophagy, ferroptosis, pyroptosis, extracellular vesicles (EVs), miRNA, lncRNA, among others. Concurrently, scholars are actively exploring corresponding therapeutic approaches encompassing novel drug development, enhancement of medical devices, and innovative diagnostic and treatment strategies [19]. However, despite these efforts, a significant portion of current research pertaining to the mechanistic understanding of myocardial I/R injury remains primarily theoretical in nature. Nonetheless, any advancements in cardiac protection warrant considerable attention.
The concept of ferroptosis, a novel form of regulated cell death, was initially proposed by Professor Dixon in 2012. At its core, ferroptosis is characterized by intracellular iron overload, which ultimately triggers the generation of lipid peroxides and subsequent oxidative stress-induced damage [20]. The maintenance of intracellular iron homeostasis is a complex process that involves the coordination of multiple steps, including iron uptake, storage, and efflux, to regulate cellular function and biological behavior. The concise mechanism entails the following: under normal conditions, extracellular Fe3+ binds to transferrin (Tf) and enters the cell via membrane transport through transferrin receptor 1 and 2 (Tfr-1) (Tfr-2). Subsequently, it undergoes reduction by STEAP3 to Fe2+ before being released into the cytoplasm through DMT1 for cellular reactions. A fraction of the released Fe2+ enters the labile iron pool (LIP) for cellular responses while another portion is stored as relatively stable Fe3+ in ferritin. Excess Fe2+ is oxidized back to Fe3+, which is then exported out of the cell by ferroportin. In cases where intracellular iron overload occurs, reactive oxygen species (ROS) can be generated through Fenton and Haber Weiss reactions. The excessive production and accumulation of ROS further lead to lipid peroxidation products formation by oxidizing lipids detrimentally. Ferroptosis ensues when Gpx-4 becomes inactive or glutathione (GSH), an antioxidant molecule crucial for Gpx-4 activity, becomes depleted resulting in ineffective clearance of harmful lipid peroxides within cells[21].
This study initially demonstrated, through animal experiments, the significant pathological structural alterations in myocardial tissue following I/R that could be visualized using TTC staining and HE staining. The iron chelator DFO effectively mitigated these adverse pathological changes in myocardial tissue by binding to excessive iron ions within tissues, preventing cellular iron overload and reducing reactive oxygen species formation, thereby averting intracellular accumulation of lipid peroxides. Ferritin serves as the primary form of intracellular iron storage with its content directly reflecting the level of iron burden. We observed a substantial increase in both serum and myocardial ferritin levels after experiencing I/R; however, this phenomenon could be reversed by administering the iron chelator DFO. Furthermore, markers of myocardial injury such as LDH along with oxidative damage markers MDA and ROS, as well as antioxidant damage markers GSH and SOD also indicate that antagonizing intracellular iron significantly improves myocardial I/R injury.
The polyunsaturated fatty acids (PUFAs) can be catalyzed by acyl-CoA synthetase long-chain family member 4 (ACSL-4) and lysophosphatidylcholine acyltransferase 3 (LPCAT3), subsequently participating in the biosynthesis of PUFA-PE, a constituent of phosphatidylethanolamine (PE). Excessive lipid peroxidation can lead to sustained deficiency of PUFAs, which in turn affects the normal structure and function of membranes, ultimately resulting in cellular ferroptosis. Moreover, iron overload also influences the sensitivity to ferroptosis regulated by ACSL-4 in tissues and cells damaged by I/R injury [22]. This is achieved through promoting the binding between exogenous iron ions with Tfr-1 and Tfr-2, leading to an influx of a large amount of iron ions into cells. Therefore, ACSL-4 has been identified as a crucial biomarker for ferroptosis, while the levels of Tfr-1 and Tfr-2 serve as key indicators reflecting intracellular iron accumulation [23]. The Xc-GSH-GPX4 pathway serves as the principal mechanism for regulating ferroptosis. System Xc- comprises carrier protein family 7 member 11 (SLC7A11) and carrier protein family 3 member 2 (SLC3A2), which facilitate the exchange of cystine and glutamate in a stoichiometric ratio of 1:1 across the membrane, subsequently enabling glutathione (GSH) synthesis. GSH, a tripeptide composed of glutamate, cysteine, and glycine, acts as an antioxidant and functions as a substrate for glutathione peroxidase-4 (GPX-4). Augmenting GSH expression can effectively inhibit ferroptosis. GPX-4 is a selenoenzyme that mitigates ROS production by generating GSH [22]. Heme oxygenase-1 (HO-1) represents a pivotal rate-limiting enzyme involved in heme degradation metabolism. It catalyzes the conversion of heme into equimolar amounts of carbon monoxide (CO), Fe2+, and biliverdin through nicotinamide adenine dinucleotide phosphate (NADPH)- cytochrome P450 (CYP) activity. Overexpression of HO-1 exerts significant detrimental effects on cells by elevating intracellular Fe2+ levels and inducing excessive ROS generation, ultimately leading to cellular damage [24]. Therefore, Gpx-4, Tfr-1, Tfr-2, Acsl-4,and HO-1 serve as direct indicators reflecting the extent of ferroptosis.
In the animal experiment section, we also employed qRT-PCR to assess the mRNA expression levels of ferroptosis-related factors HO-1, GPX-4, TFR-2, and ACSL-4. Our findings revealed a significant upregulation in the expression of pro-ferroptosis factors HO-1, TFR-2, and ACSL-4 following myocardial tissue I/R; conversely, there was a notable downregulation in the expression level of anti-ferroptosis factor GPX-4. These results strongly indicate that ferroptosis plays a pivotal role in myocardial I/R injury. Importantly, administration of iron chelator DFO effectively reversed these alterations in factor trends. In conclusion, targeting ferroptosis represents an efficacious approach for managing myocardial I/R injury.
miRNAs are a class of non-coding RNA molecules that consist of 21–24 nucleotides in length. miRNAs bind to target mRNAs through partial complementarity with the base pairs in their 3'-UTR region, thereby exerting inhibitory effects on mRNA translation and interfering with protein expression. miRNAs play pivotal roles in diverse biological processes such as cell cycle regulation, cell proliferation and apoptosis, oxidative reactions, and inflammatory responses [9]. Studies have demonstrated the crucial involvement of miRNAs in the pathological and physiological processes associated with myocardial I/R injury, including disturbances in energy metabolism, cellular apoptosis, oxidative stress-induced damage, dysregulation of autophagy function, and imbalances in iron homeostasis [10]. Moreover, miRNAs are closely linked to the occurrence and progression of heart diseases. For instance, downregulation of TNF-a expression by miRNA-130a can enhance cardiac function in rats with heart failure [25]; inhibition of ULK1 expression by miRNA-1297 can ameliorate myocardial fibrosis and subsequently improve ventricular remodeling [26]; m6a-mediated upregulation of miRNA-193a via METTL3/miRNA-193a/BCL2L2 pathway exacerbates cardiomyocyte apoptosis and inflammatory response induced by sepsis-induced cardiomyopathy [27].
Therefore, we utilized high-throughput sequencing technology to screen for miRNAs associated with ferroptosis during myocardial I/R injury. A total of 369 differentially expressed miRNAs were identified among the NC group, Model group, and DFO group. GO functional enrichment analysis and KEGG pathway enrichment analysis revealed that these differentially expressed miRNAs were primarily enriched in cytoplasmic function, membrane integrity and function, mitochondrial function integrity, lysosomal function, endoplasmic reticulum stress, peroxisomal function, lipid metabolism, oxidative-reduction reactions, inflammatory response, vesicular transport, MAPK signaling pathway, phospholipid metabolism pathway, p53 signaling pathway, peroxisome and lysosome-related signaling pathways, AMPK signaling pathway, lipid metabolism signaling pathway, HIF-1 signaling pathway etc. It is evident that all of these molecular functions and signal pathways are closely related to cell ferroptosis.
We performed further analysis on the 369 differentially expressed miRNAs and identified a total of 13 miRNAs with statistically significant differential expression (p < 0.05) between the NC and Model groups, including 6 upregulated and 7 downregulated expressions. Similarly, we observed a total of 15 differentially expressed miRNAs (11 downregulated and 4 upregulated expressions) with p < 0.05 between the Model and DFO groups. Among the NC, Model, and DFO groups, we discovered a total of 31 miRNAs exhibiting differential expression at p < 0.05; notably, among these were 8 miRNAs that demonstrated statistical significance at p < 0.01.
Hence, we aim to conduct further screening and investigation of the target miRNA from this pool of 31 differentially expressed miRNAs. Initially, considering the significance level of p < 0.01, we observed that 4 miRNAs (rno-miR-6328_R + 1, mdo-miR-152-3p_R + 3, bta-miR-2478_L-2R + 1, rno-miR-181d-3p_R + 1) exhibited very low expression levels and were thus excluded from our study. Additionally, rno-miR-671 demonstrated almost identical expression levels in both the NC group and Model group, which could not explain its association with I/R injury and was also excluded. Furthermore, the changes in expression trends of rno-miR-27a-5p and rno-miR223-3p_R + 1 could not be attributed to DFO's anti-ferroptosis effect and were therefore excluded as well. Although PC5p12265_274 appeared to have a relevant expression trend for our research purpose, no literature on its mechanism was found upon review; hence it was also excluded. Consequently, we proceeded to screen the remaining 23 candidate miRNAs, excluding those exhibiting low expression levels, miRNAs with expression trends that could not be attributed to I/R injury, miRNAs whose expression patterns did not align with the research objectives, and miRNAs lacking previous relevant studies. Ultimately, we identified miRNA-541-5p as the focal point for our subsequent investigations.
Interestingly, a statistical analysis of miRNA-541-5p expression levels revealed significant differences when comparing pairwise between the NC group, Model group, and DFO group. Specifically, there was a noteworthy increase in miRNA-541-5p expression in the Model group compared to the NC group (p < 0.05), while a substantial decrease in miRNA-541-5p expression was observed in the DFO group compared to the Model group (p < 0.05). When comparing the NC group with the Model group separately, the p-value for differential expression of miRNA-541-5p was found to be statistically significant (p < 0.05). However, when comparing the Model group with the DFO group separately, a trend towards differential expression of miRNA-541-5p was observed (p < 0.1), p = 0.052. Based on these findings, it is postulated that miRNA-541-5p may play a crucial role in the pathological progression of myocardial I/R injury, potentially attributed to DFO-mediated regulation of ferroptosis. However, it should be noted that the expression difference of miRNA-541-5p was not as significant as expected when comparing between the Model group and DFO group separately. We speculate that this may be due to prolonged reoxygenation time during modeling and a low dosage of DFO, or it could be related to a lower reactive response of miRNA-541-5p towards DFO itself. Further verification is needed to determine whether appropriately shortening the reoxygenation time or increasing the dosage of DFO can change this situation; alternatively, selecting other ferroptosis inhibitors such as Ferrostatin-1, Lipstatin-1, Dexrazoxane, MitoTEMPO can also be considered [22]. Additionally, it is important to emphasize that the primary objective of this part of the study was to screen for ferroptosis-related miRNAs in the context of myocardial I/R injury, rather than extensively investigate the regulatory mechanism between DFO and miRNA-541-5p. Therefore, further elucidation on this matter falls beyond the scope of our current investigation.
Studies have demonstrated that ectopic expression of miRNA-541-5p can ameliorate radiation-induced pulmonary fibrosis in mice by targeting Slug. Radiation itself activates MZF1, leading to the downregulation of miRNA-541-5p in alveolar epithelial cells and promoting the development of radiation-induced pulmonary fibrosis through targeted regulation of Slug [28]. As a sponge for miRNA-541-5p, circRTN4IP1 exhibits high expression levels in intrahepatic cholangiocarcinoma cells. Knockout of circRTN4IP1 upregulates miRNA-541-5p, resulting in inhibition of cell proliferation and glucose metabolism while promoting apoptosis. The primary target gene of miRNA-541-5p is HIF1A [29]. Our KEGG pathway enrichment analysis revealed HIF-1 as one of the significantly enriched signaling pathways. However, further validation is required to determine whether miRNA-541-5p exerts its proferroptotic effect through HIF1A. Robles et al. discovered a significant upregulation of miRNA-541-5p in a C57BL/6 mouse model of cerebral hemorrhage compared to the control group, indicating its potential as a biomarker for acute brain hemorrhage risk [30]. Although miRNA-541-5p has been proposed to be associated with septic cardiomyopathy in the study by Feng et al., this association is solely based on bioinformatics analysis and lacks investigation into specific underlying mechanisms [31]. Inhibition of miRNA-541-3p, which acts as a passenger strand of miRNA-541 (with miRNA-541-5p serving as the guide strand), can alleviate the impact of circIL4R knockdown on HCC cells. Upregulation of GPX-4 can mitigate the inhibitory effects of miRNA-541-3p on hepatocellular carcinoma cells (HCC) and exacerbation of ferroptosis [32].
Based on the aforementioned studies regarding miRNA-541-5p, we aim to further investigate its association with ferroptosis in myocardial cells during H/R injury. Following successful construction of vectors for miRNA-541-5p mimics and inhibitors, we initially validated the impact of varying expression levels of miRNA-541-5p on cellular viability. The results demonstrated a significant inverse correlation between H9C2 cell survival rate and miRNA-541-5p expression level. Additionally, analysis of proferroptotic markers such as ACSL-4 and HO-1 protein/mRNA expression levels, ferritin expression, and TFR-1 mRNA expression revealed a clear positive correlation with miRNA-541-5p level. Conversely, there was a notable negative correlation between miRNA-541-5p level and antioxidant damage factors SOD, GSH, as well as antiferroptosis factor GPX-4 at both protein and mRNA levels. Importantly, overexpression of miRNA-541-5p resulted in a significant increase in intracellular ROS content. These findings strongly support the existence of a positive relationship between miRNA-541-5p expression level and severity of ferroptosis in H9C2 cells during H/R injury. In conclusion, it is evident that by promoting ferroptosis processes, miRNA-541-5p exacerbates H/R injury in H9C2 cells.
Finally, we employed online miRNA-related databases such as Targetscan, miRWalk, and miRDB to predict the downstream target genes of miRNA-541-5p. Through a comprehensive search analysis, we identified 58 potential target genes which were further characterized in Table 4 with details including gene description, binding site length, miRDB score, TargetScan cumulative weight, and TargetScan total weight. Subsequent screening was performed based on specific criteria such as a binding site length exceeding 40 nucleotides, an miRDB score surpassing 80 points, and a Targetscan total weight below −0.45 points. Remarkably, only ADAM7 met all three inclusion criteria mentioned above. ADAM7 (ADAM metallopeptidase domain 7) belongs to the zinc-dependent metalloproteinase superfamily and plays a crucial role in extracellular matrix protein degradation. The expression of the ADAM7 gene has been proposed as a potential biomarker for fetuses or newborns exposed to estrogenic compounds [33]. However, a literature review revealed no research on its involvement in ferroptosis and cardiovascular diseases.
Candidate genes meeting two inclusion criteria include FNIP2, HOXD10, HCCS, and STK3. FNIP2 (folliculin interacting protein 2) forms a complex with FNIP1 and FLCN, which interacts with AMPK. FNIP1 acts as a negative regulator of AMPK; its deficiency in mice can induce left ventricular hypertrophy and glycogen accumulation cardiomyopathy [34]. Gastrin promotes ACSL4 transcription through HOXD10 (homeo box D10); inhibiting HOXD10 expression hinders ferroptosis in glioma cells. However, gastrin reverses this effect by inducing ferroptosis and inhibiting cell proliferation through the HOXD10/ACSL4-dependent pathway, thereby exerting anti-cancer effects [35]. Loss of activity in HCCS (holocytochrome c synthase) leads to respiratory chain dysfunction, abnormal cardiac cell differentiation, and reduced cell cycle activity [36]. STK3 (serine/threonine kinase 3) is a core component of the Hippo signaling pathway that regulates oxidative stress and inflammatory responses in cardiovascular diseases. Phosphorylation of KEAP1 mediated by STK3 can inhibit the expression of antioxidant genes controlled by Nrf2 in macrophages [37].
Although there is currently no relevant research on ADAM7 in the context of myocardial I/R injury, it is reassuring that our high-throughput sequencing results have revealed an association between differentially expressed miRNAs and the AMPK signaling pathway as well as the Hippo signaling pathway, based on KEGG pathway enrichment analysis. Moreover, STK3 exhibits a high miRDB score of 95, indicating that FNIP1 and STK3 are likely target genes of miRNA-541-5p. Additionally, the Nrf2/HO-1 axis demonstrates a strong correlation with ferroptosis processes [24]. Furthermore, ACSL-4, a crucial biomarker for ferroptosis, actively participates in the induction of ferroptosis through the HOXD10/ACSL4 pathway. Consequently, as a downstream target gene of miRNA-541-5p, HOXD10 holds significant promise as a potential candidate. However, these hypotheses are derived from existing research findings and require further experimental verification to elucidate the underlying mechanisms involving FNIP1, STK3, HOXD10 and miRNA-541-5p.
Naturally, this article possesses certain limitations. For instance, during the sequencing phase, we identified a total of 31 differentially expressed miRNAs; however, our study exclusively focused on miRNA-541-5p. Although some miRNAs were excluded due to their low expression levels or inconsistent trends with the research objectives outlined in this article, these particular miRNAs still hold potential for further exploration. Furthermore, our current prediction of downstream target genes for miRNA-541-5p remains speculative and necessitates extensive investigation in subsequent studies.
Conclusions
In summary, the iron chelator DFO can mitigate myocardial I/R injury by inhibiting ferroptosis. miRNA-541-5p can serve as a biomarker for myocardial I/R injury. Moreover, miRNA-541-5p can exacerbate H9C2 cell H/R injury by promoting ferroptosis. The expression level of miRNA-541-5p is positively correlated with the extent of ferroptosis in H9C2 cells. Notably, suppressing the expression of miRNA-541-5p in the context of H9C2 cells H/R injury confers a protective effect.
Supplementary Information
Acknowledgements
The authors thank the support from Dr. Yawei Ma for the further analyses and interpretation of the data in the revision process.
Abbreviations
- I/R
Ischemia–reperfusion
- H/R
Hypoxia-reoxygenation injury
- ncRNAs
Non-coding RNAs
- MDA
Malondialdehyde
- SOD
Superoxide dismutase
- DFO
Deferoxamine
- INF
Myocardial infarction area
- AAR
Myocardial damaged area
- ROS
Reactive oxygen species
- COX-2
Cyclooxygenase-2
- IRE
Iron response elements
- IREB2
Iron response element-binding protein 2
- miRNA
MicroRNA
- LDH
Lactate dehydrogenase
- Gpx-4
Glutathione peroxidase 4
- GSH
Endogenous glutathione
- TFR-1
Transferrin receptor-1
- TFR-2
Transferrin receptor-2
- HO-1
Haem oxygenase-1
- ACSL-4
Acyl-CoA synthetase long-chain familymember-4
- GSH
Endogenous glutathione
Author contributions
Writing-Original draft preparation, Z.Z.; Study protocol-B.L.; Index detection—D.C; Writing-Review & editing, Y.L.; All the authors contributed to manuscript revision, and read and approved the submitted version.
Funding
This research was supported by the National Natural Science Foundation of China (81960345), the Natural Science Foundation of Gansu Province (21JR7RA380), Natural Science Foundation of Gansu Province (24JRRA305), and the Intra-Hospital Fund of the First Hospital of Lanzhou University (ldyyyn2019-90).
Availability of data and materials
Data available on request from the corresponding authors. The datasets generated during and/or analyzed during the current study are available in the Genome Sequence Archive (GSA) repository, [https://ngdc.cncb.ac.cn/gsub/submit/bioproject/list]. Item number: PRJCA031542. No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of the First Hospital of Lanzhou University (No. LDYYLL2021-327). All animal work had been conducted according to international guidelines.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
ZhiYu Zhao, BoXia Li and DianWei Cheng have contributed equally to this work.
References
- 1.Hunter DJ, Reddy KS. Noncommunicable diseases. N Engl J Med. 2013;369(14):1336–43. [DOI] [PubMed] [Google Scholar]
- 2.Read SH, Wild SH. Prevention of premature cardiovascular death worldwide. The Lancet. 2020;395(10226):758–60. [DOI] [PubMed] [Google Scholar]
- 3.Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Investig. 2013;123(1):92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiang M, Lu Y, Xin L, Gao J, Shang C, Jiang Z, Lin H, Fang X, Qu Y, Wang Y. Role of oxidative stress in reperfusion following myocardial ischemia and its treatments. Oxid Med Cell Longev. 2021;2021:6614009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang Y, Lin X. Potential relationship between autophagy and ferroptosis in myocardial ischemia/reperfusion injury. Genes Dis. 2023;10(6):2285–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang X, Wang H, Han D, Xie E, Yang X, Wei J, Gu S, Gao F, Zhu N, Yin X. Ferroptosis as a target for protection against cardiomyopathy. Proc Natl Acad Sci. 2019;116(7):2672–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishimaru K, Ikeda M, Miyamoto HD, Furusawa S, Abe K, Watanabe M, Kanamura T, Fujita S, Nishimura R, Toyohara T. Deferasirox targeting ferroptosis synergistically ameliorates myocardial ischemia reperfusion injury in conjunction with cyclosporine A. J Am Heart Assoc. 2024;13(1):e031219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiang Q, Yi X, Zhu X-H, Wei X, Jiang D-S. Regulated cell death in myocardial ischemia–reperfusion injury. Trends Endocrinol Metab. 2023;35:219–34. [DOI] [PubMed] [Google Scholar]
- 9.Liu B, Wang B, Zhang X, Lock R, Nash T, Vunjak-Novakovic G. Cell type–specific microRNA therapies for myocardial infarction. Sci Transl Med. 2021;13(580):eabd0914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marinescu M-C, Lazar A-L, Marta MM, Cozma A, Catana C-S. Non-coding RNAs: prevention, diagnosis, and treatment in myocardial ischemia–reperfusion injury. Int J Mol Sci. 2022;23(5):2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seok H. A new member of myocardial ischemia-reperfusion (MI/R) associated miRNAs, miR-484: its potential cardiac protection role. Korean Circ J. 2020;50(3):264–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Y, Li K-S, Liu L, Li S-L. MicroRNA-132 promotes oxidative stress-induced pyroptosis by targeting sirtuin 1 in myocardial ischaemia-reperfusion injury. Int J Mol Med. 2020;45(6):1942–50. [DOI] [PubMed] [Google Scholar]
- 13.Bei Y, Lu D, Bär C, Chatterjee S, Costa A, Riedel I, Mooren FC, Zhu Y, Huang Z, Wei M. miR-486 attenuates cardiac ischemia/reperfusion injury and mediates the beneficial effect of exercise for myocardial protection. Mol Ther. 2022;30(4):1675–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jia X, Shao W, Tian S. Berberine alleviates myocardial ischemia–reperfusion injury by inhibiting inflammatory response and oxidative stress: the key function of miR-26b-5p-mediated PTGS2/MAPK signal transduction. Pharm Biol. 2022;60(1):652–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kyada S, Kamani YG, Gangannapalle M, Chenna V, Dhawan A, Gajjar R, Amin VP, Lakkimsetti M, Desai HD, Patel J. Abstract P404: statewide burden of ischemic heart disease attributable to high blood pressure in United States from 1990–2019: a benchmarking and comparative analysis for The GBD 2019. Hypertension. 2023;80(Suppl_1):AP404. [Google Scholar]
- 16.Li Y, Li Z, Liu J, Liu Y, Miao G. miR-190-5p alleviates myocardial ischemia-reperfusion injury by targeting PHLPP1. Dis Markers. 2021;2021(1):8709298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He J, Liu D, Zhao L, Zhou D, Rong J, Zhang L, Xia Z. Myocardial ischemia/reperfusion injury: mechanisms of injury and implications for management. Exp Ther Med. 2022;23(6):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Osorio-Llanes E, Villamizar-Villamizar W, Ospino Guerra MC, Díaz-Ariza LA, Castiblanco-Arroyave SC, Medrano L, Mengual D, Belón R, Castellar-López J, Sepúlveda Y. Effects of metformin on ischemia/reperfusion injury: new evidence and mechanisms. Pharmaceuticals. 2023;16(8):1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y, Li L, Wang Z, Zhang J, Zhou Z. Myocardial ischemia-reperfusion injury. Mol Mech Prev Microvasc Res. 2023;149:104565. [DOI] [PubMed] [Google Scholar]
- 20.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leng Y, Luo X, Yu J, Jia H, Yu B. Ferroptosis: a potential target in cardiovascular disease. Front Cell Dev Biol. 2022;9:813668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo Y, Lu C, Hu K, Cai C, Wang W. Ferroptosis in cardiovascular diseases: current status, challenges, and future perspectives. Biomolecules. 2022;12(3):390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu Y, Yan Y, Niu F, Wang Y, Chen X, Su G, Liu Y, Zhao X, Qian L, Liu P. Ferroptosis: a cell death connecting oxidative stress, inflammation and cardiovascular diseases. Cell Death Discov. 2021;7(1):193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryter SW. Heme oxgenase-1, a cardinal modulator of regulated cell death and inflammation. Cells. 2021;10(3):515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang Y-R, Du J-Y, Wang D-D, Yang X. miRNA-130a improves cardiac function by down-regulating TNF-α expression in a rat model of heart failure. Eur Rev Med Pharmacol Sci. 2018;22(23):8454–61. [DOI] [PubMed] [Google Scholar]
- 26.Li M-L, Li R-N, Ma Y-M, Jiang B, Chen Y-J, Hu W-X, Qv C-L, Zhang Y-J, Song Y-Y, Wang Y. MiRNA-1297 inhibits myocardial fibrosis by targeting ULK1. Eur Rev Med Pharmacol Sci. 2020;24(4):2070–6. [DOI] [PubMed] [Google Scholar]
- 27.Liang L, Liu S, Wu Q, Chen R, Jiang S, Yang Z. m6A-mediated upregulation of miRNA-193a aggravates cardiomyocyte apoptosis and inflammatory response in sepsis-induced cardiomyopathy via the METTL3/miRNA-193a/BCL2L2 pathway. Exp Cell Res. 2023;430(1):113712. [DOI] [PubMed] [Google Scholar]
- 28.Liang X, Yan Z, Wang P, Liu Y, Ao X, Liu Z, Wang D, Liu X, Zhu M, Gao S. Irradiation activates MZF1 to inhibit miR-541-5p expression and promote epithelial-mesenchymal transition (EMT) in radiation-induced pulmonary fibrosis (RIPF) by upregulating slug. Int J Mol Sci. 2021;22(21):11309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang J, Wang R, Tang R, Gu P, Han J, Huang W. CircRTN4IP1 regulates the malignant progression of intrahepatic cholangiocarcinoma by sponging miR-541-5p to induce HIF1A production. Pathol Res Pract. 2022;230:153732. [DOI] [PubMed] [Google Scholar]
- 30.Robles D, Guo D-H, Watson N, Asante D, Sukumari-Ramesh S. Dysregulation of serum microRNA after intracerebral hemorrhage in aged mice. Biomedicines. 2023;11:822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feng S, Cai K, Lin S, Chen X, Luo Y, Wang J, Lian G, Lin Z, Xie L. Exploring potential therapeutic agents for lipopolysaccharide-induced septic cardiomyopathy based on transcriptomics using bioinformatics. Sci Rep. 2023;13(1):20589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu Q, Zhou L, Yang G, Meng F, Wan Y, Wang L, Zhang L. CircIL4R facilitates the tumorigenesis and inhibits ferroptosis in hepatocellular carcinoma by regulating the miR-541-3p/GPX4 axis. Cell Biol Int. 2020;44(11):2344–56. [DOI] [PubMed] [Google Scholar]
- 33.Adachi T, Matsuno Y, Sugimura A, Takano K, Koh KB, Sakurai K, Shibayama T, Iguchi T, Mori C, Komiyama M. ADAM7 (a disintegrin and metalloprotease 7) mRNA is suppressed in mouse epididymis by neonatal exposure to diethylstilbestrol. Mol Reprod Dev Inc Gamete Res. 2003;64(4):414–21. [DOI] [PubMed] [Google Scholar]
- 34.Malik N, Ferreira B, Hollstein P, Curtis S, Trefts E, Novak SW, Yu J, Gilson R, Hellberg K, Fang L. Induction of lysosomal and mitochondrial biogenesis by AMPK phosphorylation of FNIP1. Science. 2023;380:eabj5559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao W, Lan J, Zeng Z, Yu W, Lei S. Gastrodin induces ferroptosis of glioma cells via upregulation of homeobox D10. Molecules. 2023;28(24):8062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Rahden VA, Fernandez-Vizarra E, Alawi M, Brand K, Fellmann F, Horn D, Zeviani M, Kutsche K. Mutations in NDUFB11, encoding a complex I component of the mitochondrial respiratory chain, cause microphthalmia with linear skin defects syndrome. Am J Hum Genet. 2015;96(4):640–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu H, Dai Z, Liu X, Zhou H, Wang Y. Serine/threonine kinase 3 promotes oxidative stress and mitochondrial damage in septic cardiomyopathy through inducing Kelch-like ECH-associated protein 1 phosphorylation and nuclear factor erythroid 2-related factor 2 degradation. Int J Biol Sci. 2023;19(5):1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available on request from the corresponding authors. The datasets generated during and/or analyzed during the current study are available in the Genome Sequence Archive (GSA) repository, [https://ngdc.cncb.ac.cn/gsub/submit/bioproject/list]. Item number: PRJCA031542. No datasets were generated or analysed during the current study.