Abstract
We have previously reported the structure of a chromatin remodeling complex (PYR complex) with Ikaros as its DNA binding subunit that is specifically present in adult murine and human hematopoietic cells. We now show that homozygous Ikaros “knockout” (null) mice lack the PYR complex, demonstrating the requirement for Ikaros in the formation of the complex on DNA. Heterozygous Ikaros null mice have about half as much PYR complex, indicating a dosage effect for both Ikaros and PYR complex. We also show that Ikaros null mice have multiple hematopoietic cell defects including anemia and megakaryocytic abnormalities, in addition to previously reported lymphoid and stem cell defects. The null mice also have a delay in murine embryonic to adult β-globin switching and a delay in human γ to β switching, consistent with a previously suggested role for PYR complex in this process. Lastly, cDNA array analyses indicate that several hematopoietic cell-specific genes in all blood lineages are either up- or down-regulated in 14-day embryos from Ikaros null as compared with wild-type mice. These results indicate that Ikaros and PYR complex function together in vivo at many adult hematopoietic cell-specific genes and at intergenic sites, affecting their expression and leading to pleiotropic hematopoietic defects.
Chromatin remodeling complexes are associated with both activation and repression of expression of specific eukaryotic genes. SWI/SNF containing ATPase/helicase subunits (e.g., BRG1) have been shown to remodel chromatin and activate gene transcription at the β-globin locus as well as elsewhere (1, 2). Similarly, NuRD complexes using other ATPase/helicase subunits (e.g.Mi-2β) together with histone deacetylases have been associated with chromatin deacetylation and repression of specific gene expression (3). We have recently described a SWI/SNF and NuRD-containing complex associated with the DNA binding transcription factor Ikaros that is present only in adult hematopoietic cells (4, 5). This complex binds to Ikaros-like DNA binding sites including a long polypyrimidine-rich sequence upstream of the human δ-globin gene and was thus called PYR complex. Deletion of this sequence in a human β-locus containing cosmid (carrying sequences from the human Aγ through the adult β-globin gene) in transgenic mice results in delayed human γ- to β-globin switching (4).
We now have studied Ikaros null mice whose Ikaros gene has been truncated by deletion of the two C-terminal zinc fingers required for Ikaros protein dimerization and function (6, 7). We show that Ikaros null mice completely lack PYR complex, indicating that Ikaros is required for PYR complex formation. In addition, heterozygous null mice have about half as much PYR complex as wild-type mice, indicating a dosage effect for both Ikaros and PYR complex. In this model for evaluating Ikaros and PYR complex function, we find multiple hematopoietic defects including anemia and thrombocytosis, along with previously reported lymphoid and stem cell abnormalities (8). We also show that there is a delay in mouse embryonic to adult globin switching in these null mice and delayed human γ to β switching in Ikaros null mice crossed with transgenic mice containing the human β-locus (4). cDNA chip analyses of 14-day fetal livers show changes in the expression of several hematopoietic cell-specific genes in the null mice compared with wild type in all hematopoietic lineages. These results indicate that PYR complex is required for normal hematopoiesis in vivo in most, if not all, adult hematopoietic cells and presumably acts at individual loci by remodeling chromatin to facilitate transcriptional activation or repression.
Materials and Methods
Ikaros Mice.
The generation and characteristics of Ikaros null mice have been described (7). The mice were bred and genotyped by PCR by using tail DNA and previously described primers. Materials for analysis, such as blood and tissues, were obtained from embryos at various times during gestation and from adult mice. Ikaros null mice were also crossed with transgenic mice containing a 32-kb human β-globin minilocus (wt1 line) (4) to obtain mice with the human β-globin minilocus lacking the Ikaros gene. Genotyping analysis of the transgenic null mice was performed as with the Ikaros null mice for the Ikaros gene and as with transgenic mice by using a KpnI repeat downstream of the human β-globin gene for the transgene (7). Offspring were killed at various times during gestation, and blood and tissues were obtained for analysis.
Hematologic Analysis.
Adult wild-type and Ikaros null mice were anesthetized and bled retro-orbitally into an EDTA-coated tube. Complete blood counts were performed by using a Coulter Counter. Blood smears were stained by using Wright–Giemsa stain. Supravital stains were performed by using Brilliant Cresyl Blue dye and were then used to perform reticulocyte counts. Spleen, bone marrow, and liver tissues were dissected, fixed in formalin, sectioned and mounted on slides, and stained with hematoxylin–eosin.
Biochemical Characterization.
Nuclear extracts were prepared from mouse fetal livers at day 14.5 of gestation (E14.5). Nuclei were obtained by using a sucrose cushion (9), and extracts were then prepared following a miniextract protocol (10). Protein concentrations were determined by reading the sample absorbance at 320 nm. Gel shifts were performed with 5 μg of fetal liver nuclear extract and 2 μg of double-stranded dG-dC as nonspecific competitor DNA. The end-labeled probes used were δ60ym (the PYR complex binding site) and YY1, as an extract quality control (5). Ten micrograms of fetal liver nuclear extract was run on a 4–15% gradient SDS–acrylamide gel and blotted onto a poly(vinylidene difluoride) membrane. The membrane was probed with α-Ikaros goat polyclonal antibody (Santa Cruz Biotechnology) followed by horseradish peroxidase-conjugated α-goat polyclonal secondary antibody. Chemiluminescent detection reagent (ECL, Amersham Pharmacia) was used, and the membrane was exposed to Kodak X-Omat film.
Gene Chips.
Double-stranded cDNA was synthesized from clean total RNA from E14.5 fetal livers by using Superscript Choice system (GIBCO/BRL, 18090-019). The primer used for first-strand synthesis was HPLC-purified T7-(dT)24 (Genset, San Diego). The cDNA was then cleaned by phenol-chloroform extraction and ethanol precipitation. Biotin-labeled cRNA was synthesized by in vitro transcription by using the BioArray high yield RNA transcript labeling kit (Enzo Diagnostics). The biotin-labeled cRNA was purified by using the RNeasy total RNA isolation kit cleanup protocol, and the concentration was quantitated by absorbance at 260 nm. The clean cRNA was then fragmented for target preparation in 5× fragmentation buffer (200 mM Tris-acetate, pH 8.1/500 mM KOAc/150 mM MgOAc). The target cRNA was diluted in hybridization mixture and hybridized for 16 h to a Murine Genome U74A v2 GeneChip Expression probe array (Affymetrix, 900343). The hybridized array was washed and stained automatically by a GeneChip Fluidics Station (Affymetrix) and scanned by using the HP GeneArray Scanner (Affymetrix). The data were analyzed with Microsoft exel.
Real-Time Reverse Transcriptase–PCR.
Real-time reverse transcriptase–PCR was performed on clean total RNA made from E14.5 fetal livers by using the GeneAmp Gold RNA PCR reagent kit (Perkin–Elmer). This assay accurately quantifies the amount of a specific transcript in the RNA sample by the fluorescence of a light-sensitive dye (SYBR-Green) that is incorporated into DNA during the PCR. An ABI Prism 7700 instrument (Perkin–Elmer) was used to measure the fluorescence of the incorporated dye at the elongation step of each PCR cycle. A standard curve was derived from the fluorescence detection of transcripts in known copy numbers, and each sample was normalized to its own actin control. The primers used to amplify the different transcripts were β-actin (forward, CAGGGTGTGATGGTGGGAATGG; reverse, GCTCATTGTAGAAGGTGTGGTGCC), EKLF (forward, GTACACTCACCACCCTGGGACAG; reverse, CGGGAGACTCGGAACCTGGAAAG), α-spectrin (forward, ATCGCGTTGCCGAGAGGGGTC; reverse, CAGGCGAAGTTGCTTCAGATGGG), Band 4.2 protein (forward, CATGGGGCAAGCTCTGAGCATC; reverse, GGGCATCTCTTTCCTCCACTGC), transferrin receptor (forward, CCCGGTTTAGCCTTGCTCGGCAAG; reverse, CTGGCTCAGCTGCTTGATGGTGTC), GATA-1 (forward, CATCCGGCCCAAGAAGCGAATG; reverse, GATCACGCTGGTGCTGCTGGTG), and PU.1 (forward, CCCTCCATCGGATGACTTGGTTAC; reverse, GCTTCTCCATCAGACACCTCCAGG). All sequences given here are in the 5′ to 3′ orientation. The data were analyzed by using SEQUENCE DETECTOR 1.7 software.
Primer Extension.
Total RNA was extracted from fetal livers at different time points during development by using the TRIzol reagent (GIBCO/BRL) and followed with a cleanup of the RNA by using the RNeasy total RNA isolation kit cleanup protocol (Qiagen, Chatsworth, CA). Primer extension reactions were performed as described (11) by using a mixture of four end-labeled primers complementary to mouse α-globin (GAGCACCATGGTTTCTTCCTGAGT), mouse Ey-globin (CAGCAGTAAAGTTCACCATGATGGC), mouse βh1-globin (ATAGCTGCCTTCTCCTCAGCT), and mouse β-globin (TGATGTCTGTTTCTGGGGTTGTG) (a β-globin primer designed to hybridize to both β major and β minor transcripts) for the Ikaros null mice. A mixture of two end-labeled primers complementary to the human β-globin (CCACAGGGCAGTAACGGCAGA) and the human γ-globin (CCAGCATCTTCCACATTCACC) were used for the transgenic null mice. The reactions were run on a 6% polyacrylamide/8 M Urea/1 × TBE sequencing gel, and the signal was quantified with a Molecular Dynamics PhosphorImager.
Globin Chain Electrophoresis.
Triton acid–Urea (TAU) gels were performed to separate the globin chains, as described (12). Blood was obtained from adult mice and embryos and was diluted and washed in saline buffer (1 × PBS, 3 mM EDTA). The blood cells were lysed in distilled water and quantified by absorbance at 414 nm. Equal amounts of protein of all samples were diluted in denaturation buffer (8 M Urea/10% acetic acid/10% 2-mercaptoethanol/1 mM pyroninY) and run on a 12% polyacrylamide/6 M Urea/2% Triton X-100 gel in 5% acetic acid. Protein bands were visualized by Coomassie blue stain, and the gels were fixed and dried in cellophane on a gel dryer. The protein bands were then quantified by using a Molecular Dynamics densitometer.
Results
PYR Complex Is Absent in Ikaros Null Mice.
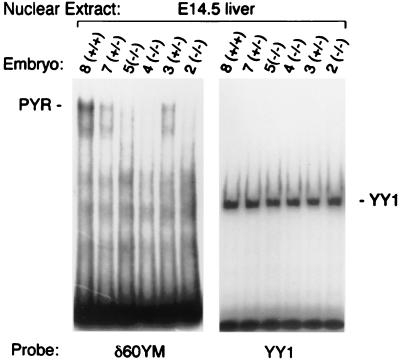
We have previously identified Ikaros as a DNA binding subunit of PYR complex (5). To determine the consequences of the lack of Ikaros on PYR complex formation, we looked at the DNA binding activity of PYR complex in vivo in mice with a targeted null mutation in the Ikaros gene (7). Gel shift experiments were performed on mouse fetal liver nuclear extracts from E14.5 embryos (Fig. 1) by using a labeled δ60ym, which is a sequence from the human β-globin locus known to bind PYR complex with highest affinity (4, 5, 13). YY1 probe was used as a control for protein degradation and DNA binding activity. This analysis shows that E14.5 fetal livers from homozygous Ikaros null mice (−/−) completely lack PYR complex DNA binding activity, whereas wild-type littermates (+/+) have normal levels of DNA binding (Fig. 1). In addition, heterozygotes (+/−) form approximately half as much complex, indicating a dosage effect relating Ikaros expression to PYR complex gel shift activity (Fig. 1). These data indicate that Ikaros is required for the DNA binding activity of PYR complex. Without Ikaros, PYR complex is incapable of binding DNA and, presumably, of performing its chromatin remodeling and transcriptional actions.
Figure 1.
Loss of PYR complex DNA binding activity. Gel shift assay of E14.5 fetal liver nuclear extract using labeled δ60ym and YY1 as probes. (Left) DNA binding activity of PYR complex is present in the wild-type mice (+/+), reduced in the heterozygotes (+/−), and completely absent in the null mice (−/−). (Right) Binding of YY1 protein to its binding site shows the quality of the protein extracts in all of the samples.
Ikaros Null Mice Have Anemia and Thrombocytosis.
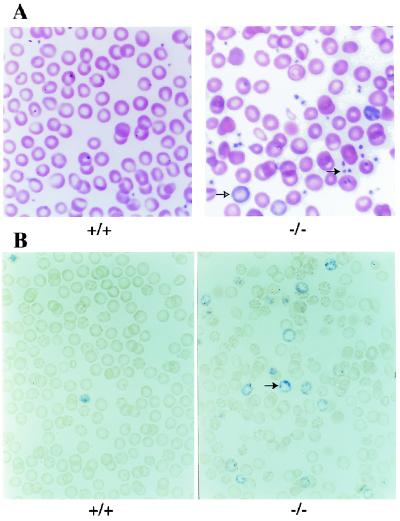
PYR complex is present by gel shifts in all adult hematopoietic lineages and absent from nonhematopoietic lineages (ref. 13 and data not shown). To characterize the effect of the lack of Ikaros and the lack of PYR complex on the different hematopoietic lineages, Ikaros null mice were analyzed hematologically. Peripheral blood smears, reticulocyte counts, and complete blood counts (CBCs) were performed. CBCs indicate that the Ikaros null mice have an anemia with decreased hemoglobin, hematocrit, and red cell values as well as an abnormal width of red cell size distribution (Table 1). Blood smears stained with Wright–Giemsa stain show that the anemia is characterized by abnormal red cell morphology including oddly shaped cells (poikilocytosis) and cells of varying size (anisocytosis) along with some polychromatophilic cells (Fig. 2A). The anemia is also accompanied by an increase in immature red cells in peripheral blood (reticulocytosis) (Fig. 2B). These data suggest that the anemia is caused, at least to some extent, by an increased destruction of red cells characterized by reticulocytosis, as blood loss in these mice is not observed.
Table 1.
Blood counts from adult Ikaros mice
| +/+ | −/− | |
|---|---|---|
| Hemoglobin (g/dL) | 14.7 ± 0.8 | 12.5 ± 1.3 |
| Hematocrit (%) | 42.8 ± 2.9 | 34.6 ± 4.2 |
| Red cell distribution width (%) | 16.2 ± 2.2 | 19.9 ± 3.2 |
| Platelet count (109/L) | 513 ± 262 | 1496 ± 691 |
| n = 10 | n = 8 |
The null mice have lower hemoglobin and hematocrits, higher red cell size distribution, and increased number of platelets than the wild-type mice.
Figure 2.
Anemia and thrombocytosis. (A) Peripheral blood smears of adult mice. Ikaros null mice have a mild anemia, abnormal red cells with polychromatophilic cells (open arrow), and increased platelets (closed arrow). (B) Reticulocyte stains of peripheral blood from adult mice. Ikaros null mice show increased numbers of reticulocytes (arrow).
CBCs also show that the null (−/−) mice have thrombocytosis. The platelet counts are consistently 3-fold higher than normal (Table 1, Fig. 2A). The other hematological parameters analyzed by the CBC seem similar in both normal and null mice. These findings show that Ikaros protein is needed for normal development of the erythroid and megakaryocytic lineages, in addition to that of normal lymphoid cells previously described (7).
Abnormal Splenic Hematopoiesis in Ikaros Null Mice.
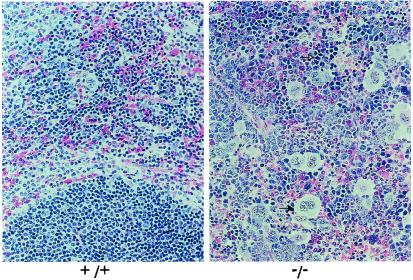
Spleens, livers, and bone marrow cores from adult mice were excised, fixed, and sectioned for examination to further characterize the hematopoietic defects in the Ikaros null mice. The spleen sections show that −/− mice have an increased number of megakaryocytes and erythroid precursors, showing extramedullary hematopoiesis, compared with +/+ mice (Fig. 3). The sections also show the lack of lymphoid cells in the spleens of −/− mice, as expected. Liver and bone marrow sections from +/+ and −/− mice look comparable (data not shown). Cell differentials from bone marrow cytospins were counted to evaluate the types of cells in the marrow. The only significant difference in the differential counts is a 2-fold increase in the earliest erythroid precursors (proerythroblast/basophilic normoblast), up to 10% of total cells in the null mice, compared with 5% of total cells in normal mice (P value = 0.004; data not shown). The proportions of other cell types in the marrow of the null mice seem unaffected.
Figure 3.
Extramedullary hematopoiesis. Spleen sections from adult mice. Ikaros null mice (−/−) have a very high number of megakaryocytes in the spleen (closed arrow). The null mice also show increased numbers of erythroid precursors in this organ (open arrow) and a lack of lymphocytes.
Delayed Mouse and Human Globin Switching.
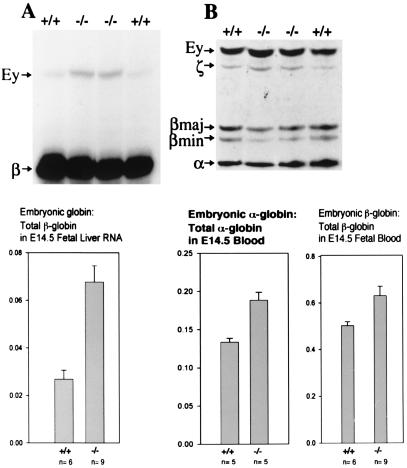
We have previously shown that PYR complex binds to a site called the PYR complex binding site, an intergenic pyrimidine-rich region upstream of the δ-globin gene within the human β-globin locus (5). We have also shown that deletion of this PYR binding site in a human mini-β-globin locus of transgenic mice carrying this cosmid leads to a delay in human γ- to β-globin switching (4). We were most interested, therefore, in characterizing the role of Ikaros in the switching process that occurs during murine development. Because mice do not have any fetal globin genes, we looked at the mouse switch from embryonic to adult globin in the Ikaros null mice. Primer extension assays and globin chain electrophoresis (TAU gels) were performed to investigate globin expression at the RNA and protein levels, respectively (Fig. 4). Primer extension analyses show a 3-fold increase (from about 2% to about 6%) in embryonic globin RNA per total β-globin mRNA in the 14.5-day fetal livers (E14.5) of Ikaros null mice compared with that of wild-type mice (Fig. 4A).
Figure 4.
Delayed mouse globin switching. Primer extension assay of fetal liver globin RNA and globin chain electrophoresis of yolk sac and fetal blood from E14.5 mice. (A Upper) Primer extension results using primers for mouse embryonic β-globin (Ey-globin and βh1) and adult β-globin. (A Lower) Embryonic globin to (embryonic + adult) globin ratios from primer extension data quantified with a phosphorimager. Ikaros null mice have relatively higher persistence of mouse embryonic globin RNA. (B Upper) Electrophoresis of globin chains using blood from E14.5 mice. (B Lower) Embryonic globin to (embryonic + adult) globin ratios from TAU gel data quantified with a densitometer. Null mice have higher levels of both embryonic α-globin and embryonic β-globin in their blood at E14.5.
At the protein level, TAU gels from the blood of E14.5 mice show an increase from about 50% in wild-type mice to about 65% embryonic globin in the null mice (Fig. 4), an observation that is consistent with delayed murine embryonic to adult globin switching. This delay was accompanied by a significant increase in the ratio of embryonic (βh1- and Ey-globin) to total β-globin (βh1-, Ey-, βmajor-, and βminor-globin) in Ikaros null mice, when compared with wild-type mice (Fig. 4).
Interestingly, there is also a delay in the embryonic α (ζ) to adult α-globin switch as well, shown by an increased ζ/ζ+ α ratio in the Ikaros null mice (Fig. 4B), suggesting that Ikaros and PYR complex facilitate switching at both the murine α- and β-globin loci.
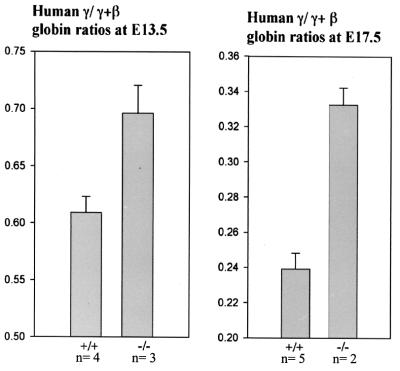
To further investigate the role of Ikaros in the human globin switch, we crossed Ikaros null mice with mice transgenic for a cosmid with sequences of the human β-globin locus, which show a developmentally normal human fetal to adult globin switch (4, 11). We examined the human globin switch in Ikaros null mice by primer extension assays. RNA analyses at E13.5 and E17.5 during development show a delay in the human fetal to adult switch, determined by the ratio of human γ/γ+ β-globin (Fig. 5). The γ/γ+β ratio at E13.5 was 0.61 m normal transgenic mice and was 0.7 in transgenic Ikaros null mice. At E17.5, the delay persisted and the ratio was 0.24 in normal as compared to 0.33 in the Ikaros null mice (Fig. 5). These data illustrate a role for Ikaros in the globin switch of both mice and humans.
Figure 5.
Delayed human globin switching. Primer extension assay of fetal liver globin RNA from E13.5 and E17.5 Ikaros null mice transgenic for the human β-locus cosmid (see Materials and Methods). Human fetal to (fetal + adult) globin ratios from primer extension data at E13.5 (Left) and E17.5 (Right). Transgenic null mice have relatively higher persistence of human fetal globin RNA at both stages during development. At E13.5: +/+, n = 4; −/−, n = 3. At E17.5: +/+, n = 5; −/−, n = 2.
Aberrant Gene Expression in Ikaros Null Mice.
The hematopoietic defects observed in the null mice presumably arise from the lack of Ikaros in the different lineages. Because Ikaros is a transcription factor with dual potential for acting as either a transcriptional activator or repressor (14, 15), we wanted to investigate the mechanism of gene regulation by Ikaros. To understand the effect of Ikaros in specific gene expression at the genomic level, gene chip analysis was performed. Biotinylated cRNA transcribed in vitro from E14.5 fetal liver cDNA was used as the target for the murine genome U74Av2 array (Affymetrix). This method allowed us to examine the variety of genes targeted by Ikaros in a hematopoietic tissue.
Gene chip assay results, shown in Table 2, demonstrate that the expression levels of several erythroid-specific genes are changed in the Ikaros null mice. The expression of EKLF, transferrin receptor, aquaporin, and band 4.2 protein is decreased in the null mice (Table 2). Embryonic βh1, x (ζ), and βh0 expression are increased in null compared with normal mice, whereas adult α and β adult globin gene expression is comparable. These data suggest that the observed delay in α- and β-globin switching is because of persistent expression of the x, βh1, and βh0 genes rather than impaired expression of the adult globin genes (Table 2). The expression of many erythroid cell-specific transcripts is unaffected, such as that of GATA-1, glycophorin A, and band 3 protein. The fact that many erythroid transcripts are unaffected makes the changes in the globin ratios and of other erythroid-specific genes in the chip data more compelling. These results indicate the requirement of Ikaros for full and normal expression of several important erythroid-specific genes.
Table 2.
Differences in expression levels of hematopoietic transcripts in E14.5 liver between Ikaros −/− and Ikaros +/+ embryos
| Class | Down-regulated genes | Unchanged genes | Up-regulated genes |
|---|---|---|---|
| Red cell membrane proteins | Aquaporin 1 (0.3)* | Erythroid alpha-spectrin† | |
| Transferrin receptor† (0.4) | Erythroid ankyrin 1 | ||
| Band 4.2† (0.6) | Glycophorin A | ||
| Rh30 (0.8) | Band 3 | ||
| Erythropoietin receptor (0.8) | Band 4.1 | ||
| Red cell enzymes | Red cell acid phosphatase (0.4) | ||
| Erythroid ALA synthase (0.7) | |||
| Erythroid nuclear proteins | EKLF† (0.6) | Friend of GATA-1 (FOG) GATA-1† | NF-E2, 45-kDa subunit (1.4) |
| Hemoglobins | Adult α-globin chain | Embryonic × globin (α-like), chain (1.3) | |
| Adult β major globin chain | Embryonic βh0 and βh1 globin chains (1.6) | ||
| Embryonic y-globin (β-like) chain | |||
| Hematopoietic | Wnt10b (<0.1) | TAL1 | MLL (1.6) |
| Ikaros† (0.1) | PU.1† (3) |
Relative null/wild-type expression ratio. This ratio is the average of E14.5 liver expression levels from five Ikaros −/− (null) and five Ikaros +/+ (wild-type) embryos. Expression ratios are shown only for transcripts that have statistically significant differences in expression (P < 0.05, t test). Unchanged genes have no statistically significant difference in expression between wild-type and Ikaros null embryos.
Results confirmed by real time reverse-transcriptase-PCR analysis.
The expression of many other hematopoietic genes is also influenced by the knockout mutation in the null mice (data not shown). Ikaros transcripts, as a control, as well as some lymphocyte-specific transcripts, such as TCRβ, are missing in the null mice, as expected. Interestingly, Notch 1 as well as many myeloid and monocytic cell-specific transcripts such as myeloid secondary granule protein and Kupffer cell receptor are significantly increased in the null mice. Taken together, these results demonstrate that many target genes are affected by Ikaros and PYR complex action in all hematopoietic lineages.
Discussion
We have previously characterized PYR complex biochemically as a macromolecular complex including SWI/SNF and NuRD subunits, and Ikaros as the DNA binding subunit by purification and sequence analysis, supershifts with subunit-specific antibodies, and immunoaffinity chromatography and immunoprecipitation (5). In this article, we show that PYR complex DNA binding activity is completely lacking in Ikaros null mice, demonstrating that Ikaros is required for PYR complex formation on DNA in vivo.
Ikaros null mice serve as a unique model to assess the cellular and molecular consequences of the absence of PYR complex. Previous studies have shown that the Ikaros null mice have severe defects in lymphocyte function including the absence of B and T cells (7). In addition, these mice have defects in stem cells and in the formation of BFU-E, a measure of early erythroid activity (8). We now show that, in addition, these mice have an anemia, presumably with a shortened red cell lifespan, as indicated by increased reticulocytosis and no evidence of blood loss. Our chip data show changes in membrane proteins that may be implicated in the anemia (Table 2); however, the precise cause(s) of the anemia remain unclear.
Abnormal megakaryocytopoiesis and increased platelet formation are evident in these mice (Table 1, Fig. 2). The cause of the thrombocytosis is undefined. In addition, the chip data indicate the increased activity of many granulocytic genes. However, we do not know whether this represents myeloid cell-autonomous events, although there is no detectable change in the expression of a number of myeloid markers in the chip analysis (data not shown). In this regard, it is of interest that PU.1 is up-regulated in Ikaros null mice (Table 2), as this factor is known to be critical to myeloid development (for review see ref. 16). We also have reported previously that both Ikaros and PYR complex are present in adult myeloid cell lines while absent in nonhematopoietic lineages, suggesting their role in adult myeloid cells (ref. 13 and unpublished observations). The results reported here and previously thus indicate that all adult murine and human hematopoietic lineages normally contain significant amounts of PYR complex (13).
The precise molecular and cellular basis for the derangements in hematopoiesis in the Ikaros null mice remains to be elucidated. There are varying extents to which the lack of Ikaros and PYR complex affect different hematopoietic cells. The lymphoid lineage is most severely affected, and the lymphoid defects are at least partially caused by defective activation of important lymphocyte-specific genes (17). There are defective stem cells, but their deficiencies remain uncharacterized. The anemia we describe here remains to be clarified, and it may be because of processes affecting red cell membranes, enzymes, and/or hemoglobin. Similarly, the increased number of platelets and the aberrant megakaryocytes in the spleen cannot yet be explained.
Presumably, Ikaros and PYR complex are required for optimal adult hematopoietic stem cell and adult hematopoietic cell-lineage development. The specific genes and proteins affected are most likely different in different cell lineages. This may be because of the activity of different isoforms of Ikaros or Ikaros-like molecules (Helios, Aiolos) (18, 19). Additionally, PYR complexes in different cell types may interact with other lineage cell-specific components at different genes; for example, in red cells, Ikaros and PYR complex may interact with GATA-1 and/or EKLF and associated other components to activate or repress erythroid-specific genes, with the formation of complex DNA/protein structures analogous to the enhanceosomes described at other genes (for review see ref. 20). Purification and characterization of the specific protein partners in addition to PYR complex at specific gene loci and in different lineages should clarify these issues. In addition, further analysis of gene arrays assessing the differences between target genes expressed in specific lineages of hematopoietic cells in null versus normal mice should continue to be informative. Although we have focused on the role of Ikaros in PYR complex as being primarily responsible for the pleiotropic phenotype of Ikaros knockout mice, it is possible that other Ikaros-containing complexes may also be involved.
Lastly, our data indicate that Ikaros and PYR complex facilitate globin switching in mice and humans. We previously reported delayed human γ- to β-globin switching in transgenic mice with a cosmid containing the human Aγ gene through the adult human β gene with a deletion of a 511-bp element containing a polypyrimidine-rich sequence with multiple Ikaros sites within it, as compared with the undeleted cosmid (4). In these studies, switching was delayed but not abolished. Thus, there are clearly other factors, presumably including EKLF, that eventually are sufficient to complete the γ- to β-globin switch. The delay in human γ to β switching in Ikaros null mice (Fig. 5) is not as great as the delay resulting from a deletion of 511 bp including the PYR complex binding site (4). This might be because the 511-bp deletion included a GATA-1 site and other potential transcription factor binding sites that may also contribute to switching.
In the Ikaros null mice, we see a delay in mouse embryonic to adult globin switching during fetal life due primarily to the relative persistence of embryonic globins in the mouse and, presumably, γ-globin in the human on the basis of our chip data (Table 2). This may reflect a primarily repressive role of PYR complex on mouse embryonic and human fetal globin expression. The presence of histone deacetylases in PYR complex (5) and the known action of the histone deacetylase inhibitor, butyrate, in increasing human γ-globin expression (21) are consistent with this idea.
Our results are consistent with a model of both murine and human hemoglobin switching in which Ikaros and PYR complex function in combination with a number of different molecules or complexes. As mentioned previously, PYR complex may be a part of the process necessary for optimal switching, eventually compensated for by the subsequent action of other molecules. EKLF, for example, which is present in mouse yolk sac cells in large amounts but does not activate the β-globin gene until fetal liver hematopoiesis (22), may require PYR complex action for its activation, perhaps by conformational changes induced by PYR complex when it first appears in adult-type cells producing adult β-globin. Our finding of decreased EKLF expression in Ikaros null mice (Table 2) supports a role for PYR complex in recruiting activators to the adult β-globin locus in adult hematopoietic cells and is consistent with this hypothesis. The requirement of SWI/SNF subunits for optimal EKLF action in in vitro studies also suggests a link between PYR complex and EKLF action (23). Mice lacking both EKLF and PYR complex should be useful in addressing this question further.
In summary, we show that PYR complex interaction with DNA requires the presence of Ikaros in vivo in Ikaros null mice. The consequences of the lack of Ikaros and PYR complex action lead to abnormalities in erythroid and megakaryocytic development, as well as previously reported lymphoid and stem cell disorders. In addition, PYR complex seems to be required for optimal embryonic to adult globin switching in mice and fetal to adult globin switching in humans. It will be of great interest to further define the role of additional molecules (coactivators and transcriptional factors) that interact with PYR complex at the promoters and enhancers of specific hematopoietic genes and at intergenic sites, which determine whether these specific hematopoietic genes are transcriptionally active, repressed, or unaffected.
Acknowledgments
We thank Katia Georgopoulos for kindly providing the Ikaros null mice and Una Terrie Collins for assistance in the manuscript preparation. This work was supported by National Institutes of Health Public Health Service Grants HL-59887, CA-65838, and DK-56635.
Abbreviations
- TAU
Triton acid–Urea
- CBC
complete blood count
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Armstrong J A, Bieker J J, Emerson B M. Cell. 1998;95:93–104. doi: 10.1016/s0092-8674(00)81785-7. [DOI] [PubMed] [Google Scholar]
- 2.Kadonaga J. Cell. 1998;92:307–313. doi: 10.1016/s0092-8674(00)80924-1. [DOI] [PubMed] [Google Scholar]
- 3.Xue Y, Wong J, Moreno G T, Young M K, Cote J, Wang W. Mol Cell. 1998;2:851–861. doi: 10.1016/s1097-2765(00)80299-3. [DOI] [PubMed] [Google Scholar]
- 4.O'Neill D, Yang J, Erdjument-Bromage H, Bornschlegel K, Tempst P, Bank A. Proc Natl Acad Sci USA. 1999;96:349–354. doi: 10.1073/pnas.96.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Neill D W, Schoetz S S, Lopez R A, Castle M, Rabinowitz L, Shor E, Krawchuk D, Goll M G, Renz M, Sellig H-P, et al. Mol Cell Biol. 2000;20:7572–7582. doi: 10.1128/mcb.20.20.7572-7582.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun L, Liu A, Georgopoulos K. EMBO J. 1996;15:5358–5369. [PMC free article] [PubMed] [Google Scholar]
- 7.Wang J H, Nichogiannopoulou A, Wu L, Sun L, Sharpe A H, Bigby M, Georgopoulos K. Immunity. 1996;5:537–549. doi: 10.1016/s1074-7613(00)80269-1. [DOI] [PubMed] [Google Scholar]
- 8.Nichogiannopoulou A, Trevisan M, Neben S, Friedrich C, Georgopoulos K. J Exp Med. 1999;190:1201–1214. doi: 10.1084/jem.190.9.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorski K, Carneiro M, Schibler U. Cell. 1986;47:767–776. doi: 10.1016/0092-8674(86)90519-2. [DOI] [PubMed] [Google Scholar]
- 10.Schreiber E, Matthias P, Muller M M, Schaffner W. Nucleic Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enver T, Raich N, Ebens A J, Papayannopoulou T, Costantini F, Stamatoyannopoulos G. Nature (London) 1990;344:309–313. doi: 10.1038/344309a0. [DOI] [PubMed] [Google Scholar]
- 12.Rovera G, Magarian C, Borun T W. Anal Biochem. 1978;85:506–518. doi: 10.1016/0003-2697(78)90248-8. [DOI] [PubMed] [Google Scholar]
- 13.O'Neill D, Bornschlegel K, Flamm M, Castle M, Bank A. Proc Natl Acad Sci USA. 1991;88:8953–8957. doi: 10.1073/pnas.88.20.8953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koipally J, Renold A, Kim J, Georgopoulos K. EMBO J. 1999;18:3090–3100. doi: 10.1093/emboj/18.11.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Molnar A, Georgopoulos K. Mol Cell Biol. 1994;14:8292–8303. doi: 10.1128/mcb.14.12.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simon M C, Olson M, Scott E, Hack A, Su G, Singh H. Curr Top Microbiol Immunol. 1996;211:113–119. doi: 10.1007/978-3-642-85232-9_11. [DOI] [PubMed] [Google Scholar]
- 17.Cortes M, Wong E, Koipally J, Georgopoulos K. Curr Opin Immunol. 1999;11:167–171. doi: 10.1016/s0952-7915(99)80028-4. [DOI] [PubMed] [Google Scholar]
- 18.Hahm K, Cobb B S, McCarty A S, Brown K E, Klug C A, Lee R, Akashi K, Weissman I L, Fisher A G, Smale S T. Genes Dev. 1998;12:782–796. doi: 10.1101/gad.12.6.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morgan B, Sun L, Avitahl N, Andrikopoulos K, Ikeda T, Gonzales E, Wu P, Neben S, Georgopoulos K. EMBO J. 1997;16:2004–2013. doi: 10.1093/emboj/16.8.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merika M, Thanos D. Curr Opin Genet Dev. 2001;11:205–208. doi: 10.1016/s0959-437x(00)00180-5. [DOI] [PubMed] [Google Scholar]
- 21.Perrine S P, Miller B A, Faller D V, Cohen R A, Vichinsky E P, Hurst D, Lubin B H, Papayannopoulou T. Blood. 1989;74:454–459. [PubMed] [Google Scholar]
- 22.Wijgerde M, Gribnau J, Trimborn T, Nuez B, Philipsen S, Grosveld F, Fraser P. Genes Dev. 1996;10:2894–2902. doi: 10.1101/gad.10.22.2894. [DOI] [PubMed] [Google Scholar]
- 23.Kadam S, McAlpine G S, Phelan M L, Kingston R E, Jones K A, Emerson B M. Genes Dev. 2000;14:2441–2451. doi: 10.1101/gad.828000. [DOI] [PMC free article] [PubMed] [Google Scholar]