Abstract
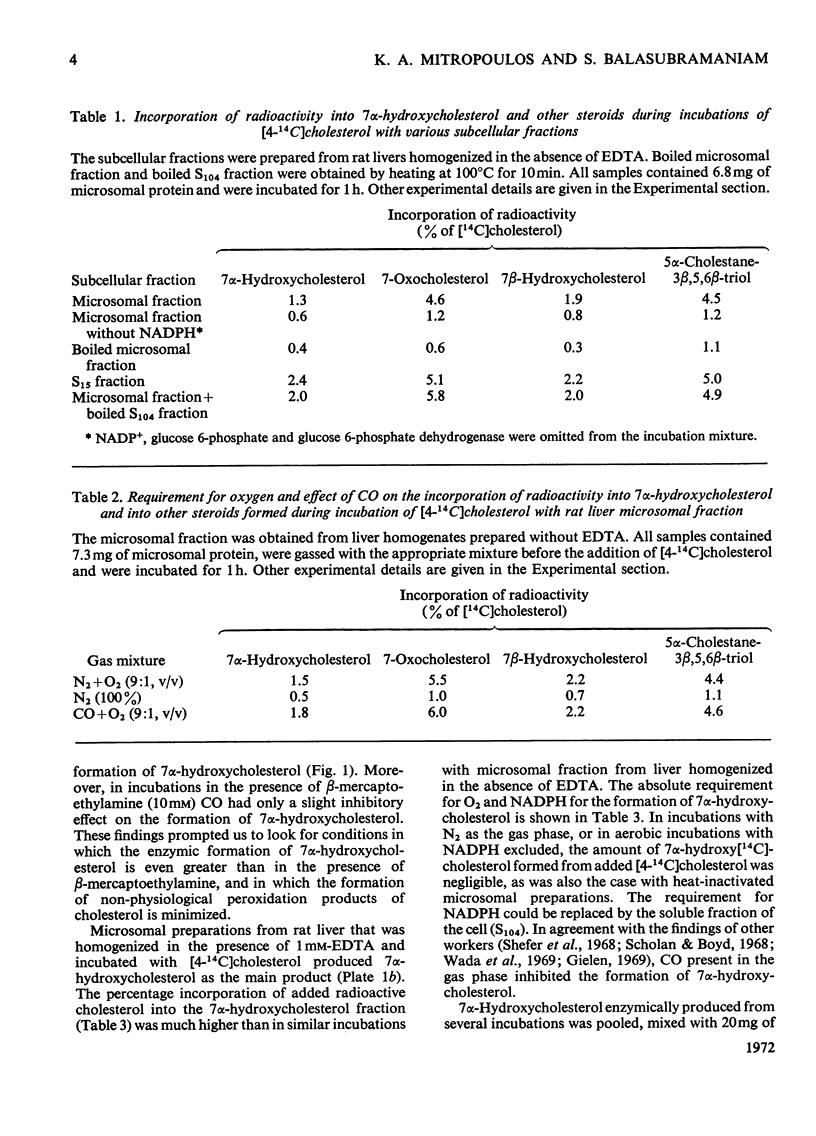
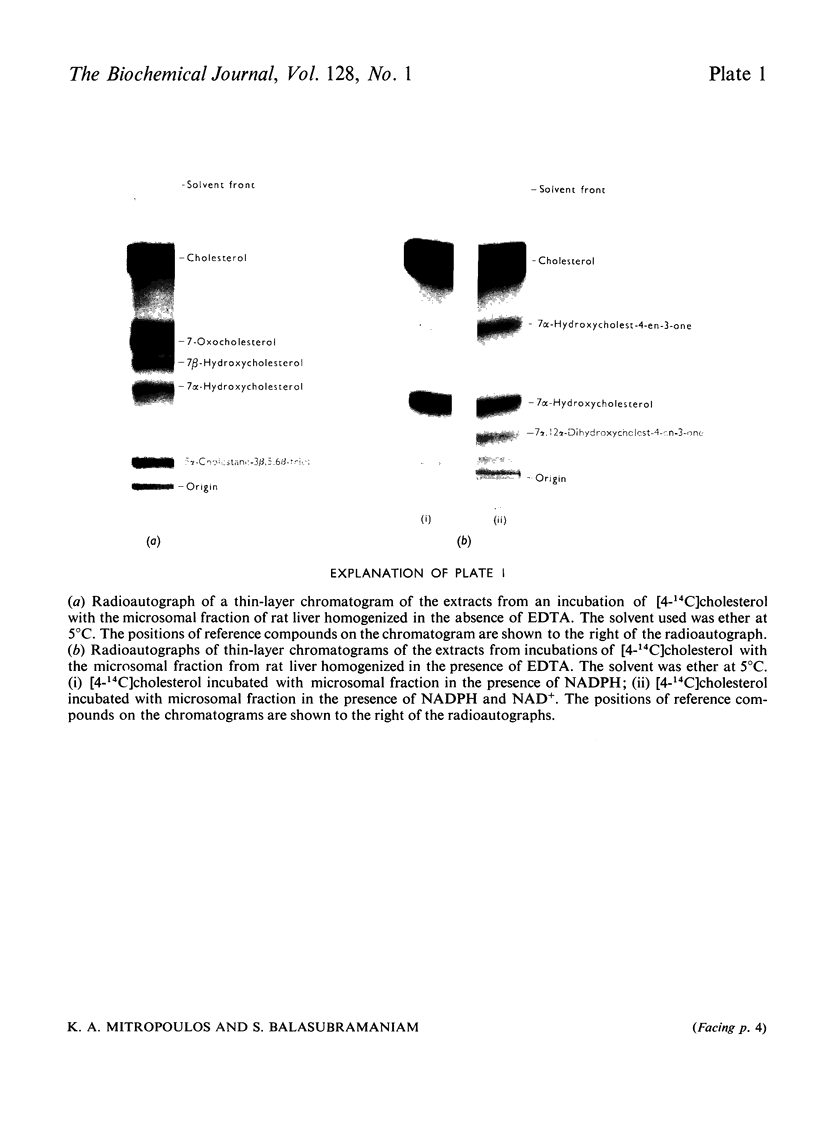
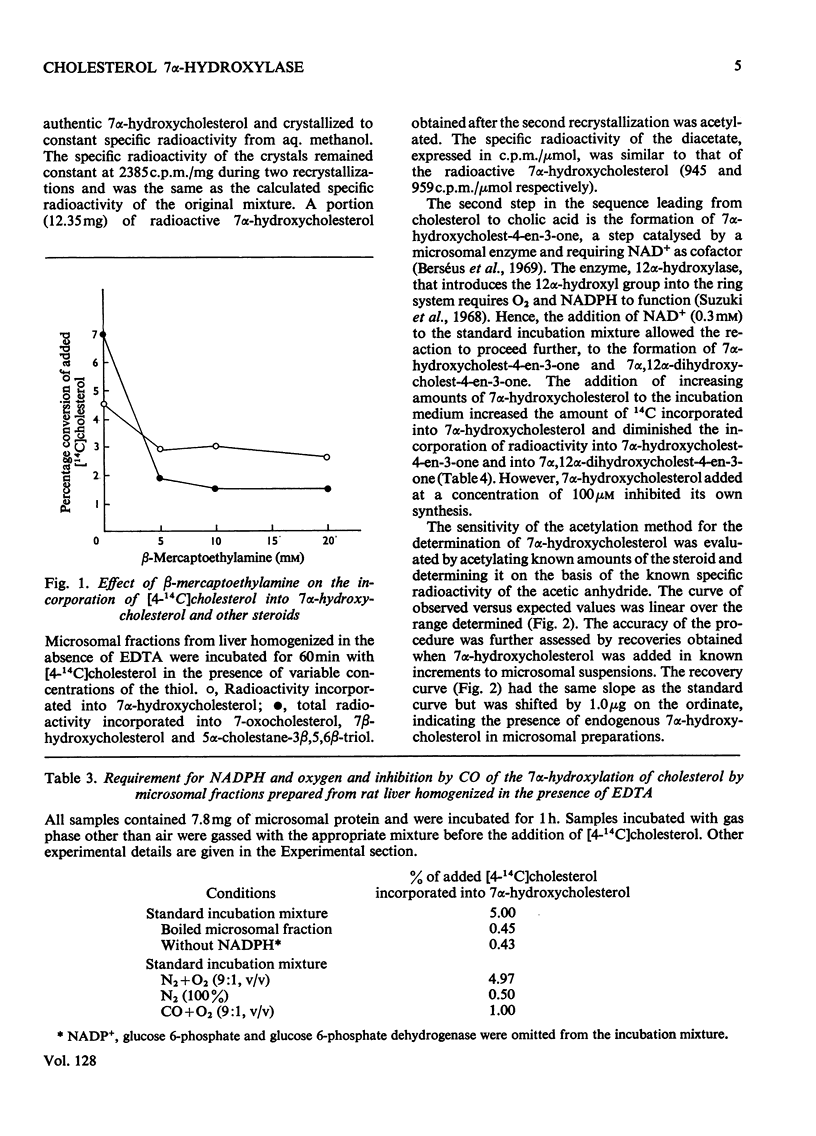
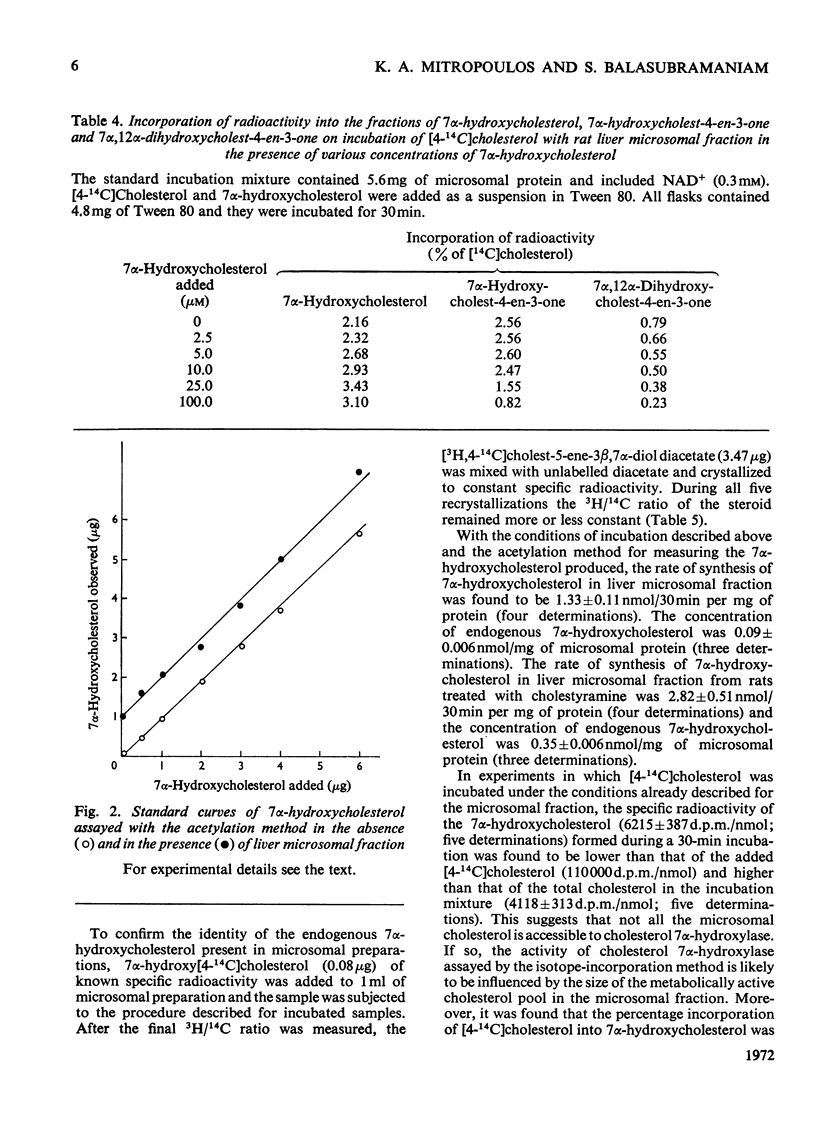
Subcellular fractions containing microsomes prepared from rat livers homogenized in the absence of EDTA catalysed the oxidation of cholesterol to 7α-hydroxycholesterol, 7-oxocholesterol, 7β-hydroxycholesterol and 5α-cholestane-3β,5,6β-triol. These reactions required native protein, molecular oxygen and NADPH. It is suggested that these compounds are formed by a peroxidation analogous to the peroxidation of fatty acids catalysed by liver microsomal preparations. Incubations of [4-14C]cholesterol with microsomal preparations from rat liver homogenized in the presence of EDTA gave 7α-hydroxy[14C]cholesterol as the main product. This reaction required molecular oxygen and NADPH, and was inhibited by CO. The mass of 7α-hydroxycholesterol formed during the incubation was measured by a double-isotope-derivative dilution procedure. This procedure was used to assay the activity of cholesterol 7α-hydroxylase and to measure low concentrations of endogenous 7α-hydroxycholesterol in liver.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clark B. R., Rubin R. T., Arthur R. J. A new micro method for determination of cholesterol in serum. Anal Biochem. 1968 Jul;24(1):27–33. doi: 10.1016/0003-2697(68)90056-0. [DOI] [PubMed] [Google Scholar]
- Gielen J., Van Cantfort J., Renson J. Dosage de la cholestérol-7-alpha-hydroxylase hépatique a l'aide de cholestérol-4-14C. Arch Int Physiol Biochim. 1968 Jul;76(3):581–582. [PubMed] [Google Scholar]
- MENDELSOHN D., MENDELSOHN L., STAPLE E. THE CATABOLISM IN VITRO OF CHOLESTEROL: FORMATION OF THE 7-EPIMERIC HYDROXYCHOLESTEROLS FROM CHOLESTEROL IN RAT LIVER. Biochim Biophys Acta. 1965 Feb 15;97:379–381. doi: 10.1016/0304-4165(65)90116-9. [DOI] [PubMed] [Google Scholar]
- Mitton J. R., Scholan N. A., Boyd G. S. The oxidation of cholesterol in rat liver sub-cellular particles. The cholesterol-7-alpha-Hydroxylase enzyme system. Eur J Biochem. 1971 Jun 29;20(4):569–579. doi: 10.1111/j.1432-1033.1971.tb01429.x. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. II. SOLUBILIZATION, PURIFICATION, AND PROPERTIES. J Biol Chem. 1964 Jul;239:2379–2385. [PubMed] [Google Scholar]
- Scholan N. A., Boyd G. S. The cholesterol 7alpha-hydroxylase enzyme system. Hoppe Seylers Z Physiol Chem. 1968 Nov;349(11):1628–1630. [PubMed] [Google Scholar]
- Shefer S., Hauser S., Mosbach E. H. 7-alpha-hydroxylation of cholestanol by rat liver microsomes. J Lipid Res. 1968 May;9(3):328–333. [PubMed] [Google Scholar]
- Suzuki M., Mitropoulos K. A., Myant N. B. The electron transport mechanism associated with 12 alpha-hydroxylation of C27 steroids. Biochem Biophys Res Commun. 1968 Mar 12;30(5):516–521. doi: 10.1016/0006-291x(68)90082-x. [DOI] [PubMed] [Google Scholar]
- Van Lier J. E., Smith L. L. Autoxidation of cholesterol via hydroperoxide intermediates. J Org Chem. 1970 Aug;35(8):2627–2632. doi: 10.1021/jo00833a031. [DOI] [PubMed] [Google Scholar]
- Wada F., Hirata K., Nakao K., Sakamoto Y. Participation of the microsomal electron transport system involving cytochrome P-450 in 7alpha-hydroxylation of cholesterol. J Biochem. 1969 Nov;66(5):699–703. [PubMed] [Google Scholar]
- Wills E. D. Lipid peroxide formation in microsomes. General considerations. Biochem J. 1969 Jun;113(2):315–324. doi: 10.1042/bj1130315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills E. D. Lipid peroxide formation in microsomes. The role of non-haem iron. Biochem J. 1969 Jun;113(2):325–332. doi: 10.1042/bj1130325. [DOI] [PMC free article] [PubMed] [Google Scholar]




