Abstract
1. Subcellular fractions of human placenta were prepared by nitrogen-bomb homogenization and differential centrifugation. 2. β-Glucuronidase from placental lysosomes was purified 2100-fold on a protein basis. 3. The lysosomal enzyme, at different stages of purification, was characterized by using 4-methylumbelliferyl β-d-glucuronide and phenolphthalein β-d-glucuronide as substrates. 4. Only one isoenzyme of β-glucuronidase was found in placenta; the enzyme in the endoplasmic reticulum appeared to be the same as the lysosomal enzyme. 5. The isoenzyme contained in normal plasma was different from that of the placenta. 6. The elevated β-glucuronidase activity found in plasma obtained during pregnancy was due to increased activity of the normal plasma isoenzyme; no contribution was made by placental isoenzyme. 7. Plasma contained a heat-stable, non-diffusible activator of placental β-glucuronidase. 8. A heat-stable competitive inhibitor of placental and plasma β-glucuronidase was also present in plasma.
Full text
PDF

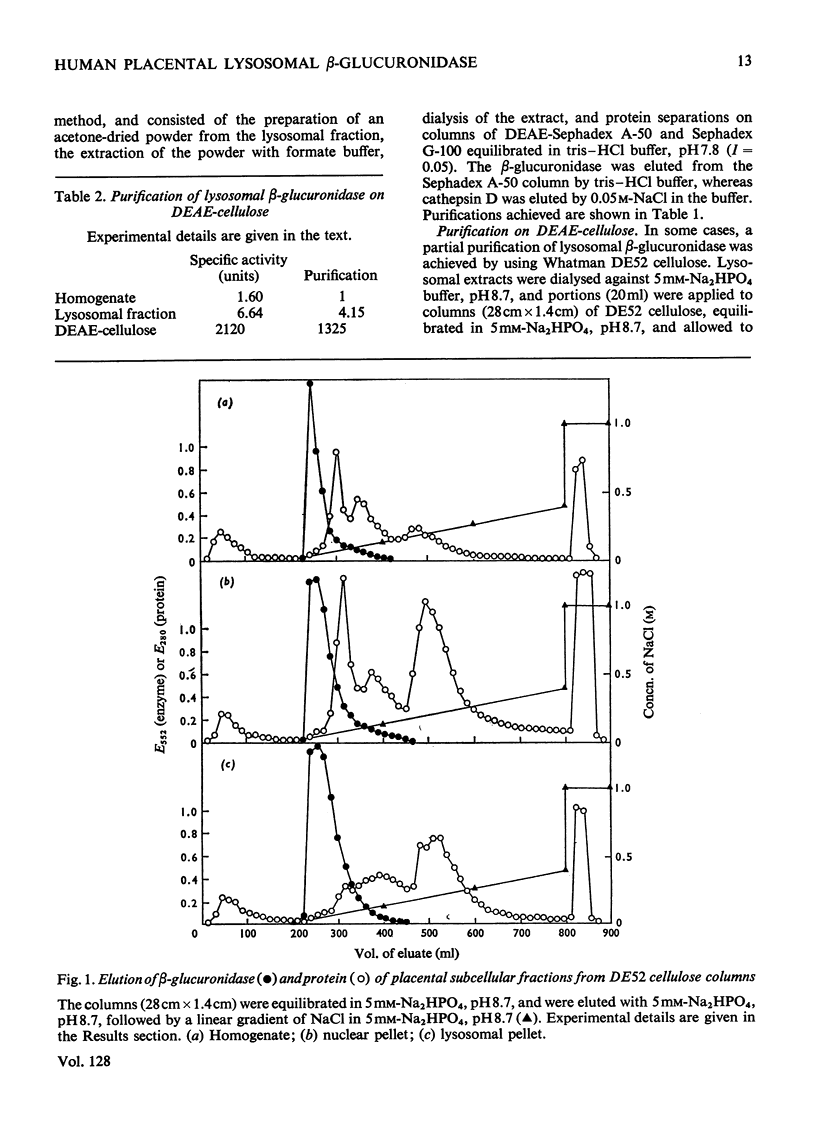
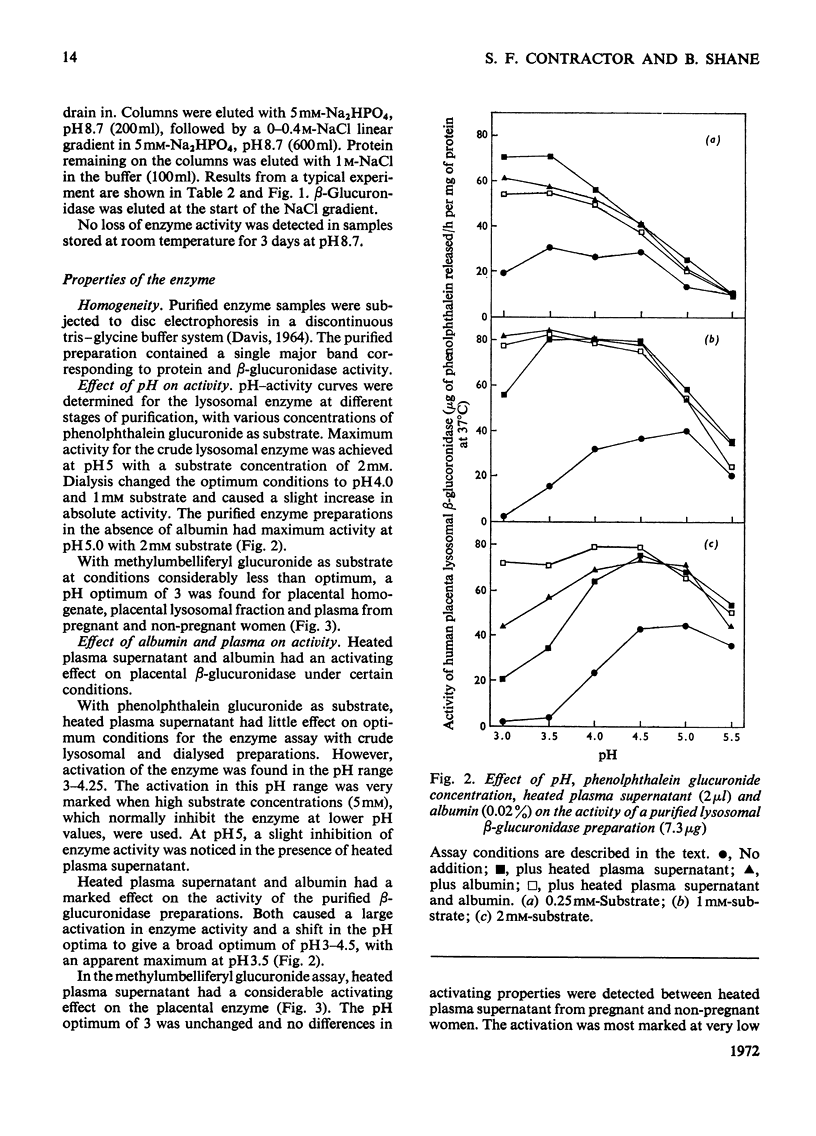
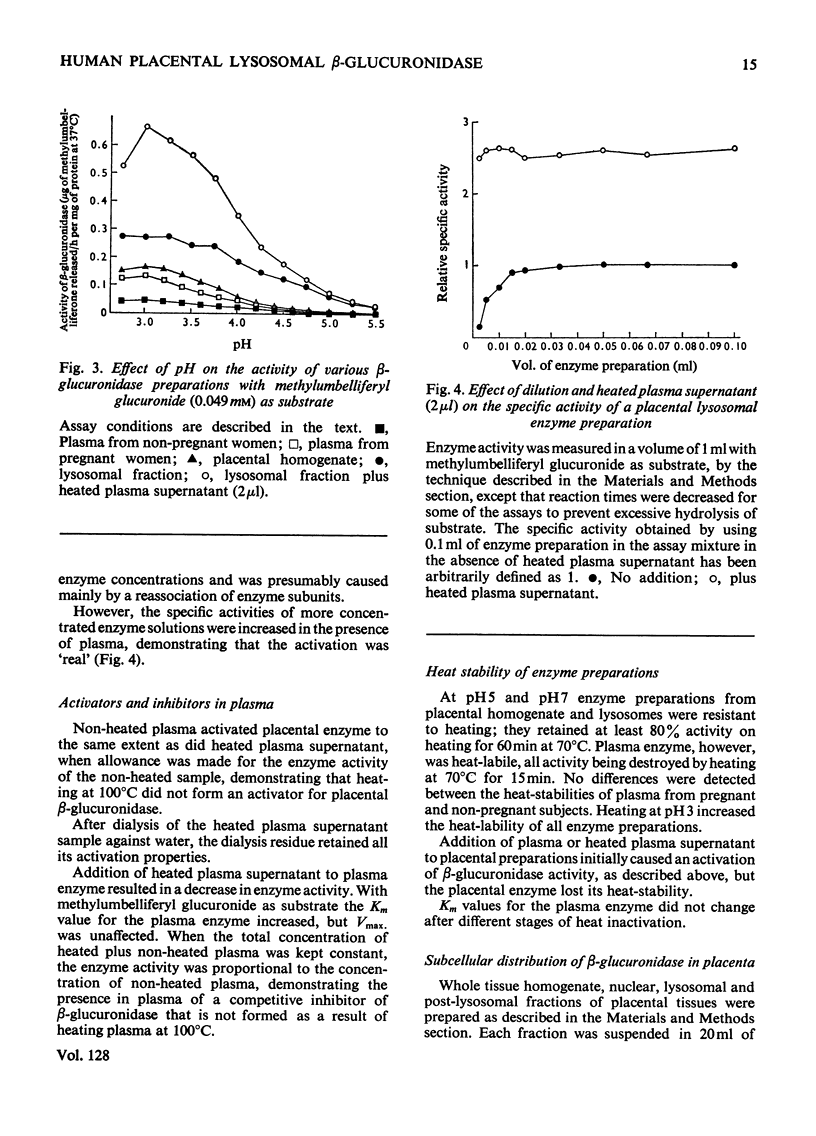



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTALOS M., GYORKEY F. Beta-glucuronidases: their significance and relation to cancer. J Am Geriatr Soc. 1963 Jan;11:21–34. doi: 10.1111/j.1532-5415.1963.tb00026.x. [DOI] [PubMed] [Google Scholar]
- Barrett A. J. Lysosomal acid proteinase of rabbit liver. Biochem J. 1967 Aug;104(2):601–608. doi: 10.1042/bj1040601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contractor S. F. Lysosomes in human placenta. Nature. 1969 Sep 20;223(5212):1274–1275. doi: 10.1038/2231274b0. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Fishman W. H., Goldman S. S., DeLellis R. Dual localization of beta-glucuronidase in endoplasmic reticulum and in lysosomes. Nature. 1967 Feb 4;213(5075):457–460. doi: 10.1038/213457a0. [DOI] [PubMed] [Google Scholar]
- Huddleston J. F., Lee G., Robinson J. C. Electrophoretic characterization of glucose dehydrogenase, beta-glucuronidase, and N-acetyl-beta-glucosaminidase from placenta and gestational serum. Am J Obstet Gynecol. 1971 Apr 1;109(7):1017–1022. doi: 10.1016/0002-9378(71)90283-3. [DOI] [PubMed] [Google Scholar]
- LEVVY G. A., MARSH C. A. Preparation and properties of beta-glucuronidase. Adv Carbohydr Chem. 1959;14:381–428. doi: 10.1016/s0096-5332(08)60227-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MILLS G. T., PAUL J., SMITH E. E. B. Studies on beta-glucuronidase. II. The preparation and properties of three-ox-spleen beta-glucuronidase fractions. Biochem J. 1953 Jan;53(2):232–245. doi: 10.1042/bj0530232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woollen J. W., Turner P. Plasma N-acetyl-beta-glucosaminidase and beta-glucuronidase in health and disease. Clin Chim Acta. 1965 Dec;12(6):671–683. doi: 10.1016/0009-8981(65)90149-x. [DOI] [PubMed] [Google Scholar]


