Abstract
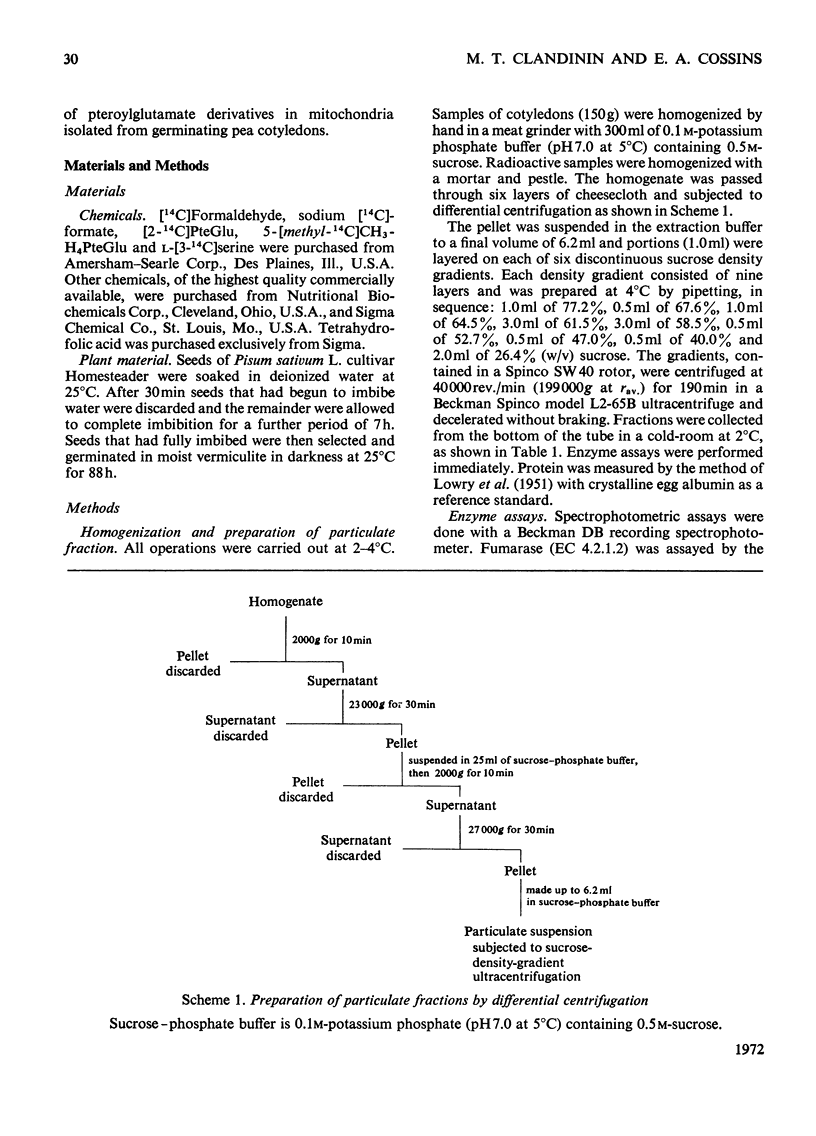
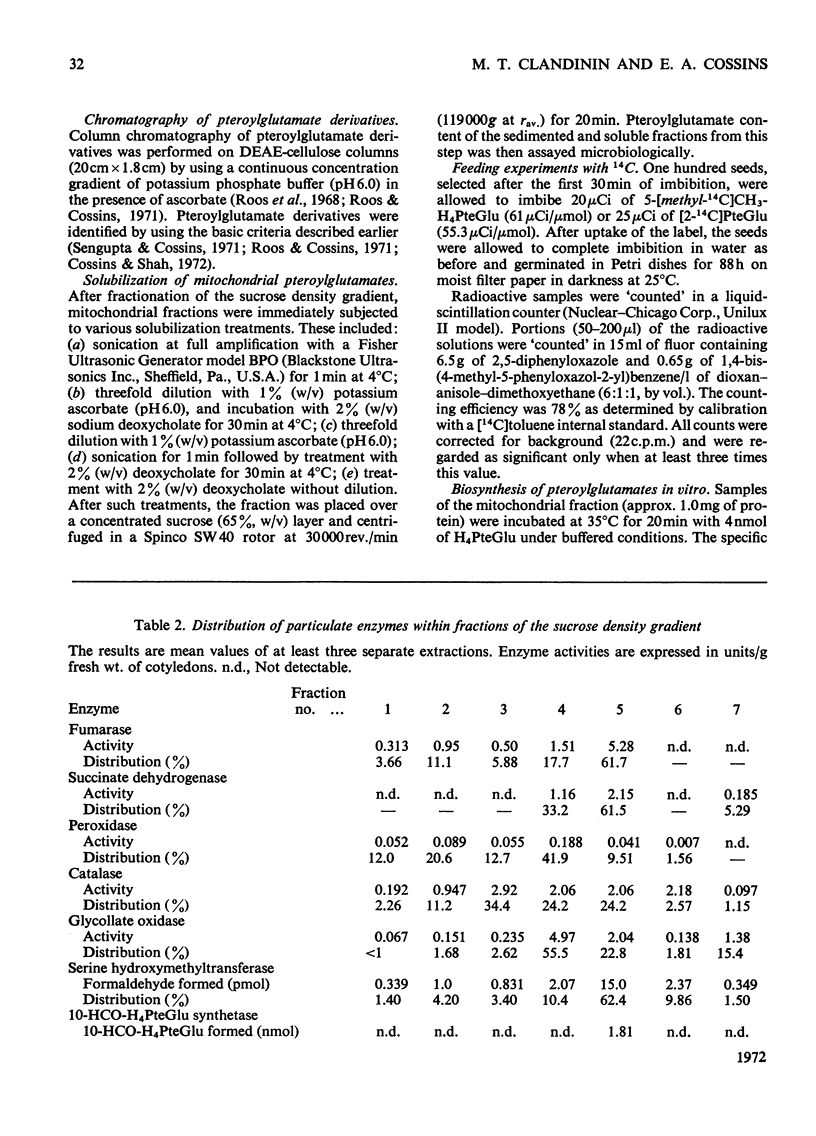
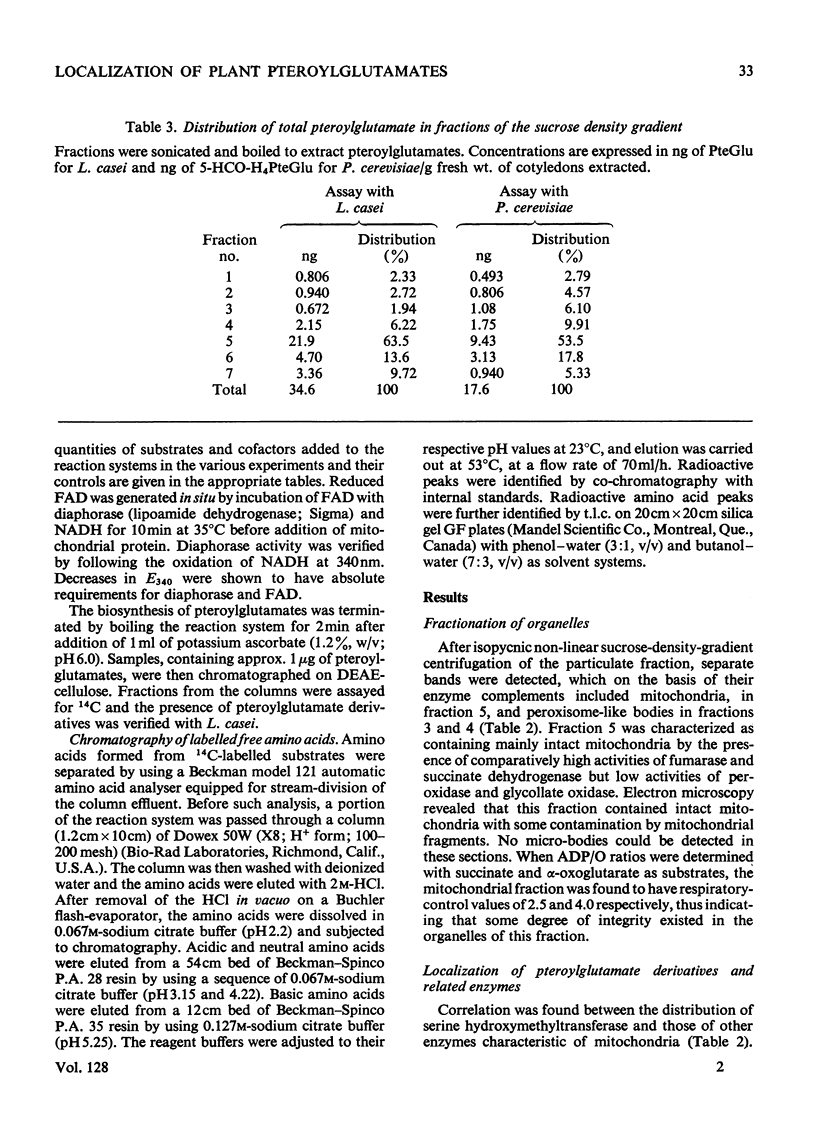
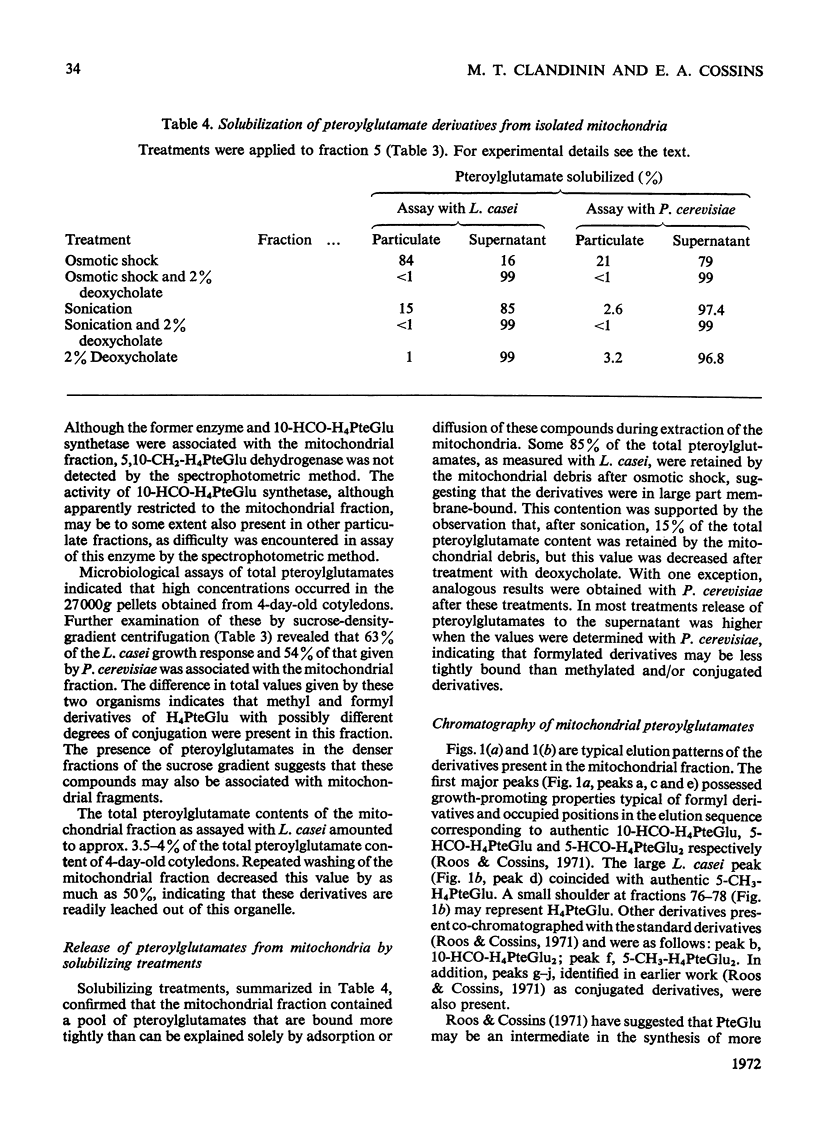
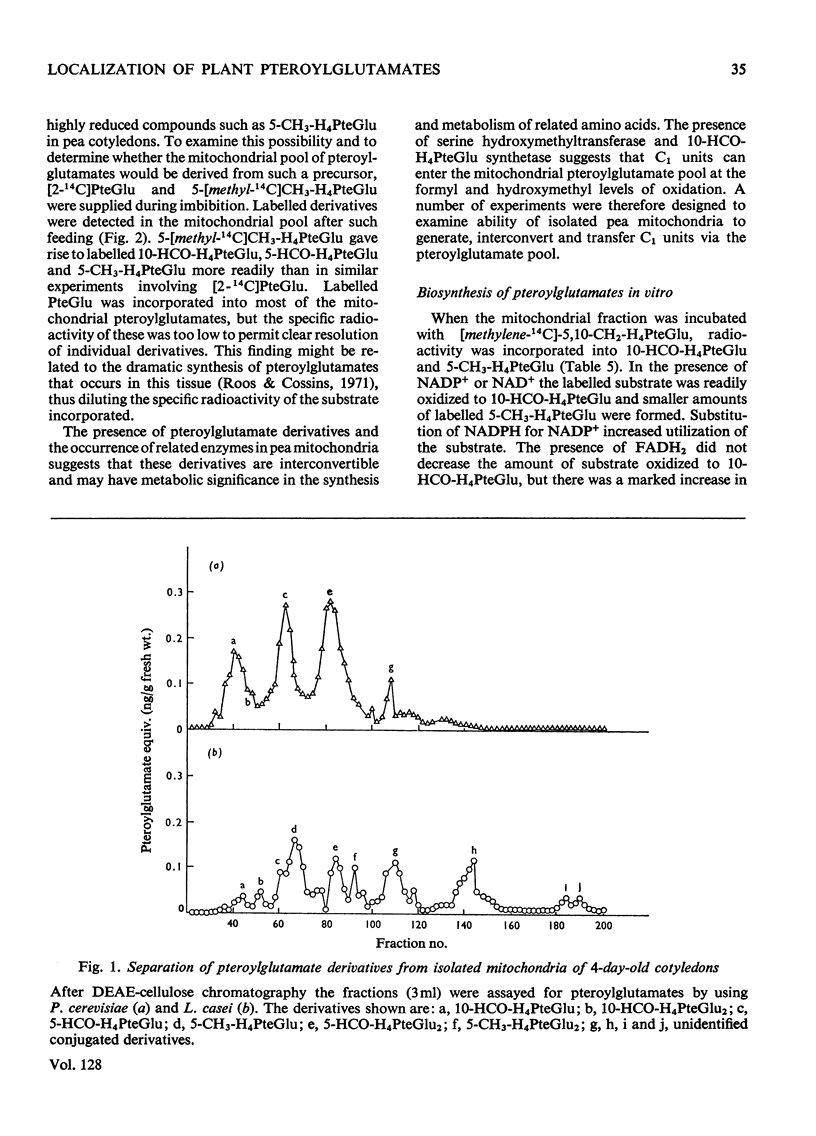
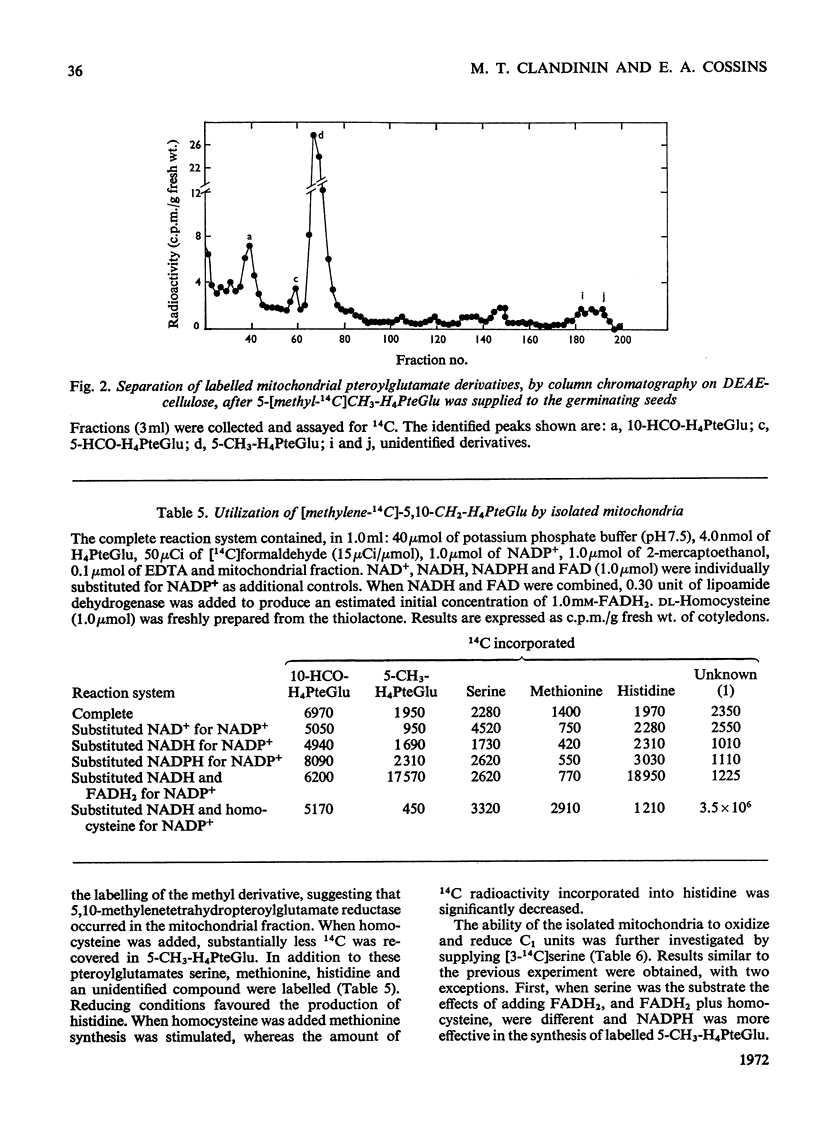
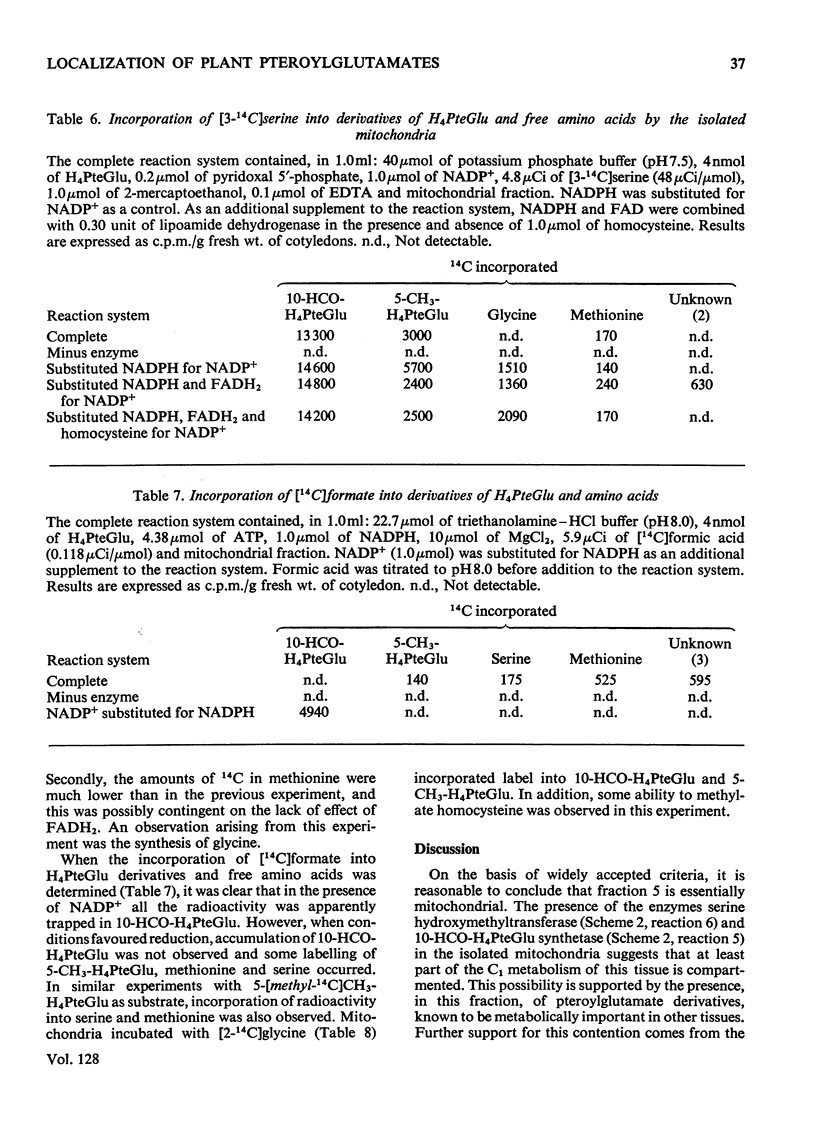
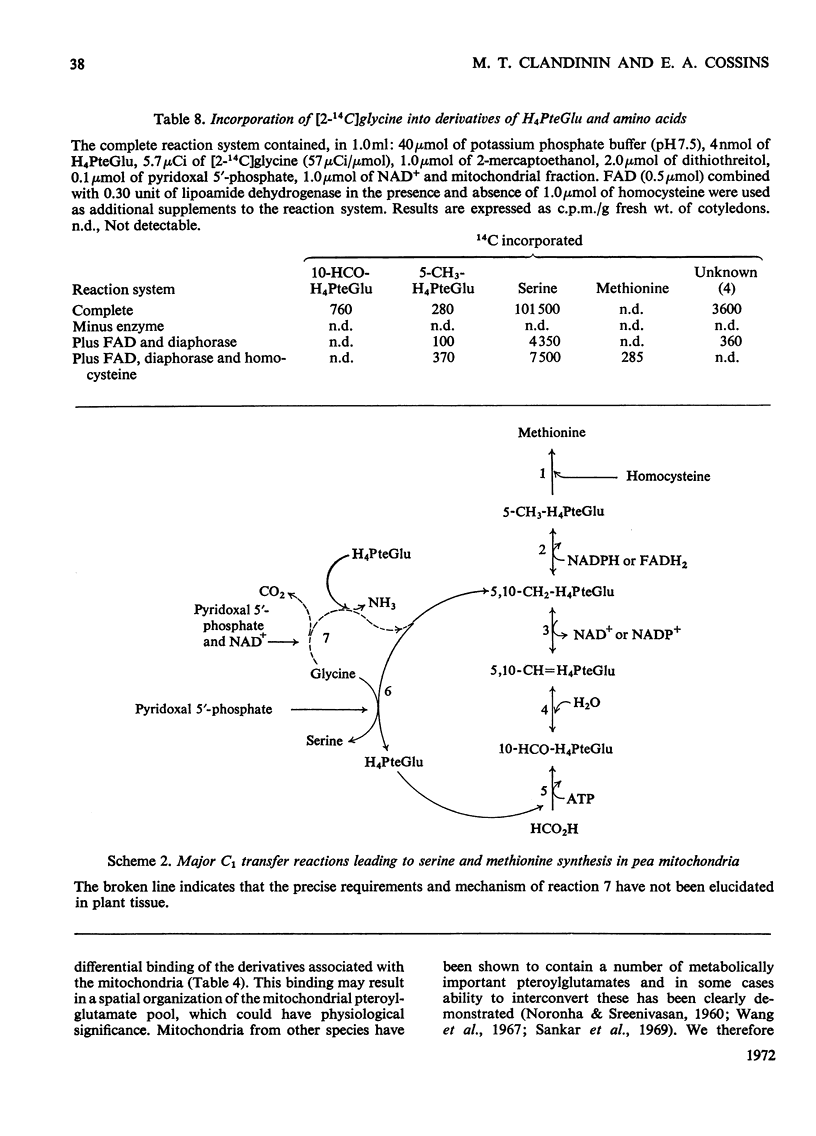
1. Mitochondria were extracted from 4-day-old pea cotyledons and purified on a sucrose density gradient. 2. Microbiological assay of the purified mitochondrial fraction with Lactobacillus casei (A.T.C.C. 7469), Streptococcus faecalis (A.T.C.C. 8043) and Pediococcus cerevisiae (A.T.C.C. 8081) revealed a discrete pool of conjugated and unconjugated derivatives of tetrahydropteroylglutamic acid. 3. Solubilization and chromatographic studies of the mitochondrial fraction demonstrated the presence of formylated and methylated derivatives, 10-formyltetrahydropteroylmonoglutamic acid, 5-formyltetrahydropteroylmonoglutamic acid and 5-formyltetrahydropteroyldiglutamic acid being the major derivatives present. 4. The principal mitochondrial pteroylglutamates were labelled when dry seeds were allowed to imbibe [2-14C]pteroylglutamic acid and 5-[methyl-14C]-methyltetrahydropteroylmonoglutamic acid. 5. The ability of isolated mitochondria to catalyse oxidation and reduction of tetrahydropteroylglutamic acid derivatives was demonstrated in feeding experiments in which [14C]formaldehyde, [3-14C]serine, sodium [14C]formate, 5-[methyl-14C]methyltetrahydropteroylmonoglutamic acid or [2-14C]-glycine served as C1 donor. In addition, 14C was incorporated into free amino acids related to C1 metabolism.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAKERMAN H. A. A method for measuring the microbiological activity of tetrahydrofolic acid and other labile reduced folic acid derivatives. Anal Biochem. 1961 Dec;2:558–567. doi: 10.1016/0003-2697(61)90023-9. [DOI] [PubMed] [Google Scholar]
- BUTTERWORTH C. E., Jr, SANTINI R., Jr, FROMMEYER W. B., Jr THE PTEROYLGLUTAMATE COMPONENTS OF AMERICAN DIETS AS DETERMINED BY CHROMATOGRAPHIC FRACTIONATION. J Clin Invest. 1963 Dec;42:1929–1939. doi: 10.1172/JCI104879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruin W. J., Nelson E. B., Tolbert N. E. Glycolate pathway in green algae. Plant Physiol. 1970 Sep;46(3):386–391. doi: 10.1104/pp.46.3.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J Biol Chem. 1955 Nov;217(1):383–393. [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- Dodd W. A., Cossins E. A. Homocysteine-dependent transmethylases catalyzing the synthesis of methionine in germinating pea seeds. Biochim Biophys Acta. 1970 Mar 24;201(3):461–470. doi: 10.1016/0304-4165(70)90166-2. [DOI] [PubMed] [Google Scholar]
- Dodd W. A., Cossins E. A. Metabolism of S-adenosylmethionine in germinating pea seeds: turnover and possible relationships between recycling of sulfur and transmethylation reactions. Arch Biochem Biophys. 1969 Sep;133(2):216–223. doi: 10.1016/0003-9861(69)90448-2. [DOI] [PubMed] [Google Scholar]
- Gregory R. P. A rapid assay for peroxidase activity. Biochem J. 1966 Dec;101(3):582–583. doi: 10.1042/bj1010582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiatt A. J. Formic Acid Activation in Plants. I. Purification, Properties and Distribution of Formyltetrahydrofolate Synthetase. Plant Physiol. 1965 Jan;40(1):184–188. doi: 10.1104/pp.40.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiatt A. J. Preparation & some properties of soluble succinic dehydrogenase from higher plants. Plant Physiol. 1961 Sep;36(5):552–557. doi: 10.1104/pp.36.5.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisaki T., Tolbert N. E. Glycolate and glyoxylate metabolism by isolated peroxisomes or chloroplasts. Plant Physiol. 1969 Feb;44(2):242–250. doi: 10.1104/pp.44.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S. M., Sagers R. D. Glycine metabolism. 3. A flavin-linked dehydrogenase associated with the glycine cleavage system in Peptococcus glycinophilus. J Biol Chem. 1967 Jan 25;242(2):297–300. [PubMed] [Google Scholar]
- Klein S. M., Sagers R. D. Glycine metabolism. I. Properties of the system catalyzing the exchange of bicarbonate with the carboxyl group of glycine in Peptococcus glycinophilus. J Biol Chem. 1966 Jan 10;241(1):197–205. [PubMed] [Google Scholar]
- Klein S. M., Sagers R. D. Glycine metabolism. II. Kinetic and optical studies on the glycine decarboxylase system from Peptococcus glycinophilus. J Biol Chem. 1966 Jan 10;241(1):206–209. [PubMed] [Google Scholar]
- Klein S. M., Sagers R. D. Glycine metabolism. IV. Effect of borohydride reduction on the pyridoxal phosphate-containing glycine decarboxylase from Peptococcus glycinophilus. J Biol Chem. 1967 Jan 25;242(2):301–305. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Motokawa Y., Hiraga K., Kochi H., Kikuchi G. Evidence for the presence of a protein-bound intermediate in the cleavage and the synthesis of glycine. Biochem Biophys Res Commun. 1970 Feb 20;38(4):771–778. doi: 10.1016/0006-291x(70)90648-0. [DOI] [PubMed] [Google Scholar]
- Motokawa Y., Kikuchi G. Glycine metabolism by rat liver mitochondria. IV. Isolation and characterization of hydrogen carrier protein, an essential factor for glycine metabolism. Arch Biochem Biophys. 1969 Dec;135(1):402–409. doi: 10.1016/0003-9861(69)90556-6. [DOI] [PubMed] [Google Scholar]
- NORONHA J. M., SREENIVASAN A. In vitro conversion of pteroylglutamic acid to citrovorum factor by rat liver enzymes. Biochim Biophys Acta. 1960 Oct 21;44:64–71. doi: 10.1016/0006-3002(60)91523-7. [DOI] [PubMed] [Google Scholar]
- Okinaka O., Iwai K. The biosynthesis of folic acid compounds in plants. 3. Distribution of the dihydropteroate-synthesizing enzyme in plants. J Vitaminol (Kyoto) 1970 Sep;16(3):196–200. doi: 10.5925/jnsv1954.16.196. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos A. J., Cossins E. A. Pteroylglutamate derivatives in Pisum sativum L. Biosynthesis of cotyledonary tetrahydropteroylglutamates during germination. Biochem J. 1971 Nov;125(1):17–26. doi: 10.1042/bj1250017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos A. J., Spronk A. M., Cossins E. A. 5-Methyltetrahydrofolic acid and other folate derivatives in germinating pea seedlings. Can J Biochem. 1968 Dec;46(12):1533–1536. doi: 10.1139/o68-227. [DOI] [PubMed] [Google Scholar]
- SANTINI R., BREWSTER C., BUTTERWORTH C. E., Jr THE DISTRIBUTION OF FOLIC ACID ACTIVE COMPOUNDS IN INDIVIDUAL FOODS. Am J Clin Nutr. 1964 Apr;14:205–210. doi: 10.1093/ajcn/14.4.205. [DOI] [PubMed] [Google Scholar]
- Sankar D. V., Geisler A., Rozsa P. W. Intracellular distribution of folic acid in mouse liver. Experientia. 1969;25(7):691–692. doi: 10.1007/BF01897562. [DOI] [PubMed] [Google Scholar]
- Sato T., Kochi H., Motokawa Y., Kawasaki H., Kikuchi G. Glycin metabolism by rat liver mitochondria. I. Synthesis of two molecules of glycine from one molecule each of serine, bicarbonate and ammonia. J Biochem. 1969 Jan;65(1):63–70. [PubMed] [Google Scholar]
- Sato T., Kochi H., Sato N., Kikuchi G. Glycine metabolism by rat liver mitochondria. 3. The glycine cleavage and the exchange of carboxyl carbon of glycine with bicarbonate. J Biochem. 1969 Jan;65(1):77–83. [PubMed] [Google Scholar]
- Shah S. P.J., Cossins E. A. Pteroylglutamates and methionine biosynthesis in isolated chloroplasts. FEBS Lett. 1970 Apr 16;7(3):267–270. doi: 10.1016/0014-5793(70)80177-6. [DOI] [PubMed] [Google Scholar]
- Tolbert N. E., Oeser A., Kisaki T., Hageman R. H., Yamazaki R. K. Peroxisomes from spinach leaves containing enzymes related to glycolate metabolism. J Biol Chem. 1968 Oct 10;243(19):5179–5184. [PubMed] [Google Scholar]
- Tolbert N. E., Oeser A., Yamazaki R. K., Hageman R. H., Kisaki T. A survey of plants for leaf peroxisomes. Plant Physiol. 1969 Jan;44(1):135–147. doi: 10.1104/pp.44.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F. K., Koch J., Stokstad E. L. Folate coenzyme pattern, folate linked enzymes and methionine biosynthesis in rat liver mitochondria. Biochem Z. 1967 Jan 27;346(5):458–466. [PubMed] [Google Scholar]
- Yoshida T., Kikuchi G. Major pathways of glycine and serine catabolism in rat liver. Arch Biochem Biophys. 1970 Aug;139(2):380–392. doi: 10.1016/0003-9861(70)90490-x. [DOI] [PubMed] [Google Scholar]
- ZELITCH I., OCHOA S. Oxidation and reduction of glycolic and glyoxylic acids in plants. I. Glycolic and oxidase. J Biol Chem. 1953 Apr;201(2):707–718. [PubMed] [Google Scholar]


