ABSTRACT
Background and Aim
Lubiprostone increases chloride and water secretion in the intestines, and several studies have demonstrated the efficacy of lubiprostone in treating functional constipation. Several new clinical trials have emerged since the previous meta‐analysis conducted in 2020. We conducted this updated meta‐analysis to assess clinical efficacy of lubiprostone in these patients.
Methods
A systematic search was conducted on MEDLINE, Cochrane, and Scopus. Randomized controlled trials published between July 2019 and June 2024 were selected. Cochrane's RoB 2 tool was used to assess the risk of bias. A meta‐analysis was performed and findings were presented using forest plots.
Results
A total of 14 studies, comprising 4550 patients, were included in the review. Only 12 studies were pooled in the meta‐analysis. Lubiprostone was associated with greater spontaneous bowel movements (SBM) per week (RR 1.454, 95% CI 1.193–1.771) and SBM within 24 h (RR 1.790, 95% CI 1.491–2.150) in patients with chronic idiopathic constipation (CIC). However, it was not associated with abdominal pain in either arm (RR 1.415, 95% CI 0.873–2.294). In opioid‐induced constipation (OIC), lubiprostone increased SBM within 24 h (RR 1.277, 95% CI 1.105–1.475) but did not significantly affect abdominal pain (RR 4.321, 95% CI 0.624–29.941). Lubiprostone improved all selected SBM‐related and abdominal pain outcomes in patients with irritable bowel syndrome with constipation (IBS‐C).
Conclusion
Lubiprostone significantly improves all SBM‐related outcomes. Owing to its good safety and efficacy profile, lubiprostone can be used in the combination regimens for management of CIC, IBS‐C, and OIC.
Keywords: chronic idiopathic constipation, constipation, irritable bowel syndrome, lubiprostone, opioid induced constipation
1. Introduction
Constipation is a common issue affecting individuals of all ages, including children and adults. In the United Kingom, around one in seven adults and one in three children experience constipation at any given time [1]. Constipation also impacts the economy heavily—the United States spends over 800 million dollars annually on laxatives [2]. In addition to its impact on quality of life, constipation reduces an individual's productivity and increases the frequency of missing work or school days [3]. Chronic idiopathic constipation (CIC) is a common functional disorder impacting up to 17% of the global population [4], with a higher prevalence among females and older individuals [5]. Most CIC patients rely on traditional laxatives for relief; the usage of laxatives is linked to age, the frequency of symptoms, and the duration of constipation [6]. Constipation is also a substantial concern in individuals with irritable bowel syndrome (IBS) [7]. Prominent symptoms of irritable bowel syndrome with constipation (IBS‐C) include abdominal pain, bloating, and straining [8]. Opioid‐induced constipation (OIC) is a frequent problem encountered by patients on long‐term opioid therapy, affecting their quality of life [9]. OIC is mainly associated with cancer pain, chronic non‐cancer pain, and opioid dependence treatment [10].
Constipation may result from mechanical obstruction of the gastrointestinal tract, a low‐fiber diet, lack of exercise, neuromuscular disorders, metabolic abnormalities, or the use of certain medications such as opiate analgesics, anticholinergic antidepressants, and calcium channel blockers [11]. Regardless of the underlying cause, the main goals of treatment are to reduce patient discomfort and return bowel function to normal [12]. For patients with functional constipation, a variety of laxatives are available, including prokinetics and osmotic, stimulant, or secretory laxatives [13]. However, existing treatment options do not adequately control IBS‐C symptoms due to high treatment costs and increased utilization of healthcare resources [14]. A novel, efficacious, and well‐tolerated treatment is necessary for constipation in patients diagnosed with CIC, IBS‐C, or OIC [15].
Lubiprostone, an emerging treatment option for functional constipation, works by increasing the release of chloride and water in the intestines [16]. It is categorized as a secretory laxative and belongs to the novel class of compounds referred to as prostones [17]. Previous investigations in healthy volunteers and constipation patients have demonstrated lubiprostone's efficacy in increasing bowel movement frequency and alleviating other constipation symptoms [18]. Several studies have investigated the efficacy of lubiprostone for the treatment of constipation; a meta‐analysis conducted in 2020 revealed positive outcomes [19]. However, results from several new, well‐designed clinical trials have been published [20, 21, 22]. We conducted this updated meta‐analysis to better analyze the efficacy and safety of Lubiprostone in CIC, IBS‐C, and OIC patients.
2. Methods
The guidelines of the Cochrane Handbook for Systematic Reviews of Interventions were used to conduct this systematic review, and reported according to the Preferred Reporting Items for Systematic Reviews and Meta‐analysis (PRISMA) statement [23]. This review has been registered with the International Prospective Register of Systematic Reviews (PROSPERO) under the identifier CRD42023443405. Our study did not require ethical approval.
2.1. Data Sources and Search Strategy
Two reviewers independently searched for eligible studies on the electronic databases and clinical trial registers: MEDLINE (PubMed), Cochrane Central Register of Controlled Trials (CENTRAL), Google Scholar, and ClinicalTrials.gov from July 2019 to June 2024. The primary keywords included “lubiprostone,” “constipation,” “chronic idiopathic constipation,” “opioid‐induced constipation,” and “irritable bowel syndrome.” Variations of the keywords were combined with the appropriate Boolean operators. A detailed overview of the search strategy is shown in Table S2. No language restriction was applied. We searched for additional articles by screening the reference lists of obtained studies and similar clinical trials.
2.2. Eligibility Criteria
We included the studies if they: (i) were randomized controlled trials (RCTs), (ii) involved patients diagnosed with CIC, OIC, or IBS‐C, (iii) reported spontaneous bowel movements and abdominal pain or discomfort as outcomes, and (iv) were available online in English. Studies were excluded due to the following reasons: (i) study designs other than randomized controlled trials, (ii) not comparing the efficacy of lubiprostone with a control group, (iii) not available in English. Additionally, we excluded editorials, reviews, conference abstracts, and experimental studies on animal models. The definitions of CIC, OIC, and IBS‐C were adopted from the original studies, provided that it was clearly mentioned in the available manuscript.
2.3. Selection Process
Mendeley Desktop 1.19.8 (Mendeley Ltd., Amsterdam, The Netherlands) was employed for the deduplication and screening of all the articles retrieved through our online search. After deduplication, two authors independently completed the first phase of screening titles and abstracts. The remaining articles were then subjected to comprehensive full‐text screening by the same authors. Any disagreements between them were resolved by a third reviewer.
2.4. Outcomes Measured
The following outcomes were measured to assess the efficacy of lubiprostone: (i) frequency of spontaneous bowel movements (SBM), assessed as mean change from baseline, frequency, and number of patients having an SBM within 24 h of the first dose of lubiprostone; (ii) full‐responder rate, defined as the number of patients presenting > 3–4 SBMs in a week; and (iii) degree of abdominal pain or discomfort, assessed as mean score and number of patients experiencing the symptoms.
SBM was defined as spontaneous bowel movement occurring 24 h or more after the use of rescue medication. Abdominal pain or discomfort was rated using a Likert scale with 0 indicating “absent,” 1 indicating “mild,” 2 indicating “moderate,” 3 indicating “severe,” and 4 indicating “very severe.” Abdominal pain as an outcome was assessed in studies having a patient population diagnosed with IBS‐C whereas abdominal discomfort was assessed in CIC and OIC trials due to the different clinical manifestations of these conditions.
2.5. Data Extraction and Quality Assessment
A spreadsheet was designed to extract relevant data from the included studies including the primary outcomes defined above, and other study characteristics such as the first author's name, year, sample size, and the disease (CIC, OIC, IBS‐C). The risk of bias was assessed using Cochrane's RoB 2, a revised tool for assessing the risk of bias in RCTs. RoB 2 uses five domains to determine the overall risk of bias: bias due to problems in the randomization process, bias due to deviations from the intended outcomes, bias due to missing outcome data, bias due to problems in the measurement of data, and bias due to selective reporting of the results. Two authors independently rated the risk of bias for each included study as low, high, or some concerns. Any disagreement between them was resolved by a third reviewer.
2.6. Data Analysis
Comprehensive Meta‐Analysis software, version 3.3 (Biostat Inc., Englewood, NJ, USA) was used for the statistical analysis. We calculated risk ratios (RR) and their 95% confidence intervals (95% CI) for dichotomous events using the Mantel–Haenszel method and a random effects model. Those studies not providing sufficient information for pooling results for continuous outcomes, such as median time to first SBM and abdominal pain and discomfort scales, were not included in the statistical analysis. Between‐study heterogeneity was assessed by Cochran's Q test and the I2 statistic; a p‐value < 0.05 was considered statistically significant. Doi plots and Luis Furuya‐Kanamori (LFK) index were used to assess publication bias because it has greater sensitivity and power than Egger's test for outcomes < 10 studies [24].
3. Results
3.1. Study Selection
We retrieved the 11 studies included in the previous meta‐analysis and searched the databases for articles published after July 2019. A total of 1203 articles were initially retrieved. After removing duplicates, the title and abstracts of 981 articles were screened, and 298 articles were selected for a full‐text review. We found three new RCTs which were relevant. In total, 14 studies met the inclusion criteria and are included in this version of the review. Figure 1 shows the PRISMA flow chart summarizing the study selection process. The studies comprised a total of 4550 patients—involving 1884 CIC patients, 1366 IBS‐C patients, and 1300 OIC patients. Of the 14 studies, 12 studies were included in the meta‐analysis. The earliest study was published in 2007 and the latest study was published in 2022.
FIGURE 1.
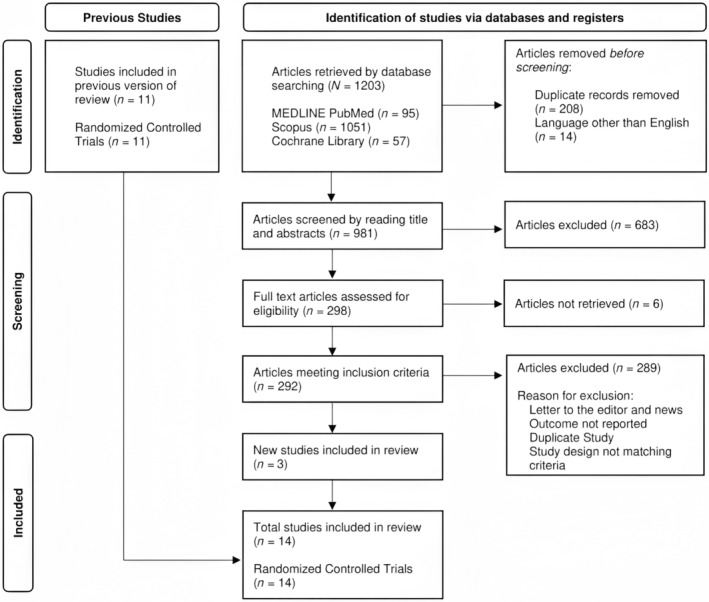
PRISMA flow diagram.
3.2. Quality of the Included Studies
Figure 2 shows the result of the quality assessment. While there were some concerns in the randomization process of all studies, the overall risk of bias was low.
FIGURE 2.
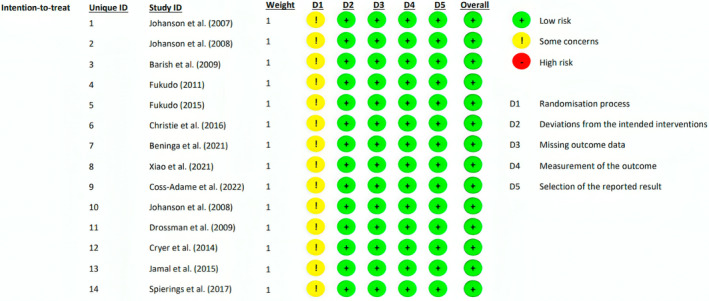
Quality assessment of the included studies.
3.3. Outcomes in CIC Studies
Among CIC studies (Table 1), five studies reported data for full‐responder rate. According to the pooled results shown in Figure 3A, lubiprostone was associated with a significantly greater full‐responder rate (RR = 1.454, 95% CI = 1.193–1.771, p = 0.000, I2 = 53%). No asymmetry was observed in the doi plot (LFK index = 0.17), suggesting no evidence of publication bias (Figure S1). Six studies reported data for SBM within 24 h. According to the pooled results shown in Figure 3B, lubiprostone significantly increased the frequency of SBM within 24 h (RR = 1.790, 95% CI = 1.491–2.150, p = 0.000, I2 = 32%). Minor asymmetry was observed in the doi plot (LFK index = 1.39), suggesting little to no evidence of publication bias (Figure S2).
TABLE 1.
Main findings in CIC lubiprostone studies.
| Outcomes | Studies | Results (lubiprostone vs. placebo) | p | |
|---|---|---|---|---|
| CIC | Mean change in the number of SBMs from baseline (4 weeks) | Fukudo, 2015 | 2.56 versus 1.62 | 0.042 |
| Xiao, 2021 | 3.14 versus 1.92 | 0.0006 | ||
| Mean SBM per week (8 weeks) | Christie, 2016 | 7.00 versus 5.27 | 0.02 | |
| Mean SBM per week (4 weeks) | Johanson, 2008 | 5.30 versus 2.91 | 0.002 | |
| Coss‐Adame, 2022 | 7.1 versus 5.6 | 0.013 | ||
| Xiao, 2021 | 4.46 versus 3.24 | 0.0006 | ||
| Barish, 2009 | 5.37 versus 3.46 | 0.0068 | ||
| Christie, 2016 | 5.77 versus 4.78 | NR | ||
| Mean SBM per week (1 week) | Johanson, 2007 | Point estimates NR, higher mean for lubiprostone (chart) | 0.02 | |
| Coss‐Adame, 2022 | 6.7 versus 5.2 | NR | ||
| Xiao, 2021 | 4.88 versus 3.22 | < 0.0001 | ||
| Johanson, 2008 | 5.69 versus 3.46 | 0.0001 | ||
| Barish, 2009 | 5.89 versus 3.99 | 0.0001 | ||
| Change in mean SBM in the first week | Fukudo, 2011 | 6.8 versus 1.5 | 0.0001 | |
| Fukudo, 2015 | 3.7 versus 1.3 | 0.001 | ||
| Coss‐Adame, 2022 | 4.9 versus 3.0 | 0.02 | ||
| Xiao, 2021 | 3.55 versus 1.90 | < 0.0001 | ||
| SBM within 24 h | Johanson, 2007 | 59.4% versus 27.3% | 0.009 | |
| Johanson, 2008 | 56.7% versus 36.9% | 0.0024 | ||
| Barish, 2009 | 61.3% versus 31.4% | 0.0001 | ||
| Fukudo, 2011 | 75.0% versus 26.2% | 0.0001 | ||
| Fukudo, 2015 | 58.1% versus 34.6% | 0.004 | ||
| Coss‐Adame, 2022 | 60.0% versus 41.5% | 0.009 | ||
| Weekly full‐responder rate a (≥ 3–4 SBM per week) (4 weeks) | Johanson, 2008 | 57.8% versus 27.9% | 0.004 | |
| Xiao, 2021 | 62.31% versus 47.29% | 0.0178 | ||
| Barish, 2009 | 60.0% versus 39.0% | 0.0022 | ||
| Fukudo, 2015 | 54.2% versus 36.7% | 0.066 | ||
| Mean abdominal discomfort score (4 weeks) | Johanson, 2008 | 1.23 versus 1.52 | 0.045 | |
| Barish, 2009 | 1.24 versus 1.47 | 0.1383 | ||
| Coss‐Adame, 2022 | 0.9 versus 1.2 | 0.004 | ||
| Mean abdominal discomfort rate (4 weeks) | Christie, 2016 | 53.0% versus 67.0% | 0.86 | |
| Mean abdominal discomfort rate (8 weeks) | Christie, 2016 | 50.0% versus 52.0% | 0.86 | |
| Overall SBM response | Beninga, 2021 (study 1 mITT population) | 18.5% versus 14.4% | 0.2245 |
Abbreviations: CIC: chronic idiopathic constipation; NR: not reported; SBM: spontaneous bowel movements.
Definition of full‐responder = ≥ 3 SBM per week.
FIGURE 3.
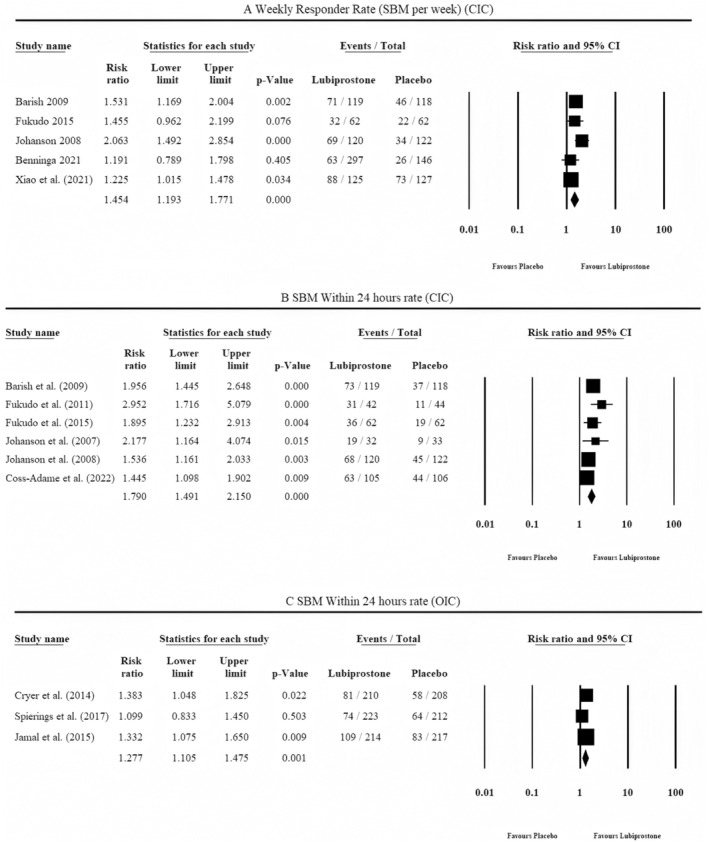
Outcome analysis in the included studies.
All CIC studies reported adverse events during treatment. Christie et al. (2016), Fukudo et al. (2011) and Fukudo et al. (2015) (Figure 4A) found no significant association of abdominal pain with either group. Johanson et al. 2007 and 2008 reported significant gastrointestinal disorders in the lubiprostone arm, and Xiao et al. noted the statistically significant association of lubiprostone administration with abdominal pain. The pooled results showed no significant association of abdominal pain with either arm (RR = 1.415, 95% CI = 0.873–2.294, p = 0.159, I2 = 29%). There was low heterogeneity between studies. No asymmetry was observed in the doi plot (LFK index = 0.15), suggesting no evidence of publication bias (Figure S3).
FIGURE 4.
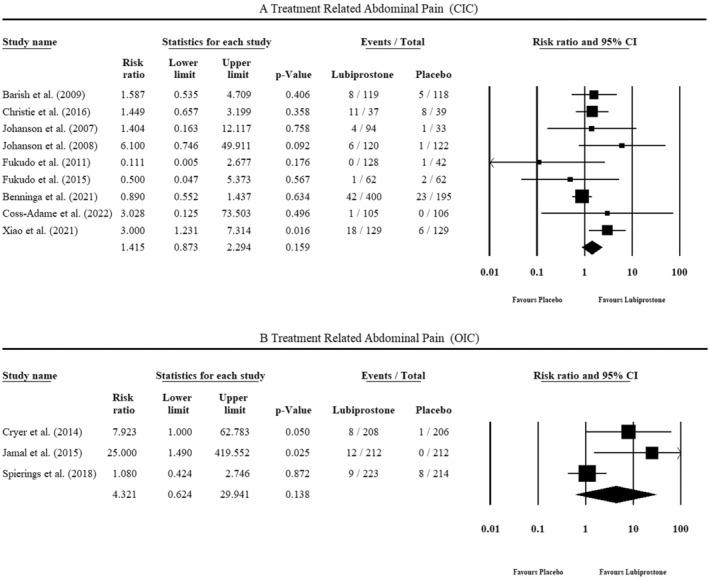
Treatment‐related abdominal pain.
3.4. Outcomes in IBS‐C Studies
In extended follow‐ups (up to 3 months) in the IBS‐C studies (Table 2), all selected SBM‐related outcomes were significantly better among lubiprostone patients. Three RCTs evaluated the mean scores for measures of abdominal pain at 1, 2, and 3 months following the start of treatment. After 2 and 3 months of taking lubiprostone, individuals in the Drossman et al. study showed more improvement in abdominal pain than those taking a placebo. After 1 and 2 months of lubiprostone medication, Johanson et al. observed a greater mean improvement in the abdominal pain score compared to the baseline.
TABLE 2.
Main findings in IBS‐C lubiprostone studies.
| Outcomes | Studies | Results (lubiprostone vs. placebo) | p | |
|---|---|---|---|---|
| IBS‐C | Overall responder rate a | Drossman, 2009 | 17.9% versus 10.1% | 0.001 |
| Weekly responder rate (weeks 2, 4, 5, 6, 10, and 12) | Drossman, 2009 | Point estimates NR, higher rate for lubiprostone (chart) | 0.030 | |
| Monthly responder rate (month 3) | Study 0431 in Drossman, 2009 | 21.3% versus 14.5% | 0.026 | |
| Study 0432 in Drossman, 2009 | 22.7% versus 14.6% | 0.026 | ||
| Drossman, 2009 (pooled results) | 22.0% versus 14.5% | 0.003 | ||
| Mean change from baseline in weekly SBM rate (month 3) | Johanson, 2008 | Point estimates NR, higher rate for lubiprostone (chart) | 0.033 | |
| Mean improvement in abdominal pain score (month 1) | Johanson, 2008 | Point estimates NR, higher mean for lubiprostone (chart) | 0.023 | |
| Drossman, 2009 | Point estimates NR, higher mean for lubiprostone | > 0.05 | ||
| Mean improvement in abdominal pain score (month 2) | Johanson, 2008 | Point estimates NR, higher mean for lubiprostone (chart) | 0.028 | |
| Drossman, 2009 | −0.43 versus −0.35 | 0.039 | ||
| Mean improvement in abdominal pain score (month 3) | Johanson, 2008 | Point estimates NR, higher mean for lubiprostone (chart) | 0.260 | |
| Drossman, 2009 | −0.45 versus −0.36 | 0.028 |
Abbreviations: IBS‐C: constipation‐predominant irritable bowel syndrome; NR: not reported; SBM: spontaneous bowel movements.
Responder rate was defined as patients achieving ≥ 3–4 SBM per week.
3.5. Outcomes in OIC Studies
Among the OIC Studies (Table 3), three trials reported data for SBM within 24 h. The pooled results in Figure 3C show that lubiprostone significantly increased the frequency of SBM within 24 h (RR = 1.277, 95% CI = 1.105–1.475, p‐value = 0.001, I2 = 0%). Minor asymmetry was observed in the doi plot (LFK index = −1.25), suggesting little to no evidence of publication bias (Figure S4). Additionally, lubiprostone yielded favorable results for SBM at week 8 (1 out of 2 RCTs) and week 12 (2 out of 3 RCTs), and overall responder rate (2 out of 3 RCTs). Publication bias could not be assessed as the number of included studies for this analysis was less than 10.
TABLE 3.
Main findings in OIC lubiprostone studies.
| Outcomes | Studies | Results (lubiprostone vs placebo) | p | |
|---|---|---|---|---|
| OIC | Mean change from baseline in SBM frequency (week 12) | Cryer, 2014 | Point estimates NR, higher mean for lubiprostone (chart) | 0.091 |
| Jamal, 2015 | Point estimates NR, higher mean for lubiprostone (chart) | 0.040 | ||
| Spierings, 2017 | 2.5 versus 2.6 | 0.956 | ||
| Mean change from baseline in SBM frequency (week 8) | Cryer, 2014 | 3.3 versus 2.4 | 0.005 | |
| Spierings, 2017 | 2.6 versus 2.4 | 0.842 | ||
| Mean change from baseline in SBM frequency (overall) | Cryer, 2014 | 2.2 versus 1.6 | 0.004 | |
| Jamal, 2015 | 3.2 versus 2.4 | 0.001 | ||
| Spierings, 2017 | 2.6 versus 2.3 | 0.224 | ||
| Overall responder rate | Jamal, 2015 | 27.1% versus 18.9% | 0.030 | |
| SBM within 24 h | Cryer, 2014 | 38.8% versus 27.8% | 0.018 | |
| Jamal, 2015 | 50.9% versus 38.2% | 0.008 | ||
| Spierings, 2017 | 33.2% versus 30.2% | 0.502 | ||
| Mean improvement in abdominal discomfort scales (overall) | Cryer, 2014 | Point estimates NR, higher mean for lubiprostone (chart) | 0.047 | |
| Jamal, 2015 | Point estimates NR, higher mean for lubiprostone (chart) | 0.127 | ||
| Spierings, 2017 | −0.5 versus −0.4 | 0.027 | ||
| Median time to first SBM | Cryer, 2014 | 28.5 versus 46.0 h | 0.053 | |
| Jamal, 2015 | 23.5 versus 37.7 h | 0.004 |
Abbreviations: NR: not reported; OIC: opioid‐induced constipation; SBM: spontaneous bowel movement.
All OIC studies also reported treatment‐associated adverse events. Cryer et al. observed a statistically significant association between the incidence of abdominal pain and lubiprostone administration (Figure 4B). Jamal et al. reported significantly higher gastrointestinal disorders in the lubiprostone arm, and the placebo group had no incidence of abdominal pain. Spierings et al. noted no significant association of gastrointestinal disorders with either group. A pooled analysis proved no significant association of abdominal pain with either group (RR = 4.321, 95% CI = 0.624–29.941, p‐value = 0.138, I2 = 69%). A moderate heterogeneity was observed between studies. Major asymmetry was observed in the doi plot (LFK index = 4.67), suggesting evidence of publication bias (Figure S5).
4. Discussion
We conducted an updated systematic review and meta‐analysis of randomized controlled trials [17, 20, 21, 22, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34] to assess the efficacy of Lubiprostone in patients of CIC, IBS‐C, and OIC. The weekly responder rate was reported by five studies [17, 20, 22, 26, 30] and the meta‐analysis favored lubiprostone over placebo among the CIC patients. Six studies [17, 21, 26, 27, 30, 31] reported SBM within 24 h among CIC patients, and the meta‐analysis favored lubiprostone over placebo. In the OIC studies [25, 29, 33], SBM was reported within 24 h, and the combined effect estimate favored lubiprostone. Among the IBS‐C studies [28, 32], a trend favoring lubiprostone was observed. Treatment‐related abdominal pain was reported by CIC studies and OIC studies; however, pooled analysis revealed that lubiprostone was not significantly associated with increased risk of abdominal pain. There was moderate heterogeneity in the CIC studies for the primary outcome which could be attributed to the differences in baseline statistics of the patients such as age, race, ethnicity, and comorbidities. For example, the mean age varied significantly across studies, ranging from 10.67 years in Benninga et al. [22] to 48.56 years in Johanson et al. [26], while the male‐to‐female ratio was also notably different, with a low proportion of males in Johanson et al. [26] (10.29%) compared to a much higher ratio in Benninga et al. [26] (84.57%).
The results of our meta‐analysis are comparable with previous literature exploring the efficacy of lubiprostone in patients of CIC, OIC, and IBS‐C. A previous meta‐analysis conducted by Passos et al. [19] demonstrated a significant improvement in weekly responder rate and SBM within 24 h rate in CIC studies, which is consistent with our findings. However, their pooled analysis did not reveal a significant association of lubiprostone with improved SBM within 24 h rate in OIC studies, which is in contrast with our findings. Furthermore, we also identified a nonsignificant association of lubiprostone with treatment‐related abdominal pain in CIC as well as OIC studies. However, the previous investigation by Passos et al. [19] did not report this outcome.
Additionally, the United States Food and Drug Administration approved lubiprostone (24 μg twice daily) for CIC after a positive trend was observed favoring lubiprostone over placebo in two randomized double‐blind placebo‐controlled clinical trials consisting of 479 patients [35]. Additionally, lubiprostone resulted in constant improvement of abdominal bloating and constipation compared to the baseline in the long term as seen in three subsequent open‐label trials comprising 871 CIC patients [35]. For IBS‐C, the approved dose of lubiprostone is 8 μg twice daily. Long‐term effects of lubiprostone use in IBS‐C have not been explored yet. Current practice is to prescribe lubiprostone for a 12‐week trial period; if the patient fails to respond to lubiprostone within this trial period, the treatment is discontinued [32]. The approval of lubiprostone was based on the results of two large multicenter, placebo‐controlled RCTs including 1154 adults, which showed a better overall response rate for 12‐week lubiprostone use. No statistically significant adverse events were observed in these trials [32]. The follow‐up open‐label trials among IBS‐C patients yielded increased efficacy of lubiprostone at 52 weeks [36]. A pooled analysis in a post hoc study [37] showed that response rates for abdominal pain and bloating are significantly higher in patients on lubiprostone as compared to placebo for IBS‐C. In the relative individual trials [28, 32] included in this review exploring the efficacy of lubiprostone in IBS‐C patients, there was a mean improvement in the symptoms of abdominal pain and bloating at about 8 weeks of treatment. For OIC, the results obtained based on individual analysis of the three included studies, Cryer et al. [33] and Jamal et al. [29] showed a positive trend in terms of overall response favoring lubiprostone over placebo. Spierings et al. [25] did not show any statistically significant improvement in responder rate associated with lubiprostone. However, the overall pooled results provided in our meta‐analysis showed a statistically significant responder rate favoring lubiprostone. Adverse effects are not common with lubiprostone use and the drug is well tolerated. Consistent with previous studies, we observed no significant relationship between abdominal pain and lubiprostone use in OIC patients [38]. Recent literature shows some other adverse effects commonly observed in patients taking lubiprostone, including headache, chest discomfort, and peripheral edema [39].
Our study has certain limitations that should be considered when interpreting the results. First, we observed some heterogeneity between the studies, possibly due to varying duration of follow‐up, different criteria for the outcomes, and discrepancies in baseline characteristics of the patients. Second, most of the included studies had short follow‐up durations. Third, we exclusively considered published articles, excluding conference abstracts and gray literature. Fourth, we limited our study to articles available in English, excluding any publications in other languages.
Although lubiprostone presents a significant success rate in improving SBM and alleviating abdominal discomfort in patients with CIC, IBS‐C, and OIC versus placebo, we suggest future studies to compare its efficacy with conventional treatment regimens. Future trials should incorporate longer follow‐up periods to better predict real‐life outcomes and adverse events. Lastly, combination regimens containing lubiprostone should be explored.
5. Conclusion
Lubiprostone significantly improves all SBM‐related outcomes and is effective for the management of CIC, IBS‐C, and OIC. We found no association of gastrointestinal adverse events with lubiprostone use. Lubiprostone demonstrated a good safety profile and can be used in combination regimens for the treatment of constipation.
Ethics Statement
Ethical approval is not applicable to this study type. All authors have read and approved the final version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Supporting information
Supplementary Figure S1: Doi plot of SBM Per week (CIC).
Supplementary Figure S2: Doi plot of SBM within 24 h (CIC).
Supplementary Figure S3: Doi plot of Treatment Related Abdominal Pain (CIC).
Supplementary Figure S4: Doi plot of SBM within 24 h (OIC).
Supplementary Figure S5: Doi plot of Treatment Related Abdominal Pain (OIC).
Supplementary Table S1: Detailed Search Strategy of Each Database.
Acknowledgments
The publication of this article was funded by Qatar National Library. Hamad Medical Corporation Open Access publishing facilitated by the Qatar National Library, as part of the Wiley Qatar National Library agreement.
Funding: The authors received no specific funding for this work.
Data Availability Statement
The data are available upon reasonable request from the corresponding author.
References
- 1. “Constipation symptoms and treatments,” accessed June 2, 2024, https://www.nhsinform.scot/illnesses‐and‐conditions/stomach‐liver‐and‐gastrointestinal‐tract/constipation.
- 2. Wilson N. and Schey R., “Lubiprostone in Constipation: Clinical Evidence and Place in Therapy,” Therapeutic Advances in Chronic Disease 6, no. 2 (2015): 40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Neri L., Basilisco G., Corazziari E., et al., “Constipation Severity Is Associated With Productivity Losses and Healthcare Utilization in Patients With Chronic Constipation,” United European Gastroenterology Journal 2, no. 2 (2014): 138–147, 10.1177/2050640614528175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Oh S. J., Fuller G., Patel D., et al., “Chronic Constipation in the United States: Results From a Population‐Based Survey Assessing Healthcare Seeking and Use of Pharmacotherapy,” American Journal of Gastroenterology 115, no. 6 (2020): 895–905, 10.14309/ajg.0000000000000614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bellini M., Gambaccini D., Usai‐Satta P., et al., “Irritable Bowel Syndrome and Chronic Constipation: Fact and Fiction,” World Journal of Gastroenterology 21, no. 40 (2015): 11362–11370, 10.3748/wjg.v21.i40.11362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shin J. E., Jung H. K., Lee T. H., et al., “Guidelines for the Diagnosis and Treatment of Chronic Functional Constipation in Korea,” Journal of Neurogastroenterology and Motility 22, no. 3 (2016): 383–411, 10.5056/jnm15185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jadallah K. A., Kullab S. M., and Sanders D. S., “Constipation‐Predominant Irritable Bowel Syndrome: A Review of Current and Emerging Drug Therapies,” World Journal of Gastroenterology 20, no. 27 (2014): 8898–8909, 10.3748/wjg.v20.i27.8898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ballou S., McMahon C., Lee H. N., et al., “Effects of Irritable Bowel Syndrome on Daily Activities Vary Among Subtypes Based on Results From the IBS in America Survey,” Clinical Gastroenterology and Hepatology 17, no. 12 (2019): 2471–2478, 10.1016/j.cgh.2019.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lang‐Illievich K. and Bornemann‐Cimenti H., “Opioid‐Induced Constipation: A Narrative Review of Therapeutic Options in Clinical Management,” Korean Journal of Pain 32, no. 2 (2019): 69–78, 10.3344/kjp.2019.32.2.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Forootan M., Bagheri N., and Darvishi M., “Chronic Constipation: A Review of Literature,” Medicine 97, no. 20 (2018): e10631, 10.1097/MD.0000000000010631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nightingale J. M., Paine P., McLaughlin J., Emmanuel A., Martin J. E., and Lal S., “The Management of Adult Patients With Severe Chronic Small Intestinal Dysmotility,” Gut 69, no. 12 (2020): 2074–2092, 10.1136/gutjnl-2020-321631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Portalatin M. and Winstead N., “Medical Management of Constipation,” Clinics in Colon and Rectal Surgery 25, no. 1 (2012): 12–19, 10.1055/s-0032-1301754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang J., Wang H. P., Zhou L., and Xu C. F., “Effect of Dietary Fiber on Constipation: A Meta Analysis,” World Journal of Gastroenterology 18, no. 48 (2012): 7378–7383, 10.3748/wjg.v18.i48.7378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jiang C., Xu Q., Wen X., and Sun H., “Current Developments in Pharmacological Therapeutics for Chronic Constipation,” Acta Pharmaceutica Sinica B 5, no. 4 (2015): 300–309, 10.1016/j.apsb.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Barrett K. E. and Keely S. J., “Chloride Secretion by the Intestinal Epithelium: Molecular Basis and Regulatory Aspects,” Annual Review of Physiology 62, no. 1 (2000): 535–572, 10.1146/annurev.physiol.62.1.535. [DOI] [PubMed] [Google Scholar]
- 16. Li F., Fu T., Tong W. D., et al., “Lubiprostone Is Effective in the Treatment of Chronic Idiopathic Constipation and Irritable Bowel Syndrome: A Systematic Review and Meta‐Analysis of Randomized Controlled Trials,” Mayo Clinic Proceedings 91, no. 4 (2016): 456–468, 10.1016/j.mayocp.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 17. Barish C. F., Drossman D., Johanson J. F., and Ueno R., “Efficacy and Safety of Lubiprostone in Patients With Chronic Constipation,” Digestive Diseases and Sciences 55 (2010): 1090–1097, 10.1007/s10620-009-1068-x. [DOI] [PubMed] [Google Scholar]
- 18.“Lubiprostone: RU 0211, SPI 0211,” Drugs in R&D 6, no. 4 (2005): 245–248, 10.2165/00126839-200506040-00009. [DOI] [PubMed] [Google Scholar]
- 19. Passos M. D., Takemoto M. L., Corradino G. C., and Guedes L. S., “Systematic Review With Meta‐Analysis: Lubiprostone Efficacy on the Treatment of Patients With Constipation,” Arquivos de Gastroenterologia 57, no. 4 (2020): 498–506, 10.1590/S0004-2803.202000000-83. [DOI] [PubMed] [Google Scholar]
- 20. Xiao Y. L., Dai N., He S. X., et al., “Efficacy and Safety of Lubiprostone for the Treatment of Functional Constipation in Chinese Adult Patients: A Multicenter, Randomized, Double‐Blind, Placebo‐Controlled Trial,” Journal of Digestive Diseases 22, no. 11 (2021): 622–629, 10.1111/1751-2980.13058. [DOI] [PubMed] [Google Scholar]
- 21. Coss‐Adame E., Remes‐Troche J. M., Rendón R. F., de la Cuesta J. T., and Díaz M. V., “Efficacy and Safety of Lubiprostone for the Treatment of Chronic Idiopathic Constipation: A Phase 3, Randomized, Placebo‐Controlled Study,” Revista de Gastroenterología de México (English Edition) 89, no. 1 (2024): 70–79, 10.1016/j.rgmxen.2023.05.006. [DOI] [PubMed] [Google Scholar]
- 22. Benninga M. A., Hussain S. Z., Sood M. R., et al., “Lubiprostone for Pediatric Functional Constipation: Randomized, Controlled, Double‐Blind Study With Long‐Term Extension,” Clinical Gastroenterology and Hepatology 20, no. 3 (2022): 602–610, 10.1016/j.cgh.2021.04.005. [DOI] [PubMed] [Google Scholar]
- 23. Page M. J., McKenzie J. E., Bossuyt P. M., et al., “The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews,” BMJ 372 (2021): 71, 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Furuya‐Kanamori L., Barendregt J. J., and Doi S. A. R., “A New Improved Graphical and Quantitative Method for Detecting Bias in Meta‐Analysis,” International Journal of Evidence‐Based Healthcare 16 (2018): 195–203. [DOI] [PubMed] [Google Scholar]
- 25. Spierings E. L., Drossman D. A., Cryer B., et al., “Efficacy and Safety of Lubiprostone in Patients With Opioid‐Induced Constipation: Phase 3 Study Results and Pooled Analysis of the Effect of Concomitant Methadone Use on Clinical Outcomes,” Pain Medicine 19, no. 6 (2018): 1184–1194, 10.1093/pm/pnx156. [DOI] [PubMed] [Google Scholar]
- 26. Johanson J. F., Morton D., Geenen J., and Ueno R., “Multicenter, 4‐Week, Double‐Blind, Randomized, Placebo‐Controlled Trial of Lubiprostone, a Locally‐Acting Type‐2 Chloride Channel Activator, in Patients With Chronic Constipation,” American Journal of Gastroenterology 103, no. 1 (2008): 170–177, 10.1111/j.1572-0241.2007.01524.x. [DOI] [PubMed] [Google Scholar]
- 27. Johanson J. F. and Ueno R., “Lubiprostone, a Locally Acting Chloride Channel Activator, in Adult Patients With Chronic Constipation: A Double‐Blind, Placebo‐Controlled, Dose‐Ranging Study to Evaluate Efficacy and Safety,” Alimentary Pharmacology and Therapeutics 25, no. 11 (2007): 1351–1361, 10.1111/j.1365-2036.2007.03320.x. [DOI] [PubMed] [Google Scholar]
- 28. Johanson J. F., Drossman D. A., Panas R., Wahle A., and Ueno R., “Clinical Trial: Phase 2 Study of Lubiprostone for Irritable Bowel Syndrome With Constipation,” Alimentary Pharmacology and Therapeutics 27, no. 8 (2008): 685–696, 10.1111/j.1365-2036.2008.03629.x. [DOI] [PubMed] [Google Scholar]
- 29. Jamal M. M., Adams A. B., Jansen J. P., and Webster L. R., “A Randomized, Placebo‐Controlled Trial of Lubiprostone for Opioid‐Induced Constipation in Chronic Noncancer Pain,” American Journal of Gastroenterology 110, no. 5 (2015): 725–732, 10.1038/ajg.2015.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fukudo S., Hongo M., Kaneko H., Takano M., and Ueno R., “Lubiprostone Increases Spontaneous Bowel Movement Frequency and Quality of Life in Patients With Chronic Idiopathic Constipation,” Clinical Gastroenterology and Hepatology 13, no. 2 (2015): 294–301, 10.1016/j.cgh.2014.08.026. [DOI] [PubMed] [Google Scholar]
- 31. Fukudo S., Hongo M., Kaneko H., and Ueno R., “Efficacy and Safety of Oral Lubiprostone in Constipated Patients With or Without Irritable Bowel Syndrome: A Randomized, Placebo‐Controlled and Dose‐Finding Study,” Neurogastroenterology and Motility 23, no. 6 (2011): 544‐e205, 10.1111/j.1365-2982.2011.01668.x. [DOI] [PubMed] [Google Scholar]
- 32. Drossman D. A., Chey W. D., Johanson J. F., et al., “Clinical Trial: Lubiprostone in Patients With Constipation‐Associated Irritable Bowel Syndrome–Results of Two Randomized, Placebo‐Controlled Studies,” Alimentary Pharmacology and Therapeutics 29, no. 3 (2009): 329–341, 10.1111/j.1365-2036.2008.03881.x. [DOI] [PubMed] [Google Scholar]
- 33. Cryer B., Katz S., Vallejo R., Popescu A., and Ueno R., “A Randomized Study of Lubiprostone for Opioid‐Induced Constipation in Patients With Chronic Noncancer Pain,” Pain Medicine 15, no. 11 (2014): 1825–1834, 10.1111/pme.12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Christie J., Shroff S., Shahnavaz N., et al., “A Randomized, Double‐Blind, Placebo‐Controlled Trial to Examine the Effectiveness of Lubiprostone on Constipation Symptoms and Colon Transit Time in Diabetic Patients,” American Journal of Gastroenterology 112, no. 2 (2017): 356–364, 10.1038/ajg.2016.531. [DOI] [PubMed] [Google Scholar]
- 35. Lang L., “The Food and Drug Administration Approves Lubiprostone for Irritable Bowel Syndrome With Constipation,” Gastroenterology 135, no. 1 (2008): 7, 10.1053/j.gastro.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 36. Chey W. D., Drossman D. A., Johanson J. F., Scott C., Panas R. M., and Ueno R., “Safety and Patient Outcomes With Lubiprostone for up to 52 Weeks in Patients With Irritable Bowel Syndrome With Constipation,” Alimentary Pharmacology and Therapeutics 35, no. 5 (2012): 587–599, 10.1111/j.1365-2036.2011.04983.x. [DOI] [PubMed] [Google Scholar]
- 37. Chang L., Chey W. D., Drossman D., et al., “Effects of Baseline Abdominal Pain and Bloating on Response to Lubiprostone in Patients With Irritable Bowel Syndrome With Constipation,” Alimentary Pharmacology and Therapeutics 44, no. 10 (2016): 1114–1122, 10.1111/apt.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Candy B., Jones L., Goodman M. L., Drake R., and Tookman A., “Laxatives or Methylnaltrexone for the Management of Constipation in Palliative Care Patients,” Cochrane Database of Systematic Reviews 19, no. 1 (2011): CD003448, 10.1002/14651858.CD003448.pub3. [DOI] [PubMed] [Google Scholar]
- 39. Lexicomp® , “Lubiprostone: Drug Information,” UpToDate Inc n.d. accessed June 12, 2024, https://www.uptodate.com.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1: Doi plot of SBM Per week (CIC).
Supplementary Figure S2: Doi plot of SBM within 24 h (CIC).
Supplementary Figure S3: Doi plot of Treatment Related Abdominal Pain (CIC).
Supplementary Figure S4: Doi plot of SBM within 24 h (OIC).
Supplementary Figure S5: Doi plot of Treatment Related Abdominal Pain (OIC).
Supplementary Table S1: Detailed Search Strategy of Each Database.
Data Availability Statement
The data are available upon reasonable request from the corresponding author.


