Abstract
High-resolution anorectal manometry (HR-ARM) is the gold standard for anorectal functional disorders’ evaluation, despite being limited by its accessibility and complex data analysis. The London Protocol and Classification were developed to standardize anorectal motility patterns classification. This proof-of-concept study aims to develop and validate an artificial intelligence model for identification and differentiation of disorders of anal tone and contractility in HR-ARM. A dataset of 701 HR-ARM exams from a tertiary center, classified according to London Classification, was used to develop and test multiple machine learning (ML) algorithms. The exams were divided in a training and testing dataset with a 80/20% ratio. The testing dataset was used for models’ evaluation through its accuracy, sensitivity, specificity, positive and negative predictive values and area under the receiving-operating characteristic curve. LGBM Classifier had the best performance, with an accuracy of 87.0% for identifying disorders of anal tone and contractility. Different ML models excelled in distinguishing specific disorders of anal tone and contractility, with accuracy over 90.0%. This is the first worldwide study proving the accuracy of a ML model for differentiation of motility patterns in HR-ARM, demonstrating the value of artificial intelligence models in optimizing HR-ARM availability while reducing interobserver variability and increasing accuracy.
Keywords: Anorectal disorders, Anorectal manometry, Artificial intelligence, Gastroenterology, Machine learning
Subject terms: Gastrointestinal diseases, Gastrointestinal system, Gastroenterology, Digestive signs and symptoms
Introduction
Functional anorectal disorders impose a significant socioeconomic burden, leading to healthcare costs and morbidity, impacting the quality of life of up to 5% of the population1. The diagnosis of these disorders can be challenging, combining symptoms and anorectal functional tests, often resulting in diagnostic delays and high disease morbidity2.
In recent years, the physiology of defecation and continence, along with associated disorders, have undergone significant evolution3. In fact, a myriad of diagnostic tools for assessing anorectal function were developed, with anorectal manometry (ARM) remaining the most widely used technique4. Technological advancements in ARM led to the development of high-resolution anorectal manometry (HR-ARM), with closely spaced sensors, a color contour display and better spatiotemporal resolution, therefore assuring more accurate diagnosis and comprehension of anorectal pathophysiology5. However, progress is hindered by the limited accessibility of the procedure, which is not uniformly available across all centers, and by the complexity of the data analysis involved. These characteristics result in high intra and interobserver variability.
In the past years, a consensus classification—the London Classification for Disorders of Anorectal Function – has come to facilitate the use of objective measures to reach a diagnosis while simultaneously standardizing both the HR-ARM protocol and anorectal motility pattern classification6. The London Classification of anorectal disfunction is divided into four parts and a single study may have relevant findings in more than one part of the classification. In fact, the London classification focuses in identifying disorders of rectoanal inhibitory reflex, anal tone and contractility, anorectal coordination and rectal sensation. The classification of anal tone and contractility is of great importance in the evaluation of anorectal symptoms. In fact, anal hypotension, defined as reduced anal resting pressure, and anal hypocontractility, considered as insufficient increase in anal squeeze pressure, are often implicated in functional disorders like fecal incontinence7,8.
The use of artificial intelligence (AI) in Medicine has experienced exponential growth, particularly in fields heavily reliant on imaging and big data, and Gastroenterology is not an exception9. From capsule endoscopy to upper endoscopy and colonoscopy, artificial intelligence models have proved their role in increasing the diagnostic accuracy of Gastroenterology exams10–12. In the functional studies field, there have been works about automatic identification of motility patterns in esophageal manometry13–15. However, when it comes to functional anorectal disorders, it remains uncharted territory, with only a few research articles published16.
Therefore, this study aims to develop and validate an AI model for the identification and differentiation of disorders of anal tone and contractility during HR-ARM, based on the London Classification.
Methods
Study design
A total of 701 HR-ARM exams performed at a reference center for functional anorectal disorders (Hospital Universitario La Princesa) were retrospectively reviewed for development of the model. All the procedures were performed with Solar™ GI High-Resolution Anorectal Manometry (Laborie™, Enschede, The Netherlands). Data from these examinations were retrieved after analysis and consensus by 2 experts in ARM. In cases of disagreement between the experts, a third expert was consulted to achieve the final diagnosis. Our study respected the Declaration of Helsinki and was developed with a non-interventional nature. The study was approved by the ethics committee of Hospital Universitario La Princesa (No. 4977, October 6th, 2022). Omission of potentially identifying information of the subjects was ensured and each patient received a random number assignment to obtain effective data anonymization for researchers involved in the CNN. A legal team with Data Protection Officer (DPO) certification was responsible for the non-traceability of the data in conformity with general data protection regulation (GDPR).
High resolution anorectal manometry procedure
All HR-ARM procedures were performed with Solar™ GI High-Resolution Anorectal Manometry HRAM Water Perfused Catheters, with either 8, 12 or 24 single use pressure channels, MMS G-90550. (Laborie™, Enschede, The Netherlands. All the procedures were performed in order to fulfill the steps of the International Anorectal Physiology Working Group (IAPWG) protocol and London classification6. Bowel preparation was generally not necessary, but occasionally a tap water enema administered the day before the test was used, at the discretion of the prescribing physician The exam was initially performed in left lateral decubitus. After insertion, a 3-min stabilization period took place, with measurement of resting pressure for 60 s (in order to determine anal resting pressure). Subsequently, the patient was asked to take thrice a 5 s short squeeze (measuring the squeeze pressure). A 60-s squeeze took place to measure the endurance squeeze time, before asking the patient to cough twice (with a 30-s interval). Later, the patient was asked to perform a 15 s push maneuver thrice with 30-s interval between them. This maneuver was used to evaluate the variation in rectal and anal pressure. After that, a rectal balloon was inflated with increasing volumes, and the patient was asked to identify the first constant sensation volume, the desire to defecate volume and the maximum tolerated volume. The balloon was also inflated with increasing volumes in order to identify the physiological rectoanal inhibitory reflex. Finally, the patient was asked to expulse a previously inflated balloon. If the patient could not expulse the balloon in left lateral decubitus, the maneuver was repeated in the sitting position. All the exams were classified according to the London classification for anorectal disorders.
Our group focused on developing a model capable of identifying and accurately differentiating disorders of anal tone and contractility according to the London Classification for functional anorectal disorders. The later included anal hypertension, anal hypotension with normal contractility, anal normotension with hypocontractility, and combined anal hypotension with hypocontractility. Anal hypertension was defined if the anal resting pressure was above the upper limit of normal for gender and age. On the other hand, anal hypotension was based on an anal resting pressure under the lower limit of normality. If anal squeeze pressure was under the normal limit of normality, the patient was classified as having hypocontractility.
Data acquisition and preparation
A total of 701 HR-ARM anonymized exams went for independent review by two gastroenterologists. In case of disagreement (98 out of 701 exams), a third gastroenterologist was consulted to review the cases and make the final diagnosis. Each reviewer had access to the report generated by Solar™ GI High-Resolution Anorectal Manometry (Laborie™) with annotations and conclusions. Each exam was labelled according to the major and minor findings of disorders of anal tone and contractility in London Classification, namely: “Anal Hypertension” (anal resting pressure above the upper limit of normality), “ Anal Normotension with Contractility” (anal resting pressure within the normal range and anal squeeze pressure under the lower limit of normality), “Anal Hypotension with Normal Contractility” (anal resting pressure under the lower limit of normality and anal squeeze pressure within the normal range) and “Combined Anal Hypotension and Hypocontractility (anal resting pressure under the lower limit of normality and anal squeeze pressure under the lower limit of normality). Each finding was classified as “Yes”, “No” or “Inconclusive” by two gastroenterologists, with a review by a third expert gastroenterologist in cases of discordance. The classification “Disorder of Anal Tone and Contractility” was considered positive in cases where at least a specific disorder was present.
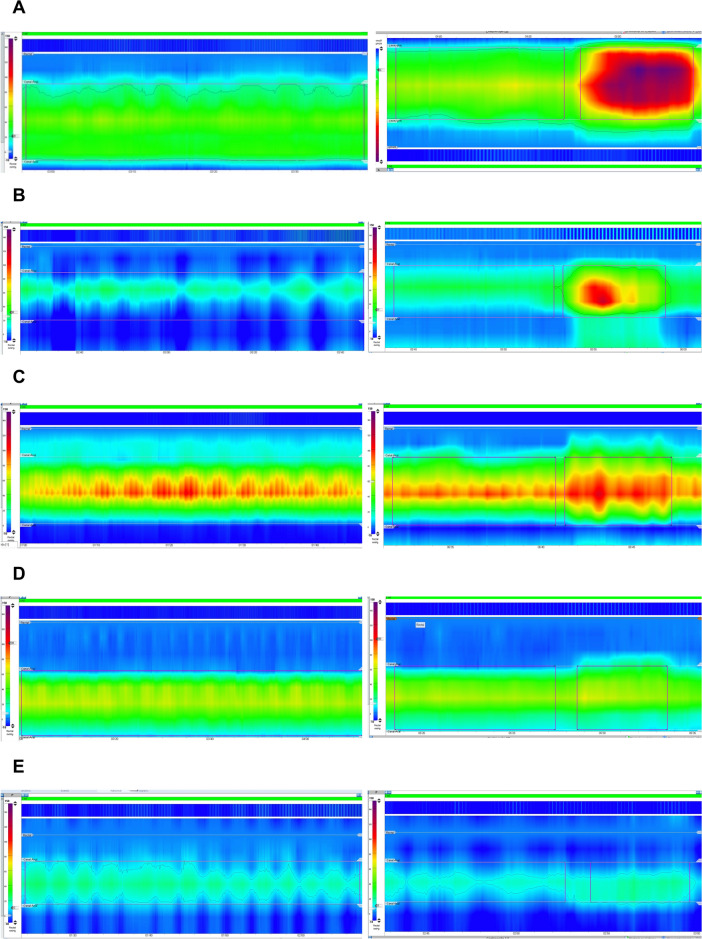
To classify the presence or absence of disorders of anal tone and contractility, the tabular time series data of both the 60-s resting period and squeeze maneuvers was extracted from each exam (Fig. 1). To ensure maneuver initial data points, the 10 s immediately before the squeeze maneuvers were included. We targeted the pressure signals of each exam/maneuver. The resulting data set comprises a set of collected signal features along with the target value. All data splits used for model training were stratified by the target value.
Fig. 1.
Evaluation of the resting pressure and squeeze maneuver, with examples of a normal exam (A); anal hypotension with normal contractility (B); anal hypertension (C); anal normotension with hypocontractility (D); combined anal hypotension and hypocontractility (E).
Model selection and tuning
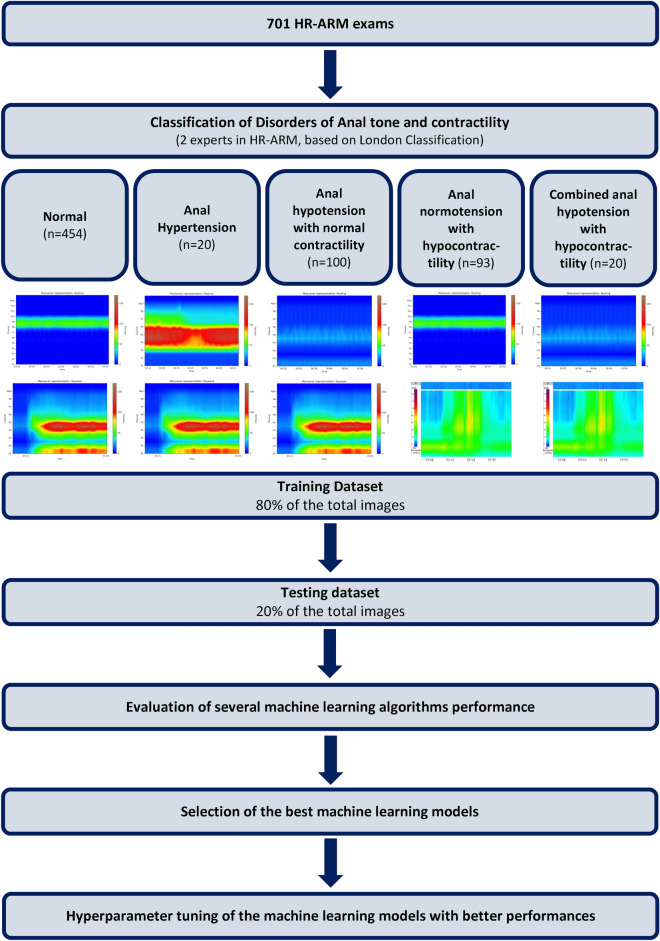
The study aimed to select the model that best identified “Disorders of Anal Tone and Contractility”. Additionally, this study aimed to specifically identify patterns of “Anal Hypertension”, “Anal Normotension with Hypocontractility”, “Anal Hypotension with Normal Contractility” and “Combined Anal Hypotension and Hypocontractility”. Thus, our group trained multiple machine learning (ML) classifiers to understand which best fits our dataset. In this context 80% of the exams were used in the training phase, while the remaining 20% of the exams (using data stratification) were used for evaluating model’s performance. Our group trained XGBClassifier, LGBMClassifier AdaBoostClassifier, GaussianNB, BernoulliNB, BaggingClassifier, Perceptron, DecisionTreeClassifier, ExtraTreesClassifier, RandomForestClassifier, LogisticRegression, SGDClassifier, NearestCentroid, ExtraTreeClassifier, SVC, PassiveAggressiveClassifier, LinearSVC, KNeighborsClassifier, LinearDiscriminantAnalysis, RidgeClassifierCV, RidgeClassifier, QuadraticDiscriminantAnalysis, LabelSpreading, LabelPropagation, CalibratedClassifierCV. In order to establish baseline comparisons our group used a Dummy classifier. Figure 2 summarizes the study design.
Fig. 2.
Study design for the detection and differentiation of disorders of anal tone and contractility in HR-ARM exams. HR-ARM–high resolution anorectal manometry.
Additionally, our group has split the data and trained these models 10 times to select the top 5 based on weighted F1-score and area under the receiver operating curve (AUC-ROC) metrics. To find the model architecture that best suits the data, the top 3 models were fine-tuned using a genetic algorithm hyperparameter search. Finally, the architecture of the best-fine-tuned model was selected and used to train the classifier five times.
The analyses were performed with a computer equipped with a 2.1-GHz Intel Xeon Gold 6130 processor (Intel, Santa Clara, CA) and a double NVIDIA Quadro RTX 8000 graphic processing unit (NVIDIA, Santa Clara, CA).
Model performance and statistical analysis
The models were evaluated through their accuracy, F1-weight score, sensitivity, specificity, positive and negative predictive values, in differentiating disorders of anal tone and contractility in HR-ARM. Furthermore, the discriminating performance of each model was evaluated using receiver operating curves. The classification produced by the models was evaluated according to the current gold standard of the diagnosis established by two expert gastroenterologists.
Results
Study population
A total of 701 HR-ARM exams were used for development of the model. Out of the total 701 HR-ARM exams, 233 patients had a disorder of anal tone and contractility, 454 had normal anal tone and contractility, and 14 had an inconclusive exam. On the other hand, our dataset had 20 exams that fulfilled the criteria for anal hypertension, 100 with anal hypotension with normal contractility, 93 with anal normotension with hypocontractility, and 20 with combined anal hypotension with hypocontractility.
Models’ accuracies during fivefold stratified training
Table 1 describes the overall performance of the 5 ML algorithms that achieved the best results in identifying disorders of anal tone and contractility. The LGBMClassifier was the model that achieved better performance, with a F1 weight score of 85.0%. All tested ML algorithms outperformed the dummy model.
Table 1.
F1 weight score and area under the ROC curve of the best machine learning models for detection of disorders of anal tone and contractility.
| Model | F1 weight score (Mean ± SD) | Area under the ROC curve (Mean ± SD) |
|---|---|---|
| LGBMClassifier | 85.0 ± 3.5 | 82.0 ± 4.3 |
| XGBClassifier | 84.5 ± 2.7 | 80.5 ± 4.7 |
| RandomForestClassifier | 83.0 ± 3.2 | 79.5 ± 4.3 |
| ExtraTreesClassifier | 82.0 ± 3.5 | 77.0 ± 4.1 |
| AdaBoostClassifier | 78.5 ± 3.8 | 74.5 ± 4.3 |
SD: standard deviation, ROC curve: receiver operating characteristic curve.
Hyperparameter tuning and models’ performance
To achieve maximal performance, the hyperparameters of the best-fine-tuned model (LGBMClassifier) were tuned 5 times. LGBMClassifier achieved an accuracy of 87.0% and a F1 weight score of 85.0% for identification of disorders of anal tone and contractility.
Differentiation of specific patterns of disorders of anal tone and contractility
Our group trained different ML algorithms to differentiate specific disorders of anal tone and contractility, namely: anal hypotension with normal contractility, combined anal hypotension and hypocontractility, anal normotension with hypocontractility and anal hypertension. Table 2 summarizes the ML models that achieved the best performance in detecting specific disorders of anal tone and contractility. LGBMClassifier identified anal hypotension with normal contractility with a F1 weight score of 91.0%. On the other hand, AdaBoostClassifier had a F1 weight score of 97.0% for combined anal hypotension and hypocontractility. Anal normotension with hypocontractility was detected with 90.0% F1 weight score by LGBM Classifier. Finally, DecisionTreeClassifier excelled in the detection of anal hypertension, with a F1 weight score of 97.0%.
Table 2.
Accuracy of the best machine learning model after fine-tuning for identification of specific disorders of anal tone and contractility.
| Disorder | Model | F1 weight score (Mean ± SD) |
|---|---|---|
| Anal hypotension with normal contractility | LGBMClassifier | 91.0 ± 2.4 |
| Combined anal hypotension and hypocontractility | AdaBoostClassifier | 97.0 ± 0.7 |
| Anal normotension with hypocontractility | LGBMClassifier | 90.0 ± 2.1 |
| Anal hypertension | DecisionTreeClassifier | 97.0 ± 0.4 |
SD: standard deviation.
Discussion
The increasing integration of AI across Medicine is prompting individuals to adapt and transform their work practices17. Gastroenterology is not an exception to this trend. In fact, the research and development of AI models in Gastroenterology, using numerous methodologies, is increasing exponentially. These developments are driven by the need to address various challenges, such as resource management and providing high-quality patient care17.
To the best of our knowledge, this is the first study to evaluate the performance of ML models in the automatic identification and differentiation of disorders of anal tone and contractility in HR-ARM by using the London classification. It should be noted that two studies have already been conducted using AI models and anorectal manometry; however, they used different types of ARM. One study used conventional ARM, while the other one used 3-dimensional high-definition anal manometry16,18. Additionally, both studies were performed with ARM exams that did not fulfill the London Classification protocol, and the results may not be replicable to the most current practice for HR-ARM exams.
Functional anorectal disorders are a significant health problem with high economical burden19. The Rome IV diagnostic criteria for disorders of gut-brain interaction focus in achieving a more accurate and replicable diagnosis of functional disorders, namely anorectal functional disorders20. Nevertheless, a symptomatic approach is insufficient to accurately understand the pathophysiology of functional anorectal disorders, which is essential for deciding the most appropriate treatment. HR-ARM, like previously explained, allows for the diagnosis and monitoring of functional anorectal disorders by measuring the tone and coordination of anorectal muscles. The London Protocol and Classification was created to standardize the classification and, therefore, reduce the interobserver variability in anorectal functional disorders21. Nevertheless, significant interobserver variability subsists, and the intrinsic limitations of the exam hinder its widespread approach, limiting patient access to the best diagnosis and treatment for functional anorectal disorders.
The present study focused in accurately identifying disorders of anal tone and contractility in HR-ARM exams. In fact, anal hypotension and anal hypocontractility are commonly implied in fecal incontinence7. Indeed, fecal incontinence is associated with anal sphincter disfunction, which can be objectively identified with HR-ARM22. In the matter of fact, anal resting pressure traduces the internal anal sphincter function, whereas squeeze pressure reflects external anal sphincter function. Therefore, the Part II of London Classification is a measure of the internal and external anal sphincter function, and accurate identification of these disorders is of utmost importance for guiding clinical practice.
In fact, HR-ARM poses challenges for most of the Gastroenterology community. The results’ interpretation is difficult and time-consuming. Additionally, interobserver variability hinders its diagnostic accuracy, impacting data acquisition, analysis and interpretation.23 These challenges exam´s accessibility unequal across different centers, an issue that has been under efforts to change for years.24 AI has the potential to enhance accessibility to this exam by facilitating the evaluation of the data and, perhaps, making it more cost-efficient. Furthermore, interoperability, a key point of interest, can be achieved using AI models.
This study has some highlights that deserve acknowledgement, especially the methodology involved in it and the results obtained. This study assesses the performance of multiple ML algorithms classifiers in HR-ARM. It also used a large data set comprising 701 HR-ARM exams, independent review by two gastroenterologists, performed at worldwide reference center in functional anorectal disorders, with all exams performed in accordance with London Classification and Protocol for Disorders of Anorectal Function.
The model was trained using 80% of the dataset, and its performance was evaluated using the remaining 20%. It was ensured a balanced distribution of the exams through stratification. After training the models, the ones with better results were fine-tuned to obtain high-performance results. In this context, LGBM Classifier identified disorders of anal tone and contractility with an accuracy of 87.0%. These results suggest that AI algorithms will most certainly assist Digestive Health experts in obtaining the right diagnosis for differentiation of disorders of tone and contractility. We consider that this work offers great advantages to achieve quality and rapid training in the acquisition of knowledge in Neurogastroenterology and Motility, considering the training objectives in this area of knowledge25.
Despite the outstanding results, it is important to discuss the limitations of HR-ARM. Indeed, results are directly influenced by both patient cooperation and anatomic variations, depending heavily on the medical team to interpret them. The subjective nature of motility exams interpretation creates the need for a reliable AI model. In fact, ML models are commonly understood as black box models, as a significant part of the decision process is not fully understood by the clinician26,27. In this context, the concept of explainable AI has been gaining interest in the development of AI models in Gastroenterology28. Indeed, the incomprehension in the decision process of an AI model limits the tolerance to machine error. In this context, is specifically important to create a trustworthy and explainable model, that promotes confidence through understanding of its decision proces29.
Nevertheless, the development of explainable AI models is specifically difficult in functional disorders, in line with the subjectiveness of the exam findings. The developed classifications for functional esophageal and anorectal disorders, the Chicago and London classifications, have appeared as expert consensus that aim to standardize both the exam protocol and interpretation6,30. Despite, there is still a significant variability in exam interpretation. Additionally, despite software capacity to automate measurement and classification of values such as the resting or contraction pressure, these values are sustainable to patient cooperation and operator marking interference, being error prone. Therefore, AI appears as a solution for augmenting diagnostic accuracy while standardizing exam interpretation. Our group believes that explainable AI is a necessary step for this implementation, and the following studies will focus on the development of specific explainable AI mechanisms to increase the trustworthiness and reliability of our model.
Some drawbacks should be acknowledged in this study. Firstly, the research study was conducted data HR-ARM data from a single center. In order to address the interoperability challenge, the incorporation of a large dataset from different HR-ARM devices is fundamental to develop a model proficient in every device. Secondly, it is important to consider the inclusion of different centers with distinct demographic contexts, with the aim of developing a model that is accurate in different ethnic and geographical contexts, ultimately diminishing the impact of demographic bias. Thirdly, the models only assess Part II of the London Classification, which limits their applicability in clinical practice. Despite the promising results observed, the next step of our work, will be the evaluating a model capable of providing automatic prediction for all parts of the London Classification. Considering the integration of clinical data, the automatic detection and differentiation of motility patterns is the first phase in AI-enhanced HR-ARM. Ultimately, future studies will focus on incorporating patient symptoms, medical history, age, and gender into multimodal AI prediction tools (as the same manometry finding can have a completely different interpretation depending on whether it is observed in a young male without a relevant medical history or a sexagenarian female with a history of complicated labor). Nevertheless, a first step must be made in the incorporation of AI into the anorectal motility field, and this study constitutes the first landmark in the process. Future studies will focus on multicenter, multi-device models, capable not only of interpreting data from all parts of the London Classification but also of including patient clinical data in the decision-making, transforming the analysis, availability, and accuracy of HR-ARM.
In conclusion, this is the first worldwide proof-of-concept study that aims to develop and validate an AI model for the identification and differentiation of disorders of anal tone and contractility in HR-ARM. Our group believes that AI-assisted HR-ARM can revolutionize the way diagnostics are conducted and favor a widespread of this exam, enabling equal access to healthcare.
Author contributions
M.M. (Miguel Mascarenhas) and F.M.: equal contribution in study design, image extraction, drafting of the manuscript, and critical revision of the manuscript. J.M., T.R., P.C., M.M. (Miguel Martins), M.J.A.: bibliographic review, image extraction, critical revision of the manuscript. J.C., J.F.: construction and development of the machine learning models, statistical analysis, critical revision of the manuscript. G.M., C.S.: study design, critical revision of the manuscript. All authors approved the final version of the manuscript.
Data availability
If the reader needs some data, please contact the corresponding author. A portion of the data may not be provided, in order to respect the Declaration of Helsinki principles and general data protection regulation.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Miguel Mascarenhas and Francisco Mendes contributed equally to the work.
References
- 1.Heitmann, P. T. et al. Understanding the physiology of human defaecation and disorders of continence and evacuation. Nat. Rev. Gastroenterol. Hepatol.18, 751–769. 10.1038/s41575-021-00487-5 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Rao, S. S. et al. Functional anorectal disorders. Gastroenterology10.1053/j.gastro.2016.02.009 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Bharucha, A. E. et al. Review of the indications, methods, and clinical utility of anorectal manometry and the rectal balloon expulsion test. Neurogastroenterol. Motil.34, e14335. 10.1111/nmo.14335 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heinrich, H. & Misselwitz, B. High-resolution anorectal manometry - new insights in the diagnostic assessment of functional anorectal disorders. Visc. Med.34, 134–139. 10.1159/000488611 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bredenoord, A. J. & Hebbard, G. S. Technical aspects of clinical high-resolution manometry studies. Neurogastroenterol. Motil.24(Suppl 1), 5–10. 10.1111/j.1365-2982.2011.01830.x (2012). [DOI] [PubMed] [Google Scholar]
- 6.Carrington, E. V. et al. The international anorectal physiology working group (IAPWG) recommendations: Standardized testing protocol and the London classification for disorders of anorectal function. Neurogastroenterol. Motil.32, e13679. 10.1111/nmo.13679 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rasijeff, A. M. P. et al. Systematic review and meta-analysis of anal motor and rectal sensory dysfunction in male and female patients undergoing anorectal manometry for symptoms of faecal incontinence. Colorectal Dis.24, 562–576. 10.1111/codi.16047 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carrington, E. V., Knowles, C. H., Grossi, U. & Scott, S. M. High-resolution anorectal manometry measures are more accurate than conventional measures in detecting anal hypocontractility in women with fecal incontinence. Clin. Gastroenterol. Hepatol.17, 477-485.e479. 10.1016/j.cgh.2018.06.037 (2019). [DOI] [PubMed] [Google Scholar]
- 9.Kroner, P. T. et al. Artificial intelligence in gastroenterology: A state-of-the-art review. World J. Gastroenterol.27, 6794–6824. 10.3748/wjg.v27.i40.6794 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mascarenhas, M. et al. Deep learning and minimally invasive endoscopy: Automatic classification of pleomorphic gastric lesions in capsule endoscopy. Clin. Transl. Gastroenterol.14, e00609. 10.14309/ctg.0000000000000609 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Popovic, D. et al. The importance of artificial intelligence in upper gastrointestinal endoscopy. Diagnostics (Basel)10.3390/diagnostics13182862 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hassan, C. et al. Performance of artificial intelligence in colonoscopy for adenoma and polyp detection: A systematic review and meta-analysis. Gastrointest. Endosc.93, 77–85. 10.1016/j.gie.2020.06.059 (2021). [DOI] [PubMed] [Google Scholar]
- 13.Fass, O., Rogers, B. D. & Gyawali, C. P. Artificial intelligence tools for improving manometric diagnosis of esophageal dysmotility. Curr. Gastroenterol. Rep.26, 115–123. 10.1007/s11894-024-00921-z (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Popa, S. L. et al. Automatic diagnosis of high-resolution esophageal manometry using artificial intelligence. J. Gastrointestin. Liver Dis.31, 383–389. 10.15403/jgld-4525 (2022). [DOI] [PubMed] [Google Scholar]
- 15.Kou, W. et al. Deep learning-based artificial intelligence model for identifying swallow types in esophageal high-resolution manometry. Neurogastroenterol. Motil.34, e14290. 10.1111/nmo.14290 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saraiva, M. M. et al. Artificial intelligence and anorectal manometry: Automatic detection and differentiation of anorectal motility patterns-a proof-of-concept study. Clin. Transl. Gastroenterol.14, e00555 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhagat, S. V. & Kanyal, D. Navigating the future: The transformative impact of artificial intelligence on hospital management- a comprehensive review. Cureus16, e54518. 10.7759/cureus.54518 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levy, J. J. et al. Video-based deep learning to detect dyssynergic defecation with 3D high-definition anorectal manometry. Dig. Dis. Sci.68, 2015–2022. 10.1007/s10620-022-07759-3 (2023). [DOI] [PubMed] [Google Scholar]
- 19.Nag, A. et al. The humanistic and economic burden of chronic idiopathic constipation in the USA: A systematic literature review. Clin. Exp. Gastroenterol.13, 255–265. 10.2147/ceg.S239205 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drossman, D. A. & Hasler, W. L. Rome IV-functional GI disorders: Disorders of gut-brain interaction. Gastroenterology150, 1257–1261. 10.1053/j.gastro.2016.03.035 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Scott, S. M. & Carrington, E. V. The London classification: Improving characterization and classification of anorectal function with anorectal manometry. Curr. Gastroenterol. Rep.22, 55. 10.1007/s11894-020-00793-z (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Townsend, D. C. et al. Pathophysiology of fecal incontinence differs between men and women: A case-matched study in 200 patients. Neurogastroenterol. Motil.28, 1580–1588. 10.1111/nmo.12858 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Carrington, E. V. et al. Methods of anorectal manometry vary widely in clinical practice: Results from an international survey. Neurogastroenterol Motil29, e13016. 10.1111/nmo.13016 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Kruk, M. E. et al. High-quality health systems in the sustainable development goals era: Time for a revolution. Lancet Glob. Health6, e1196–e1252. 10.1016/s2214-109x(18)30386-3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gyawali, C. P. et al. Curriculum for neurogastroenterology and motility training: A report from the joint ANMS-ESNM task force. Neurogastroenterol. Motil.30, e13341. 10.1111/nmo.13341 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Poon, A. I. F. & Sung, J. J. Y. Opening the black box of AI-medicine. J. Gastroenterol. Hepatol.36, 581–584. 10.1111/jgh.15384 (2021). [DOI] [PubMed] [Google Scholar]
- 27.Mascarenhas, M. et al. The future of minimally invasive capsule panendoscopy: Robotic precision, wireless imaging and AI-driven insights. Cancers (Basel)10.3390/cancers15245861 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dong, Z. et al. Explainable artificial intelligence incorporated with domain knowledge diagnosing early gastric neoplasms under white light endoscopy. NPJ Digit. Med.6, 64. 10.1038/s41746-023-00813-y (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mascarenhas, M. et al. Smart endoscopy is greener endoscopy: Leveraging artificial intelligence and blockchain technologies to drive sustainability in digestive health care. Diagnostics (Basel)10.3390/diagnostics13243625 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fox, M. R. et al. Chicago classification version 4.0((c)) technical review: Update on standard high-resolution manometry protocol for the assessment of esophageal motility. Neurogastroenterol. Motil.33, e14120. 10.1111/nmo.14120 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
If the reader needs some data, please contact the corresponding author. A portion of the data may not be provided, in order to respect the Declaration of Helsinki principles and general data protection regulation.