Abstract
An increasing number of superficial non-ampullary duodenal epithelial tumors (SNADETs) have been detected recently owing to the development of endoscopic imaging technology and increased awareness of this disease. Endoscopic resection is the first-line treatment for SNADETs, with methods including cold snare polypectomy (CSP), conventional endoscopic mucosal resection (cEMR), underwater EMR (uEMR), and endoscopic submucosal dissection (ESD). Here, we review the current status and recent advances in endoscopic resection for SNADETs. Endoscopic resection in the duodenum is more difficult and has a higher risk of adverse events than that in other organs owing to specific anatomical disadvantages. SNADETs ≤10 mm in size are candidates for CSP, cEMR, and uEMR. Among these lesions, suspected carcinoma lesions should not be treated using CSP because of their low curability. cEMR or uEMR is considered for lesions sized 10 to 20 mm, whereas piecemeal EMR or ESD is considered for tumors >20 mm in size. In particular, ESD or surgical resection should be considered for suspected carcinoma lesions >30 mm in size. The treatment plan should be selected on a case-to-case basis, considering the balance between the risk of adverse events and the necessity of en bloc resection.
Keywords: Cold snare polypectomy, Duodenal neoplasms, Endoscopic mucosal resection, Endoscopic submucosal dissection
INTRODUCTION
Superficial non-ampullary duodenal epithelial tumors (SNADETs) are defined as tumors originating from the non-ampullary region of the duodenum and consist of dysplastic glandular epithelium. SNADETs are uncommon, with an estimated prevalence of 0.3% to 4.6%.1,2 Recently, the detection rate of SNADETs has been increasing owing to the widespread use of health checkup endoscopy, development of endoscopic imaging technology, and increased awareness of this disease.3 Approximately 60% of SNADETs occur in patients with familial adenomatous polyposis (FAP), and sporadic occurrence is rare.4 The natural history of SNADETs is not well understood owing to their rarity; nevertheless, SNADETs are considered precancerous lesions because the adenoma-adenocarcinoma sequence has been reported in the duodenum.5 Therefore, early diagnosis and management are essential.
Previously, the traditional treatment strategy for SNADETs was radical surgical resection, such as pancreaticoduodenectomy. However, endoscopic resection is currently regarded as the first-line treatment for SNADETs owing to the high risk of morbidity and mortality associated with duodenal surgical resection.6 Endoscopic resection methods include cold snare polypectomy (CSP), conventional endoscopic mucosal resection (cEMR), underwater EMR (uEMR), and endoscopic submucosal dissection (ESD).7
The duodenum has specific anatomical features, causing it to be one of the most dangerous and difficult areas in the digestive tract for endoscopic procedures.8 Furthermore, most endoscopists have limited experience in endoscopic resection in the duodenum, contrasting with the extensive experience in such procedures in the colon; therefore, outcomes of endoscopic resection for SNADETs vary by country and institution.8 For these reasons, strategies for endoscopic resection of SNADETs have not yet been standardized. Recently, the European Society of Gastrointestinal Endoscopy (ESGE) issued the first clinical practice guidelines for SNADETs.9 Herein, we reviewed the current status and recent advances in endoscopic resection for SNADETs.
CONSIDERATIONS BEFORE ENDOSCOPIC RESECTION OF SNADETS
1. Anatomical features of the duodenum
Endoscopic resection in the duodenum is more difficult and has a higher risk of adverse events compared with other organs owing to specific duodenal anatomical features, including: (1) narrow and tortuous duodenal lumen, which restricts the reverse method of manipulation; (2) rich blood supply; (3) abundant Brunner’s gland in the submucosal layer, leading to difficult mucosal lifting; (4) a thin proper muscular layer, which is associated with a high risk of perforation; (5) poor endoscope maneuverability owing to the long distance of the duodenum from the mouth and its C-loop structure; and (6) exposure to bile and pancreatic juice from the duodenal papillae, which cause more severe and fatal adverse events compared with endoscopic resection in other organs.10
2. Indication for endoscopic resection of SNADETs
Similar to the colorectal adenoma-adenocarcinoma sequence, duodenal adenoma is the precursor of duodenal carcinoma.5 Okada et al.11 reported that approximately 21% of low-grade dysplasia progressed to high-grade dysplasia or noninvasive carcinoma over a 6-month follow-up, and high-grade dysplasia diagnosed at the first biopsy and a lesion diameter of ≥20 mm were significant predictive factors for progression to adenocarcinoma. Patients with FAP are known to have a high prevalence of duodenal adenomas, and such adenomas have been reported to slowly progress to cancer.12 Sporadic non-ampullary duodenal cancers occur either by the adenoma-adenocarcinoma sequence or de novo.5
Determining which SNADET should be treated is crucial to reduce the risk of progression to invasive cancer. The ESGE recommends that all duodenal adenomas should be considered for endoscopic resection since they have a high likelihood of progressing to invasive carcinomas.9 Endoscopic resection plays a role as a treatment and diagnostic modality in SNADETs. First, endoscopic forceps biopsies have low diagnostic accuracy for SNADETs, whereas the diagnostic accuracy of endoscopic biopsy is 68% to 74%, and 20.3% of biopsy-proven adenomas are histologically diagnosed as carcinomas after resection.3,13 We previously reported a histopathologic discrepancy rate of 19.0% between endoscopic forceps biopsies and endoscopic resection specimens in SNADETs.14 In addition, preoperative endoscopic forceps biopsies may induce scarring and fibrosis in the submucosal layer, causing difficulty in subsequent endoscopic resection and necessitating conversion to more invasive methods, increasing the risk of adverse events.13,15 The ESGE suggests that if endoscopic features are suggestive of superficial duodenal adenoma, the use of biopsy for histological assessment should be limited prior to endoscopic resection.9
In addition to duodenal adenoma, early duodenal cancer without lymph node metastasis (LNM) is a good candidate for endoscopic resection.16 No established definition of early duodenal cancer or indications for endoscopic resection of early duodenal cancer regarding invasion depth and LNM risk exist. A previous study reported the lack of LNM in 34 surgically resected intramucosal duodenal cancers; however, LNM was observed in five of 12 submucosal duodenal cancers.17 Nishio et al.18 reported that among 42 patients with duodenal cancer who underwent lymph node dissection, LNM was found in none of the all 15 patients with pTis-T2.18 However, only two cases with pT1b and one with T2 were included in that study. Therefore, the authors suggested that lymph node dissection could be omitted in Tis-T1a duodenal cancers. The evidence remains weak owing to the low incidence and small number of reported cases of SNADETs; nonetheless, endoscopic resection of intramucosal non-ampullary duodenal cancer may be an ideal replacement treatment modality for surgical resection. Accordingly, all patients with duodenal cancer should undergo abdominal computed tomography before endoscopic resection to evaluate the presence of lymph node or distant metastases.
ENDOSCOPIC RESECTIONTECHNIQUES FOR SNADETS
1. Cold snare polypectomy
CSP is an endoscopic procedure that uses only a snare with mechanical strangulation, without submucosal injection or electrocautery (Fig. 1). Tappero et al.19 first described CSP of colorectal polyps, and it has become a standard procedure for small colorectal lesions <10 mm.20,21 Endoscopists have recognized the cold approach as a potential therapeutic option for SNADETs, based on the development of the cold snaring technique for colorectal lesions and the lower rate of adverse events compared with hot snare techniques.22 Regarding its advantages, CSP is easy and safe, with a low incidence of adverse events. Hamada et al.23 reported that 332 duodenal adenomas were resected using CSP in 10 FAP patients, without adverse events, except for one intraprocedural arterial bleeding that occurred during resection and was easily managed using hemoclips; most of these lesions were sized <10 mm. Okimoto et al.24 reported that intraprocedural bleeding occurred in 30 of 46 lesions (65.2%) during CSP and all cases were successfully managed using hemoclips. Other studies reported a lack of perforation or delayed bleeding after CSP (Table 1).24-26
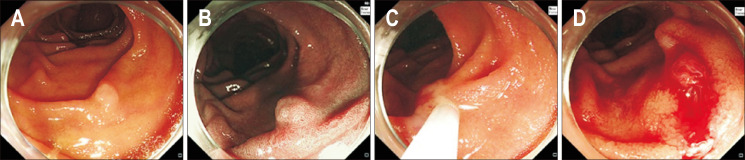
Fig. 1.
Cold snare polypectomy of a duodenal epithelial tumor. (A) A 6 mm white, slightly elevated lesion (0-IIa) located in the second portion of the duodenum. (B) Narrow-band imaging. (C) After snaring the entire lesion along with the surrounding mucosa, resection was performed without a high-frequency device. (D) The lesion was completely resected.
Table 1.
Summary of Cold Snare Polypectomy Outcomes for Duodenal Epithelial Tumors
| Year | Author | No. of lesions | Tumor size, mm | En bloc resection | R0 resection | Immediate | Delayed | Recurrence | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Perforation | Bleeding | Perforation | ||||||||
| 2017 | Maruoka et al.25 | 30 | 4 (2−6) | 29 (96.7) | 17 (68.0) | 0 | 0 | 0 | 0 | |
| 2018 | Hamada et al.23 | 332 | NA | NA | NA | 0 | 0 | 0 | NA | |
| 2022 | Takizawa et al.26 | 21 | 8 (3−10) | 17 (81.0) | NA | 0 | 0 | 0 | 1 (5.5) | |
| 2022 | Okimoto et al.24 | 37 | 4 (2−7) | 36 (97.3) | 26 (70.3) | 0 | 0 | 0 | 1 (2.7) | |
| 2022 | Kato et al.29 | 187 | 5.5±2.4 | 149 (79.1) | 64 (40.5) | 0 | 1 (0.5) | 0 | NA | |
Data are presented as median (range), number (%), or mean±SD.
NA, not applicable.
Nevertheless, CSP may have inferior curability to hot snare techniques because of the absence of electrocautery, which can eradicate neoplastic tissue around the resected site. A previous study on colorectal polyps reported that CSP had a lower resection depth and a higher incomplete resection rate than cEMR.27 Therefore, CSP should not be used for suspected carcinoma lesions. Nevertheless, despite the low rate of complete resection, several studies of CSP for SNADETs have reported a low incidence of recurrence.24-26 Notably, recurrence rates are significantly associated with lesion size and not with the resection technique.24
Therefore, CSP can be indicated for SNADETs <10 mm, although the ESGE recommends CSP for adenomas <6 mm.9 In particular, CSP can be appropriate for FAP patients with numerous and small duodenal polyps because it is simple and safe.23
2. Conventional endoscopic mucosal resection
According to the ESGE guidelines, cEMR is the first choice of endoscopic resection for SNADETs.9 cEMR involves submucosal injection to create a lift between the lesion and the muscularis propria, allowing for subsequent resection using a snare combined with electrocautery (Fig. 2). cEMR is familiar to endoscopists, and most of them can perform this procedure without difficulty.28 cEMR increases the chance of complete resection of lesions. A high-volume multicenter study showed that the en bloc and R0 resection rates of cEMR were inferior to those of ESD; however, the adverse event rate of cEMR was significantly lower than that of ESD.29 Basically, en bloc resection rates vary with lesion size. Lesions ≤20 mm can be removed en bloc using cEMR at approximately 80% to 90% (Table 2).29-38 However, en bloc resection rates of cEMR decrease in lesions >20 mm.29,36 In these cases, ESD can be considered as the first treatment to achieve a high en bloc resection rate; however, considering the high incidence of adverse events with ESD in the duodenum, 20- to 30-mm sized lesions could be acceptable for piecemeal cEMR. Piecemeal cEMR tends to increase the recurrence rate;36 nevertheless, Nonaka et al.37 reported no residual recurrence during a median follow-up period of 51 months in a population with a high rate of piecemeal cEMR. Furthermore, most local recurrences can be treated by another endoscopic procedure.29,36 The decision to use cEMR for lesions >30 mm should be carefully considered owing to considerable major adverse events and recurrence after cEMR.39 Although these adverse events have a lower incidence than those of ESD, they cannot be completely avoided. Several studies have reported higher adverse event rates after endoscopic resection in the duodenum than in other areas of the digestive tract, with bleeding and perforation occurring in approximately ~12% and ~3% of cases, respectively (Table 2). In summary, SNADETs ≤20 mm can be safely and reliably removed using cEMR.
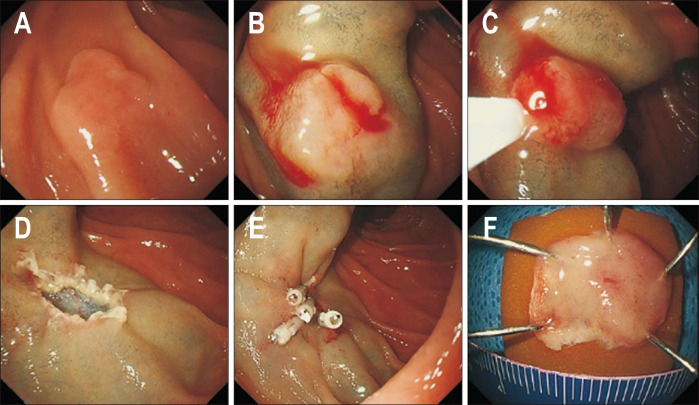
Fig. 2.
Conventional endoscopic mucosal resection of a duodenal epithelial tumor. (A) A 10 mm white, slightly elevated lesion (0-IIa) located in the second portion of the duodenum. (B) A saline solution containing small amounts of epinephrine and indigo carmine dye was injected beneath the lesion. (C) Snare resection was performed using a high-frequency device. (D) The lesion was completely resected. (E) The defect was completely closed by clipping. (F) The resected specimen.
Table 2.
Summary of Conventional Endoscopic Mucosal Resection Outcomes for Duodenal Epithelial Tumors
| Year | Author | No. of lesions | Tumor size, mm | En bloc resection | R0 resection | Immediate | Delayed | Recurrence | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Perforation | Bleeding | Perforation | ||||||||
| 2015 | Nonaka et al.37 | 113 | 12 (3−50) | 71 (62.8) | 38 (33.6) | 0 | 14 (12.4) | 0 | 0 | |
| 2017 | Hoteya et al.31 | 55 | 9.4±5.3 | 43 (78.2) | 33 (60.0) | 1 (1.8) | 4 (7.3) | 0 | 2 (3.6) | |
| 2018 | Yahagi et al.30 | 146 | 9.8±6.3 | 139 (95.2) | 123 (82.2) | 1 (0.7) | 2 (1.4) | 0 | NA | |
| 2018 | Tomizawa et al.36 | 166 | 20 (7−55) | 88 (53.0) | NA | 0 | 8 (4.8) | 0 | 32 (23.0) | |
| 2019 | Hara et al.33 | 136 | 9 (7−14) | 121 (89.0) | 93 (68.4) | 0 | 0 | 0 | 4 (2.9) | |
| 2020 | Kiguchi et al.32 | 167 | 9.9±4.1 | 161 (96.4) | 133 (79.6) | 0 | 1 (0.6) | 0 | NA | |
| 2020 | Kuroki et al.34 | 157 | 10.7±7.2 | 152 (96.8) | 141 (89.8) | 1 (0.6) | 4 (2.5) | 4 (2.5) | 2 (1.3) | |
| 2021 | Hirasawa et al.35 | 59 | 11.0±7.1 | 50 (85.8) | 42 (71.2) | 1 (1.7) | 1 (1.7) | 0 | NA | |
| 2022 | Cho et al.38 | 58 | 12 (4−20) | 39 (67.2) | 36 (62.1) | 0 | 0 | 1 (1.7) | 0 | |
| 2022 | Kato et al.29 | 1,324 | 11.3±7.7 | 1150 (86.8) | 784 (61.2) | 10 (0.8) | 35 (2.6) | 2 (0.2) | NA | |
Data are presented as median (range), number (%), or mean±SD.
NA, not applicable.
Cap-assisted EMR or EMR with a ligation device has been proposed as an alternative to cEMR, considering the flat morphology of duodenal lesions (Fig. 3).40,41 Kimoto et al.41 reported a cap-assisted EMR study on 228 SNADETs, with high en bloc and R0 resection rates (99.6% and 97.4%, respectively), a low adverse event rate (3.1%) without perforation, and no recurrence. This technique would be feasible and effective for SNADETs, particularly for small sizes (≤10 mm).
Fig. 3.
Endoscopic mucosal resection of a duodenal epithelial tumor using a ligation device. (A) A 6 mm slightly elevated lesion (0-IIa) located in the second portion of the duodenum. (B) A saline solution containing small amounts of epinephrine and indigo carmine dye was injected beneath the lesion. (C) The lesion was aspirated into the ligation device, and then the elastic band was deployed. (D) Snare resection was performed using a high-frequency device. (E) The lesion was completely resected. (F) The resected specimen.
Recently, a prospective study evaluated the efficacy and safety of thermal ablation on the defect margin after EMR (EMR-T) to reduce residual or recurrent adenomas in duodenal lateral spreading adenomas (≥10 mm).42 EMR was performed for all visible lesions, followed by ablation of defect margins by snare-tip soft coagulation, aiming to create a 2 to 3 mm rim of the completely ablated denatured tissue. EMR-T significantly reduced the recurrence rate compared with cEMR (2.3% vs 17.6%, p=0.01).42 In addition, precutting EMR, which is a technical modification of ESD, has been applied to SNADETs. This procedure involves making a circumferential incision along the margin of the lesion using a dual knife, causing the sufficiently elevated lesion to be fully captured by a snare.43 As a result, precutting EMR was comparable to ESD for SNADETs, demonstrating a lower intraoperative perforation rate and shorter procedure time compared with ESD.43
3. Cold snare endoscopic mucosal resection
cEMR, including submucosal injection and electrocautery, is associated with a risk of delayed bleeding, post-polypectomy syndrome, and perforation owing to thermal injury. Cold snare-EMR (CS-EMR) is an attractive option because it does not require electrocautery, reducing these risks. Furthermore, unlike CSP, which is indicated for SNADETs <10 mm, CS-EMR can be performed for large SNADETs. A recent meta-analysis including 1,137 sessile serrated colorectal lesions >10 mm demonstrated that CS-EMR had a significantly lower rate of delayed bleeding than cEMR.44 Therefore, performing the CS-EMR technique for large SNADETs seems reasonable.
Wang et al.45 reported that CS-EMR had lower intraprocedural and postprocedural bleeding rates than cEMR in 50 duodenal adenomas >15 mm. However, intraprocedural perforation occurred in two cases of 30- and 60-mm-sized adenomas treated using CS-EMR. Capturing large amounts of mucosa for cold snare excision with failed cutting and amputating the ensnared tissue against the tip of the endoscope may create a shearing force on the relatively fixed but thin duodenal muscle layer, resulting in perforation.45 A systematic review and meta-analysis of CS-EMR for non-ampullary duodenal polyps showed a significantly lower rate of delayed bleeding than cEMR.46 Other studies also reported a low incidence of adverse events after CS-EMR (Table 3).45,47-49 Recurrence rates after CS-EMR for large SNADETs are as 12% to 46%.45,47,48 Furthermore, most residual or recurrent lesions can be removed successfully using endoscopic resection, without surgical referral. A large tumor size is correlated with higher recurrence.45,47,48 Considering low rates of adverse events, CS-EMR might be another treatment option for large duodenal adenomas in some situations such as high risk for delayed bleeding.
Table 3.
Summary of Cold Snare Endoscopic Mucosal Resection Outcomes for Duodenal Epithelial Tumors
| Year | Author | No. of lesions | Tumor size, mm | Immediate | Delayed | Recurrence | ||
|---|---|---|---|---|---|---|---|---|
| Perforation | Bleeding | Perforation | ||||||
| 2015 | Choksi et al.49 | 15 | 24 (10−60) | 0 | 1 (6.7) | 0 | NA | |
| 2022 | Dang et al.48 | 39 | 20 (10−70) | 0 | 0 | 0 | 18 (46.2) | |
| 2022 | Repici et al.47 | 33 | 31.5±9.7 | 0 | 0 | 0 | 4 (12.1) | |
| 2023 | Wang et al.45 | 50 | 30 (19−40) | 2 (4.0) | 2 (4.0) | 0 | 10 (24.4) | |
Data are presented as median (range), number (%), or mean±SD.
NA, not applicable
4. Underwater endoscopic mucosal resection
uEMR is a relatively new EMR technique involving filling the duodenal lumen with water instead of submucosal injection (Fig. 4). Binmoeller et al. first reported uEMR for colorectal polyps in 201250 and for duodenal adenoma in 2013.51 Duodenal folds (Kerckring folds) do not contain muscularis propria.52 The muscularis propria remains flat under water immersion along the long axis and maintains the circular shape during endoscopic ultrasound examination, whereas the mucosal and the submucosal layers tend to float in a water-filled lumen.53 Unlike cEMR using air, water immersion can maintain the thickness of the duodenal wall, reduce thermal injury, and cause superficial lesions to float up, simulating protruded lesions (buoyancy effect). Therefore, the lesion can be easily snared and removed, with a low risk of muscularis propria entrapment by snaring.53
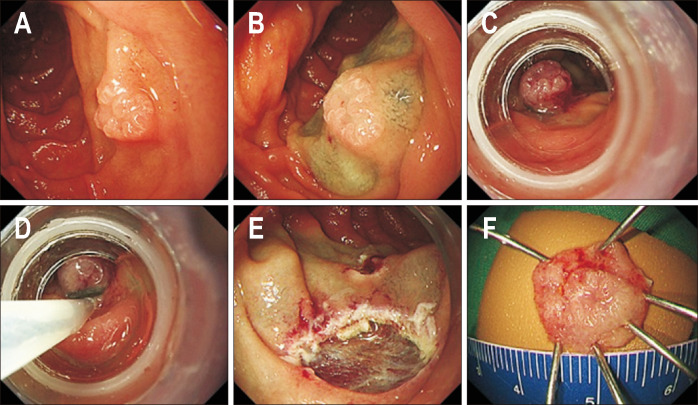
Fig. 4.
Underwater endoscopic mucosal resection of a duodenal epithelial tumor. (A) A 10 mm white, slightly elevated lesion (0-IIa) located in the second portion of the duodenum. (B) After complete air deflation, the lumen was filled with water. (C) After snaring the entire lesion along with the surrounding mucosa, resection was performed with a high-frequency device. (D) The lesion was completely resected. (E) The defect was completely closed by clipping. (F) The resected specimen.
Omitting submucosal injection causes uEMR to be a low-labor procedure and shortens the procedure time compared to cEMR.54-56 Prophylactic suturing after uEMR is easier than that after cEMR because post-uEMR ulcers are small, and the surrounding mucosa is soft without submucosal injection.57 Furthermore, uEMR reduces the influence of fibrosis on the submucosa by eliminating injection, enabling the resection of lesions with scars. Even a small forceps biopsy can cause severe submucosal fibrosis in the duodenum, causing difficulty in subsequent endoscopic resection and necessitating conversion to more invasive methods in some cases.13 Kiguchi et al.32 reported that uEMR was less frequently converted to ESD compared to cEMR. Several studies reported that uEMR showed favorable efficacy and safety (Table 4).32,35,57-60 However, uEMR achieved decreased en bloc resection and increased recurrence rates for lesions >20 mm, similar to cEMR.29,61
Table 4.
Summary of Underwater Endoscopic Mucosal Resection Outcomes for Duodenal Epithelial Tumors
| Year | Author | No. of lesions | Tumor size, mm | En bloc resection | R0 resection | Immediate | Delayed | Recurrence | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Perforation | Bleeding | Perforation | ||||||||
| 2018 | Yamasaki et al.57 | 31 | 12.0±7.3 | 27 (87.1) | 19 (61.3) | 0 | 0 | 0 | 1 (3.2) | |
| 2020 | Kiguchi et al.32 | 90 | 10.3±4.1 | 78 (86.7) | 60 (66.7) | 0 | 2 (2.2) | 0 | NA | |
| 2020 | Iwagami et al.61 | 162 | 10 (2−40) | 110 (67.9) | NA | 0 | 2 (1.2) | 1 (0.6) | 7 (4.5) | |
| 2021 | Hirasawa et al.35 | 67 | 8.1±3.7 | 62 (92.5) | 51 (76.1) | 0 | 3 (4.5) | 0 | NA | |
| 2021 | Furukawa et al.59 | 28 | 8 (2−20) | 27 (96.4) | 20 (71.4) | 0 | 0 | 0 | 0 | |
| 2022 | Kato et al.29 | 579 | 11.0±7.5 | 455 (78.6) | 316 (56.0) | 3 (0.5) | 12 (2.1) | 1 (0.2) | NA | |
| 2022 | Yamasaki et al.58 | 166 | 9.8±4.7 | 149 (89.8) | 111 (66.9) | 0 | 2 (1.2) | 0 | 4 (2.7) | |
| 2023 | Tanaka et al.60 | 96 | 8.1±4.3 | 90 (93.8) | 65 (67.7) | 0 | 1 (1) | 0 | NA | |
| 2023 | Hashiguchi et al.63 |
25 (uEMR) 22 (uEMR-SIM) |
8.4±3.1 7.2±4.4 |
22 (88.0) 22 (100) |
12 (48.0) 20 (90.0) |
0 0 |
0 0 |
0 0 |
0 0 |
|
Data are presented as mean±SD, number (%), or median (range).
NA, not applicable; uEMR, underwater endoscopic mucosal resection; uEMR-SIM, uEMR with submucosal injection and marking.
Conversely, continuously maintaining water in the duodenal lumen is difficult. Large-volume water injections are associated with the risk of aspiration pneumonia.57 Furthermore, endoscopic visibility may be reduced owing to intestinal peristalsis, bile, and blood. The use of sterile water heated to 37°C and antiperistalsis agents is helpful to overcome this drawback.53 Recently, gel immersion endoscopic resection has been reported as an alternative method to uEMR.62 Continuously maintaining gelatinous liquid in the duodenal lumen, without additional infusion, is easier than maintaining water; therefore, gel immersion endoscopic resection demonstrates a significantly shorter procedure time than uEMR.62
Another disadvantage of uEMR is that the floating center can hinder the visualization of the anal side of the lesions, increasing the risk of positive horizontal margins. Recently, a partial submucosal injection technique combining uEMR for SNADETs has been reported; the difficult side (typically the anal side) of the lesion is locally injected to recognize sufficient margins before resection.64 A study of partial submucosal injection technique combining uEMR for 30 SNADETs reported en bloc resection and R0 resection rates of 97% and 83%, respectively.64 A technique using uEMR with submucosal injection and marking has also been introduced; markings around the lesion and submucosal injection to expand, rather than lift, the lesion were performed before water immersion.63 This resection method achieved a significantly higher en bloc resection rate than cEMR and a significantly higher R0 resection rate than uEMR.
5. Endoscopic submucosal dissection
ESD has been introduced for SNADETs >20 mm, with en bloc resection rates >90% (Table 5).29-31,35,65 ESD is also considered for lesions that cannot be removed using cEMR, such as non-lifting lesions or those for which en bloc resection is required (Fig. 5).66 ESD has the advantage of a high en bloc resection rate (>90%), even for lesions >20 mm.30,65,67 Many previous studies have shown that duodenal ESD can reach a high R0 resection rate of 83.7% to 96.0%29-31,65 and a low recurrence rate of ~1.0%.29,31 However, ESD has not been widely accepted as a standard treatment owing to the high risk of adverse events: bleeding rates are up to 14.3%, perforation rates are 1.7% to 28.6%, and emergency surgery rates are up to 2.5% (Table 5).29-31 Moreover, ESD outcomes vary by country and institution. Most duodenal ESD studies have been published in Asia (Japan and Korea), and data from Western countries are limited. The largest series from Europe showed relatively low en bloc and R0 resection rates and a high recurrence rate (29.7%, 19.4%, and 14.7%, respectively) compared with previous studies from Asian expert centers.68 In addition, there is no differences in long-term outcomes and survival between cEMR and ESD.31,68-70
Table 5.
Summary of Endoscopic Submucosal Dissection Outcomes for Duodenal Epithelial Tumors
| Year | Author | No. of lesions | Tumor size (mm) | En bloc resection | R0 resection | Immediate | Delayed | Recurrence | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Perforation | Bleeding | perforation | ||||||||
| 2017 | Hoteya et al.31 |
49 (large) 25 (small) |
31.3±12.6 11.6±4.1 |
48 (98.0) 25 (100) |
41 (83.7) 24 (96.0) |
14 (28.6) 6 (24.0) |
7 (14.3) 4 (16.0) |
1 (2.0) 0 |
0 0 |
|
| 2018 | Yahagi et al.30 | 174 | 27.4±16.1 | 171 (98.3) | 148 (85.1) | 24 (13.7) | 9 (5.2) | 3 (1.7) | NA | |
| 2021 | Hirasawa et al.35 | 64 | 21.4±10.2 | 63 (98.4) | 61 (95.3) | 12 (18.6) | 3 (4.7) | 2 (3.1) | NA | |
| 2022 | Kato et al.29 | 1,017 | 20.6±13.5 | 964 (94.8) | 790 (78.7) | 95 (9.3) | 48 (4.7) | 23 (2.3) | 56 (0.4) | |
| 2024 | Seya et al.65 | 100 | 17 (5−76) | 100 (100) | 93 (93.0) | 1 (1.0) | 3 (3.0) | 4 (4.0) | 1 (1.0) | |
Data are presented as mean±SD, number (%), or median (range).
NA, not applicable.
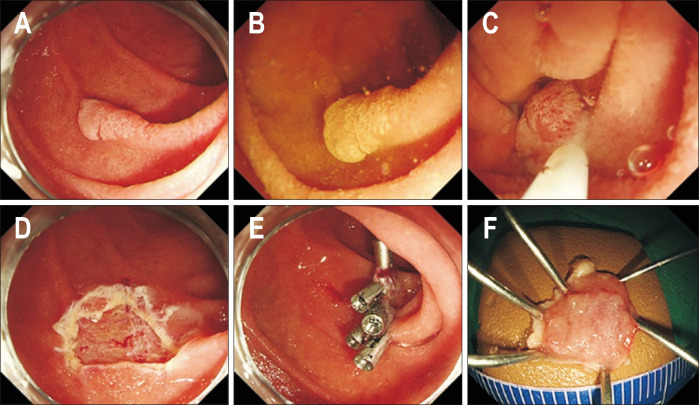
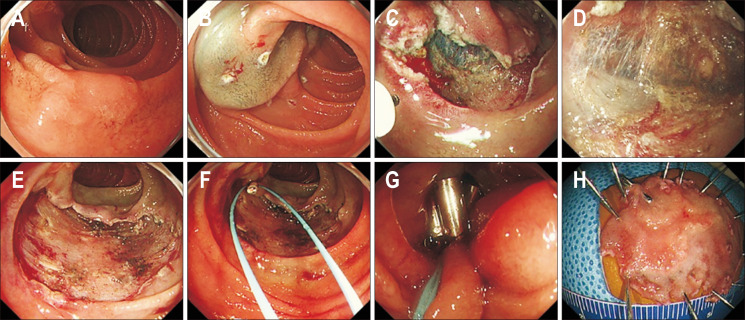
Fig. 5.
Endoscopic submucosal dissection of a duodenal epithelial tumor. (A) A 30 mm, slightly elevated lesion (0-IIa) located in the second portion of the duodenum. (B) Circumferential marking was performed, and a saline solution containing small amounts of epinephrine and indigo carmine dye was injected beneath the lesion. (C, D) Mucosal incision and submucosal dissection were performed. (E) The lesion was completely resected. (F, G) The resected area was completely closed using an endo loop and clips. (H) The resected specimen.
Therefore, duodenal endoscopic resection should focus on safety rather than outcomes of en bloc and R0 resection rates. We suggest that ESD should be performed in high-volume centers, and the lesion should be carefully selected. The pocket-creation method of ESD for SNADETs was introduced to overcome difficulties of ESD, including difficult locations and high rates of perforation. En bloc resection rate in the pocket-creation method group was 100%, even for lesions in the duodenal angles, and perforation was less frequent than in the conventional ESD group.71
HOW TO PREVENT ADVERSE EVENTS OF ENDOSCOPIC RESECTION FOR SNADETS
The ESGE recommends that the high rate of adverse events, such as immediate or delayed bleeding or perforation with duodenal endoscopic resection, may be reduced by mucosal defect closure techniques, such as endoscopic clipping or over-the-scope clip (OTSC) clipping, and by noncontact hemostatic measures.9 Several studies have shown favorable outcomes of prophylactic procedures to prevent delayed bleeding. A retrospective study of 50 patients with duodenal cEMR reported that prophylactic argon plasma coagulation therapy on the resection defect may lower the risk of delayed bleeding.72 Another study including 37 duodenal adenomas treated with cEMR reported a significantly lower delayed bleeding rate in patients treated with prophylactic clipping or prophylactic argon plasma coagulation than in the no prophylaxis group.73 Nonaka et al.37 also showed decrease in the delayed bleeding rate from 32 % to 7 % with prophylactic clipping. Therefore, prophylactic procedures should be considered to prevent delayed bleeding.
Furthermore, mucosal defect closure techniques can help prevent delayed perforation. Kato et al.74 reported that complete mucosal defect closure after duodenal ESD significantly decreased delayed adverse events. Closure of post-ESD defects is another strategy to enhance the safety of duodenal ESD. Various closure methods have been reported to date. Endoscopic clipping is the most convenient and standard method.75 Small defects can be closed using clips (Fig. 4E). A large defect can be closed by combining an endo loop and clips; opening an endo loop along the defect margin, deploying clips on both edges of the defect, and tightening the endo loop (Fig. 5F and G).74 String clip suturing is also available for large defects and involves deploying a clip with string at the distal edge of the defect and placing a second one at the opposite side to anchor the string.76 In addition, endoscopic suturing is another option for closing large mucosal defects after endoscopic resection.77
Recently, an OTSC system was introduced, which enables full-thickness defect closure by its strong holding and grasping forces. Complete defect closure by OTSC prevents delayed perforation,78 and additional use of conventional clip after OTSC is useful to reduce the risk of delayed bleeding.79 Covering the wound with PGA (polyglycolic acid) sheets and fibrin glue can be used as an alternative to suturing to prevent delayed perforation after duodenal ESD.80,81 The mucosal defect is covered with several PGA sheets, and fibrin glue (fibrinogen and thrombin) is subsequently applied using each spray tube. This constitutes a simple and easy procedure compared with clip closure and can remain on the defect site for more than a week.
CONCLUSIONS
Several endoscopic procedures are available for the treatment of SNADETs; however, treatment strategies for SNADETs have not been standardized owing to their rarity. Accordingly, the final decision is frequently made based on the endoscopist’s personal experience. Adverse events that occur after endoscopic resection are challenging. Therefore, more consensus guidelines are required to establish a standard strategy for SNADETs.
SNADETs ≤10 mm in size are candidates for CSP, cEMR, or uEMR, based on previous reports (Table 6). Among these lesions, suspected carcinoma lesions should not be treated using CSP because of their low curability. cEMR or UEMR can be considered for lesions sized 10 to 20 mm. Piecemeal EMR (including CS-EMR) and ESD are the options for lesions >20 mm. In particular, ESD or surgical resection should be considered for suspected carcinoma lesions >30 mm. The treatment plan should be selected on a case-to-case basis, considering the balance between the risk of adverse events and necessity of en bloc resection.
Table 6.
Recommendation for Endoscopic Resection of Duodenal Epithelial Tumors
| Based on histology and endoscopic findings | Tumor size | Treatment method |
|---|---|---|
| Lesions of suspected adenoma |
≤10 mm 10−20 mm >20 mm |
Cold snare polypectomy Underwater EMR Conventional EMR Underwater EMR Conventional EMR Piecemeal EMR ESD |
| Lesions of suspected carcinoma |
≤20 mm 20−30 mm >30 mm |
Underwater EMR Conventional EMR ESD Piecemeal EMR ESD Surgery |
EMR, endoscopic mucosal resection; ESD, endoscopic submucosal dissection.
Footnotes
CONFLICTS OF INTEREST
G.H.K. is an editorial board member of the journal but was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
REFERENCES
- 1.Jepsen JM, Persson M, Jakobsen NO, et al. Prospective study of prevalence and endoscopic and histopathologic characteristics of duodenal polyps in patients submitted to upper endoscopy. Scand J Gastroenterol. 1994;29:483–487. doi: 10.3109/00365529409092458. [DOI] [PubMed] [Google Scholar]
- 2.Kawasaki A, Tsuji K, Uedo N, et al. Non-atrophic gastric mucosa is an independently associated factor for superficial non-ampullary duodenal epithelial tumors: a multicenter, matched, case-control study. Clin Endosc. 2023;56:75–82. doi: 10.5946/ce.2022.059.7a284aa2ea2c4d7c970d6ff005bdb5eb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goda K, Kikuchi D, Yamamoto Y, et al. Endoscopic diagnosis of superficial non-ampullary duodenal epithelial tumors in Japan: multicenter case series. Dig Endosc. 2014;26 Suppl 2:23–29. doi: 10.1111/den.12277. [DOI] [PubMed] [Google Scholar]
- 4.Johnson MD, Mackey R, Brown N, Church J, Burke C, Walsh RM. Outcome based on management for duodenal adenomas: sporadic versus familial disease. J Gastrointest Surg. 2010;14:229–235. doi: 10.1007/s11605-009-1091-4. [DOI] [PubMed] [Google Scholar]
- 5.Sellner F. Investigations on the significance of the adenoma-carcinoma sequence in the small bowel. Cancer. 1990;66:702–715. doi: 10.1002/1097-0142(19900815)66:4<702::AID-CNCR2820660419>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 6.Cameron JL, Riall TS, Coleman J, Belcher KA. One thousand consecutive pancreaticoduodenectomies. Ann Surg. 2006;244:10–15. doi: 10.1097/01.sla.0000217673.04165.ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suwa T, Takizawa K, Kawata N, et al. Current treatment strategy for superficial nonampullary duodenal epithelial tumors. Clin Endosc. 2022;55:15–21. doi: 10.5946/ce.2021.141.2ba125688db545e0b6f08de7f98bb0d6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao Z, Jiao Y, Yang S, et al. Endoscopic diagnosis and treatment of superficial non-ampullary duodenal epithelial tumors: a review. J Transl Int Med. 2023;11:206–215. doi: 10.2478/jtim-2023-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vanbiervliet G, Moss A, Arvanitakis M, et al. Endoscopic management of superficial nonampullary duodenal tumors: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy. 2021;53:522–534. doi: 10.1055/a-1442-2395. [DOI] [PubMed] [Google Scholar]
- 10.Uozumi T, Abe S, Makiguchi ME, et al. Complications of endoscopic resection in the upper gastrointestinal tract. Clin Endosc. 2023;56:409–422. doi: 10.5946/ce.2023.024.83a54e20fdc149a4967c104d16bff028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okada K, Fujisaki J, Kasuga A, et al. Sporadic nonampullary duodenal adenoma in the natural history of duodenal cancer: a study of follow-up surveillance. Am J Gastroenterol. 2011;106:357–364. doi: 10.1038/ajg.2010.422. [DOI] [PubMed] [Google Scholar]
- 12.Brosens LA, Keller JJ, Offerhaus GJ, Goggins M, Giardiello FM. Prevention and management of duodenal polyps in familial adenomatous polyposis. Gut. 2005;54:1034–1043. doi: 10.1136/gut.2004.053843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kinoshita S, Nishizawa T, Ochiai Y, et al. Accuracy of biopsy for the preoperative diagnosis of superficial nonampullary duodenal adenocarcinoma. Gastrointest Endosc. 2017;86:329–332. doi: 10.1016/j.gie.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Kim DM, Kim GH, Lee BE, et al. Histopathologic discrepancies between endoscopic forceps biopsy and endoscopic resection specimens in nonampullary duodenal epithelial tumors. Medicine (Baltimore) 2021;100:e28307. doi: 10.1097/MD.0000000000028307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amoyel M, Belle A, Dhooge M, et al. Endoscopic management of non-ampullary duodenal adenomas. Endosc Int Open. 2022;10:E96–E108. doi: 10.1055/a-1723-2847.dd4b96bf9483440d985d2705aafd3f4a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bourke MJ. Endoscopic resection in the duodenum: current limitations and future directions. Endoscopy. 2013;45:127–132. doi: 10.1055/s-0032-1326177. [DOI] [PubMed] [Google Scholar]
- 17.Yoshimizu S, Kawachi H, Yamamoto Y, et al. Clinicopathological features and risk factors for lymph node metastasis in early-stage non-ampullary duodenal adenocarcinoma. J Gastroenterol. 2020;55:754–762. doi: 10.1007/s00535-020-01696-6. [DOI] [PubMed] [Google Scholar]
- 18.Nishio K, Kimura K, Eguchi S, et al. Prognostic factors and lymph node metastasis patterns of primary duodenal cancer. World J Surg. 2022;46:163–171. doi: 10.1007/s00268-021-06339-2. [DOI] [PubMed] [Google Scholar]
- 19.Tappero G, Gaia E, De Giuli P, Martini S, Gubetta L, Emanuelli G. Cold snare excision of small colorectal polyps. Gastrointest Endosc. 1992;38:310–313. doi: 10.1016/S0016-5107(92)70422-2. [DOI] [PubMed] [Google Scholar]
- 20.Ferlitsch M, Moss A, Hassan C, et al. Colorectal polypectomy and endoscopic mucosal resection (EMR): European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline. Endoscopy. 2017;49:270–297. doi: 10.1055/s-0043-102569. [DOI] [PubMed] [Google Scholar]
- 21.Giri S, Jearth V, Darak H, Sundaram S. Outcomes of thin versus thick-wire snares for cold snare polypectomy: a systematic review and meta-analysis. Clin Endosc. 2022;55:742–750. doi: 10.5946/ce.2022.141.779cd36408e04f9d83bf262cbb6280c6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Benito Sanz M, Hernández L, Garcia Martinez MI, et al. Efficacy and safety of cold versus hot snare polypectomy for small (5-9 mm) colorectal polyps: a multicenter randomized controlled trial. Endoscopy. 2022;54:35–44. doi: 10.1055/a-1327-8357. [DOI] [PubMed] [Google Scholar]
- 23.Hamada K, Takeuchi Y, Ishikawa H, et al. Safety of cold snare polypectomy for duodenal adenomas in familial adenomatous polyposis: a prospective exploratory study. Endoscopy. 2018;50:511–517. doi: 10.1055/s-0043-124765. [DOI] [PubMed] [Google Scholar]
- 24.Okimoto K, Maruoka D, Matsumura T, et al. Long-term outcomes of cold snare polypectomy for superficial non-ampullary duodenal epithelial tumors. J Gastroenterol Hepatol. 2022;37:75–80. doi: 10.1111/jgh.15666. [DOI] [PubMed] [Google Scholar]
- 25.Maruoka D, Matsumura T, Kasamatsu S, et al. Cold polypectomy for duodenal adenomas: a prospective clinical trial. Endoscopy. 2017;49:776–783. doi: 10.1055/s-0043-107028. [DOI] [PubMed] [Google Scholar]
- 26.Takizawa K, Kakushima N, Tanaka M, et al. Cold snare polypectomy for superficial non-ampullary duodenal epithelial tumor: a prospective clinical trial (pilot study) Surg Endosc. 2022;36:5217–5223. doi: 10.1007/s00464-021-08899-9. [DOI] [PubMed] [Google Scholar]
- 27.Hirose R, Yoshida N, Murakami T, et al. Histopathological analysis of cold snare polypectomy and its indication for colorectal polyps 10-14 mm in diameter. Dig Endosc. 2017;29:594–601. doi: 10.1111/den.12825. [DOI] [PubMed] [Google Scholar]
- 28.Gotoda T. Endoscopic resection of early gastric cancer: the Japanese perspective. Curr Opin Gastroenterol. 2006;22:561–569. doi: 10.1097/01.mog.0000239873.06243.00. [DOI] [PubMed] [Google Scholar]
- 29.Kato M, Takeuchi Y, Hoteya S, et al. Outcomes of endoscopic resection for superficial duodenal tumors: 10 years' experience in 18 Japanese high volume centers. Endoscopy. 2022;54:663–670. doi: 10.1055/a-1640-3236. [DOI] [PubMed] [Google Scholar]
- 30.Yahagi N, Kato M, Ochiai Y, et al. Outcomes of endoscopic resection for superficial duodenal epithelial neoplasia. Gastrointest Endosc. 2018;88:676–682. doi: 10.1016/j.gie.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 31.Hoteya S, Furuhata T, Takahito T, et al. Endoscopic submucosal dissection and endoscopic mucosal resection for non-ampullary superficial duodenal tumor. Digestion. 2017;95:36–42. doi: 10.1159/000452363. [DOI] [PubMed] [Google Scholar]
- 32.Kiguchi Y, Kato M, Nakayama A, et al. Feasibility study comparing underwater endoscopic mucosal resection and conventional endoscopic mucosal resection for superficial non-ampullary duodenal epithelial tumor <20 mm. Dig Endosc. 2020;32:753–760. doi: 10.1111/den.13524. [DOI] [PubMed] [Google Scholar]
- 33.Hara Y, Goda K, Dobashi A, et al. Short- and long-term outcomes of endoscopically treated superficial non-ampullary duodenal epithelial tumors. World J Gastroenterol. 2019;25:707–718. doi: 10.3748/wjg.v25.i6.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuroki K, Sanomura Y, Oka S, et al. Clinical outcomes of endoscopic resection for superficial non-ampullary duodenal tumors. Endosc Int Open. 2020;8:E354–E359. doi: 10.1055/a-0998-3708.ff636284471f4eee8d85de4108974195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirasawa K, Ozeki Y, Sawada A, et al. Appropriate endoscopic treatment selection and surveillance for superficial non-ampullary duodenal epithelial tumors. Scand J Gastroenterol. 2021;56:342–350. doi: 10.1080/00365521.2020.1867896. [DOI] [PubMed] [Google Scholar]
- 36.Tomizawa Y, Ginsberg GG. Clinical outcome of EMR of sporadic, nonampullary, duodenal adenomas: a 10-year retrospective. Gastrointest Endosc. 2018;87:1270–1278. doi: 10.1016/j.gie.2017.12.026. [DOI] [PubMed] [Google Scholar]
- 37.Nonaka S, Oda I, Tada K, et al. Clinical outcome of endoscopic resection for nonampullary duodenal tumors. Endoscopy. 2015;47:129–135. doi: 10.1055/s-0034-1390774. [DOI] [PubMed] [Google Scholar]
- 38.Cho JH, Lim KY, Lee EJ, Lee SH. Clinical outcomes of endoscopic resection of superficial nonampullary duodenal epithelial tumors: as 10-year retrospective, single-center study. World J Gastrointest Surg. 2022;14:329–340. doi: 10.4240/wjgs.v14.i4.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Probst A, Freund S, Neuhaus L, et al. Complication risk despite preventive endoscopic measures in patients undergoing endoscopic mucosal resection of large duodenal adenomas. Endoscopy. 2020;52:847–855. doi: 10.1055/a-1144-2767. [DOI] [PubMed] [Google Scholar]
- 40.Jamil LH, Kashani A, Peter N, Lo SK. Safety and efficacy of cap-assisted EMR for sporadic nonampullary duodenal adenomas. Gastrointest Endosc. 2017;86:666–672. doi: 10.1016/j.gie.2017.02.023. [DOI] [PubMed] [Google Scholar]
- 41.Kimoto Y, Sawada R, Banjoya S, et al. Efficacy and safety of cap-assisted endoscopic mucosal resection for superficial duodenal epithelial neoplasia ≤10 mm. Endosc Int Open. 2023;11:E976–E982. doi: 10.1055/a-2161-2212.02f3d3fc034942edbc27762ad8d36ba8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sidhu M, Fritzsche JA, Klein A, et al. Outcomes of thermal ablation of the defect margin after duodenal endoscopic mucosal resection (with videos) Gastrointest Endosc. 2021;93:1373–1380. doi: 10.1016/j.gie.2020.11.024. [DOI] [PubMed] [Google Scholar]
- 43.Chen D, Fu S, Shen J. Efficacy and safety of precutting endoscopic mucosal resection versus endoscopic submucosal dissection for non-ampullary superficial duodenal lesions. Clin Res Hepatol Gastroenterol. 2024;48:102304. doi: 10.1016/j.clinre.2024.102304. [DOI] [PubMed] [Google Scholar]
- 44.Thoguluva Chandrasekar V, Aziz M, Patel HK, et al. Efficacy and safety of endoscopic resection of sessile serrated polyps 10 mm or larger: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2020;18:2448–2455. doi: 10.1016/j.cgh.2019.11.041. [DOI] [PubMed] [Google Scholar]
- 45.Wang H, Sidhu M, Gupta S, et al. Cold snare EMR for the removal of large duodenal adenomas. Gastrointest Endosc. 2023;97:1100–1108. doi: 10.1016/j.gie.2023.01.040. [DOI] [PubMed] [Google Scholar]
- 46.Mohamed MF, Ahmed K, Rajadurai S, et al. Efficacy and safety of cold snare endoscopic mucosal resection (CS-EMR) for nonampullary duodenal polyps: systematic review and meta-analysis. J Clin Gastroenterol. 2024;58:580–587. doi: 10.1097/MCG.0000000000001898. [DOI] [PubMed] [Google Scholar]
- 47.Repici A, Capogreco A, Spadaccini M, et al. Cold versus hot EMR for large duodenal adenomas. Gut. 2022;71:1763–1765. doi: 10.1136/gutjnl-2022-327171. [DOI] [PubMed] [Google Scholar]
- 48.Dang DT, Suresh S, Vance RB, et al. Outcomes of cold snare piecemeal EMR for nonampullary small-bowel adenomas larger than 1 cm: a retrospective study. Gastrointest Endosc. 2022;95:1176–1182. doi: 10.1016/j.gie.2021.12.018. [DOI] [PubMed] [Google Scholar]
- 49.Choksi N, Elmunzer BJ, Stidham RW, Shuster D, Piraka C. Cold snare piecemeal resection of colonic and duodenal polyps ≥1 cm. Endosc Int Open. 2015;3:E508–E513. doi: 10.1055/s-0034-1392214.9d2edf12878049d798c708cfe5b68d0e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Binmoeller KF, Weilert F, Shah J, Bhat Y, Kane S. "Underwater" EMR without submucosal injection for large sessile colorectal polyps (with video) Gastrointest Endosc. 2012;75:1086–1091. doi: 10.1016/j.gie.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 51.Binmoeller KF, Shah JN, Bhat YM, Kane SD. "Underwater" EMR of sporadic laterally spreading nonampullary duodenal adenomas (with video) Gastrointest Endosc. 2013;78:496–502. doi: 10.1016/j.gie.2013.03.1330. [DOI] [PubMed] [Google Scholar]
- 52.Miura Y, Osawa H, Nomoto Y, Yamamoto H. Anatomical features of duodenal folds: a key feature to consider during endoscopic resection of duodenal neoplasms. VideoGIE. 2021;6:529–532. doi: 10.1016/j.vgie.2021.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maida M, Sferrazza S, Murino A, et al. Effectiveness and safety of underwater techniques in gastrointestinal endoscopy: a comprehensive review of the literature. Surg Endosc. 2021;35:37–51. doi: 10.1007/s00464-020-07907-8. [DOI] [PubMed] [Google Scholar]
- 54.Lee JG, Lee SP, Jang HJ, Kae SH. Underwater versus conventional endoscopic mucosal resection for superficial non-ampullary duodenal epithelial tumors: a systematic review and meta-analysis. Dig Dis Sci. 2023;68:1482–1491. doi: 10.1007/s10620-022-07715-1. [DOI] [PubMed] [Google Scholar]
- 55.Lv XH, Luo R, Lu Q, Deng K, Yang JL. Underwater versus conventional endoscopic mucosal resection for superficial non-ampullary duodenal epithelial tumors ≤20 mm: a systematic review and meta-analysis. Dig Liver Dis. 2023;55:714–720. doi: 10.1016/j.dld.2022.09.001. [DOI] [PubMed] [Google Scholar]
- 56.Garg R, Singh A, Aggarwal M, et al. Underwater endoscopic mucosal resection for 10 mm or larger nonpedunculated colorectal polyps: a systematic review and meta-analysis. Clin Endosc. 2021;54:379–389. doi: 10.5946/ce.2020.276.abbf13186c8245fe9f3d153292e6000f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamasaki Y, Uedo N, Takeuchi Y, et al. Underwater endoscopic mucosal resection for superficial nonampullary duodenal adenomas. Endoscopy. 2018;50:154–158. doi: 10.1055/s-0043-119214. [DOI] [PubMed] [Google Scholar]
- 58.Yamasaki Y, Uedo N, Akamatsu T, et al. Nonrecurrence rate of underwater EMR for ≤20-mm nonampullary duodenal adenomas: a multicenter prospective study (D-UEMR study) Clin Gastroenterol Hepatol. 2022;20:1010–1018. doi: 10.1016/j.cgh.2021.06.043. [DOI] [PubMed] [Google Scholar]
- 59.Furukawa M, Mitoro A, Ozutumi T, et al. Efficacy of underwater endoscopic mucosal resection for superficial non-ampullary duodenal epithelial tumor. Clin Endosc. 2021;54:371–378. doi: 10.5946/ce.2020.147.1e36bcb2d886460190e8d196fec0471b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tanaka H, Urabe Y, Takemoto H, et al. Can underwater endoscopic mucosal resection be an alternative to conventional endoscopic mucosal resection for superficial non-ampullary duodenal epithelial tumors? DEN Open. 2024;4:e312. doi: 10.1002/deo2.312.dec447262c7f4a33af15d33cb4c8119c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Iwagami H, Takeuchi Y, Yamasaki Y, et al. Feasibility of underwater endoscopic mucosal resection and management of residues for superficial non-ampullary duodenal epithelial neoplasms. Dig Endosc. 2020;32:565–573. doi: 10.1111/den.13541. [DOI] [PubMed] [Google Scholar]
- 62.Miyakawa A, Kuwai T, Sakuma Y, et al. A feasibility study comparing gel immersion endoscopic resection and underwater endoscopic mucosal resection for superficial nonampullary duodenal epithelial tumors. Endoscopy. 2023;55:261–266. doi: 10.1055/a-1924-4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hashiguchi K, Yamaguchi N, Shiota J, et al. 'Underwater endoscopic mucosal resection with submucosal injection and marking' for superficial non-ampullary duodenal epithelial tumors to achieve R0 resection: a single-center case series. Scand J Gastroenterol. 2023;58:813–821. doi: 10.1080/00365521.2023.2171315. [DOI] [PubMed] [Google Scholar]
- 64.Takatori Y, Kato M, Masunaga T, et al. Feasibility study of partial submucosal injection technique combining underwater EMR for superficial duodenal epithelial tumors. Dig Dis Sci. 2022;67:971–977. doi: 10.1007/s10620-021-06925-3. [DOI] [PubMed] [Google Scholar]
- 65.Seya M, Dohi O, Iwai N, et al. Short- and long-term outcomes of endoscopic submucosal dissection and laparoscopic and endoscopic cooperative surgery for superficial non-ampullary duodenal epithelial tumors. Surg Endosc. 2024;38:1784–1790. doi: 10.1007/s00464-023-10666-x. [DOI] [PubMed] [Google Scholar]
- 66.Kakushima N, Yoshida M, Yabuuchi Y, et al. Present status of endoscopic submucosal dissection for non-ampullary duodenal epithelial tumors. Clin Endosc. 2020;53:652–658. doi: 10.5946/ce.2019.184.38bc6ff96922494f85e3b2fb6124990c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hwang KL, Kim GH, Lee BE, Lee MW, Baek DH, Song GA. Long-term outcomes of endoscopic resection for non-ampullary duodenal epithelial tumors: a single-center experience. Turk J Gastroenterol. 2020;31:49–57. doi: 10.5152/tjg.2020.19156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pérez-Cuadrado-Robles E, Quénéhervé L, Margos W, et al. Comparative analysis of ESD versus EMR in a large European series of non-ampullary superficial duodenal tumors. Endosc Int Open. 2018;6:E1008–E1014. doi: 10.1055/a-0577-7546.d3b0d2951e4e4ad083fc299e63032f7c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Na HK, Kim DH, Ahn JY, et al. Clinical outcomes following endoscopic treatment for sporadic nonampullary duodenal adenoma. Dig Dis. 2020;38:364–372. doi: 10.1159/000504249. [DOI] [PubMed] [Google Scholar]
- 70.Park SM, Ham JH, Kim BW, et al. Feasibility of endoscopic resection for sessile nonampullary duodenal tumors: a multicenter retrospective study. Gastroenterol Res Pract. 2015;2015:692492. doi: 10.1155/2015/692492.50673f83b27344929be159e942f1e574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miura Y, Shinozaki S, Hayashi Y, Sakamoto H, Lefor AK, Yamamoto H. Duodenal endoscopic submucosal dissection is feasible using the pocket-creation method. Endoscopy. 2017;49:8–14. doi: 10.1055/s-0042-116315. [DOI] [PubMed] [Google Scholar]
- 72.Aschmoneit-Messer I, Richl J, Pohl J, Ell C, May A. Prospective study of acute complication rates and associated risk factors in endoscopic therapy for duodenal adenomas. Surg Endosc. 2015;29:1823–1830. doi: 10.1007/s00464-014-3871-5. [DOI] [PubMed] [Google Scholar]
- 73.Lépilliez V, Chemaly M, Ponchon T, Napoleon B, Saurin JC. Endoscopic resection of sporadic duodenal adenomas: an efficient technique with a substantial risk of delayed bleeding. Endoscopy. 2008;40:806–810. doi: 10.1055/s-2008-1077619. [DOI] [PubMed] [Google Scholar]
- 74.Kato M, Ochiai Y, Fukuhara S, et al. Clinical impact of closure of the mucosal defect after duodenal endoscopic submucosal dissection. Gastrointest Endosc. 2019;89:87–93. doi: 10.1016/j.gie.2018.07.026. [DOI] [PubMed] [Google Scholar]
- 75.An JY, Kim BW, Kim JS, Park JM, Kim TH, Lee J. The use of endoscopic clipping in preventing delayed complications after endoscopic resection for superficial non-ampullary duodenal tumors. Clin Endosc. 2021;54:563–569. doi: 10.5946/ce.2020.109.9f0d754e4935421f943edc00095246a5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yahagi N, Nishizawa T, Akimoto T, Ochiai Y, Goto O. New endoscopic suturing method: string clip suturing method. Gastrointest Endosc. 2016;84:1064–1065. doi: 10.1016/j.gie.2016.05.054. [DOI] [PubMed] [Google Scholar]
- 77.Chung J, Wang K, Podboy A, Gaddam S, K Lo S. Endoscopic suturing for the prevention and treatment of complications associated with endoscopic mucosal resection of large duodenal adenomas. Clin Endosc. 2022;55:95–100. doi: 10.5946/ce.2020.281.636c0168fdfd422bad75bcaecf1c5867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tashima T, Ohata K, Sakai E, et al. Efficacy of an over-the-scope clip for preventing adverse events after duodenal endoscopic submucosal dissection: a prospective interventional study. Endoscopy. 2018;50:487–496. doi: 10.1055/s-0044-102255. [DOI] [PubMed] [Google Scholar]
- 79.Ohata K, Sakai E, Suzuki Y, et al. Risk factors of delayed bleeding after endoscopic resection of superficial non-ampullary duodenal epithelial tumors and prevention by over-the-scope and conventional clipping. Dig Endosc. 2021;33:390–398. doi: 10.1111/den.13729. [DOI] [PubMed] [Google Scholar]
- 80.Doyama H, Tominaga K, Yoshida N, Takemura K, Yamada S. Endoscopic tissue shielding with polyglycolic acid sheets, fibrin glue and clips to prevent delayed perforation after duodenal endoscopic resection. Dig Endosc. 2014;26 Suppl 2:41–45. doi: 10.1111/den.12253. [DOI] [PubMed] [Google Scholar]
- 81.Takimoto K, Imai Y, Matsuyama K. Endoscopic tissue shielding method with polyglycolic acid sheets and fibrin glue to prevent delayed perforation after duodenal endoscopic submucosal dissection. Dig Endosc. 2014;26 Suppl 2:46–49. doi: 10.1111/den.12280. [DOI] [PubMed] [Google Scholar]