Abstract
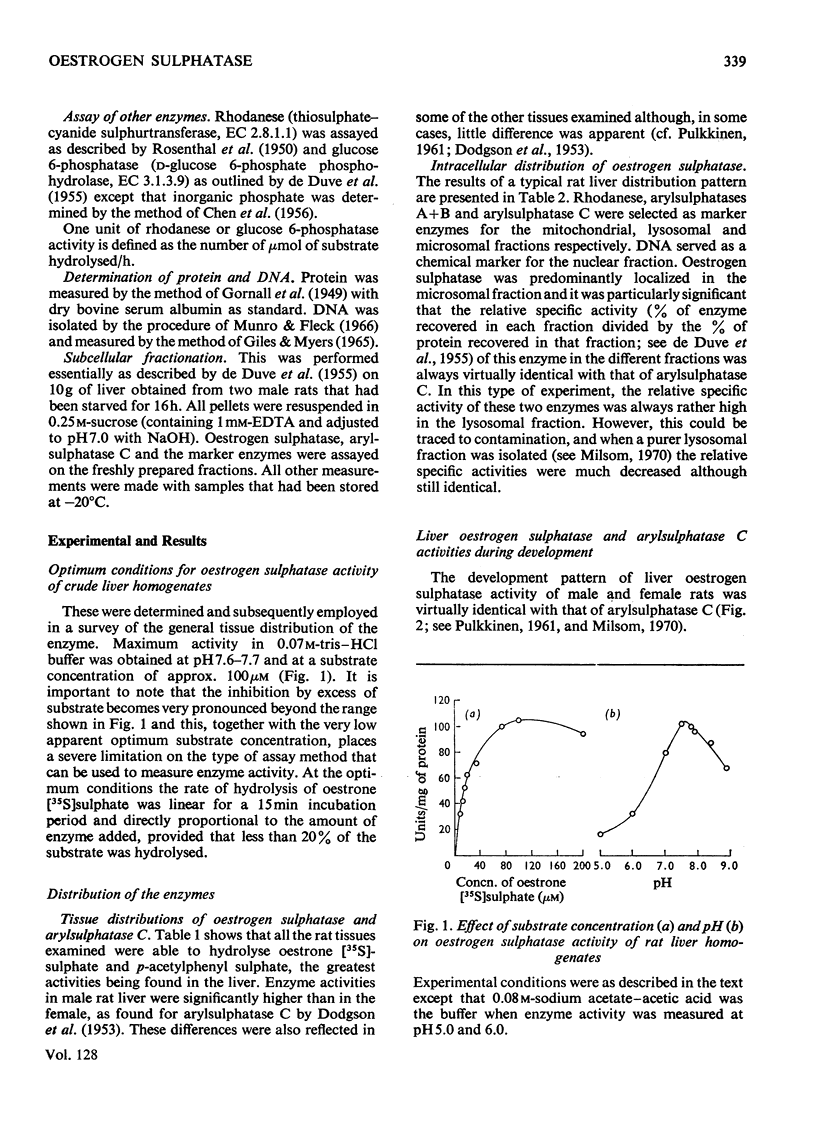
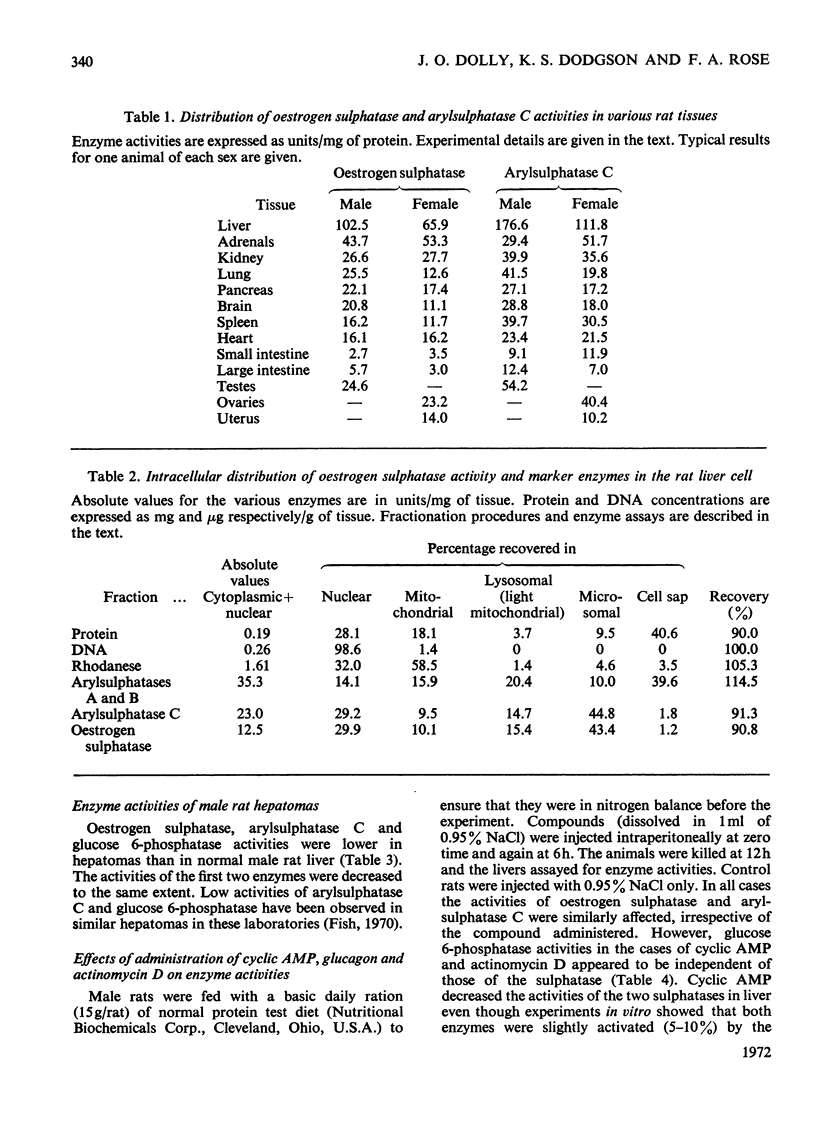
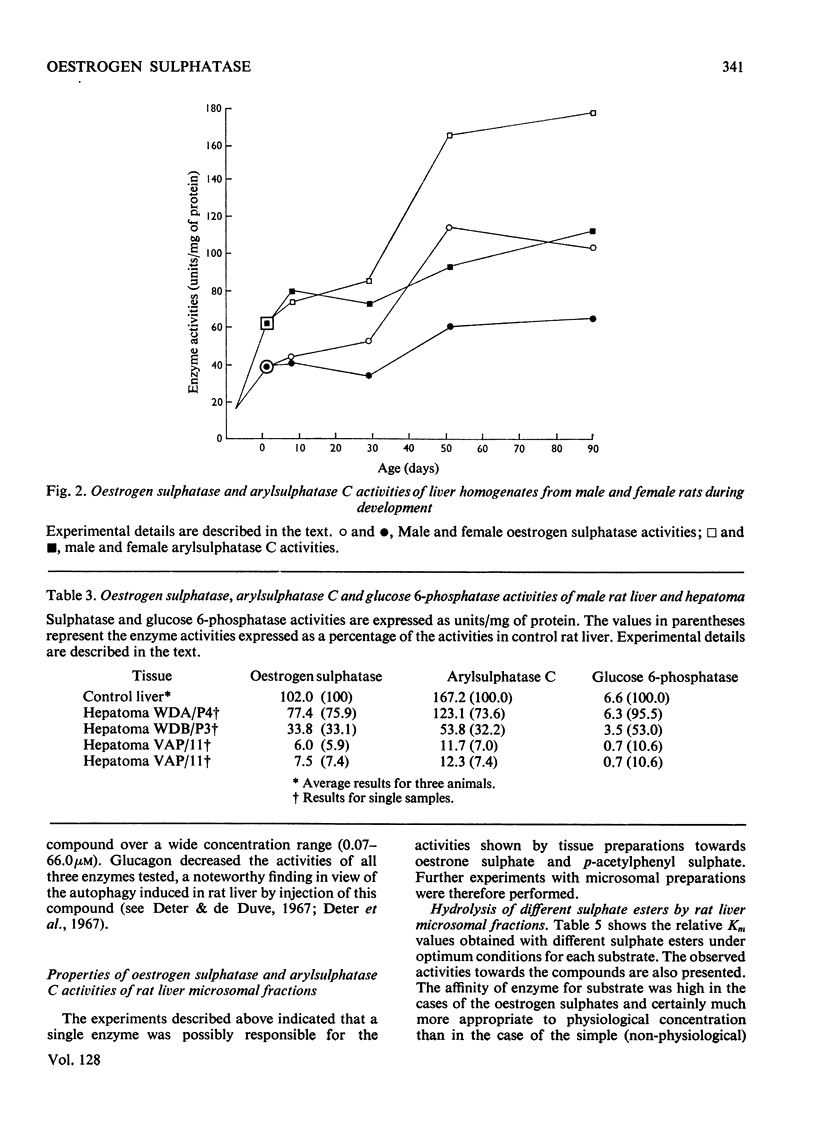
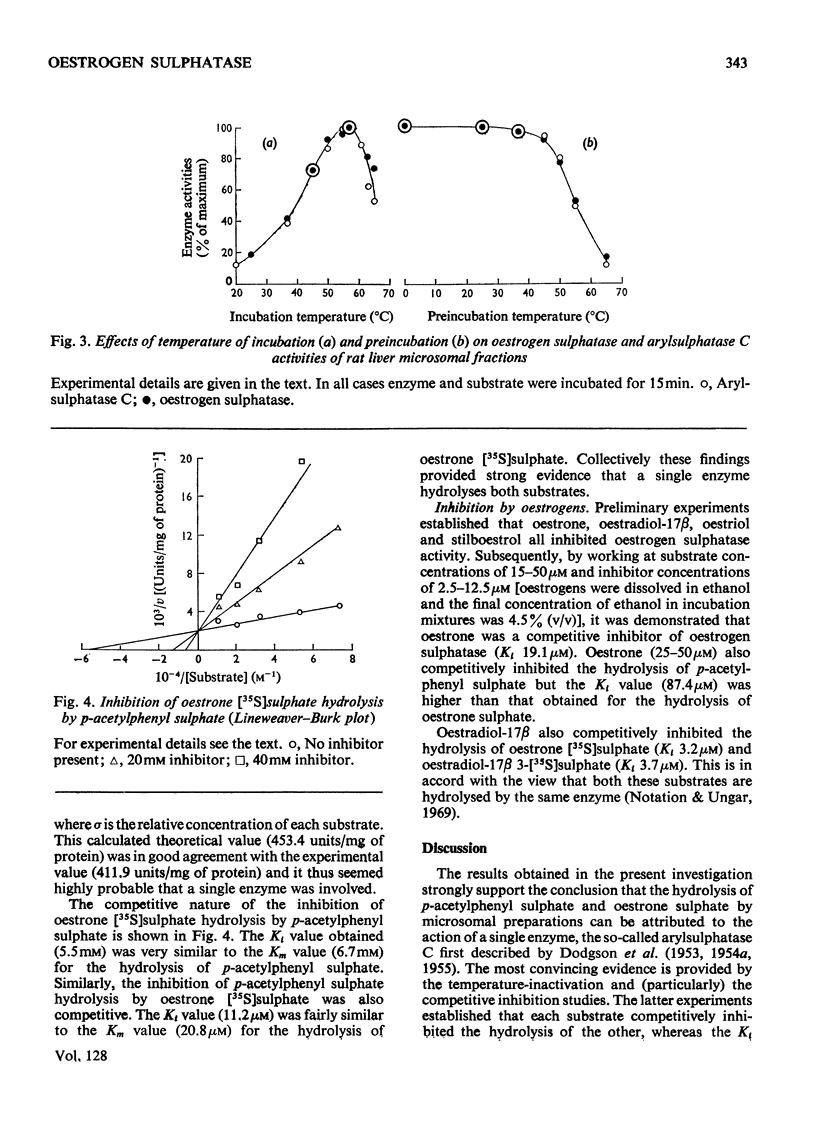
Detailed studies on the hydrolysis of p-acetylphenyl sulphate and oestrone sulphate by rat liver preparations strongly indicate that arylsulphatase C and oestrogen sulphatase are the same enzyme. Liver is the richest source of both enzymes, which have identical intracellular distributions, being localized mainly in the microsomal fraction. Low oestrogen sulphatase and arylsulphatase C activities were present in foetal liver and these increased at a similar rate after birth. The activities of the enzymes in an ethionine-induced hepatoma were similarly low. Results of heat inactivation, mixed-substrate and competitive-inhibition experiments employing liver microsomal fractions were also consistent with one enzyme being involved. Oestradiol-17β 3-sulphate was also hydrolysed by microsomal preparations and activity towards both this substrate and oestrone sulphate was inhibited by oestrone and oestradiol-17β. The physiological significance of this inhibition is discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGSON K. S., MELVILLE T. H., SPENCER B., WILLIAMS K. Studies on sulphatases. VIII. The arylsulphatase of a strain of Alcaligenes metalcaligenes isolated from intertidal mud. Biochem J. 1954 Oct;58(2):182–187. doi: 10.1042/bj0580182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGSON K. S., ROSE F. A., SPENCER B. Studies on sulphatases. 16. A soluble preparation of arysulphatase C of rat-liver microsomes. Biochem J. 1957 Jun;66(2):357–363. doi: 10.1042/bj0660357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGSON K. S., ROSE F. A., SPENCER B., THOMAS J. Studies on sulphatases. 17. The action of surface-active agents on the arylsulphatase C of rat liver. Biochem J. 1957 Jun;66(2):363–368. doi: 10.1042/bj0660363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGSON K. S., ROSE F. A., TUDBALL N. Studies on sulphatases. 23. The enzymic desulphation of tyrosine O-sulphate. Biochem J. 1959 Jan;71(1):10–15. doi: 10.1042/bj0710010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGSON K. S., SPENCER B. Studies on sulphatases. I. The choice of substrate for the assay of rat-liver arylsulphatase. Biochem J. 1953 Feb;53(3):444–451. doi: 10.1042/bj0530444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGSON K. S., SPENCER B., THOMAS J. Studies on sulphatases. 6. The localization of arylsulphatase in the rat-liver cell. Biochem J. 1954 Feb;56(2):177–181. doi: 10.1042/bj0560177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGSON K. S., SPENCER B., THOMAS J. Studies on sulphatases. II. The assay of the arylsulphatase activity of rat tissues. Biochem J. 1953 Feb;53(3):452–457. doi: 10.1042/bj0530452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGSON K. S., SPENCER B., THOMAS J. Studies on sulphatases. IX. The arylsulphatases of mammalian liver. Biochem J. 1955 Jan;59(1):29–37. doi: 10.1042/bj0590029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGSON K. S., SPENCER B. The impure nature of nitrocatechol sulphate. Biochim Biophys Acta. 1956 Jul;21(1):175–175. doi: 10.1016/0006-3002(56)90112-3. [DOI] [PubMed] [Google Scholar]
- DODGSON K. S., SPENCER B., WYNN C. H. Studies on sulphatases. 12. The arylsulphatases of human tissues. Biochem J. 1956 Mar;62(3):500–507. doi: 10.1042/bj0620500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deter R. L., De Duve C. Influence of glucagon, an inducer of cellular autophagy, on some physical properties of rat liver lysosomes. J Cell Biol. 1967 May;33(2):437–449. doi: 10.1083/jcb.33.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolly J. O., Curtis C. G., Dodgson K. S., Rose F. A. Metabolism of sodium oestrone ( 35 S)sulphate in the rat. Biochem J. 1971 Jun;123(2):261–266. doi: 10.1042/bj1230261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French A. P., Warren J. C. Properties of steroid sulphatase and arylsulphatase activities of human placenta. Biochem J. 1967 Oct;105(1):233–241. doi: 10.1042/bj1050233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French A. P., Warren J. C. Sulfatase activity in the human placenta. Steroids. 1966 Jul;8(1):79–85. doi: 10.1016/0039-128x(66)90120-6. [DOI] [PubMed] [Google Scholar]
- HANAHAN D. J., EVERETT N. B. The metabolism of S35-sodium estrone sulfate in the adult female rat. J Biol Chem. 1950 Aug;185(2):919–925. [PubMed] [Google Scholar]
- Hug G., Schubert W. K. Lysosomes in type II glycogenosis. Changes during administration of extract from Aspergillus niger. J Cell Biol. 1967 Oct;35(1):C1–C6. doi: 10.1083/jcb.35.1.c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASTER R. W. POSSIBLE SYNTHESIS OF POLYRIBONUCLEOTIDES OF KNOWN BASE-TRIPLET SEQUENCES. Nature. 1965 Apr 3;206:93–93. doi: 10.1038/206093b0. [DOI] [PubMed] [Google Scholar]
- Mattock P., Jones J. G. Partial purification and properties of an enzyme from rat liver that catalyses the sulphation of L-tyrosyl derivatives. Biochem J. 1970 Mar;116(5):797–803. doi: 10.1042/bj1160797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milson D. W., Rose F. A., Dodgson K. S. The specific assay of arylsulphatase C, a rat liver microsomal marker enzyme. Biochem J. 1972 Jun;128(2):331–336. doi: 10.1042/bj1280331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro H. N. The determination of nucleic acids. Methods Biochem Anal. 1966;14:113–176. doi: 10.1002/9780470110324.ch5. [DOI] [PubMed] [Google Scholar]
- Notation A. D., Ungar F. Rat testis steroid sulfatase. 2. Kinetic study. Steroids. 1969 Aug;14(2):151–159. doi: 10.1016/0039-128x(69)90030-0. [DOI] [PubMed] [Google Scholar]
- PULKKINEN M. O., PAUNIO I. HYDROLYSIS OF AROMATIC STEROID SULPHATES DURING PREGNANCY. Ann Med Exp Biol Fenn. 1963;41:283–285. [PubMed] [Google Scholar]
- Pack B. A., Brooks S. C. Metabolism of estrogens and their sulfates in rat uterine minces. Endocrinology. 1970 Nov;87(5):924–933. doi: 10.1210/endo-87-5-924. [DOI] [PubMed] [Google Scholar]
- ROSENTHAL O., ROGERS C. S., VARS H. M., FERGUSON C. C. Arginase, adenosinepyrophosphatase, and rhodanese levels in the liver of rats. J Biol Chem. 1950 Aug;185(2):669–680. [PubMed] [Google Scholar]
- ROY A. B. Comparative studies on the liver sulphatases. Biochem J. 1958 Mar;68(3):519–528. doi: 10.1042/bj0680519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWERS J., RODESCH F. M'ETABOLISME DES OESTROG'ENES PAR LE PLACENTA HUMAIN. II. HYDROLYSE DE L'OESTRIOL-3-SULFATE ET L'OESTRONE-3-SULFATE PAR LE PLACENTA IN VITRO. Ann Endocrinol (Paris) 1963 Sep-Oct;24:931–935. [PubMed] [Google Scholar]
- Sandberg E. C., Jenkins R. C. Aryl and steroid sulfatase in the human ovary. Biochim Biophys Acta. 1966 Jan 11;113(1):190–193. doi: 10.1016/s0926-6593(66)80137-6. [DOI] [PubMed] [Google Scholar]
- Sunshine G. H., Williams D. J., Rabin B. R. Role for steroid hormones in the interaction of ribosomes with the endoplasmic membranes of rat liver. Nat New Biol. 1971 Mar 31;230(13):133–136. doi: 10.1038/newbio230133a0. [DOI] [PubMed] [Google Scholar]
- VIALA R., GIANETTO R. The binding of sulphatase by rat-liver particles as compared to that of acid phosphatase. Can J Biochem Physiol. 1955 Sep;33(5):839–844. [PubMed] [Google Scholar]
- WENGLE B. STUDIES ON ESTER SULPHATES. 21. ON SULPHATE CONJUGATION IN FOETAL HUMAN TISSUE EXTRACTS. Acta Soc Med Ups. 1964;69:105–124. [PubMed] [Google Scholar]
- Zuckerman N. G., Hagerman D. D. The hydrolysis of estrone sulfate by rat kidney microsomal sulfatase. Arch Biochem Biophys. 1969 Dec;135(1):410–415. doi: 10.1016/0003-9861(69)90557-8. [DOI] [PubMed] [Google Scholar]


