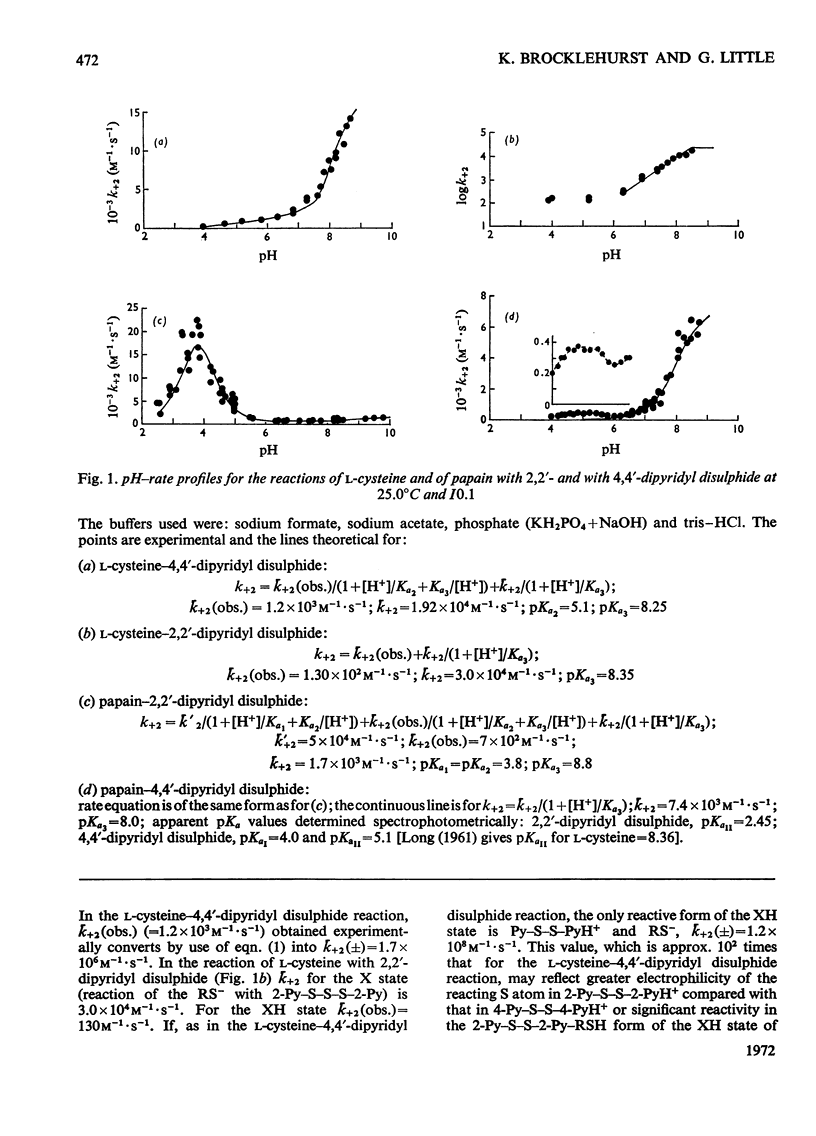
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brocklehurst K., Little G. A novel reactivity of papain and a convenient active site titration in the presence of other thiols. FEBS Lett. 1970 Jul 29;9(2):113–116. doi: 10.1016/0014-5793(70)80327-1. [DOI] [PubMed] [Google Scholar]
- Drenth J., Jansonius J. N., Koekoek R., Sluyterman L. A., Wolthers B. G. IV. Cysteine proteinases. The structure of the papain molecule. Philos Trans R Soc Lond B Biol Sci. 1970 Feb 12;257(813):231–236. doi: 10.1098/rstb.1970.0022. [DOI] [PubMed] [Google Scholar]
- Husain S. S., Lowe G. Evidence for histidine in the active site of papain. Biochem J. 1968 Aug;108(5):855–859. doi: 10.1042/bj1080855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little G., Brocklehurst K. Kinetics of the reversible reaction of papain with 5,5'-dithiobis-(2-nitrobenzoate) dianion: evidence for nucleophilic reactivity in the un-ionized thiol group of cysteine-25 and for general acid catalysis by histidine-159 of the reaction of the 5-mercapto-2-nitrobenzoate dianion with the papain-5-mercapto-2-nitrobenzoate mixed disulphide. Biochem J. 1972 Jun;128(2):475–477. doi: 10.1042/bj1280475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITAKER J. R., BENDER M. L. KINETICS OF PAPAIN-CATALYZED HYDROLYSIS OF ALPHA-N-BENZOYL-L-ARGININE ETHYL ESTER AND ALPHA-N-BENZOYL-L-ARGININAMIDE. J Am Chem Soc. 1965 Jun 20;87:2728–2737. doi: 10.1021/ja01090a034. [DOI] [PubMed] [Google Scholar]



