Abstract
We show that a signal from the germ line represses growth in the nematode Caenorhabditis elegans. Laser-microbeam ablation of cells that give rise to the germ line causes adults to become giant. Ablation of these cells in self-sterile mutant worms also causes gigantism, suggesting that the germ line represses growth because it is the source of a growth-antagonizing signal rather than because of a sink of resources required for reproduction. The C. elegans germ line also emits a signal that represses longevity. This longevity-repressing signal requires the activity of DAF-16, a forkhead/winged-helix transcription factor, but we find that that the growth-repressing signal does not. The growth-repressing signal also does not require the activity of DBL-1, a transforming growth factor β-related protein that promotes growth in worms. By ablating the germ-line precursors of other species of free-living nematodes, we also found that both the growth-repressing and longevity-repressing signals are evolutionarily variable. Some species have both signals; others have just one or the other. We suggest that variation in germ-line signaling contributes to body size and life-history diversity in the nematodes.
An axiom of evolutionary life-history theory is that reproduction exacts a cost in somatic investment (1–3). Gametes, the reasoning goes, require materials (proteins, lipids, and the like) that would otherwise be available for building or maintaining somatic tissue (4, 5). In support of this idea, many experiments in many organisms have shown that longevity and growth “trade off” with reproduction if reproduction is altered by genetic, surgical, or environmental manipulations (2–8). Similarly, phenotypic and genetic correlations between various measures of somatic and reproductive investment are often negative, just as predicted by theory (2–4, 7–11).
The existence of tradeoffs between somatic and reproductive investment seems indisputable. What is less clear is that such tradeoffs are the direct consequence of conflicts in resource allocation. It could be that tradeoffs stem from molecular signals that inversely regulate somatic and reproductive investment (5, 6). Just such a tradeoff appears to exist in the free-living nematode Caenorhabditis elegans. Surgical ablation of germ-line precursor cells in larval worms causes an elimination of reproduction and an increase in adult longevity of more than 60% (12)—precisely the kind of result that has been classically taken as evidence for a fecundity cost to survivorship (6). However, other ways of manipulating reproductive output in this worm (mating levels, sterility mutations, chemical inhibition) do not show such a cost (6, 13, 14). Furthermore, ablation of the entire gonad (germ line as well as the somatic tissue that contain them) causes no increase in longevity (12, 14). The increased longevity of germ-line-ablated animals seems, then, not to be caused by the elimination of reproduction per se. Hsin and Kenyon (12) resolve these paradoxical results by postulating that the larval gonad is the source of two signals, one from the somatic gonad that promotes longevity and one from the germ line that represses longevity, and that these signals are of roughly equal magnitude. The molecular nature of the germ-line signal is unknown, but was hinted at by the observation that it requires daf-16, a forkhead-transcription factor known to be a target of insulin-like signaling (15, 16).
These findings caused us to wonder whether the larval gonad of C. elegans might also influence another aspect of somatic investment, growth. We therefore asked what consequences manipulating reproductive investment had for adult body size in hermaphrodites. We did this manipulation by ablating the gonads of larval worms. Finding that such ablations caused gigantism in adults, we then asked whether the gonad's influence over growth was due to a molecular signal or the direct effects of reproduction. Finally, we examined the effects of larval gonadectomy on growth and longevity in six other species of nematode to determine whether the gonadal regulation of growth and longevity found in C. elegans was conserved within the Nematoda.
Materials and Methods
Strains and Culture Conditions.
We studied free-living terrestrial nematodes belonging to two families, Rhabditidae and Diplogasteridae, in the order Rhabditida. We used two wild-type C. elegans N2 strains: one derived from the Caenorhabditis Genetics Center (CGC, Minneapolis) strain in 1996 and kept in continuous culture in the Leroi laboratory since its inception, and another also ultimately derived from the CGC and used by the Kenyon laboratory in their studies (ref. 12 and C. Kenyon, personal communication). We also used Caenorhabditis briggsae (AF16), Oscheius myriophila (BW290), Oscheius sp. (CEW1), Rhabditis sp. (PS1191), Pristionchus pacificus (PS1843), and Pristionchus maupasi (PS321). The following mutant C. elegans strains were used: LG I, fer-6 (hc6), and daf-16 (mu86); and LG V: dbl-1 (nk3). hc6 is a temperature-sensitive hypomorph; mu86 and nk3 are null alleles. All nematodes were cultured by using standard C. elegans methods on agar plates seeded with Escherichia coli (OP50) and incubated at 20°C.
Laser Microsurgery.
Hatchlings were synchronized over a period of 1 h, and the nematodes were transferred to an agar pad containing 0.1 M NaN3 as an anaesthetic. They were examined under Normarski DIC optics using a Nikon E600 microscope attached, via the epifluorescence port, to a VSL-337ND-S nitrogen laser with a coumarin (440 nm) dye module. The four-cell gonad was identified, and ablations were performed on approximately half of the nematodes on the slide. The remaining half was used as intact controls. The nematodes were unmounted and each was transferred to a separate fresh 50-mm plate and grown at 20°C. Successful ablations were confirmed after 72 h by examining the nematodes on their plates under a dissecting microscope and under 100× magnification. Intact control and ablated nematodes were transferred to fresh plates daily for 7 days after reaching adulthood.
Body Size and Longevity Analysis.
Maximum volume was estimated from area and length measurements taken with OBJECT-IMAGE 1.62N3 and calculated by assuming a cylinderical worm. All growth assays were longitudinal. Longevity was determined by daily mortality assays. Worms that ceased to move or respond to gentle prodding were taken as dead; those that committed “suicide” by crawling off the plate or that died as “bags of worms” were censored, a proportion that varied between 0 and 13% among treatments. Treatments were compared by log-rank tests using JMP 3.2.2 (SAS Institute, Cary, NC).
Results
The C. elegans Germ Line Is the Source of a Signal That Regulates Body Size.
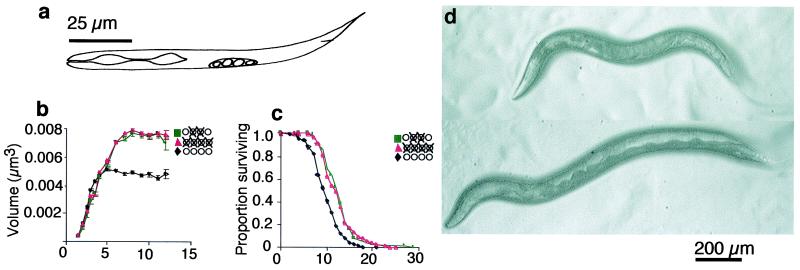
The C. elegans gonad arises from four cells visible in the hatchling worm. Two of these, Z2 and Z3, will give rise to the germ line, and two, Z1 and Z4, will give rise to the somatic gonad (Fig. 1a). When we ablated the germ-line precursors, Z2 and Z3, we found that the adults become giant. Unablated control hermaphrodites have a volume of 0.0058 mm3 at adulthood, but germ-line-ablated worms were 0.0086 mm3, a relative increase in body size of 46% (Fig. 1 b and d). Because this increase in volume is caused by both increased length and width, ablating the germ line appears to cause nearly proportionate gigantism (Fig. 1d). All of the relative increase in body size occurs during adulthood 100 h after hatching. Thus, the germ line has a repressive effect on adult growth.
Figure 1.
Growth and survival of wild-type N2 grown at 20°C after ablation of germ-line and somatic precursor cells in hatchling nematodes. (a) The four precursor cells seen in a hatchling. Z1 and Z4 give rise to the somatic gonad and Z2 and Z3 give rise to the germ line. Growth (b, volume, mm3) and survival (c, days) of N2 hermaphrodites in which the germ-line precusors, Z2 and Z3 (solid line) were ablated or else left intact (♦). ■, Z2/3(−); ▴, Z1–4(−). n, total number of nematodes observed, followed by the number of independent experiments performed in parentheses; v, mean maximum body size (±SE); l = mean longevity (±SE). P values are based on a comparison of ablated and intact animals. (b) Intact control, n = 194(3), v = 0.0058 mm3 (± 0.00008); Z2/3(−), n = 65(2), v = 0.0084 mm3 (± 0.0002), P < 0.0001. (c) Intact control, n = 366(3), l = 9.9 (± 0.2); Z2/3(−), n = 67(2), l = 12.9 (± 0.5), P < 0.0001. (d) A wild-type hermaphrodite with intact gonads (Upper) and a germ-line-ablated hermaphrodite (Lower), both grown at 20°C for 11 days from hatching.
This finding parallels the recent observation that the germ line has a repressive effect on longevity (12). The longevity-repressing effect of the germ line is, however, antagonized by a longevity-enhancing effect of the somatic gonad, so that ablating the entire gonad has no net effect on longevity (12, 14). We asked whether the somatic gonad has a similar positive effect on growth. Because it is not possible to ablate just the somatic precursor cells, Z1 and Z4, which flank Z2 and Z3 in the hatchling worm's gonadal primordium without grossly disrupting germ-line development as well, we ablated all four gonadal precursor cells. These worms grew into adults devoid of a gonad and a body size no larger than that of germ-line-ablated worms [Z2/Z3(−), n = 65(2), v = 0.0084 mm3 (± 0.0002); Z1–Z4 (−), n = 110(3), v = 0.0086 mm3 (± 0.0001), P = 0.2]. In contrast to its effects on longevity, the only effect of the gonad on body size in worms appears to be a repressive one of the germ line.
Why does the presence of the germ line repress adult growth? One possibility is that germ-line-ablated hermaphrodites grow larger because they do not pay the reproductive costs of laying the 300-odd eggs that they normally would, a volume 1.7 times that of an adult worm. However, there is much evidence in C. elegans that increased longevity—be it caused by gonadectomy or mutations—is not caused by a decrease in reproduction but rather by an interruption of signaling pathways that control adult longevity (12–14). To test whether the gigantism of germ-line-ablated worms was caused by their lack of reproduction, we ablated Z2 and Z3 in the temperature-sensitive sterile mutant, fer-6 (hc6), which possess an intact and functioning gonad, but is self-sterile at 25°C because of dysfunctional spermatozoa and so does not lay eggs (17). We reasoned that if the gigantism of germ-line-ablated worms is caused by a cost of reproduction, then no further increase in body size should be seen in worms that have essentially no reproductive expenditure to begin with. We found that at the nonpermissive temperature, 25°C, fer-6 (hc6) worms also became giant (Fig. 2a). We conclude that the repressive effect of the germ line on body size is not caused by a cost of reproduction, but is probably caused by a signal that is a negative regulator of adult body size. Consistent with the idea that the germ-line longevity effect is caused not by a cost of reproduction, but by a repressive signal, we found that the longevity of germ-line-ablated fer-6 (hc6) worms was 29% greater than unablated controls (Fig. 2b).
Figure 2.
Responses of fer-6, daf-16, and dbl-1 mutants to ablation of germ-line precursors, Z2/3. Intact control (⧫) and Z2/3(−) (solid line). (a and b) fer-6 (hc6), control, and Z2/3(−) nematodes were reared at 25°C. Intact control, n = 29(1), v = 0.0031 mm3 (± 0.0002); Z2/3(−), n = 26(1), v = 0.0044 mm3 (± 0.0003), P < 0.0001; intact control, n = 29(1), l = 8.7 (± 0.4); Z2/3(−), n = 29(1), l = 11.0 (± 0.6), P = 0.0003. A wild-type N2 experiment was run alongside at 25°C; intact control, n = 35(1), v = 0.0074 mm3 (± 0.0002); Z2/3(−), n = 55(1), v = 0.0084 mm3 ± (0.0002), P < 0.0001; intact control, n = 29(1), l = 9.9 ± 0.4; Z2/3(−), n = 55(1), l = 15.3 (± 0.8), P < 0.0001. (c and d) daf-16 (mu86), intact control, n = 51(2), v = 0.0086 mm3 (± 0.0004); Z2/Z3(−), n = 59(2), v = 0.0114 mm3 (± 0.0002), P < 0.0001; intact control, n = 51(2), l = 10.8 (± 0.4); Z2/3(−), n = 59(2), l = 11.7 (± 0.4), P = 0.3. In one experiment, all of the gonadal precursor cells Z1-Z4(−) were ablated as well: n = 28(1), v = 0.0108 mm3 (± 0.0003), l = 11.6 (± 0.6). Z2/3(−) versus Z1-Z4(−), P = 0.8 for body size and P = 0.8 for survival. (e and f) dbl-1 (nk3), intact control, n = 44(2), v = 0.0035 mm3 (± 0.0001); Z2/3(−), n = 50(2), v = 0.0055 mm3 (± 0.0001), P < 0.0001; intact control, n = 48(2), l = 14.3 (± 1.1); Z2/3(−), n = 55(2), l = 20.2 (± 1.4), P < 0.001.
The Growth-Repressing Signal Is daf-16 Independent.
We next wanted to know the signal that represses body size and the signal that represses longevity were one and the same. The longevity signal has been shown to be repressed by the null daf-16 mutation, mu86 (12). We therefore carried out ablation experiments in worms carrying the same null mutation. We confirm that germ-line-ablated daf-16(0) worms do not have increased longevity relative to unablated controls, but find that they become giant (Fig. 2 c and d). We conclude that the effects of the germ line on body size and longevity are genetically separable.
The Growth-Repressing Signal Is Independent of Transforming Growth Factor (TGF)-β Signaling.
Mutations in many genes affect body size in C. elegans, although few gigantism mutations are known. The best understood growth control pathway in this worm is the TGF-β growth pathway (18–21). The TGF-β ligand DBL-1 is not likely to be the germ-line signal itself because it is not known to be expressed in the gonad and impairing its function causes dwarfism (18, 19), whereas removal of the germ line causes gigantism (Fig. 2 e and f). However, it is possible that the germ-line signal normally inhibits DBL-1 activity. If so, ablation of the germ line might cause an increase in DBL-1 activity and hence gigantism.
Two lines of evidence suggest that this model is incorrect. First, worms that overexpress DBL-1 are not giant (that is, they are not larger in volume) but rather are just longer than wild type, a distinctive phenotype called Lon (18, 19). Second, we ablated the germ line in worms carrying the null dbl-1 mutation, nk3. If the germ-line body size effect depended on TGF-β signaling, dbl-1(0) worms should show no increase in body size. We found, however, that germ-line-ablated dbl-1(0) worms also become giant, with body sizes 60% greater than unablated controls, an absolute size increase comparable to wild type (Fig. 2e). We therefore propose that adult body size in C. elegans is regulated by at least two independent mechanisms: a somatic TGF-β-dependent pathway that promotes growth and a germ-line-dependent signal that antagonizes growth.
Germ-Line Signals That Affect Body Size and Longevity Are Evolutionarily Variable.
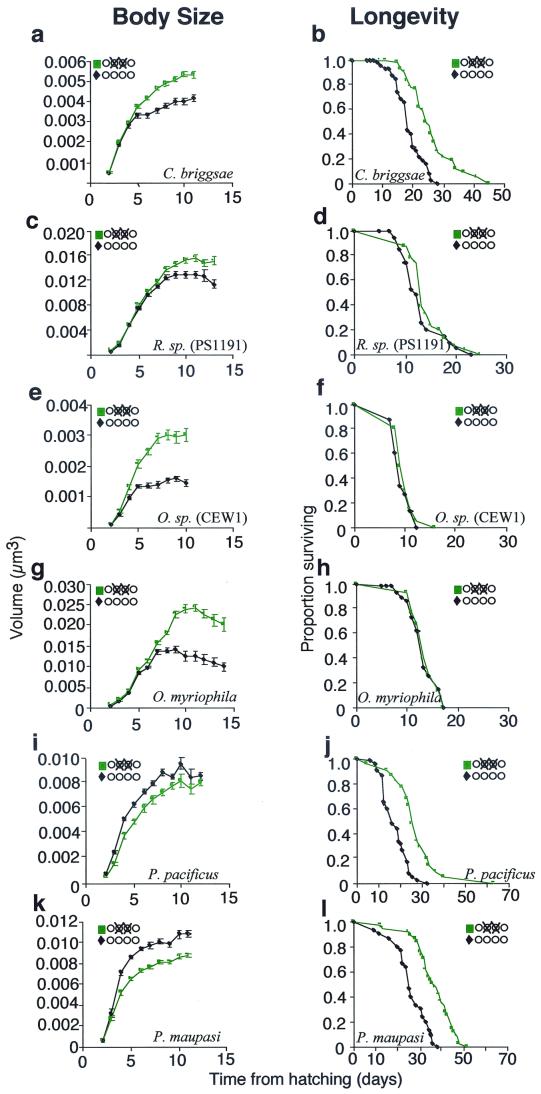
In our original experiment, in which we ablated the germ-line precursors of the standard wild-type strain, N2, we also looked at longevity. We found that germ-line ablation increased longevity by only 2.8 days or 32% [intact control, 8.7 ± 0.3 days; germ line (−), 11.5 ± 0.6, P < 0.001, n =115]. This finding was a surprise because Hsin and Kenyon (12) had shown that germ-line ablation of N2 causes mean longevity to increase by 12 days (64%). We requested Kenyon's N2 and repeated the experiment in both N2 strains simultaneously. We confirmed that germ-line ablation in our N2 causes mean longevity to increase by about 3 days, but found that identical treatment of Kenyon's N2 causes a mean longevity increase of 8 days (70%). We also found that germ-line ablation in Kenyon's N2 causes adult body size to increase by only by 0.0011 mm3, or 15%, far less than the 0.0026 mm3 or 46% that we found in our N2. We also found that unablated hermaphrodites of Kenyon's N2 are slightly (2 days) longer lived than ours and 0.0012 mm3 or 22% larger. Because both N2s are derived from a common ancestor, the C. elegans Genetics Center N2, it appears that body size and longevity as well the response to germ-line ablation, has evolved rapidly in these strains.
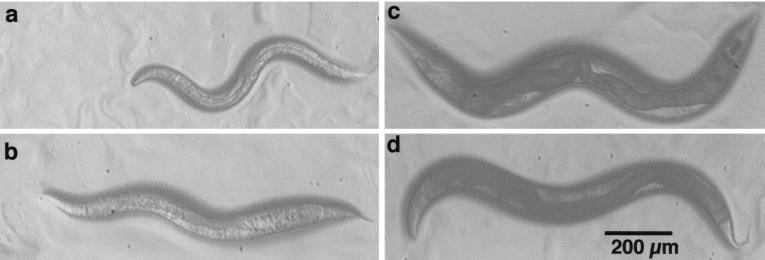
This surprising result prompted us to ask whether other species of free-living nematodes would show even greater variation in their responses to germ-line ablation. Hsin and Kenyon (12) suggested that the gonad longevity signals that they identified in C. elegans might be conserved throughout the Nematoda, but they examined only one other species, P. pacificus. We therefore examined six other species related in varying degrees to C. elegans (Fig. 3). Like C. elegans, all of these species have hatchlings in which four gonad precursors cells are visible, the two median cells being larger than the flanking pair, and presumably homologous to Z2 and Z3. We found that, as in C. elegans, ablation of these cells causes worms to grow up with an empty somatic gonad (data not shown). Germ-line ablation in Caenorhabditis briggsae (AF16) causes significant increases in both longevity and body size. But germ-line ablation in three species, Oscheius myriophila (BW190), Oscheius sp. (CEW1), and their relative Rhabditis sp. (PS1191), caused no significant increase in longevity but large increases in body size (Fig. 3). The increase in relative body size was particularly pronounced in Oscheius sp. (CEW1); germ-line-ablated animals were nearly twice as large as those with intact gonads (Fig. 4). In contrast to Oscheius and Rhabditis, we found that germ-line ablation of two species of Pristionchus, P. pacificus (PS1843) and P. maupasi (PS321), caused a significant increase in longevity, but no increase in adult body size (Figs. 3 and 4), indeed, the body size of germ-line-ablated worms of these species tended to be slightly smaller than those of worms with intact gonads. We attribute this to the slimming effect of having a gonad devoid of eggs.
Figure 3.
Response of selected free-living nematode species to ablation of germ-line precursor cells, Z2/3. Intact control worms (♦); Z2/3(−) worms (▪). (a and b) Caenorhabditis briggsae (AF16), intact control n = 35(2); v = 0.0044 mm3 (± 0.0001); Z2/3(−), n = 44(2), v = 0.0056 mm3 (± 0.0001), P < 0.0001; intact control, n = 45(2), l = 18.6 ± (0.8); Z2/3(−), n = 44(2), l = 26.2 (± 1.2), P < 0.0001. (c and d) Rhabditis sp. (PS1191), intact control, n = 25(1), v = 0.0138 mm3 (± 0.0005); Z2/3(−), n = 27(1), v = 0.0158 mm3 (± 0.0005), P = 0.007; intact control, n = 34 (1), l = 12.9 (± 0.8); Z2/3(−), n = 31(1), l = 14.2 (± 0.7), P = 0.2. (e and f) O. sp. (CEW1), intact control, n = 15(1), v = 0.0017 mm3 (± 0.0003); Z2/3(−), n = 20(1), v = 0.0033 mm3 (± 0.0008), P < 0.0001; intact control, n = 15(1), l = 9.2 (± 0.4); Z2/3(−), n = 20(1), l = 9.9 (± 0.4), P = 0.2. (g and h) O. myriophila (BW290), intact control, n = 54(1), v = 0.0200 mm3 (± 0.0008); Z2/3(−), n = 30(1), v = 0.0260 mm3 (± 0.00009), P < 0.0001; intact control, n = 54 (1); l = 12.9 (± 0.4); Z2/3(−), n = 30(1); l = 13.4 (± 0.5), P = 0.6. (i and j) P. pacificus (PS1843), intact control, n = 42(2), v = 0.0095 mm3 (± 0.0002); Z2/3(−), n = 21(2), v = 0.0083 mm3 (± 0.0004), P < 0.0001; intact control, n = 42(2), l = 17.1 (± 0.9); Z2/3(−), n = 21(2), l = 27.1 (± 2.5), P < 0.0001. (k and l) P. maupasi (PS321), intact control, n = 30(2), v = 0.0119 mm3 (± 0.0003); Z2/3(−), n = 36(2), v = 0.0097 mm3 (± 0.0002), P < 0.0001; intact control, n = 30(2), l = 25.6 (± 1.4); Z2/3(−), n = 36(2), l = 36.3 (± 1.5), P < 0.0001.
Figure 4.
Intact (Upper) and Z2/3(−) ablated (Lower) 11-day-old nematodes of O. sp. (CEW1; a and b) and P. pacificus (c and d).
Discussion
Gonadectomy experiments provide a classic line of evidence for the idea that fecundity exacts a somatic cost (4, 8, 31). Our finding that germ-line-ablated C. elegans hermaphrodites become giant is consistent with this idea. However, our finding that gigantism also occurs in germ-line-ablated worms that carry a sterility mutation (and which therefore gain few, if any, additional somatic resources relative to unablated worms) suggests that the immediate cause of the gigantism is not the absence of fecundity per se, but merely the absence of the germ line. We propose, then, that the C. elegans germ line is the source of a growth-repressing signal. Additional evidence for this signal comes from the finding that some nematode species (of the genus Pristionchus) do not increase in body size when their germ lines are ablated despite having lifetime self fecundities comparable to those that do (of the genera Caenorhabditis, Oscheius, and Rhabditis; data not shown).
Our results parallel the recent finding that the germ line is the source of a longevity-repressing signal (12). However, because the longevity signal is daf-16-repressible and the growth-repressing signal is not, either the somatic targets of these signals or possibly the signals themselves are different. Our survey of nematode species provides additional evidence for this hypothesis because not only are these signals genetically separable, they are evolutionarily separable as well. We speculate that the roles of these germ-line signals are to regulate growth and longevity in response to environmental variation (6, 12).
Such signals are likely not unique to nematodes. Around 335 B.C. the Greek biologist Aristotle noted that mammals grow up to be unusually large when gonadectomized as juveniles (23), an observation that has often been confirmed since (24–27). Men who have been castrated in childhood frequently grow unusually tall—the so called “eunuchoid” phenotype shown by Chinese and Ottoman court eunuchs, 18th century Italian castrati, and modern hypogonadal patients (28). Such men have unusually long limbs because of continued linear growth after age 20, which, in turn, is caused by a failure of long-bone growth plates to fuse in late adolescence (28). This finding implies that the human gonad is the source of a signal required for growth plate fusion. The signaling molecule itself is thought to be estrogen because testes are a major source of serum estrogen and men with estrogen-signaling deficiencies are also eunuchoid (29–31). Both estrogen and the unknown nematode gonadal signal are candidates for the systemic growth-repressing signals that have been proposed to regulate adult body size in metazoans (32, 33). However, gonadal signals in both nematodes and mammals can only be part of the growth-stopping mechanism because the absence of either permits extended, but not indefinite, adult growth.
At least one study has also noted that N2 strains from different laboratories differ in mean longevity (34). Our finding that the effect of gonad ablation on body size and longevity also varies among strains emphasizes the need to standardize genetic background when studying the genetically complex traits such as longevity and body size. On the other hand, this finding also suggests that microevolutionary variation might reveal important insights into the regulation of life-history in nematodes (35, 36).
The Rhabditid genera Caenorhabditis, Oscheius, and Rhabditis are closely related (37), but the Diplogasterid genus Pristionchus is thought to have separated from the lineage leading to C. elegans 100 million years ago (38). Our survey shows that neither the germ-line growth-signaling pathway nor the germ-line longevity signaling pathway are conserved within the Nematoda; the genera Oscheius and Rhabditis lack the longevity signal, Pristionchus lacks the body size signal, but Caenorhabditis has both. Our survey is not extensive enough to indicate whether the last common ancestor of these species (a primitive Secernentean) possessed either signal. In failing to show increased longevity in response to germ-line ablation, Oscheius and Rhabditis species mimic the effects of daf-16(0) mutations. These species may lack daf-16, but because daf-16 is an important component of the dauer formation pathway (14, 15) and all of the species studied here are capable of forming dauers (data not shown), a more likely explanation is that they lack the longevity signal itself or its receptor. A direct test of this hypothesis must, however, await the molecular identification of the germ-line longevity signal. Our study shows evolutionary variation in the signaling pathways that control growth and senescence. Recent cell-ablation studies have revealed that the intercellular signals responsible for vulval specification also vary greatly within the nematode taxa studied here (37–40). We speculate that the gain and loss of intercellular signaling pathways may be a major device by which diversity in cell fate, body size, and life history is produced in the course of evolution.
Acknowledgments
We thank Lynn Carta, Paul Sternberg, and Cynthia Kenyon for strains, as well as the Caenorhabditis Genetics Center, which is funded by the National Institutes for Health National Center for Research Resources. We thank Naoto Ueno, Simon Tuck, Marie-Anne Félix, an anonymous reviewer, and members of the Leroi laboratory for comments on the manuscript, and David Fitch, Lynn Carta, and Cynthia Kenyon for answering queries about methods and taxonomy. This study was supported by Biotechnology and Biological Sciences Research Council (U.K.) and Royal Society (U.K.) grants (to A.M.L.).
Abbreviation
- TGF-β
transforming growth factor β
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Williams G C. Am Nat. 1966;100:687–690. [Google Scholar]
- 2.Stearns S. The Evolution of Life-Histories. New York: Oxford Univ. Press; 1992. [Google Scholar]
- 3.Roff D A. The Evolution of Life-Histories. New York: Chapman & Hall; 1992. [Google Scholar]
- 4.Bell G, Koufopanou V. Oxford Surv Evol Biol. 1986;3:83–131. [Google Scholar]
- 5.Rose M R, Bradley T J. Oikos. 1998;83:443–451. [Google Scholar]
- 6.Leroi A M. Trends Ecol Evol. 2001;16:24–29. doi: 10.1016/s0169-5347(00)02032-2. [DOI] [PubMed] [Google Scholar]
- 7.Reznick D. Oikos. 1985;44:257–267. [Google Scholar]
- 8.Sgrò C M, Partridge L. Science. 1999;286:2521–2524. doi: 10.1126/science.286.5449.2521. [DOI] [PubMed] [Google Scholar]
- 9.Westendorp R G, Kirkwood T B L. Nature (London) 1998;396:743–746. doi: 10.1038/25519. [DOI] [PubMed] [Google Scholar]
- 10.Roff D A. J Evol Biol. 2000;13:434–445. [Google Scholar]
- 11.Reznick D, Nunnev L, Tessier A. Trends Ecol Evol. 2000;15:421–425. doi: 10.1016/s0169-5347(00)01941-8. [DOI] [PubMed] [Google Scholar]
- 12.Hsin H, Kenyon C. Nature (London) 1999;399:362–366. doi: 10.1038/20694. [DOI] [PubMed] [Google Scholar]
- 13.Gems D, Riddle D L. Nature (London) 1996;376:723–725. doi: 10.1038/379723a0. [DOI] [PubMed] [Google Scholar]
- 14.Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R A. Nature (London) 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- 15.Ogg S, Paradis S, Gottlieb S, Patterson G I, Lee L, Tissenbaum H A, Ruvkun G. Nature (London) 1997;389:994–999. doi: 10.1038/40194. [DOI] [PubMed] [Google Scholar]
- 16.Lin K, Dorman J B, Rodan A, Kenyon C. Science. 1997;278:1319–1322. doi: 10.1126/science.278.5341.1319. [DOI] [PubMed] [Google Scholar]
- 17.Argon Y, Ward S. Genetics. 1980;96:413–433. doi: 10.1093/genetics/96.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morita K, Chow K L, Ueno N. Development (Cambridge, UK) 1999;126:1337–1347. doi: 10.1242/dev.126.6.1337. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki Y, Yandell M D, Roy P J, Krishna S, Savage-Dunn C, Ross R M, Padgett R W, Wood W B. Development (Cambridge, UK) 1999;126:241–250. doi: 10.1242/dev.126.2.241. [DOI] [PubMed] [Google Scholar]
- 20.Patterson G I, Padgett R W. Trends Genet. 2000;16:27–33. doi: 10.1016/s0168-9525(99)01916-2. [DOI] [PubMed] [Google Scholar]
- 21.Flemming A J, Shen Z Z, Cunha A, Emmons S W, Leroi A M. Proc Natl Acad Sci USA. 2000;97:5285–5290. doi: 10.1073/pnas.97.10.5285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamb M J. J Insect Physiol. 1964;10:4871. [Google Scholar]
- 23.Aristotle Historia Animalium. trans. Thompson, D. W. (1910) in Works of Aristotle, eds. Smith, J. A. & Ross, W. D. (Oxford Univ. Press, Oxford). pp. 631b–632a.
- 24.Singh D, Prakash P, Goel V D. Indian Vet J. 1987;64:560–562. [Google Scholar]
- 25.Salmeri K R, Bloomberg M S, Scruggs S L, Shille V. J Am Vet Med Assoc. 1991;198:1193–1203. [PubMed] [Google Scholar]
- 26.Root M V, Johnston S D, Olson P N. Vet Radiol Ultrasound. 1997;38:42–47. doi: 10.1111/j.1740-8261.1997.tb01601.x. [DOI] [PubMed] [Google Scholar]
- 27.Fettman M J, Stanton C A, Banks L L, Johnson D G, Hamar D W, Hegstad R L, Johnston S. Res Vet Sci. 1997;62:131–136. doi: 10.1016/s0034-5288(97)90134-x. [DOI] [PubMed] [Google Scholar]
- 28.Wilson J D, Roehrborn C. J Clin Endocrinol Metab. 1999;84:4324–4331. doi: 10.1210/jcem.84.12.6206. [DOI] [PubMed] [Google Scholar]
- 29.Smith E P, Boyd J, Frank G R, Takahashi H, Cohen R M, Specker B, Williams T C, Lubahn D B, Korach K S. N Engl J Med. 1994;331:1056–1061. doi: 10.1056/NEJM199410203311604. [DOI] [PubMed] [Google Scholar]
- 30.Sharpe R M. Trends Endocrinol Metab. 1998;9:371–377. doi: 10.1016/s1043-2760(98)00089-7. [DOI] [PubMed] [Google Scholar]
- 31.Lee P A, Witchel S F. Curr Opin Ped. 1997;9:431–436. doi: 10.1097/00008480-199708000-00020. [DOI] [PubMed] [Google Scholar]
- 32.Conlon I, Raff M. Cell. 1999;96:235–244. doi: 10.1016/s0092-8674(00)80563-2. [DOI] [PubMed] [Google Scholar]
- 33.Day S J, Lawrence P A. Development (Cambridge, UK) 2000;127:2977–2987. doi: 10.1242/dev.127.14.2977. [DOI] [PubMed] [Google Scholar]
- 34.Gems D, Riddle D L. J Gerontol A Biol Sci. 2000;55:B215–B219. doi: 10.1093/gerona/55.5.b215. [DOI] [PubMed] [Google Scholar]
- 35.Delattre M, Félix M-A. BioEssays. 2001;23:807–819. doi: 10.1002/bies.1116. [DOI] [PubMed] [Google Scholar]
- 36.Knight C G, Azevedo R B R, Leroi A M. Evolution (Lawrence, Kans) 2001;55:1795–1804. doi: 10.1111/j.0014-3820.2001.tb00828.x. [DOI] [PubMed] [Google Scholar]
- 37.Félix M-A. J Exp Zool. 1999;285:3–18. doi: 10.1002/(sici)1097-010x(19990415)285:1<3::aid-jez2>3.3.co;2-a. [DOI] [PubMed] [Google Scholar]
- 38.Eizinger A, Sommer R J. Science. 1997;278:452–455. doi: 10.1126/science.278.5337.452. [DOI] [PubMed] [Google Scholar]
- 39.Sigrist C B, Sommer R J. Dev Genes Evol. 1999;209:451–459. doi: 10.1007/s004270050278. [DOI] [PubMed] [Google Scholar]
- 40.Sommer R J. Curr Opin Genet Dev. 2000;10:443–448. doi: 10.1016/s0959-437x(00)00110-6. [DOI] [PubMed] [Google Scholar]