Abstract
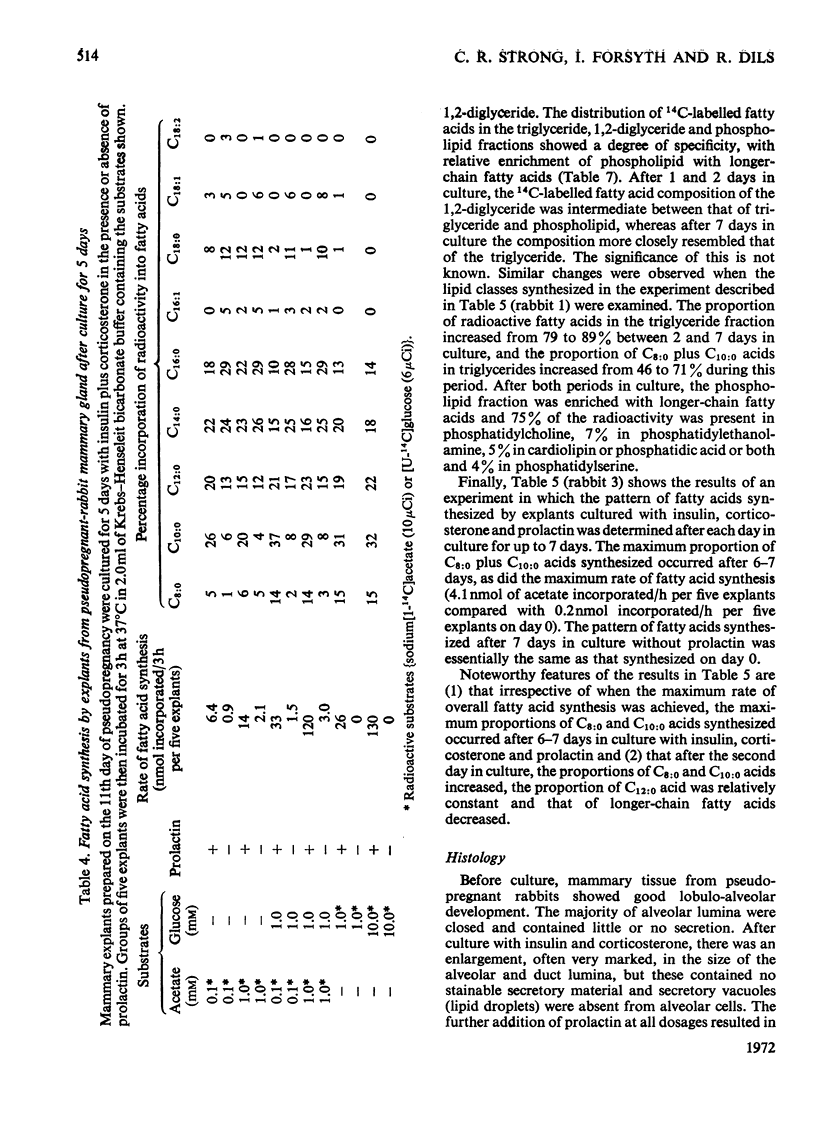
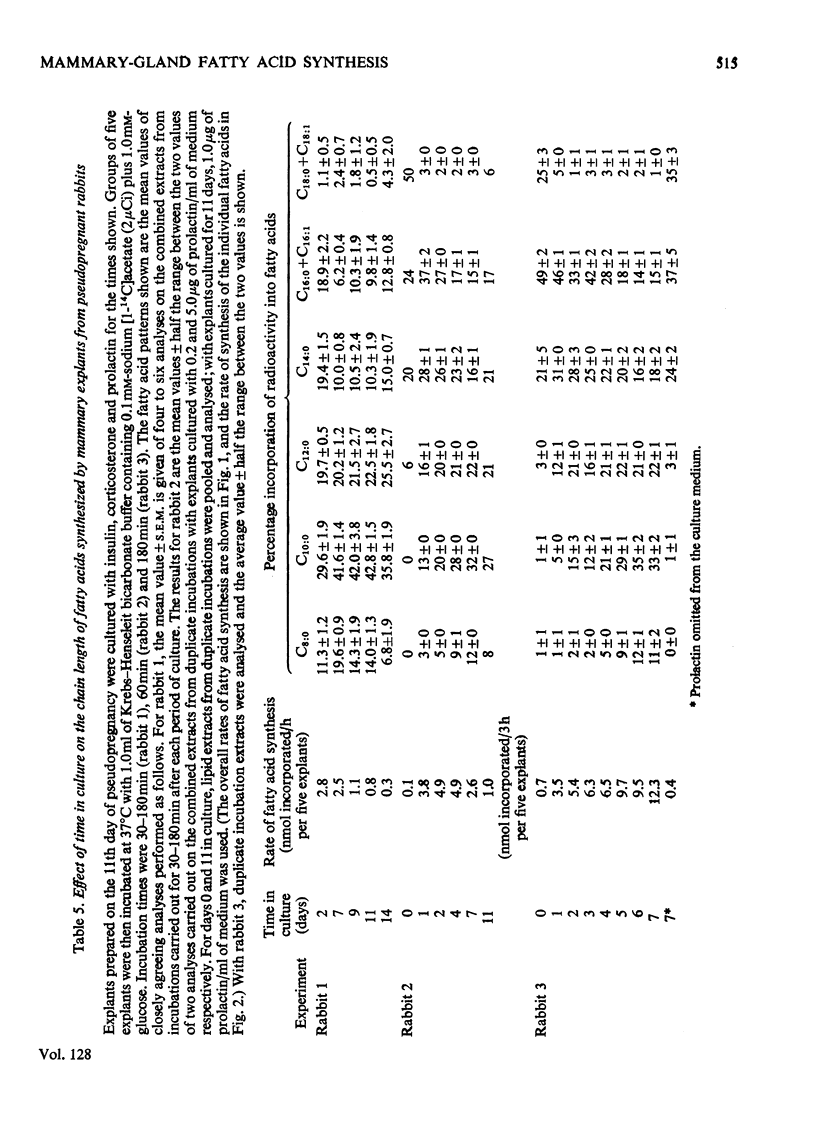
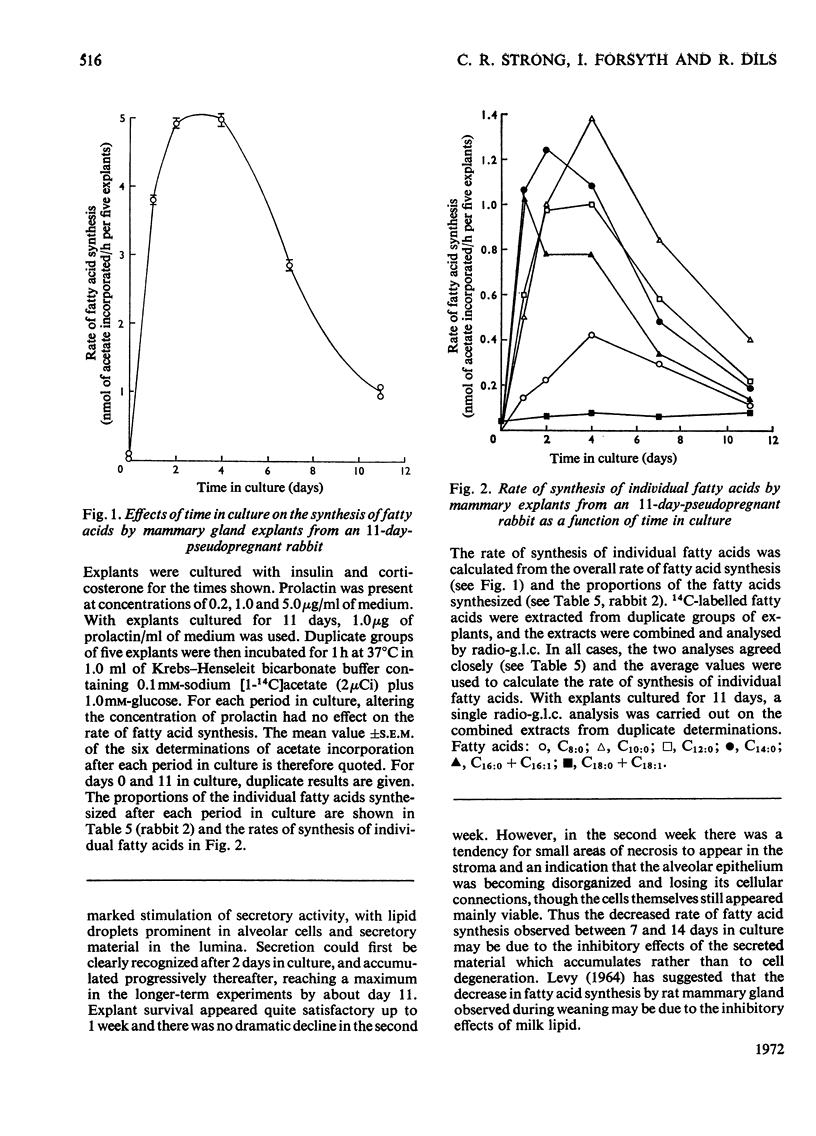
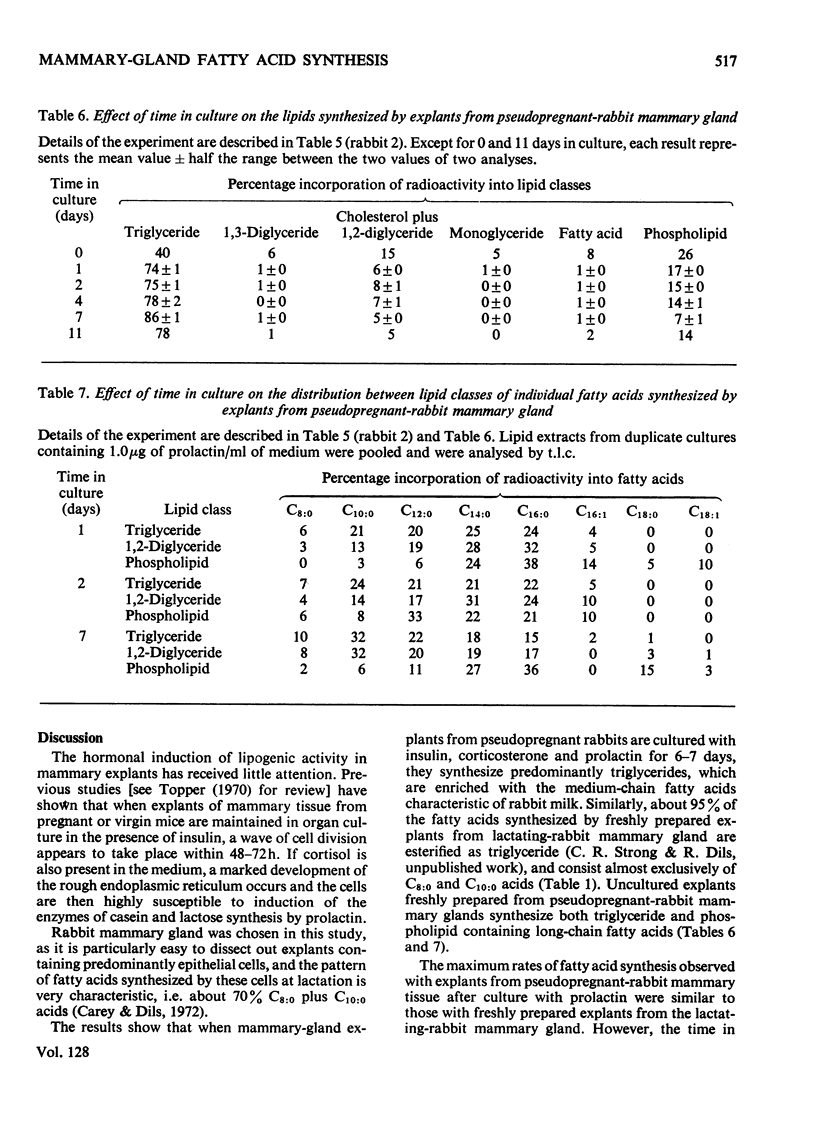
1. When freshly prepared explants from pseudopregnant-rabbit mammary gland were incubated with sodium [1-14C]acetate plus glucose, they synthesized triglyceride and phospholipid containing long-chain fatty acids. Explants cultured with insulin and corticosterone also synthesized these products. The addition of prolactin to this culture medium increased the rate of fatty acid synthesis up to 40-fold and the explants synthesized predominantly triglyceride enriched with C8:0 and C10:0 fatty acids characteristic of rabbit milk. 2. The maximum rates of fatty acid synthesis obtained by explants from pseudopregnant-rabbit mammary gland after culture with insulin, corticosterone and prolactin were similar to those observed with freshly prepared explants from lactating-rabbit mammary gland. The time in culture required to attain these maximum rates varied between animals, and did not appear to be connected with the time required (6–7 days) to synthesize the maximum proportions of C8:0 and C10:0 acids. 3. As the pattern of short- and medium-chain milk fatty acids is characteristic for many species, the techniques described to determine the time-course for the development of this pattern can be used to investigate hormonal response.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnawell E. B. A comparative study of the responses of mammary tissues from several mammalian species to hormones in vitro. J Exp Zool. 1965 Nov;160(2):189–206. doi: 10.1002/jez.1401600206. [DOI] [PubMed] [Google Scholar]
- Bolton C. E., Bolton A. E. Effect of prolactin on pathways of glucose oxidation in explants from rabbit mammary gland. FEBS Lett. 1970 Sep 6;9(3):177–179. doi: 10.1016/0014-5793(70)80348-9. [DOI] [PubMed] [Google Scholar]
- Carey E. M., Dils R. Fatty acid biosynthesis. V. Purification and characterisation of fatty acid synthetase from lactating-rabbit mammary gland. Biochim Biophys Acta. 1970 Sep 8;210(3):371–387. doi: 10.1016/0005-2760(70)90033-0. [DOI] [PubMed] [Google Scholar]
- Carey E. M., Dils R. Fatty acid biosynthesis. VI. Specificity for termination of fatty acid biosynthesis by fatty acid synthetase from lactating-rabbit mammary gland. Biochim Biophys Acta. 1970 Sep 8;210(3):388–399. doi: 10.1016/0005-2760(70)90034-2. [DOI] [PubMed] [Google Scholar]
- Carey E. M., Dils R. The pattern of fatty acid synthesis in lactating rabbit mammary gland studied in vivo. Biochem J. 1972 Feb;126(4):1005–1007. doi: 10.1042/bj1261005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsyth I. A., Myres R. P. Human prolactin. Evidence obtained by the bioassay of human plasma. J Endocrinol. 1971 Sep;51(1):157–168. doi: 10.1677/joe.0.0510157. [DOI] [PubMed] [Google Scholar]
- Forsyth I. A. Reviews of the progress of dairy science. Section A. Physiology. Organ culture techniques and the study of hormone effects on the mammary gland. J Dairy Res. 1971 Oct;38(3):419–444. [PubMed] [Google Scholar]
- LEVY H. R. THE EFFECTS OF WEANING AND MILK ON MAMMARY FATTY ACID SYNTHESIS. Biochim Biophys Acta. 1964 Jun 15;84:229–238. doi: 10.1016/0926-6542(64)90052-6. [DOI] [PubMed] [Google Scholar]
- Mayne R., Barry J. M. Biochemical changes during development of mouse mammary tissue in organ culture. J Endocrinol. 1970 Jan;46(1):61–70. doi: 10.1677/joe.0.0460061. [DOI] [PubMed] [Google Scholar]
- Mills E. S., Topper Y. J. Some ultrastructural effects of insulin, hydrocortisone, and prolactin on mammary gland explants. J Cell Biol. 1970 Feb;44(2):310–328. doi: 10.1083/jcb.44.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti R. L., Abraham S. Effects of insulin on glucose metabolism by explants of mouse mammary gland maintained in organ culture. Biochim Biophys Acta. 1966 Aug 24;124(2):280–288. doi: 10.1016/0304-4165(66)90191-7. [DOI] [PubMed] [Google Scholar]
- POPJAK G., HUNTER G. D., FRENCH T. H. Biosynthesis of milk-fat in the rabbit from acetate and glucose; the mode of conversion of carbohydrate into fat. Biochem J. 1953 May;54(2):238–247. doi: 10.1042/bj0540238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter R. E. Feedback effects of thyroxine on the hypothalamus and pituitary of goldfish, Carassius auratus. J Endocrinol. 1971 Sep;51(1):31–39. doi: 10.1677/joe.0.0510031. [DOI] [PubMed] [Google Scholar]
- SMITH S., DILS R. FACTORS EFFECTING THE CHAIN-LENGTH OF FATTY ACIDS SYNTHESISED BY LACTATING-RABBIT MAMMARY GLANDS. Biochim Biophys Acta. 1964 Dec 2;84:776–778. doi: 10.1016/0926-6542(64)90044-7. [DOI] [PubMed] [Google Scholar]
- Smith S., Dils R. Factors affecting the chain length of fatty acids synthesised by lactating-rabbit mammary glands. Biochim Biophys Acta. 1966 Feb 1;116(1):23–40. doi: 10.1016/0005-2760(66)90089-0. [DOI] [PubMed] [Google Scholar]
- Smith S., Watts R., Dils R. Quantitative gas-liquid chromatographic analysis of rodent milk triglycerides. J Lipid Res. 1968 Jan;9(1):52–57. [PubMed] [Google Scholar]
- Strong C., Dils R., Forsyth I. A. The effects of prolactin on fatty acid synthesis by rabbit mammary gland in vitro. J Endocrinol. 1971 Oct;51(2):32–33. [PubMed] [Google Scholar]
- Topper Y. J. Multiple hormone interactions in the development of mammary gland in vitro. Recent Prog Horm Res. 1970;26:287–308. doi: 10.1016/b978-0-12-571126-5.50011-x. [DOI] [PubMed] [Google Scholar]


