Abstract
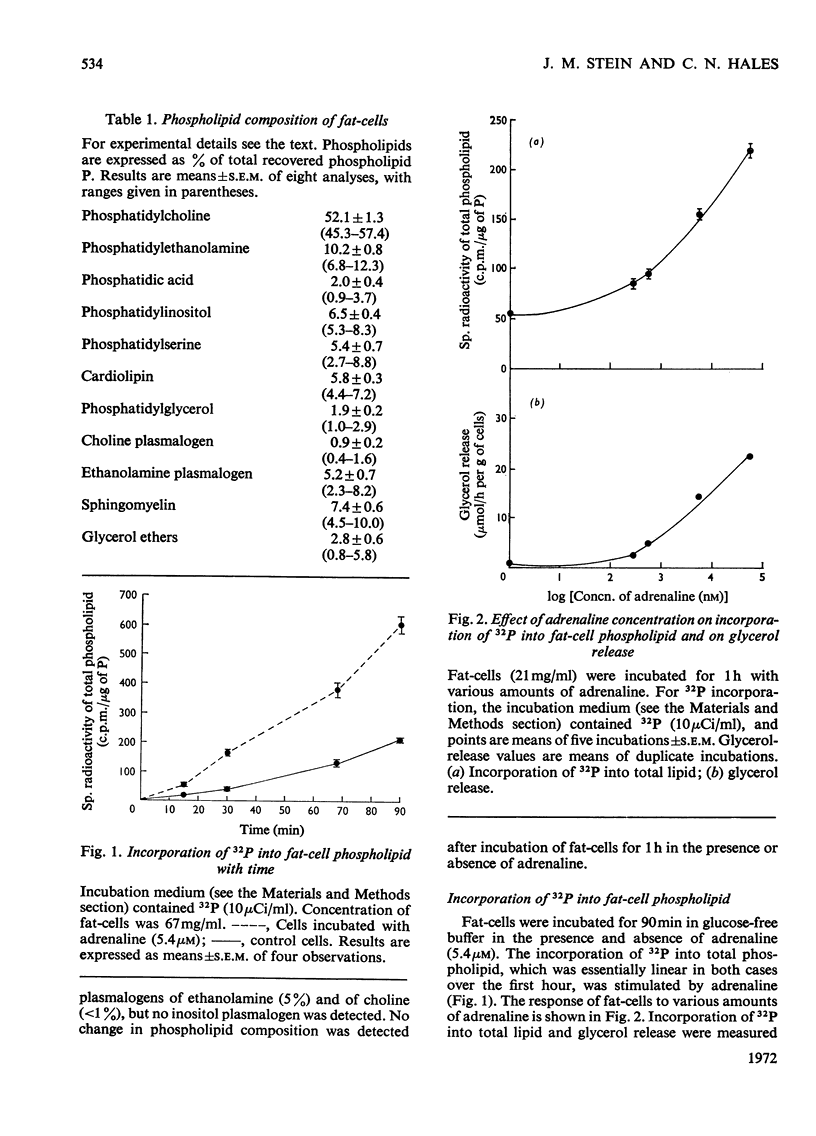
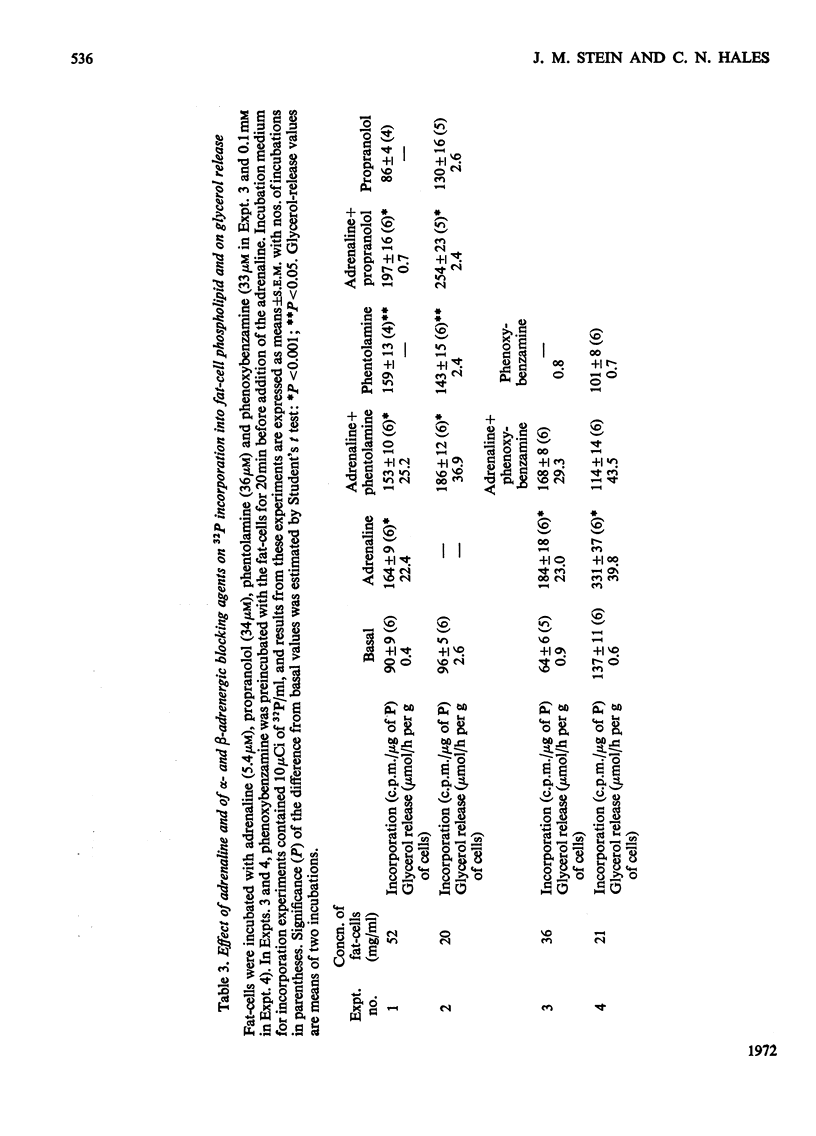
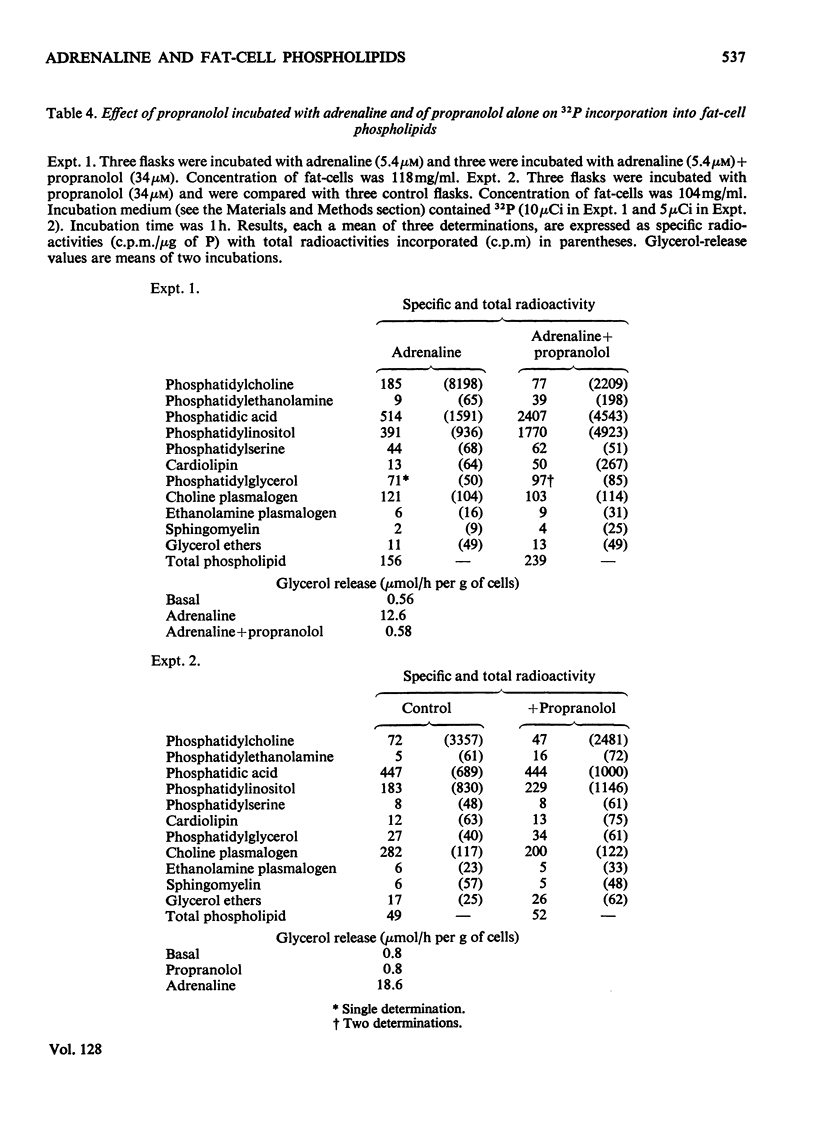
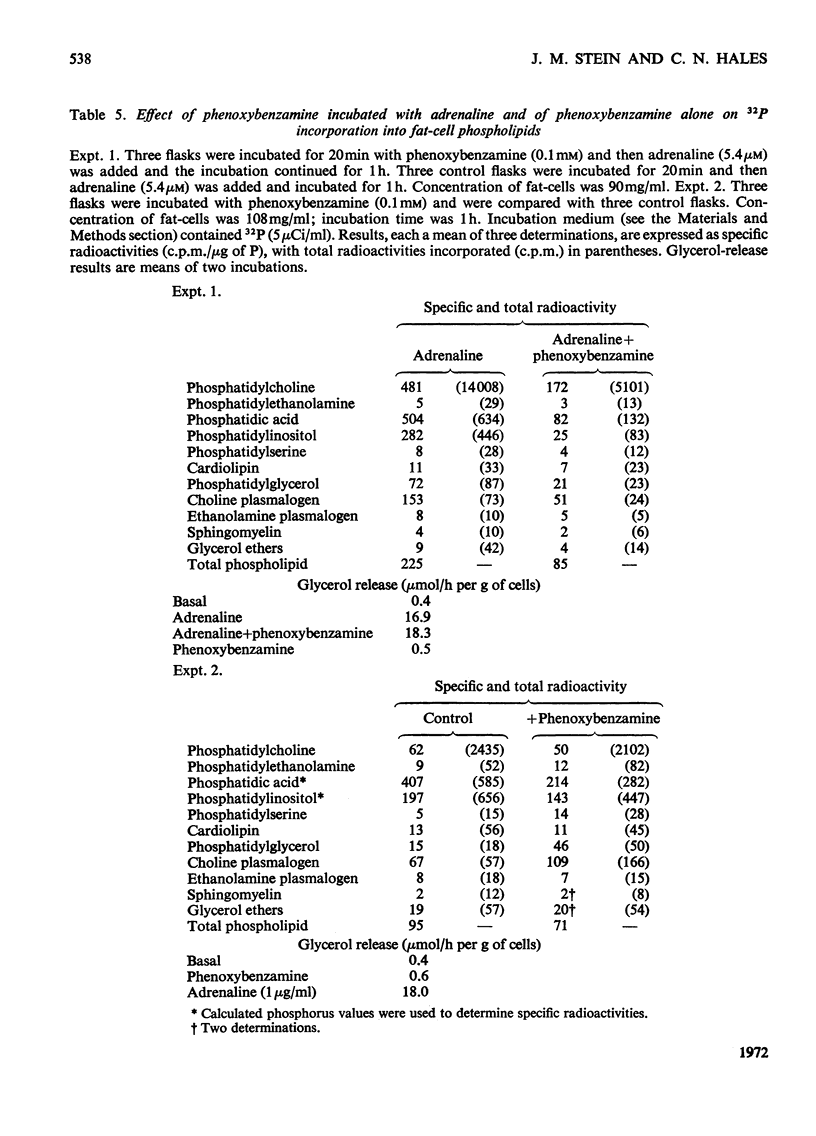
1. The phospholipid composition of fat-cells prepared from rat epididymal fat-pad was determined. 2. The incorporation of [32P]Pi into the phospholipids of fat-cells incubated in glucose-free medium and the effect of adrenaline and of α- and β-adrenergic blocking agents, were studied. 3. Incorporation of [32P]Pi into fat-cell phospholipid increased with time; incubation with adrenaline resulted in increased incorporation that was related to the concentration of adrenaline. 4. The pattern of incorporation of [32P]Pi into the individual phospholipids of fat-cells after incubation for 1h was determined; adrenaline (5.4μm) resulted in increased incorporation into phosphatidylcholine. 5. Incubation of fat-cells with propranolol (34μm) and adrenaline (5.4μm) resulted in abolition of adrenaline-stimulated lipolysis; there was a decrease in the specific radioactivity of phosphatidylcholine and an increase in the specific radioactivity of phosphatidylethanolamine, phosphatidic acid, phosphatidylinositol and cardiolipin compared with cells incubated with adrenaline alone. 6. Incubation of fat-cells with phenoxybenzamine (0.1mm) and adrenaline (5.4μm) resulted in stimulation of lipolysis, and in diminished specific radioactivities of phosphatidylcholine, phosphatidic acid, phosphatidylinositol, phosphatidylglycerol and choline plasmalogen compared with cells stimulated with adrenaline alone.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BELLEAU B., TRIGGLE D. J. BLOCKADE OF ADRENERGIC ALPHA-RECEPTORS BY A CARBONIUM ION. J Med Pharm Chem. 1962 May;91:636–639. doi: 10.1021/jm01238a023. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- BORGSTROM B. Investigation on lipid separation methods. Separation of phospholipids from neutral fat and fatty acids. Acta Physiol Scand. 1952 Jun 6;25(2-3):101–110. doi: 10.1111/j.1748-1716.1952.tb00862.x. [DOI] [PubMed] [Google Scholar]
- Bihler I., Jeanrenaud B. ATP content of isolated fat cells. Effects of insulin, ouabain, and lipolytic agents. Biochim Biophys Acta. 1970 May 5;202(3):496–506. doi: 10.1016/0005-2760(70)90120-7. [DOI] [PubMed] [Google Scholar]
- Blecher M. Effects of insulin and phospholipase A on glucose transport across the plasma membrane of free adipose cells. Biochim Biophys Acta. 1967 Jun 6;137(3):557–571. doi: 10.1016/0005-2760(67)90137-3. [DOI] [PubMed] [Google Scholar]
- Burke G. Effects of adrenergic blocking agents on basal and stimulated thyroid function. Metabolism. 1969 Nov;18(11):961–967. doi: 10.1016/0026-0495(69)90036-5. [DOI] [PubMed] [Google Scholar]
- Burns T. W., Langley P. E. Lipolysis by human adipose tissue: the role of cyclic 3',5'-adenosine monophosphate and adrenergic receptor sites. J Lab Clin Med. 1970 Jun;75(6):983–997. [PubMed] [Google Scholar]
- Butcher R. W., Ho R. J., Meng H. C., Sutherland E. W. Adenosine 3',5'-monophosphate in biological materials. II. The measurement of adenosine 3',5'-monophosphate in tissues and the role of the cyclic nucleotide in the lipolytic response of fat to epinephrine. J Biol Chem. 1965 Nov;240(11):4515–4523. [PubMed] [Google Scholar]
- Butcher R. W., Sutherland E. W. The effects of the catecholamines, adrenergic blocking agents, prostaglandin E1, and insulin on cyclie AMP levels in the rat epididymal fat pad in vitro. Ann N Y Acad Sci. 1967 Feb 10;139(3):849–859. doi: 10.1111/j.1749-6632.1967.tb41255.x. [DOI] [PubMed] [Google Scholar]
- Clausen T. Measurement of 32P activity in a liquid scintillation counter without the use of scintillator. Anal Biochem. 1968 Jan;22(1):70–73. doi: 10.1016/0003-2697(68)90260-1. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Interaction of insulin with the cell membrane: the primary action of insulin. Proc Natl Acad Sci U S A. 1969 Jun;63(2):450–457. doi: 10.1073/pnas.63.2.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Torrontegui G., Berthet J. The action of adrenalin and glucagon on the metabolism of phospholipids in rat liver. Biochim Biophys Acta. 1966 Jun 1;116(3):467–476. doi: 10.1016/0005-2760(66)90116-0. [DOI] [PubMed] [Google Scholar]
- De Torrontegui G., Berthet J. The action of insulin on the incorporation of [32P]phosphate in the phospholipids of rat adipose tissue. Biochim Biophys Acta. 1966 Jun 1;116(3):477–481. doi: 10.1016/0005-2760(66)90117-2. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- GARLAND P. B., RANDLE P. J. A rapid enzymatic assay for glycerol. Nature. 1962 Dec 8;196:987–988. doi: 10.1038/196987a0. [DOI] [PubMed] [Google Scholar]
- Hepp D., Challoner D. R., Williams R. H. Studies on the action of insulin in isolated adipose tissue cells. I. Stimulation of incorporation of 32P-labeled inorganic phosphate into mononucleotides in the absence of glucose. J Biol Chem. 1968 Aug 10;243(15):4020–4026. [PubMed] [Google Scholar]
- Jarett L., Reuter M., McKeel D. W., Smith R. M. Loss of adenyl cyclase hormone receptors during purification of fat cell plasma membranes. Endocrinology. 1971 Nov;89(5):1186–1190. doi: 10.1210/endo-89-5-1186. [DOI] [PubMed] [Google Scholar]
- Jungalwala F. B., Dawson R. M. Phospholipid synthesis and exchange in isolated liver cells. Biochem J. 1970 Apr;117(3):481–490. doi: 10.1042/bj1170481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENNEDY E. P. Biosynthesis of complex lipids. Fed Proc. 1961 Dec;20:934–940. [PubMed] [Google Scholar]
- KIYASU J. Y., KENNEDY E. P. The enzymatic synthesis of plasmalogens. J Biol Chem. 1960 Sep;235:2590–2594. [PubMed] [Google Scholar]
- KLAINER L. M., CHI Y. M., FREIDBERG S. L., RALL T. W., SUTHERLAND E. W. Adenyl cyclase. IV. The effects of neurohormones on the formation of adenosine 3',5'-phosphate by preparations from brain and other tissues. J Biol Chem. 1962 Apr;237:1239–1243. [PubMed] [Google Scholar]
- Kerkof P. R., Tata J. R. The subcellular distribution of 32P-labelled phospholipids, 32P-labelled ribonucleic acid and 125I-labelled odoprotein in pig thyroid slices. Effect in vitro of thyrotrophic hormone and dibutyryl-3',5'-(cyclic)-adeosine onophosphate. Biochem J. 1969 May;112(5):729–739. doi: 10.1042/bj1120729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEAL R. S., GREENBAUM A. L. Incorporation of phosphorylcholine labelled with phosphorus-32 into the liver phospholipids of rats treated with growth hormone. Nature. 1961 May 6;190:548–549. doi: 10.1038/190548a0. [DOI] [PubMed] [Google Scholar]
- McMurray W. C., Dawson R. M. Phospholipid exchange reactions within the liver cell. Biochem J. 1969 Mar;112(1):91–108. doi: 10.1042/bj1120091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry M. C., Hales C. N. Factors affecting the permeability of isolated fat-cells from the rat to [42K] potassium and [36Cl] chloride ions. Biochem J. 1970 Apr;117(3):615–621. doi: 10.1042/bj1170615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Rodbell M., Jones A. B., Chiappe de Cingolani G. E., Birnbaumer L. The actions of insulin and catabolic hormones on the plasma membrane of the fat cells. Recent Prog Horm Res. 1968;24:215–254. doi: 10.1016/b978-1-4831-9827-9.50011-3. [DOI] [PubMed] [Google Scholar]
- Rodbell M., Jones A. B. Metabolism of isolated fat cells. 3. The similar inhibitory action of phospholipase C (Clostridium perfringens alpha toxin) and of insulin on lipolysis stimulated by lipolytic hormones and theophylline. J Biol Chem. 1966 Jan 10;241(1):140–142. [PubMed] [Google Scholar]
- Rodbell M. Metabolism of isolated fat cells. II. The similar effects of phospholipase C (Clostridium perfringens alpha toxin) and of insulin on glucose and amino acid metabolism. J Biol Chem. 1966 Jan 10;241(1):130–139. [PubMed] [Google Scholar]
- SRIBNEY M., KENNEDY E. P. The enzymatic synthesis of sphingomyelin. J Biol Chem. 1958 Dec;233(6):1315–1322. [PubMed] [Google Scholar]
- Scott T. W., Jay S. M., Freinkel N. Further studies on the action of pituitary thyrotropin on the individual phosphatides of thyroid tissue. Endocrinology. 1966 Sep;79(3):591–600. doi: 10.1210/endo-79-3-591. [DOI] [PubMed] [Google Scholar]
- Shephard E. H., Hübscher G. Phosphatidate biosynthesis in mitochondrial subfractions of rat liver. Biochem J. 1969 Jun;113(2):429–440. doi: 10.1042/bj1130429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turtle J. R., Kipnis D. M. An adrenergic receptor mechanism for the control of cyclic 3'5' adenosine monophosphate synthesis in tissues. Biochem Biophys Res Commun. 1967 Sep 7;28(5):797–802. doi: 10.1016/0006-291x(67)90388-9. [DOI] [PubMed] [Google Scholar]


