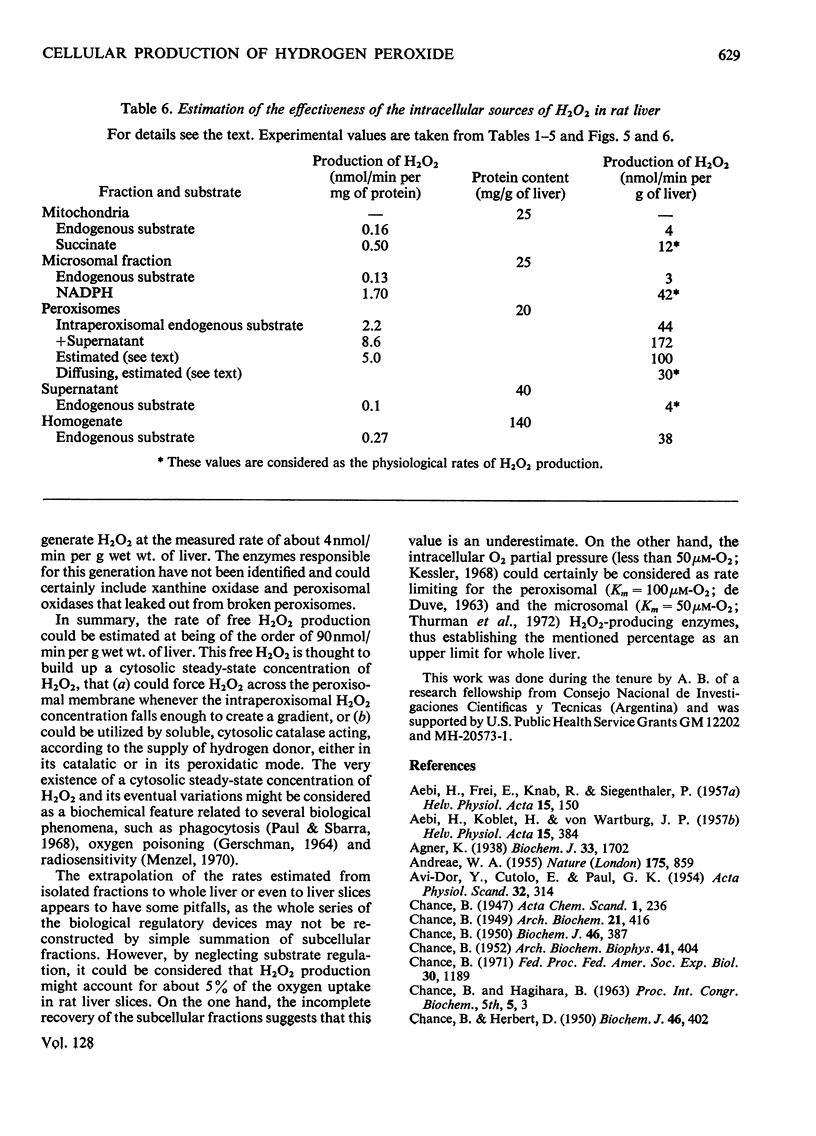
Abstract
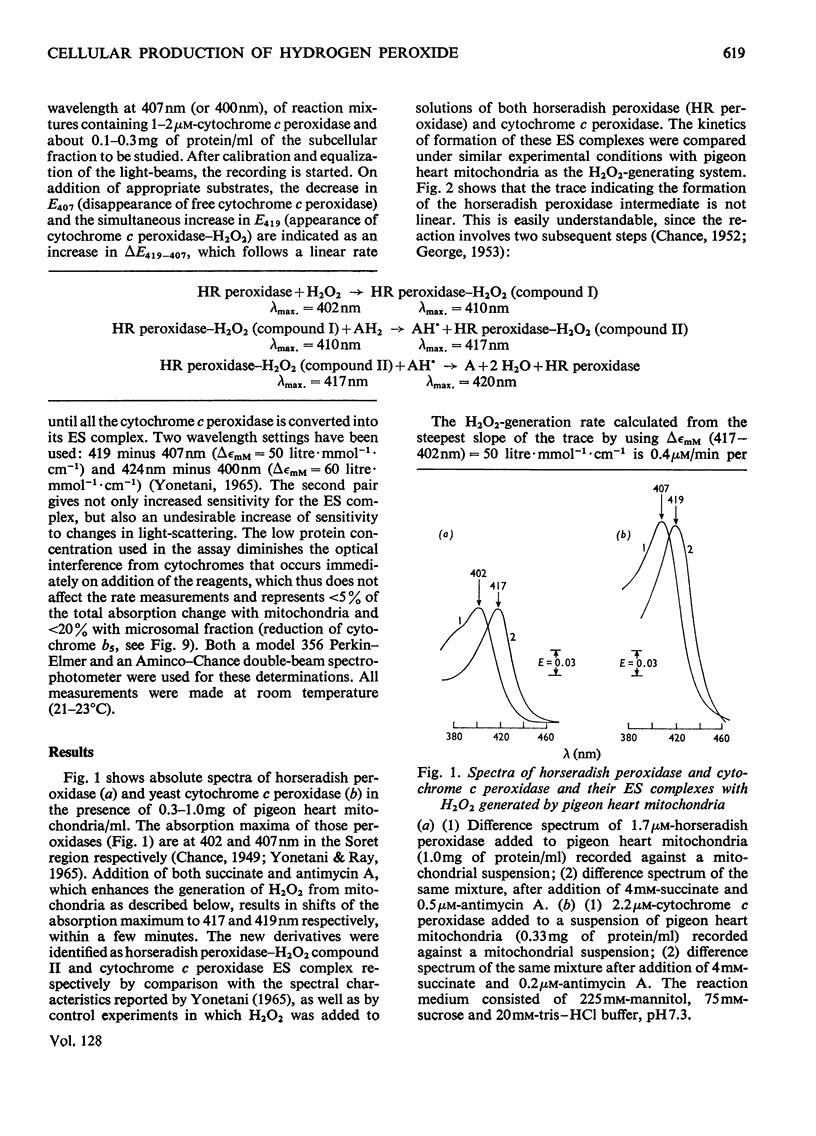
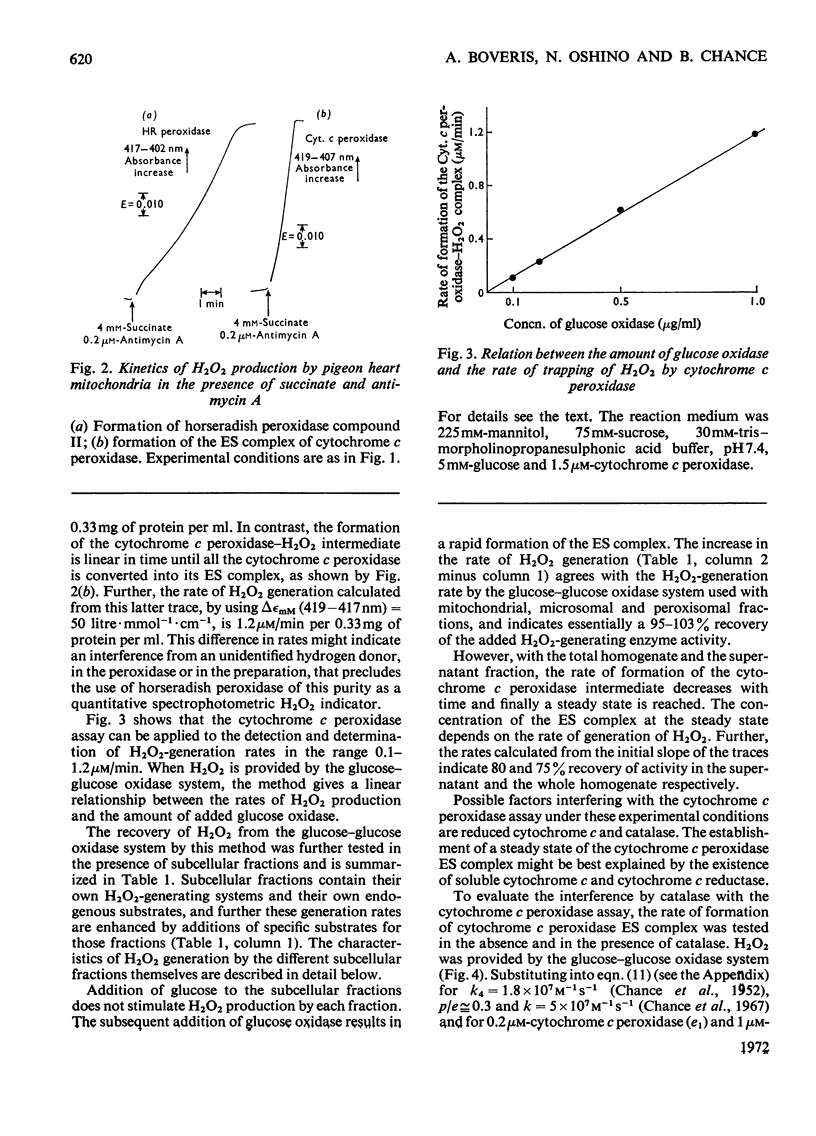
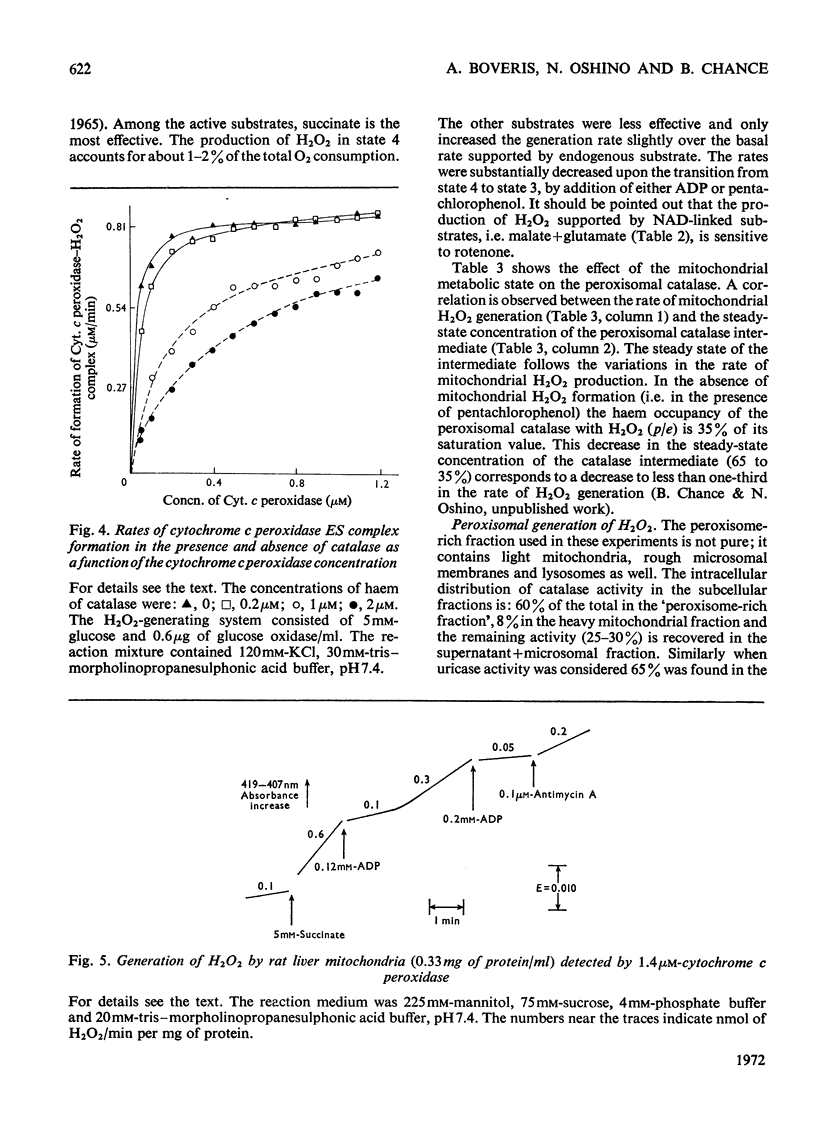
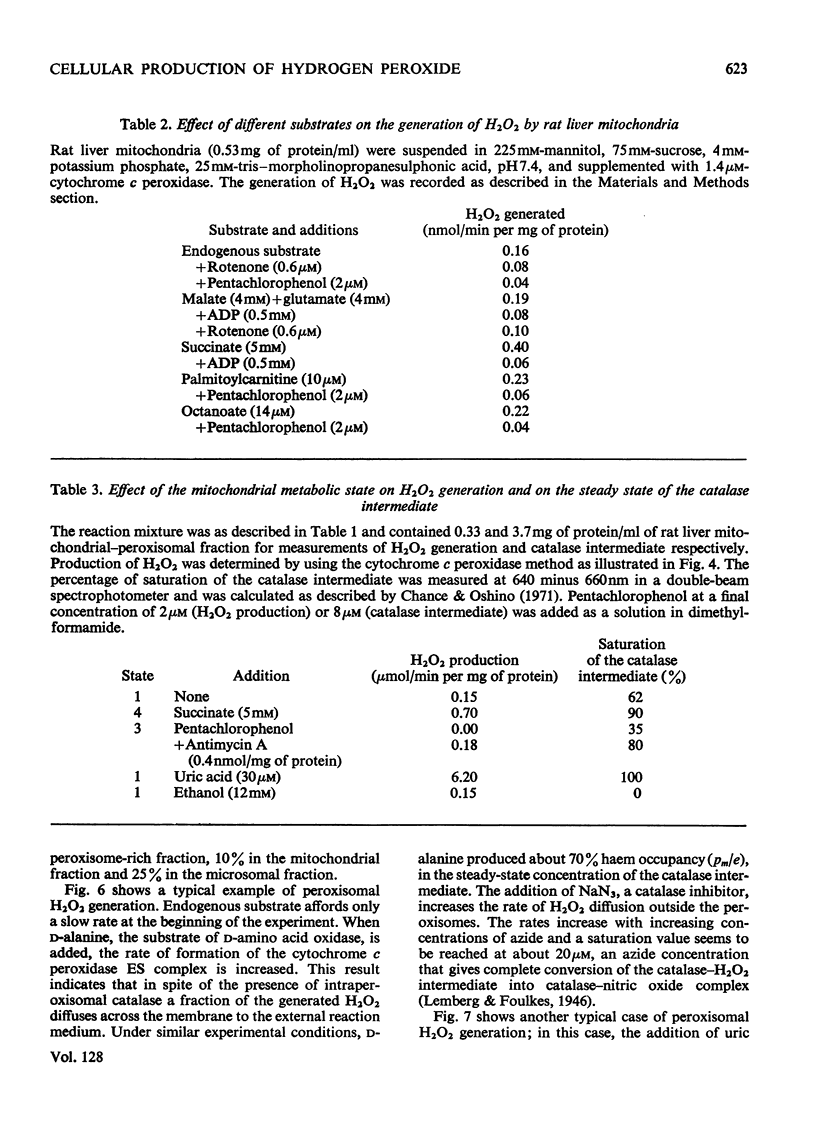
1. The enzyme–substrate complex of yeast cytochrome c peroxidase is used as a sensitive, specific and accurate spectrophotometric H2O2 indicator. 2. The cytochrome c peroxidase assay is suitable for use with subcellular fractions from tissue homogenates as well as with pure enzyme systems to measure H2O2 generation. 3. Mitochondrial substrates entering the respiratory chain on the substrate side of the antimycin A-sensitive site support the mitochondrial generation of H2O2. Succinate, the most effective substrate, yields H2O2 at a rate of 0.5nmol/min per mg of protein in state 4. H2O2 generation is decreased in the state 4→state 3 transition. 4. In the combined mitochondrial–peroxisomal fraction of rat liver the changes in the mitochondrial generation of H2O2 modulated by substrate, ADP and antimycin A are followed by parallel changes in the saturation of the intraperoxisomal catalase intermediate. 5. Peroxisomes supplemented with uric acid generate extraperoxisomal H2O2 at a rate (8.6–16.4nmol/min per mg of protein) that corresponds to 42–61% of the rate of uric acid oxidation. Addition of azide increases these H2O2 rates by a factor of 1.4–1.7. 6. The concentration of cytosolic uric acid is shown to vary during the isolation of the cellular fractions. 7. Microsomal fractions produce H2O2 (up to 1.7nmol/min per mg of protein) at a ratio of 0.71–0.86mol of H2O2/mol of NADP+ during the oxidation of NADPH. H2O2 is also generated (6–25%) during the microsomal oxidation of NADH (0.06–0.025mol of H2O2/mol of NAD+). 8. Estimation of the rates of production of H2O2 under physiological conditions can be made on the basis of the rates with the isolated fractions. The tentative value of 90nmol of H2O2/min per g of liver at 22°C serves as a crude approximation to evaluate the biochemical impact of H2O2 on cellular metabolism.
Full text
PDF


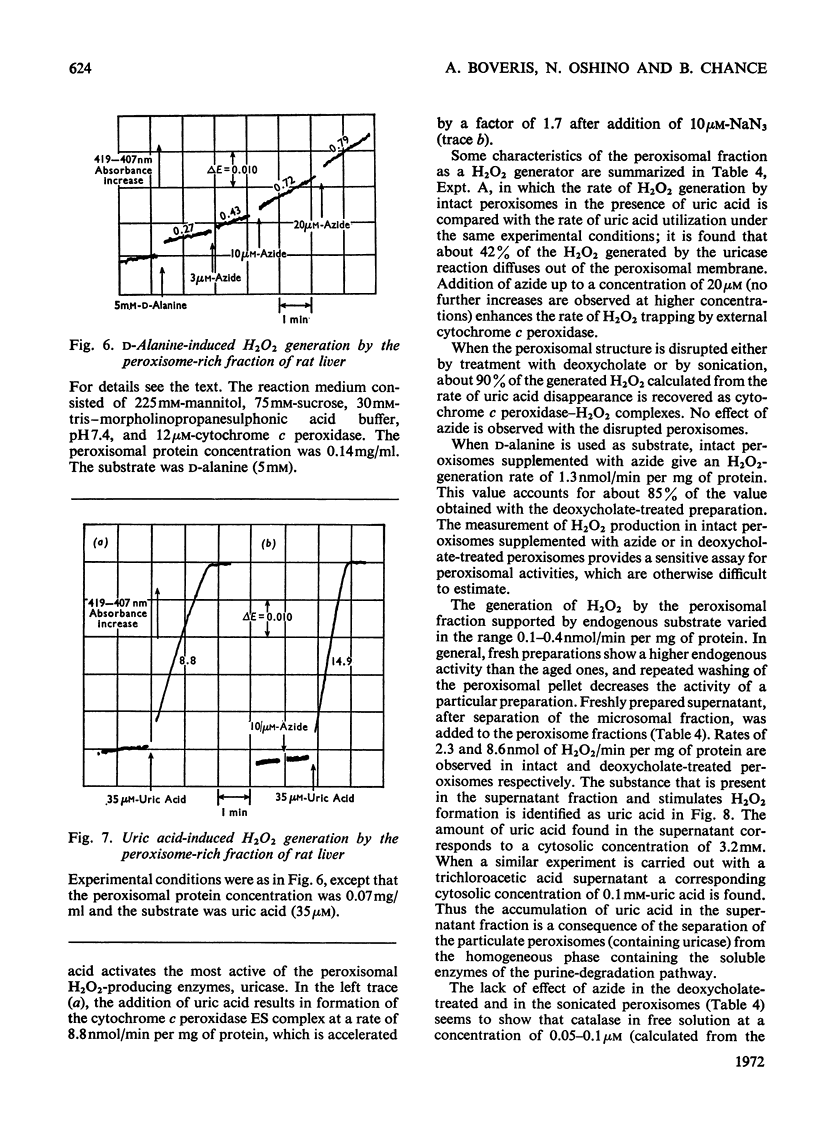
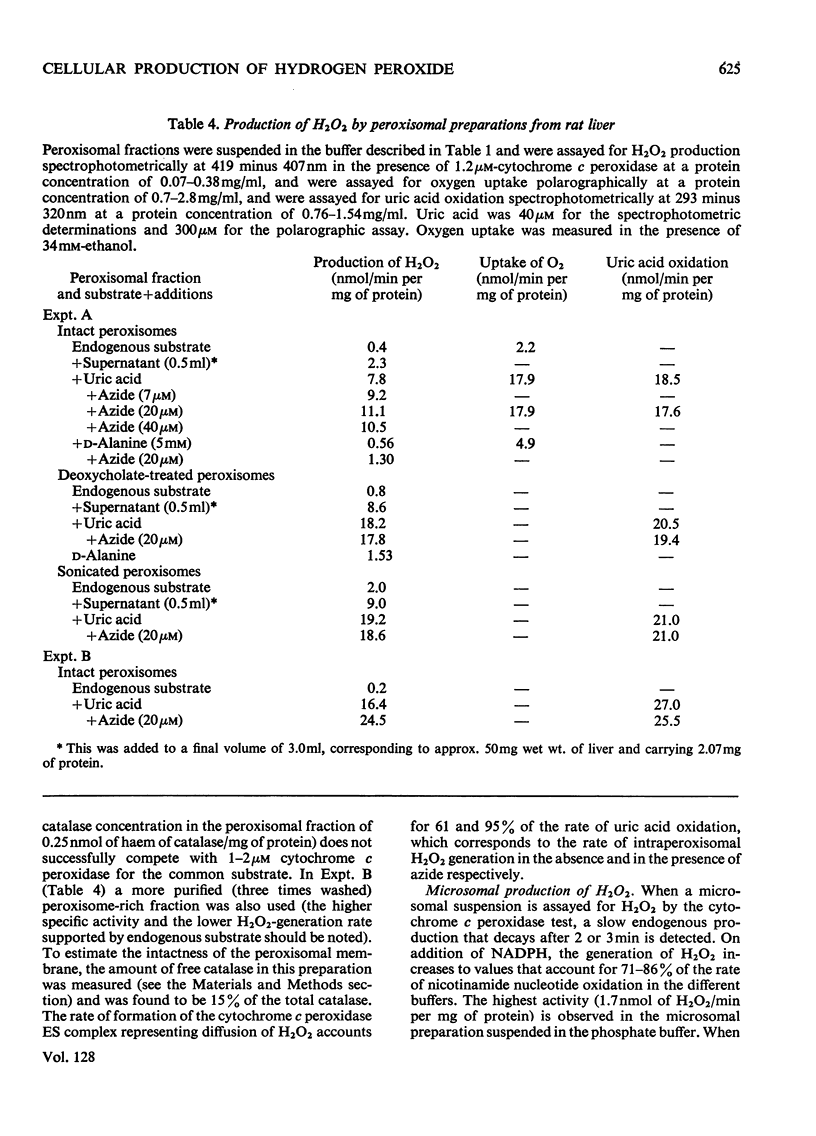
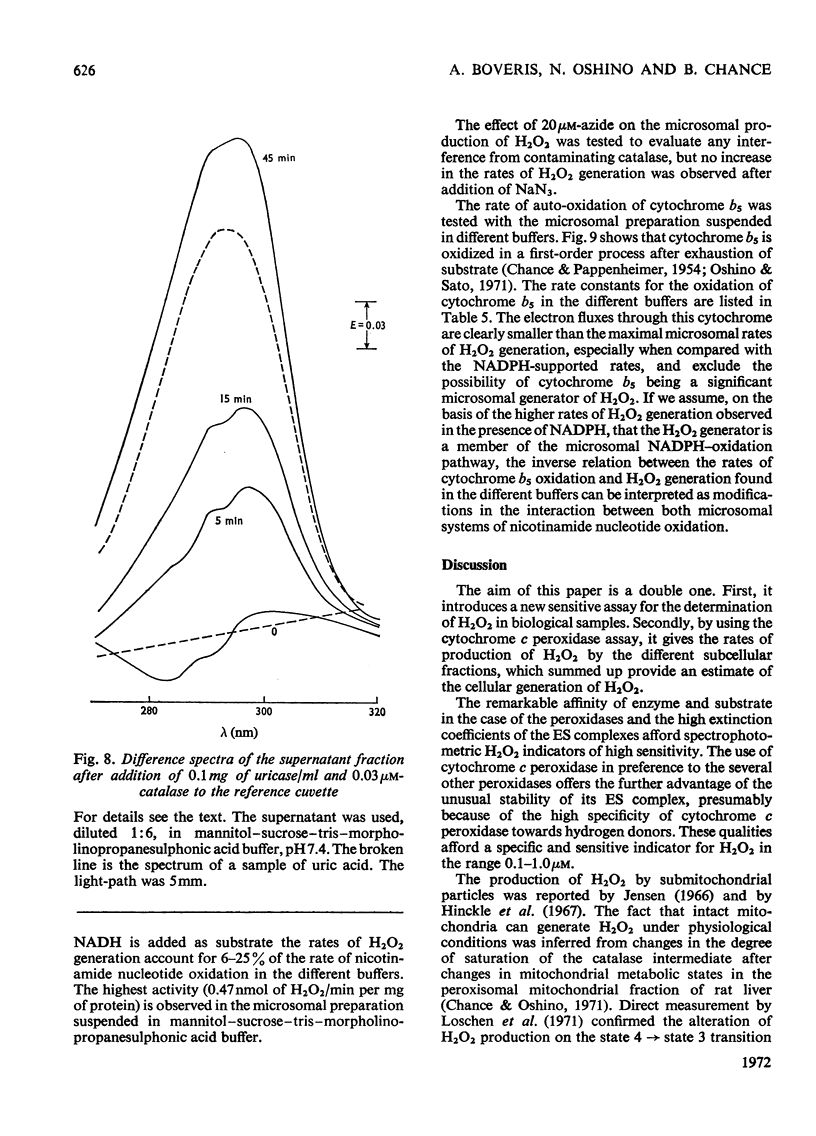
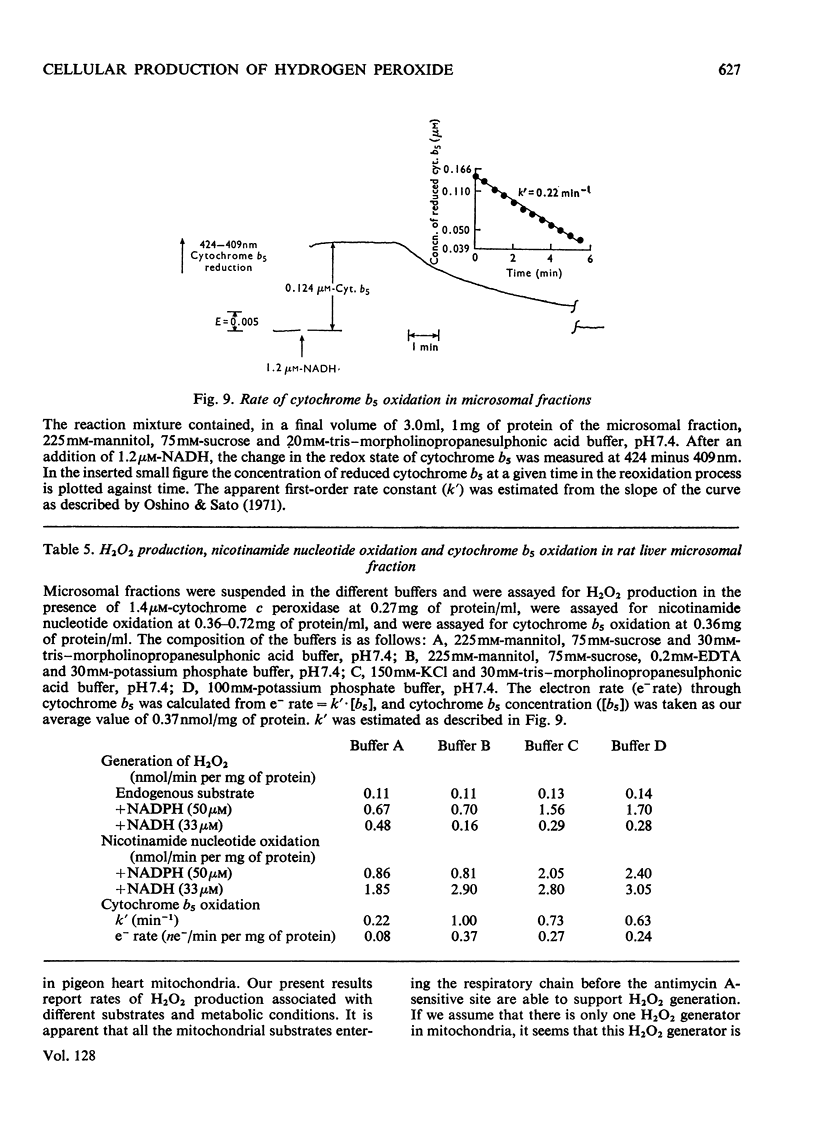


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AEBI H., FREI E., KNAB R., SIEGENTHALER P. Untersuchungen über die Formiatoxydation in der Leber. Helv Physiol Pharmacol Acta. 1957;15(1):150–167. [PubMed] [Google Scholar]
- AEBI H., KOBLET H., VON WARTBURG J. P. Uber den Mechanismus der biologischen Methanoloxydation. Helv Physiol Pharmacol Acta. 1957;15(3):384–399. [PubMed] [Google Scholar]
- ANDREAE W. A. A sensitive method for the estimation of hydrogen peroxide in biological materials. Nature. 1955 May 14;175(4463):859–860. doi: 10.1038/175859a0. [DOI] [PubMed] [Google Scholar]
- AVI-DOR Y., CUTOLO E., PAUL K. G. The assay of hydrogen peroxide in small quantities with horse radish peroxidase as catalyst. Acta Physiol Scand. 1954 Dec 31;32(4):314–319. doi: 10.1111/j.1748-1716.1954.tb01179.x. [DOI] [PubMed] [Google Scholar]
- Agner K. The preparation and properties of a highly active catalase from horse liver. Biochem J. 1938 Oct;32(10):1702–1706. doi: 10.1042/bj0321702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANCE B., GREENSTEIN D. S., ROUGHTON F. J. W. The mechanism of catalase action. I. Steady-state analysis. Arch Biochem Biophys. 1952 Jun;37(2):301–321. doi: 10.1016/0003-9861(52)90194-x. [DOI] [PubMed] [Google Scholar]
- CHANCE B., HERBERT D. The enzymesubstrate compounds of bacterial catalase and peroxides. Biochem J. 1950 Apr;46(4):402–414. doi: 10.1042/bj0460402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANCE B., PAPPENHEIMER A. M., Jr Kinetic and spectrophotometric studies of cytochrome b5 in midgut homogenates of cecropia. J Biol Chem. 1954 Aug;209(2):931–943. [PubMed] [Google Scholar]
- CHANCE B. The reactions of catalase in the presence of the notatin system. Biochem J. 1950 Apr;46(4):387–402. doi: 10.1042/bj0460387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANCE B. The spectra of the enzyme-substrate complexes of catalase and peroxidase. Arch Biochem Biophys. 1952 Dec;41(2):404–415. doi: 10.1016/0003-9861(52)90469-4. [DOI] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- Chance B., Oshino N. Kinetics and mechanisms of catalase in peroxisomes of the mitochondrial fraction. Biochem J. 1971 Apr;122(2):225–233. doi: 10.1042/bj1220225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Duve C., Baudhuin P. Peroxisomes (microbodies and related particles). Physiol Rev. 1966 Apr;46(2):323–357. doi: 10.1152/physrev.1966.46.2.323. [DOI] [PubMed] [Google Scholar]
- De Duve C. The separation and characterization of subcellular particles. Harvey Lect. 1965;59:49–87. [PubMed] [Google Scholar]
- Deisseroth A., Dounce A. L. Catalase: Physical and chemical properties, mechanism of catalysis, and physiological role. Physiol Rev. 1970 Jul;50(3):319–375. doi: 10.1152/physrev.1970.50.3.319. [DOI] [PubMed] [Google Scholar]
- Dixon M. The acceptor specificity of flavins and flavoproteins. 3. Flavoproteins. Biochim Biophys Acta. 1971 Mar 2;226(2):269–284. doi: 10.1016/0005-2728(71)90094-6. [DOI] [PubMed] [Google Scholar]
- GEORGE P. The chemical nature of the second hydrogen peroxide compound formed by cytochrome c peroxidase and horseradish peroxidase. 2. Formation and decomposition. Biochem J. 1953 Sep;55(2):220–230. doi: 10.1042/bj0550220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILLETTE J. R., BRODIE B. B., LA DU B. N. The oxidation of drugs by liver microsomes: on the role of TPNH and oxygen. J Pharmacol Exp Ther. 1957 Apr;119(4):532–540. [PubMed] [Google Scholar]
- Goodman J. I., Tephly T. R. The role of hepatic microbody and soluble oxidases in the peroxidation of methanol in the rat and monkey. Mol Pharmacol. 1968 Sep;4(5):492–501. [PubMed] [Google Scholar]
- HOCHSTEIN P., ERNSTER L. ADP-ACTIVATED LIPID PEROXIDATION COUPLED TO THE TPNH OXIDASE SYSTEM OF MICROSOMES. Biochem Biophys Res Commun. 1963 Aug 14;12:388–394. doi: 10.1016/0006-291x(63)90111-6. [DOI] [PubMed] [Google Scholar]
- Hinkle P. C., Butow R. A., Racker E., Chance B. Partial resolution of the enzymes catalyzing oxidative phosphorylation. XV. Reverse electron transfer in the flavin-cytochrome beta region of the respiratory chain of beef heart submitochondrial particles. J Biol Chem. 1967 Nov 25;242(22):5169–5173. [PubMed] [Google Scholar]
- JACOBSEN E. The metabolism of ethyl alcohol. Nature. 1952 Apr 19;169(4303):645–647. doi: 10.1038/169645a0. [DOI] [PubMed] [Google Scholar]
- Jensen P. K. Antimycin-insensitive oxidation of succinate and reduced nicotinamide-adenine dinucleotide in electron-transport particles. I. pH dependency and hydrogen peroxide formation. Biochim Biophys Acta. 1966 Aug 10;122(2):157–166. doi: 10.1016/0926-6593(66)90057-9. [DOI] [PubMed] [Google Scholar]
- KESTON A. S., BRANDT R. THE FLUOROMETRIC ANALYSIS OF ULTRAMICRO QUANTITIES OF HYDROGEN PEROXIDE. Anal Biochem. 1965 Apr;11:1–5. doi: 10.1016/0003-2697(65)90034-5. [DOI] [PubMed] [Google Scholar]
- Keilin D., Hartree E. F. Properties of azide-catalase. Biochem J. 1945;39(2):148–157. doi: 10.1042/bj0390148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber C. S., DeCarli L. M. Hepatic microsomal ethanol-oxidizing system. In vitro characteristics and adaptive properties in vivo. J Biol Chem. 1970 May 25;245(10):2505–2512. [PubMed] [Google Scholar]
- Loschen G., Flohé L., Chance B. Respiratory chain linked H(2)O(2) production in pigeon heart mitochondria. FEBS Lett. 1971 Nov 1;18(2):261–264. doi: 10.1016/0014-5793(71)80459-3. [DOI] [PubMed] [Google Scholar]
- Menzel D. B. Toxicity of ozone, oxygen, and radiation. Annu Rev Pharmacol. 1970;10:379–394. doi: 10.1146/annurev.pa.10.040170.002115. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- Orme-Johnson W. H., Ziegler D. M. Alcohol mixed function. Oxidase activity of mammalian liver microsomes. Biochem Biophys Res Commun. 1965 Oct 8;21(1):78–82. doi: 10.1016/0006-291x(65)90429-8. [DOI] [PubMed] [Google Scholar]
- Oshino N., Sato R. Stimulation by phenols of the reoxidation microsomal bound cytochrome b5 and its implication to fatty acid desaturation. J Biochem. 1971 Jan;69(1):169–180. doi: 10.1093/oxfordjournals.jbchem.a129445. [DOI] [PubMed] [Google Scholar]
- PRICE V. E., STERLING W. R., TARANTOLA V. A., HARTLEY R. W., Jr, RECHCIGL M., Jr The kinetics of catalase synthesis and destruction in vivo. J Biol Chem. 1962 Nov;237:3468–3475. [PubMed] [Google Scholar]
- Paul B., Sbarra A. J. The role of the phagocyte in host-parasite interactions. 13. The direct quantitative estimation of H2O2 in phagocytizing cells. Biochim Biophys Acta. 1968 Feb 1;156(1):168–178. doi: 10.1016/0304-4165(68)90116-5. [DOI] [PubMed] [Google Scholar]
- Poole B. Biogenesis and turnover of rat liver peroxisomes. Ann N Y Acad Sci. 1969 Dec 19;168(2):229–243. doi: 10.1111/j.1749-6632.1969.tb43112.x. [DOI] [PubMed] [Google Scholar]
- Scholz R., Thurman R. G., Williamson J. R., Chance B., Bücher T. Flavin and pyridine nucleotide oxidation-reduction changes in perfused rat liver. I. Anoxia and subcellular localization of fluorescent flavoproteins. J Biol Chem. 1969 May 10;244(9):2317–2324. [PubMed] [Google Scholar]
- Sies H., Chance B. The steady state level of catalase compound I in isolated hemoglobin-free perfused rat liver. FEBS Lett. 1970 Dec;11(3):172–176. doi: 10.1016/0014-5793(70)80521-x. [DOI] [PubMed] [Google Scholar]
- Thurman R. G., Ley H. G., Scholz R. Hepatic microsomal ethanol oxidation. Hydrogen peroxide formation and the role of catalase. Eur J Biochem. 1972 Feb;25(3):420–430. doi: 10.1111/j.1432-1033.1972.tb01711.x. [DOI] [PubMed] [Google Scholar]
- Yonetani T., Ray G. S. Studies on cytochrome c peroxidase. I. Purification and some properties. J Biol Chem. 1965 Nov;240(11):4503–4508. [PubMed] [Google Scholar]
- Yonetani T. Studies on cytochrome c peroxidase. II. Stoichiometry between enzyme, H2O2, and ferrocytochrome c and enzymic determination of extinction coefficients of cytochrome c. J Biol Chem. 1965 Nov;240(11):4509–4514. [PubMed] [Google Scholar]


