Abstract
Island ecosystems are particularly vulnerable to exotic species. Here we show how an introduced prey has led to the wholesale restructuring of an island food web, including the near extinction of an endemic carnivore. Introduced pigs, by providing abundant food, enabled golden eagles to colonize the California Channel Islands. Eagles preyed heavily on the island fox, whose resulting decline toward extinction released populations of the competitively inferior island skunk. The presence of exotic pigs led to major ecosystem shifts by indirectly causing predation to replace competition as the dominant force shaping these island communities.
Exotic species and apex predators are important drivers of ecosystem change and have been increasingly recognized as major concerns in the conservation of terrestrial ecosystems (1, 2). In cases where exotic species have caused biodiversity loss, their effects are typically manifested through direct species interactions in the form of predation, competition, or hybridization. A well-known example is the decimation of avifauna on the island of Guam caused by heightened predation from the exotic brown tree snake (Boiga irregularis) (3). Although it is clear that introduced species can have direct, negative impacts on native taxa, indirect interactions with community-level consequences are less well documented (4, 5). Select plant species provide the best examples. By altering resource availability or modifying habitat, exotic plants can change characteristics of entire ecosystems (6–8).
Apex predators can reduce biodiversity directly through predation, and their influence can also cascade through trophic levels, changing community structure (2, 9). Further, polyphagous predators, through apparent competition, may alter community topology by asymmetrically impacting prey species and excluding those that are more vulnerable (10). Recently, functional models of apparent competition have linked exotic predators to both native and exotic prey, suggesting that the introduction of a novel prey species can indirectly cause the extinction of indigenous prey (11, 12). This form of apparent competition, termed hyperpredation, occurs when an indigenous prey species experiences an increase in predation pressure caused by an exotic predator that is sustained by an abundant exotic prey. Although this process has been inferred from studies documenting the decline of native species (13, 14), empirical evidence for such an interaction is lacking.
Here we document a case of apparent competition involving an exotic species, the feral pig (Sus scrofa), an apex predator, the golden eagle (Aquila chrysaetos), and two endemic carnivores, the island fox (Urocyon littoralis) and island spotted skunk (Spilogale gracilis amphiala). By acting as an abundant prey, pigs enabled native, mainland golden eagles to colonize the California Channel Islands, and through hyperpredation, indirectly caused a rapid decline in the native fox populations (15). This colonization event not only restructured the trophic hierarchy on the islands—eagles became the apex predator and both the fox and the skunk became prey—but it also altered the competitive relations between the fox and the skunk and caused a radical change in the carnivore community. The unique aspect of this interaction is that it involves a change in both predatory and competitive relations between three native predators that was indirectly driven by the presence of a single exotic prey.
Methods
Study Area.
The California Channel Islands are a group of eight islands located off the coast of southern California. Six of the islands are inhabited by the endemic island fox (16). Island foxes are the largest native carnivore on the islands, feeding predominately on mice, insects, and fruits (16, 17). On two islands, Santa Cruz and Santa Rosa, the island spotted skunk co-occurs with the fox. Smaller in size, skunks are strict carnivores, feeding exclusively on mice and insects (18). Feral pigs are also found on Santa Cruz Island, where they have been present for over 150 years (19). Golden eagles were historically transient visitors to the islands but have recently colonized the islands and have successfully nested (15).
Trapping.
We began a demographic study of the fox, and secondarily the skunk, on Santa Cruz Island in 1993 (20). Foxes and skunks were live-captured on Santa Cruz Island on two grids (13 km apart) from 1993 to 1999 with a hiatus in 1994 (site 1 = 390 trap nights per year, site 2 = 300 trap nights per year). Mark–recapture data were used to estimate grid population size, density (foxes per km2), and capture success (no. captures per trap night) of foxes. Only capture success was recorded for skunks. Because fox capture success is correlated (P < 0.01) with number of foxes captured (Rs = 0.98), estimates of grid population size (Rs = 0.96), and density (Rs = 0.95), we assume that capture success is a good indicator of both fox and skunk population size.
Isotope Analysis.
We used stable isotope ratios (δ13C and δ15N) as an index of prey consumption by eagles, foxes, and skunks (21). We analyzed golden eagle breast feathers, plasma blood samples (fox, skunk, and pig), whole arthropods, rodent tails, and fruits from Santa Cruz Island. Isotope values were determined by using a Carlo Erba model NA 2500 elemental analyzer coupled to a Finnigan Delta Plus isotopic ratio mass spectrometer at the Colorado Plateau Stable Isotope Laboratory, Northern Arizona University (Flagstaff, AZ). Prey item isotope signatures were corrected for consumer fractionation (+1‰ for δ13C and +3‰ for δ15N) (22). Seabird isotope signatures are from the Farallon Islands, located 500 km north of the Channel Islands (23). Differences in marine versus terrestrial isotope signatures and low variability in seabird values are sufficient to conclude that seabirds contributed little to the eagle diet (22, 24). Fox, piglet, and seabird remains were reported in an eagle nest on Santa Cruz Island and thus are confirmed prey (15). At least three eagles live-captured on Santa Cruz smelled of skunk, also confirming skunks as prey items. The remaining samples are confirmed prey of either foxes or skunks (16, 18). The mechanistic model (see below) proved robust to the parameters derived (φ and σ) from the isotope model.
Estimates of Competition.
Estimates of resource competition between foxes and skunks (βsf and βfs) were determined from field metabolic rates for foxes (males = 1,426.1 kJ/day and females = 1,310.2 kJ/day) and skunks (males = 527.0 kJ/day and females = 438.9 kJ/day) based on allometry (fox: male = 2.00 ± 0.23 kg, female = 1.81 ± 0.22 kg, n = 77 for both sexes; skunk: male = 620 ± 40 g, n = 5, female = 500 ± 40 g, n = 4; refs. 20, 25, and 26). These estimates were converted to daily food consumption [gram of dry matter (DM) per day] by using estimates of metabolizable energy for omnivores (14.0 kJ/g of DM) and carnivores (16.8 kJ/g of DM), respectively. We determined a relative index of animal food in the diet of the fox (0.77 animal and 0.23 plant) by using the isotope model, and assumed that skunks were completely carnivorous (18). We then converted dry animal matter to fresh animal matter (FM) consumed (3.33 g FM/1 g DM) by a pair of foxes (500.58 g FM/day) and a pair of skunks (181.58 g FM/day). Energetic estimates of resource competition were calculated with these values (i.e., βsf = 500.48/181.58 = 2.76, βfs = 181.58/500.48 = 0.36).
The Model.
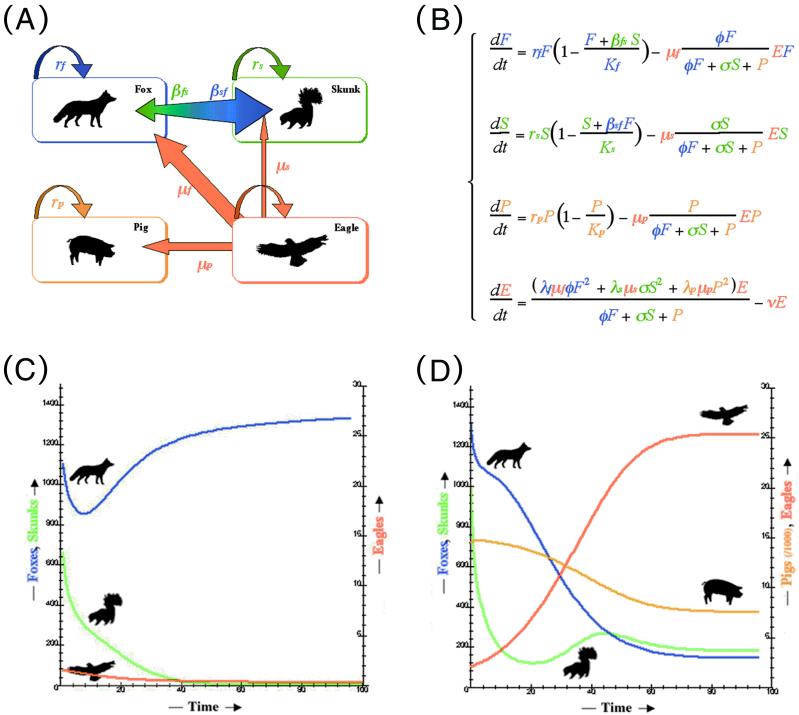
We combined a Lotka–Volterra competition and predation model, where one predator (golden eagle = E) has the choice between three prey (fox = F, skunk = S, or piglet = P). Details of comparable models of apparent competition without resource competition are found elsewhere (11, 12, 27). Each prey population i is characterized by its intrinsic growth rate (ri), its carrying capacity (Ki), an energetic measure of resource competition (βij—for foxes and skunks only), a predation rate by eagles (μi), and a term of eagle preference for foxes (φ) and skunks (σ) relative to piglets. If φ or σ is greater than 1, eagles prey more often on foxes or skunks than on piglets, respectively. Eagle mortality rate is ν, and the rate at which prey i are turned into new predators is given by λi (see Fig. 3B).
Figure 3.
Schematic representation of interspecific dynamics on Santa Cruz Island (A): fox (blue), skunk (green), pig (orange), and their predator the golden eagle (red); corresponding set of equations (B), and resulting simulations (C and D). (C) Foxes and skunks together without pigs are not sufficient to sustain an eagle population; any dispersing eagle disappears without colonizing, leaving the dominant foxes to outcompete skunks. (D) When pigs are present, they provide enough prey for dispersing eagles to colonize and breed. The growing eagle population drives the endemic fox toward extinction, which releases skunks from fox competition. The time scale is 15 years.
The model was parameterized as follows: rf = 0.32, rp = 0.78, Kf = 1,544; Ks = 2,490, Kp = 15,189, F(0) = 1,312, P(0) = 13,827 (15, 28). The proportion of piglets < 1.0 year old (51%) (28) was used to calculate preference coefficients. We divided the ratio of prey i in the eagle diet by Ki, to account for prey abundance, and then divided the values obtained for foxes and skunks by that obtained for piglets. This yielded the preference of an eagle for a fox (φ = 8.1) or a skunk (σ = 3.1) relative to a piglet. Because data were missing, we assumed rs = rf and S(0) = 1,000. Mortality rate of eagles was set at ν = 0.09 (29). Eagles kill ≈132.1 prey per year or 0.34 foxes (or piglets) per day outside of the 70-day breeding season, and 0.45 during the breeding season (15). We divided predation rate by Ki to correct for relative abundance yielding μf = 0.086 and μp = 0.019. Because skunks are ≈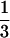 the weight of a fox, μs = 0.159. λ was estimated as a measure of energy and time investments necessary for a breeding pair of eagles to produce an adult eagle. We obtained λ by multiplying the inverse of the number of prey killed per year and τ, the time necessary for a pair of eagles to produce an adult eagle (comparable to a generation time of 5 years), yielding 7.7 × 10−4 for foxes and piglets and 2.5 × 10−4 for skunks.
the weight of a fox, μs = 0.159. λ was estimated as a measure of energy and time investments necessary for a breeding pair of eagles to produce an adult eagle. We obtained λ by multiplying the inverse of the number of prey killed per year and τ, the time necessary for a pair of eagles to produce an adult eagle (comparable to a generation time of 5 years), yielding 7.7 × 10−4 for foxes and piglets and 2.5 × 10−4 for skunks.
Results and Discussion
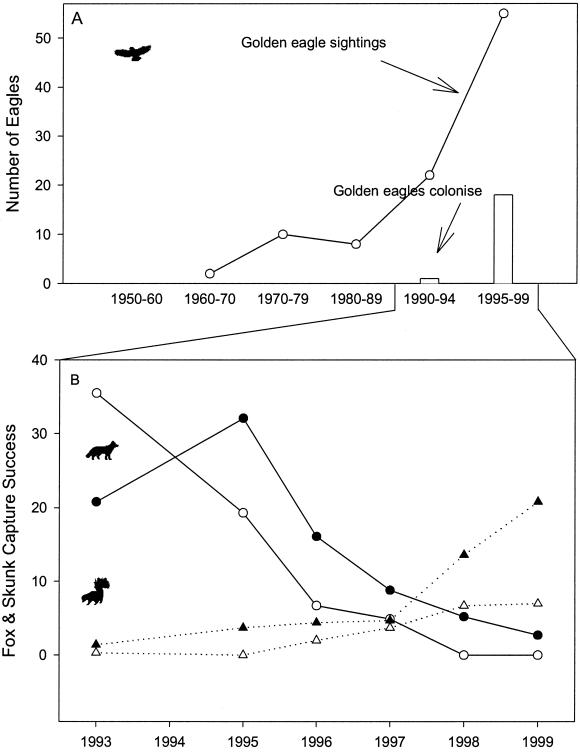
In the first year of our study, fox capture success was high (28.3% ± 8.7%) and skunk capture success was low (0.8% ± 1.0), a condition congruent with a previous study (26). After 1993, the fox population began to decline precipitously, by 1999 fox capture success, density, and estimated population size were the lowest ever recorded on Santa Cruz Island (4.3% ± 1.9%, 0–2.4 foxes per km2, N̂ = 133, respectively). The decline of foxes coincided with a 17-fold increase in the capture success of the island skunk (1999 = 13.9% ± 8.5%; Fig. 1B). Capture success of skunks was inversely correlated with capture success of foxes (Fig. 1B) and the number of skunk captures reflected the number of fox captures on an annual basis (Model II regression: F = 6.8, P < 0.03, n = 12).
Figure 1.
(A) Changes in resident golden eagles (white bars) and golden eagle sightings (line) on the northern Channel Islands. (B) Concurrent changes in island fox (solid line) and skunk (dotted line) capture success at two sites on Santa Cruz Island. Fox and skunk trends are statistically significant over time (repeated-measures ANOVA: fox, F = 166.8, P < 0.001; skunk, F = 33.3, P < 0.001; n = 6 at both sites), and capture success is inversely correlated [site 1: Rp = −0.77, P < 0.001 (open symbols), site 2: Rp = −0.64, P < 0.001 (filled symbols), n = 36 at both sites].
The fox decline and coincident increase in skunks was not restricted to Santa Cruz Island. Fox populations declined on all three northern Channel Islands (San Miguel, Santa Rosa, and Santa Cruz) and skunk capture success was greater than fox capture success on Santa Rosa in both 1998 (5.8% vs. 4.8%, n = 132 trap nights) and 2000 (15.0% vs. 1.6%, n = 1,115 trap nights) (refs. 20 and 30, and T. Coonan, personal communication).
The change in relative carnivore abundance prompted further study, starting by examining the competitive relationship between the fox and skunk. A previous study suggested that foxes were competitively dominant because foxes and skunks overlapped in resource utilization and foxes occurred at much higher densities (18). To investigate the role of resource competition in structuring the abundance of these two carnivores, we estimated dietary overlap by using stable isotopes (21) and then energetically determined consumption of similar foods (25) (see Estimates of Competition). An average fox consumes nearly 3 times the daily amount of mice and insects as a skunk (500.48 vs. 181.58 g FM/day), and energetically derived estimates of competition were highly asymmetric. Thus, comparative ecology and respective energetic demands on the environment are sufficient to predict that foxes should be competitively dominant to skunks and support the premise that competition by foxes negatively influenced skunk numbers.
Although resource competition may have been the primary force dictating carnivore abundance on the islands before the decline in foxes, it could not explain why the fox populations had declined. Disease was initially suspected as a contributing agent, but the distribution of micro- and macroparasites was incongruent with fox demographic patterns, suggesting that disease played no role (15, 30, 32). Further, a generalist pathogen like rabies or distemper, the most common causes of disease-driven declines in carnivores (33), is unlikely to be responsible for the decline in foxes because a decline in the population size of skunks would be expected as well. Starvation could also be dismissed because prey abundance increased on San Miguel during the period of fox decline (30) and because skunk populations increased on both Santa Cruz and Santa Rosa Islands. If foxes had starved, so likely would have skunks, whose diet overlaps that of foxes. An alternative explanation was the presence of a novel apex predator that had an asymmetrical effect on the two species.
Multiple lines of evidence pointed to predation by golden eagles as the cause of the decline in foxes (15). First, an abrupt decline in the population size of foxes coincided with an increase in eagle sightings among the northern Channel Islands (Fig. 1). Second, physical evidence from a total of 28 fox carcasses discovered on two islands implicated eagle predation as the primary cause of fox mortality. Third, golden eagles recently colonized Santa Cruz Island, most likely in 1994, and the first golden eagle nest recorded for the Channel Islands in 1999 contained remains of foxes. And finally, from fall 1999 to spring 2001, a total of 21 individual golden eagles had been observed on Santa Cruz Island, 14 of which were live-captured and translocated as part of a conservation strategy to save the fox from extinction (34).
Although predation by eagles was the cause of the fox decline and competitive release strongly implicated in the changes in abundance of the fox and skunk, observations of eagle predation on piglets led us to hypothesize that the presence of feral pigs had played a role in these dynamics (15). Because such processes are often difficult to highlight empirically (4), we expanded on a previous mechanistic model (12, 15) and parameterized the model with independent data sets to elucidate the community-level dynamics of the native, exotic, and colonizing species.
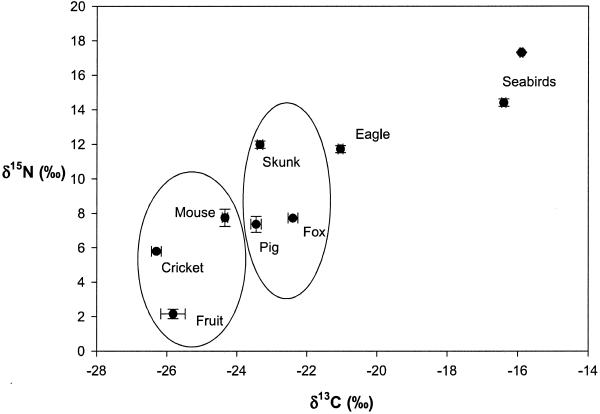
We estimated predation rates of golden eagles from climate data and a time–energy budget (15) and used stable isotope signatures as a heuristic tool to index prey consumption by eagles on their main prey: foxes, piglets, and skunks (Fig. 2) (21). At the onset of the fox decline (1994 and 1995), foxes contributed most (0.51) to the relative composition of the golden eagle diet, followed by piglets (0.34) and skunks (0.15). We then simultaneously explored the competitive relationship between foxes and skunks and the predatory relationship between eagles and their prey by using a combination of the Lotka–Volterra models of competition and predation (12, 35) (Fig. 3 A and B).
Figure 2.
Carbon and nitrogen isotope ratios (mean ± 1 SE) of food web components on Santa Cruz Island. Isotope signatures increase with rank in the trophic web. Known fox and skunk prey [deer mice (Peromyscus maniculatus), Jerusalem crickets (Stenopelmatus fuscus), and toyon fruits (Heteromeles arbutifolia)—foxes only]. Seabirds contribute little to eagle diet (see Methods). Known fox/skunk prey and known eagle prey (foxes, piglets, and skunks) were significantly different from each other (represented by circles, K-nearest neighbor randomization test; Bonferroni correction, n = 5 for each sample, P < 0.05).
The model predicted that foxes are likely to drive skunks to near extinction in absence of a mediating force, such as predation by eagles (Fig. 3C). This result is corroborated by the initially high densities of foxes and low densities of skunks on Santa Cruz and Santa Rosa Islands and may explain the historic extinction of skunks on San Miguel, the smallest island of the group (36). The model also illuminated the role of exotic pigs in the colonization of the islands by golden eagles and in the subsequent change in community dynamics. The eagle population cannot be maintained in the absence of pigs. Pigs, by acting as an abundant food, permitted the colonization of the islands by eagles and indirectly caused the decline of foxes (Fig. 3D). Skunks then increased as a result of the decline in their dominant competitor. The model results were also concordant with recent field estimates of fox and eagle abundance (145 vs. 133 foxes, 25 vs. 21 eagles, respectively). In sum, golden eagles impacted the pig population little, drove the foxes to near extinction through hyperpredation, and indirectly caused an increase in skunks by means of competitive release (Fig. 3).
To examine the robustness of the model to error in parameter estimation, we evaluated the sensitivity of the species specific growth rates to all parameters in the model. The sensitivity analysis showed that varying the value of the parameters by ± 10% did not result in significant changes in the output values, confirming the robustness of the model. Moreover, this analysis allowed us to examine whether the presence of pigs was the driving force behind the eagle colonization event by comparing the effect of changing the species specific parameters (λi, μi, ri, Ki, φ, and σ). The percent change in eagle population size was much greater with respect to those parameters associated with piglets (−9.6% to +12.5%) compared with those associated with either foxes (−0.1% to +0.2%) or skunks (−0.1% to +0.1%). Furthermore, without pigs, eagles could not have colonized the islands unless both fox and skunk population growth rates were unrealistically high (i.e., >200% of their current values) and even so, attained a simulated population size of only four eagles after 100 years. In conclusion, the sensitivity analysis suggests that the model is robust to variation in the parameter estimates and confirms that without pigs, eagles could never have colonized the islands or increased in population size to that observed.
The differential impact of predation on the three prey species is likely a consequence of differences in natural history. Pigs produce a large number of piglets annually, are capable of producing young in any season, and escape predation by growing beyond the size range typically preyed upon by eagles (0.5–4 kg) (37, 38). In contrast, less than 60% of mature female foxes on Santa Cruz Island annually produced pups and on average weaned only 1.5 pups (±0.42) (20). Skunks are also affected little, because they behaviorally avoid predation by being almost entirely nocturnal. On Santa Cruz, skunks were never active during the day (n = 107 locations), whereas foxes were active in 60% of 592 diurnal locations (18). Thus, foxes are neither fecund nor large and are active during the day, making them more vulnerable to avian predators.
If nocturnality decreases the vulnerability of skunks to eagle predation, we hypothesized that individual variation in diurnal activity of foxes should be correlated with mortality events. On Santa Cruz, diurnal activity and the order in which radio-collared foxes were killed was significantly correlated (Rs = 0.61, P < 0.05) (20). Diurnal activity of 11 radio-collared foxes killed by eagles was also greater than that of 4 collared foxes alive at the end of the study (23%, ± 15.5% vs. 8%, ± 9.1%). Similar to other prey species that relearned predator avoidance and escape behaviors (39), foxes killed later in the study may have learned to avoid eagles by becoming more nocturnal, or perhaps were simply less active during the day than foxes that were killed earlier.
These community dynamics are of broad ecological and conservation significance. Prior studies of exotic vertebrates have focused primarily on their direct impacts on biodiversity with little empirical support for indirect community-level interactions (4, 40). Past studies of predation ecology have focused on predator–prey interactions and, more recently, the rippling effects of trophic cascades (9, 31, 41). The impact of asymmetric predation by means of apparent competition and its disruption of interspecific resource competition have largely been overlooked (10). Our study links introduced species with apex predators and shows how an exotic vertebrate prey can induce a trophic reorganization that causes apparent competition to replace resource competition as a dominant biotic force structuring vertebrate communities. These dynamics highlight the importance of the application of contingent theory and functional frameworks in ecology and conservation (10, 14) and suggest that apparent competition, driven by the introduction of exotic species, may be an under-appreciated mechanism contributing to the loss of global biodiversity.
Acknowledgments
We thank L. Laughrin of the University of California, Santa Cruz Island Reserve and T. J. Coonan of Channel Islands National Park for logistical support. G. Koch and B. Hungate provided technical support on the isotope analyses. D. Garcelon provided skunk serum samples. T. Gorton assisted with figures. The paper was improved by comments from D. Croll, J. Diamond, J. Estes, R. Holt, D. Macdonald, E. Marcus, C. Vilà, T. Williams, E. Zavaleta, and two anonymous reviewers. We especially thank J. Estes for stimulating discussion and for his unwavering support and guidance throughout this research. Funding was provided by the National Geographic Society, the University of California, Los Angeles, the National Park Service, New Mexico Agricultural Experiment Station, and the Institute for Wildlife Studies (to G.W.R.) and from the Switzer Foundation (to C.J.D.).
Abbreviations
- DM
dry matter
- FM
fresh matter
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
See commentary on page 554.
References
- 1.Mooney H A, Hobbs R J. Invasive Species in a Changing World. Washington, DC: Island; 2000. [Google Scholar]
- 2.Terborgh J, Estes J A, Paquet P, Ralls K, Boyd-Heger D, Miller B J, Noss R F. In: Continental Conservation: Scientific Foundations of Regional Reserve Networks. Soulé M E, Terborgh J, editors. Washington, DC: Island Press; 1999. pp. 39–64. [Google Scholar]
- 3.Savidge J A. Ecology. 1987;68:660–668. [Google Scholar]
- 4.Parker I M, Simberloff D, Lonsdale W M, Goodell K, Wonham M, Kareiva P M, Williamson M H, Holle B V, Moyle P B, Byers J E, Goldwasser L. Biol Invasions. 1999;1:3–19. [Google Scholar]
- 5.Simberloff D, Holle B V. Biol Invasions. 1999;1:21–32. [Google Scholar]
- 6.D'Antonio C M, Vitousek P M. Annu Rev Ecol Syst. 1992;23:63–87. [Google Scholar]
- 7.Vitousek P M, Walker L R. Ecol Monogr. 1989;59:247–266. [Google Scholar]
- 8.Vivrette N J, Muller C H. Ecol Monogr. 1977;47:301–318. [Google Scholar]
- 9.Estes J A, Tinker M T, Williams T M, Doak D F. Science. 1998;282:473–476. doi: 10.1126/science.282.5388.473. [DOI] [PubMed] [Google Scholar]
- 10.Holt R D, Lawton J H. Annu Rev Ecol Syst. 1994;25:495–520. [Google Scholar]
- 11.Courchamp F, Langlais M, Sugihara G. Biol Cons. 1999;89:219–225. [Google Scholar]
- 12.Courchamp F, Langlais M, Sugihara G. J Anim Ecol. 2000;69:154–164. [Google Scholar]
- 13.Smith A P, Quin D G. Biol Cons. 1996;77:243–267. [Google Scholar]
- 14.Zavaleta E S, Hobbs R J, Mooney H A. Trends Ecol Evol. 2001;16:454–459. [Google Scholar]
- 15.Roemer G W, Coonan T J, Garcelon D K, Bascompte J, Laughrin L. Anim Cons. 2001;4:307–318. [Google Scholar]
- 16.Moore C M, Collins P W. Mamm Spec. 1995;489:1–7. [Google Scholar]
- 17.Roemer G W, Smith D A, Garcelon D K, Wayne R K. J Zool (London) 2001;255:1–14. [Google Scholar]
- 18.Crooks K R, Van Vuren D. Oecologia (Berlin) 1995;104:301–307. doi: 10.1007/BF00328365. [DOI] [PubMed] [Google Scholar]
- 19.Junak S, Ayers T, Scott R, Wilken D, Young D. A Flora of Santa Cruz Island. Santa Barbara, CA: Santa Barbara Botanic Gardens; 1995. [Google Scholar]
- 20.Roemer G W. Ph.D. thesis. Los Angeles: Univ. of California; 1999. [Google Scholar]
- 21.Ben-David M, Flynn R W, Schell D M. Oecologia (Berlin) 1997;111:280–291. doi: 10.1007/s004420050236. [DOI] [PubMed] [Google Scholar]
- 22.Kelly J F. Can J Zool. 2000;78:1–27. [Google Scholar]
- 23.Sydeman W J, Hobson K A, Pyle P, McLaren E B. Condor. 1997;99:327–336. [Google Scholar]
- 24.Schoeninger M J, Deniro M J. Geochim Cosmochim Acta. 1984;40:625–639. [Google Scholar]
- 25.Nagy K A, Girard I A, Brown T K. Annu Rev Nutr. 1999;19:247–277. doi: 10.1146/annurev.nutr.19.1.247. [DOI] [PubMed] [Google Scholar]
- 26.Crooks K. Southwest Nat. 1994;39:257–262. [Google Scholar]
- 27.Holt R D. Theor Popul Biol. 1977;12:197–229. doi: 10.1016/0040-5809(77)90042-9. [DOI] [PubMed] [Google Scholar]
- 28.Sterner J D, Barrett R H. Trans West Sec Wildl Soc. 1991;27:47–53. [Google Scholar]
- 29.Buehler D A, Fraser J D, Seegar J K D, Therres G D. J Wildl Management. 1991;55:608–613. [Google Scholar]
- 30.Coonan T J, Schwemm C A, Roemer G W, Austin G. In: Proceedings of the Fifth Channel Islands Symposium. Browne D R, Mitchell K, Chaney L, editors. Camarillo, CA: U.S. Department of the Interior, Minerals Management Service; 2000. pp. 289–297. [Google Scholar]
- 31.Pace M L, Cole J J, Carpenter S R, Ketchell J. Trends Ecol Evol. 1999;14:483–488. doi: 10.1016/s0169-5347(99)01723-1. [DOI] [PubMed] [Google Scholar]
- 32.Roemer G W, Coonan T J, Garcelon D K, Starbird C H, McCall J W. J Wildl Dis. 2000;36:723–728. doi: 10.7589/0090-3558-36.4.723. [DOI] [PubMed] [Google Scholar]
- 33.Funk S M, Fiorello C V, Cleaveland S, Gompper M E. In: Carnivore Conservation. Gittleman J L, Funk S M, MacDonald D W, Wayne R K, editors. Cambridge, U.K.: Cambridge Univ. Press; 2001. pp. 443–466. [Google Scholar]
- 34.Coonan T J. Recovery Plan for the Island Fox. Ventura, CA: Channel Islands National Park; 2001. [Google Scholar]
- 35.Holt R D. Am Nat. 1984;124:377–406. doi: 10.1086/284280. [DOI] [PubMed] [Google Scholar]
- 36.Walker P L. In: The California Islands. Proceedings of a Multidisciplinary Symposium. Power D M, editor. Santa Barbara, CA: Santa Barbara Museum of Natural History; 1980. pp. 703–717. [Google Scholar]
- 37.Baber D W, Coblentz B E. J Mamm. 1986;67:512–525. [Google Scholar]
- 38.Watson J. The Golden Eagle. London: Poysner; 1997. [Google Scholar]
- 39.Berger J, Swenson J E, Persson I-L. Science. 2001;291:1036–1039. doi: 10.1126/science.1056466. [DOI] [PubMed] [Google Scholar]
- 40.Ebenhard T. Swedish Wildl Res Viltrevy. 1988;13:1–107. [Google Scholar]
- 41.Sih A, Crawly P, McPeek M, Petranka J, Strohmeier K. Annu Rev Ecol Syst. 1985;16:269–311. [Google Scholar]