Abstract
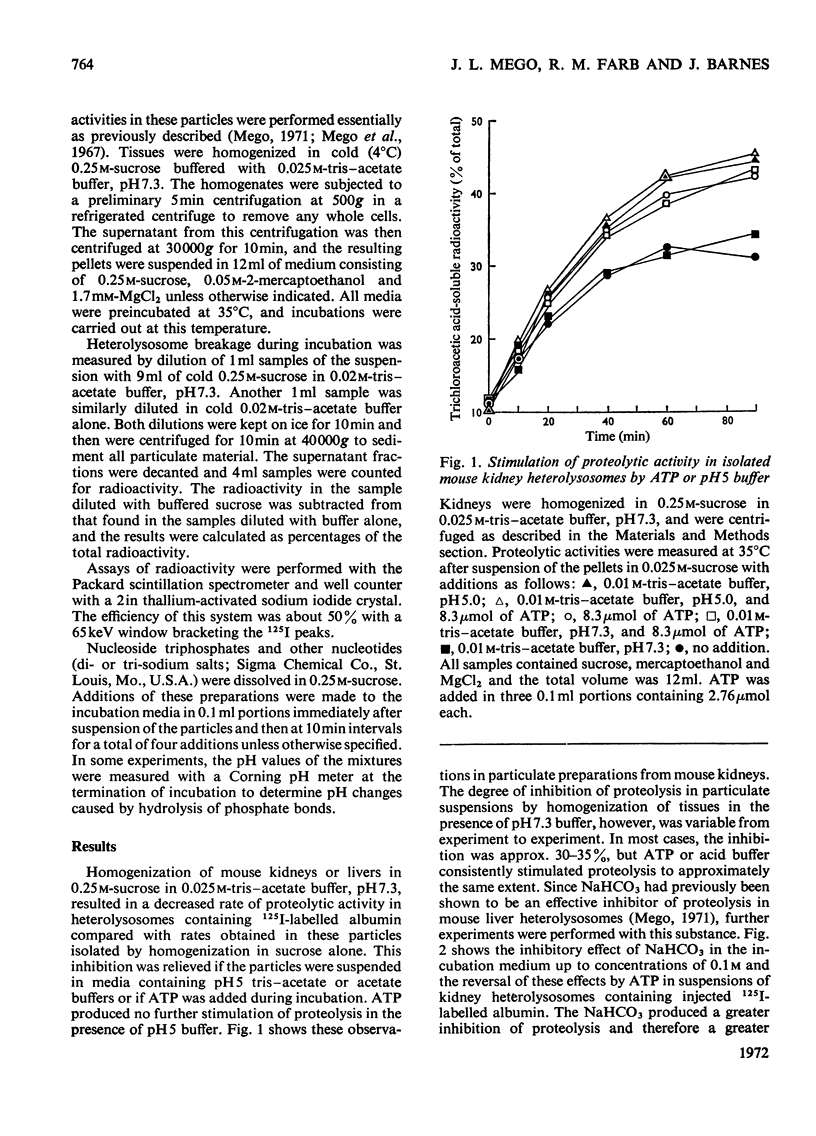
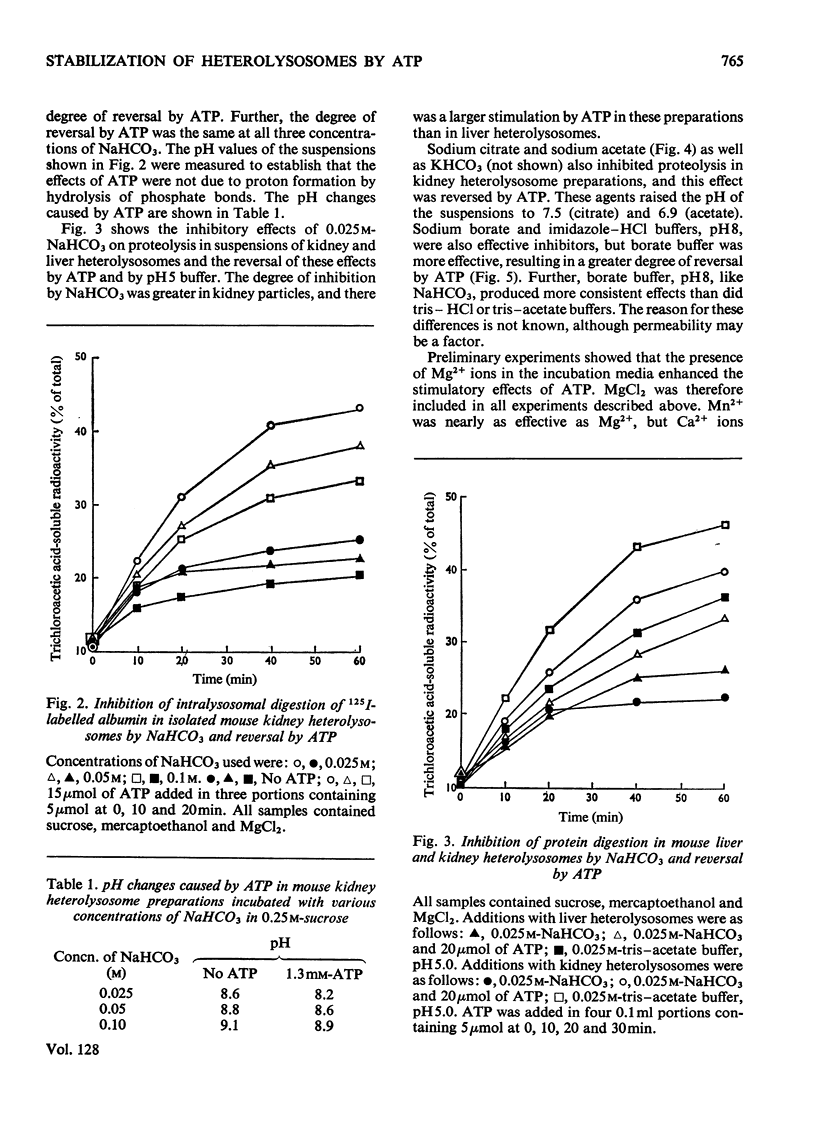
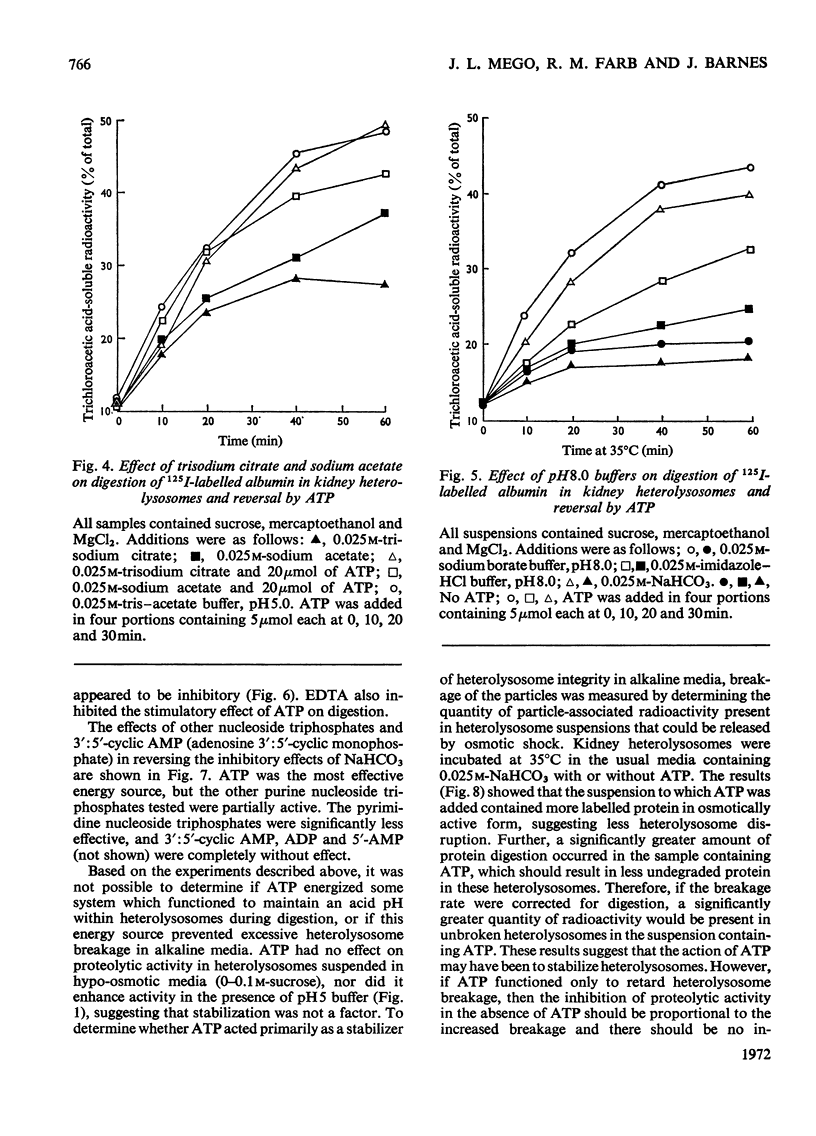
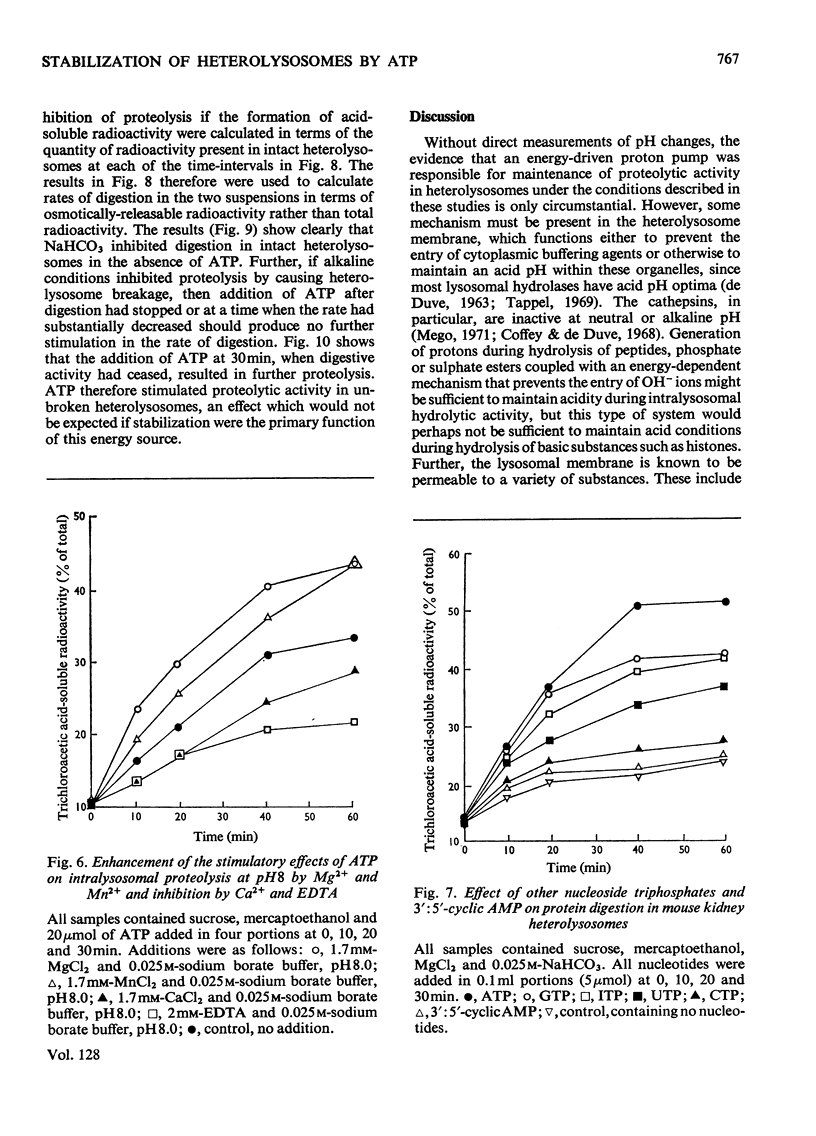
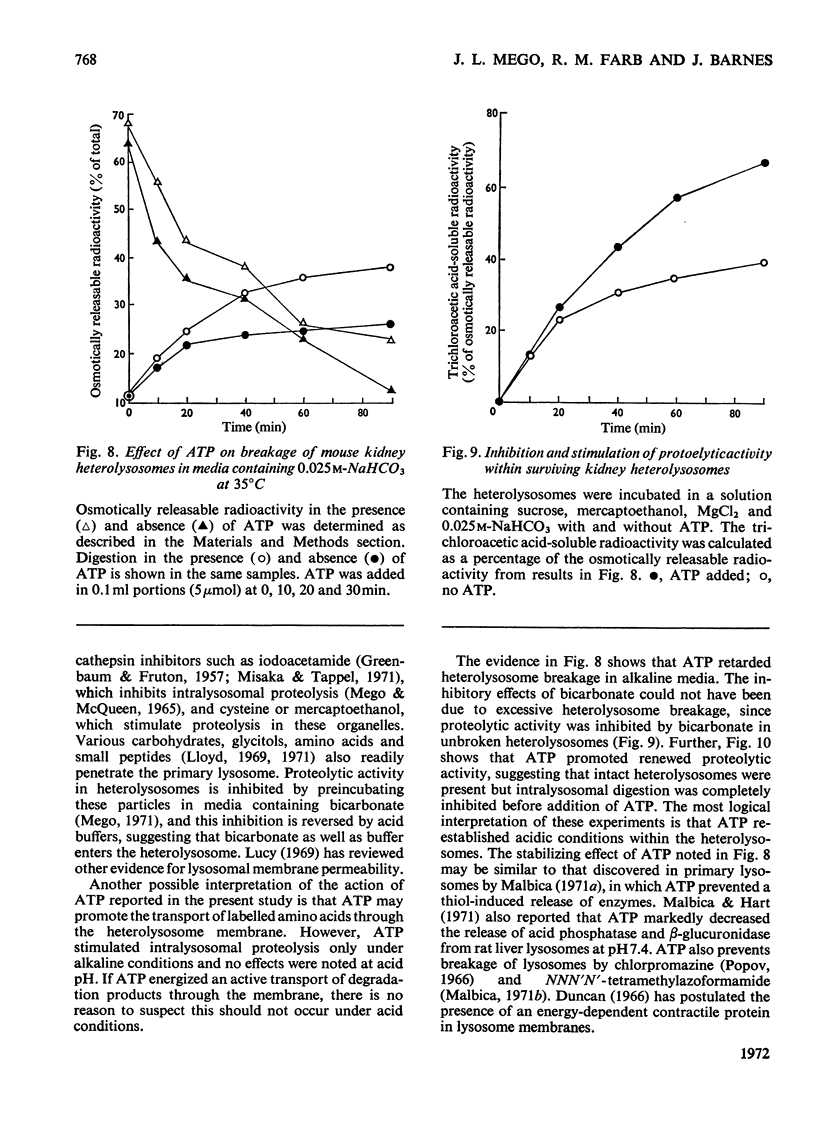
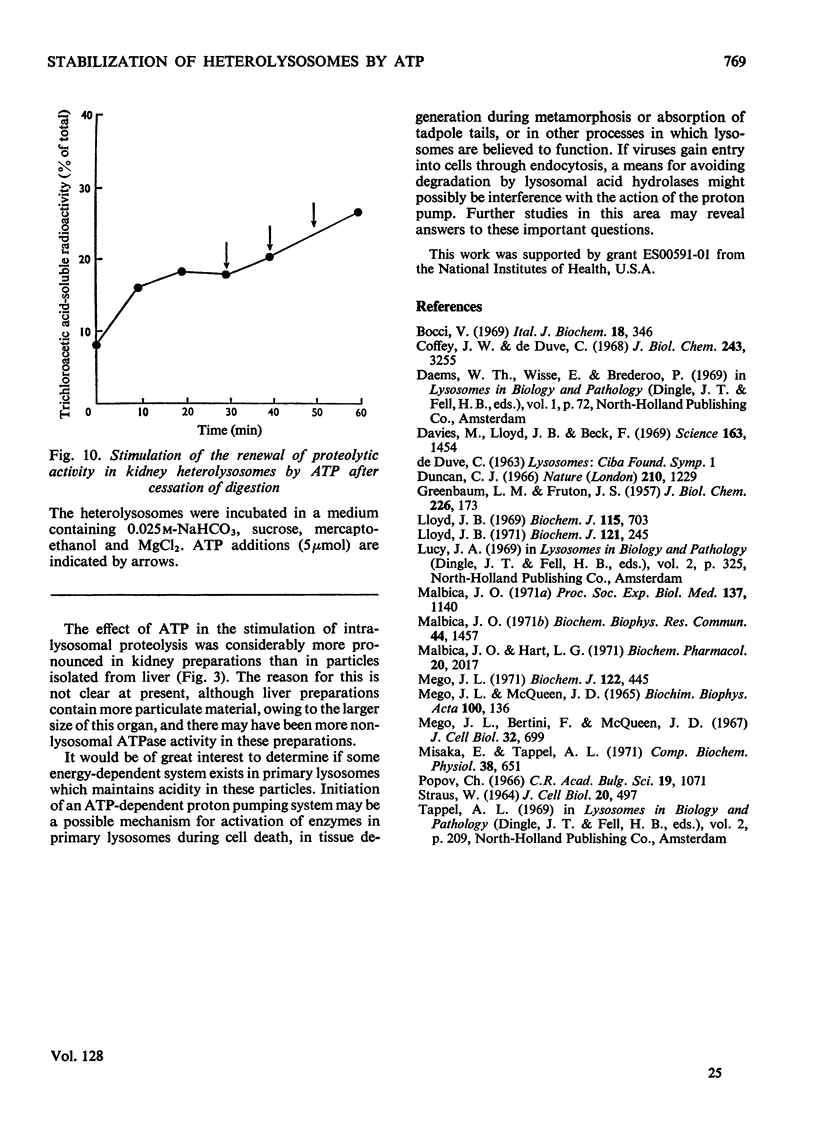
1. Homogenization of mouse kidneys or livers in 0.25m-sucrose buffered with tris–acetate, pH7.3, resulted in a decreased rate of proteolysis within isolated heterolysosomes containing injected 125I-labelled albumin when these particles were incubated at 35°C. Proteolysis in mouse kidney or liver heterolysosomes isolated from homogenates made in 0.25m-sucrose buffered at pH7.3 was stimulated by pH5 buffer or by additions of ATP. 2. A greater inhibition of proteolysis was produced by including bicarbonate or pH8 borate buffers in the incubation media, and this inhibition was also reversed by ATP. 3. Other nucleoside triphosphates were not as effective as ATP, but GTP and ITP were more effective than CTP or UTP. ADP, AMP, or adenosine 3′:5′-cyclic monophosphate were completely without effect. 4. Although ATP prevented some heterolysosome breakage in media containing bicarbonate, the primary effect appeared to be to promote proteolytic activity. 5. These observations are consistent with the presence of a proton pump in the heterolysosome membrane, which functions to maintain intralysosomal pH in alkaline media.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bocci V. Factors affecting protein iodination using chloramine T and the assessment of homogeneity of serum 131-I-proteins. Ital J Biochem. 1969 Sep-Oct;18(5):346–375. [PubMed] [Google Scholar]
- Coffey J. W., De Duve C. Digestive activity of lysosomes. I. The digestion of proteins by extracts of rat liver lysosomes. J Biol Chem. 1968 Jun 25;243(12):3255–3263. [PubMed] [Google Scholar]
- Davies M., Lloyd J. B., Beck F. Protein digestion in isolated lysosomes inhibited by intralysosomal trypan blue. Science. 1969 Mar 28;163(3874):1454–1456. doi: 10.1126/science.163.3874.1454. [DOI] [PubMed] [Google Scholar]
- Duncan C. J. Properties and stabilization of the lysosomal membrane. Nature. 1966 Jun 18;210(5042):1229–1230. doi: 10.1038/2101229a0. [DOI] [PubMed] [Google Scholar]
- GREENBAUM L. M., FRUTON J. S. Purification and properties of beef spleen cathepsin B. J Biol Chem. 1957 May;226(1):173–180. [PubMed] [Google Scholar]
- Lloyd J. B. A study of permeability of lysosomes to amino acids and small peptides. Biochem J. 1971 Jan;121(2):245–248. doi: 10.1042/bj1210245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd J. B. Studies on the permeability of rat liver lysosomes to carbohydrates. Biochem J. 1969 Dec;115(4):703–707. doi: 10.1042/bj1150703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEGO J. L., MCQUEEN J. D. THE UPTAKE AND DEGRADATION OF INJECTED LABELED PROTEINS BY MOUSE-LIVER PARTICLES. Biochim Biophys Acta. 1965 Apr 12;100:136–143. doi: 10.1016/0304-4165(65)90436-8. [DOI] [PubMed] [Google Scholar]
- Malbica J. O., Hart L. G. Effect of adenosine triphosphate and some anti-inflammatory agents on a purified lysosomal fraction having high acid phosphatase and labile -glucuronidase activity. Biochem Pharmacol. 1971 Aug;20(8):2017–2026. doi: 10.1016/0006-2952(71)90401-1. [DOI] [PubMed] [Google Scholar]
- Malbica J. O., Kimura K. K. Effect of ATP on cysteine and other thiol-induced labilization of rat liver lysosomes. Proc Soc Exp Biol Med. 1971 Sep;137(4):1140–1144. doi: 10.3181/00379727-137-35743. [DOI] [PubMed] [Google Scholar]
- Malbica J. O. The effect of N,N,N'-tetramethylazoformamide (TMAF) on lysosomal stability in the presence and absence of anti-inflammatory agents. Biochem Biophys Res Commun. 1971 Sep 17;44(6):1457–1463. doi: 10.1016/s0006-291x(71)80249-8. [DOI] [PubMed] [Google Scholar]
- Mego J. L., Bertini F., McQueen J. D. The use of formaldehyde-treated 131-I-albumin in the study of digestive vacuoles and some properties of these particles from mouse liver. J Cell Biol. 1967 Mar;32(3):699–707. doi: 10.1083/jcb.32.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mego J. L. The effect of pH on cathepsin activities in mouse liver heterolysosomes. Biochem J. 1971 May;122(4):445–452. doi: 10.1042/bj1220445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misaka E., Tappel A. L. Inhibition studies of cathepsins A, B, C and D from rat liver lysosomes. Comp Biochem Physiol B. 1971 Apr 15;38(4):651–662. doi: 10.1016/0305-0491(71)90268-9. [DOI] [PubMed] [Google Scholar]
- Popov C. Protecting liver lysosomes from the injurious effect of chlorpromazine with ATP. C R Acad Bulg Sci. 1966;19(11):1071–1074. [PubMed] [Google Scholar]
- STRAUS W. CYTOCHEMICAL OBSERVATIONS ON THE RELATIONSHIP BETWEEN LYSOSOMES AND PHAGOSOMES IN KIDNEY AND LIVER BY COMBINED STAINING FOR ACID PHOSPHATASE AND INTRAVENOUSLY INJECTED HORSERADISH PEROXIDASE. J Cell Biol. 1964 Mar;20:497–507. doi: 10.1083/jcb.20.3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]


