Abstract
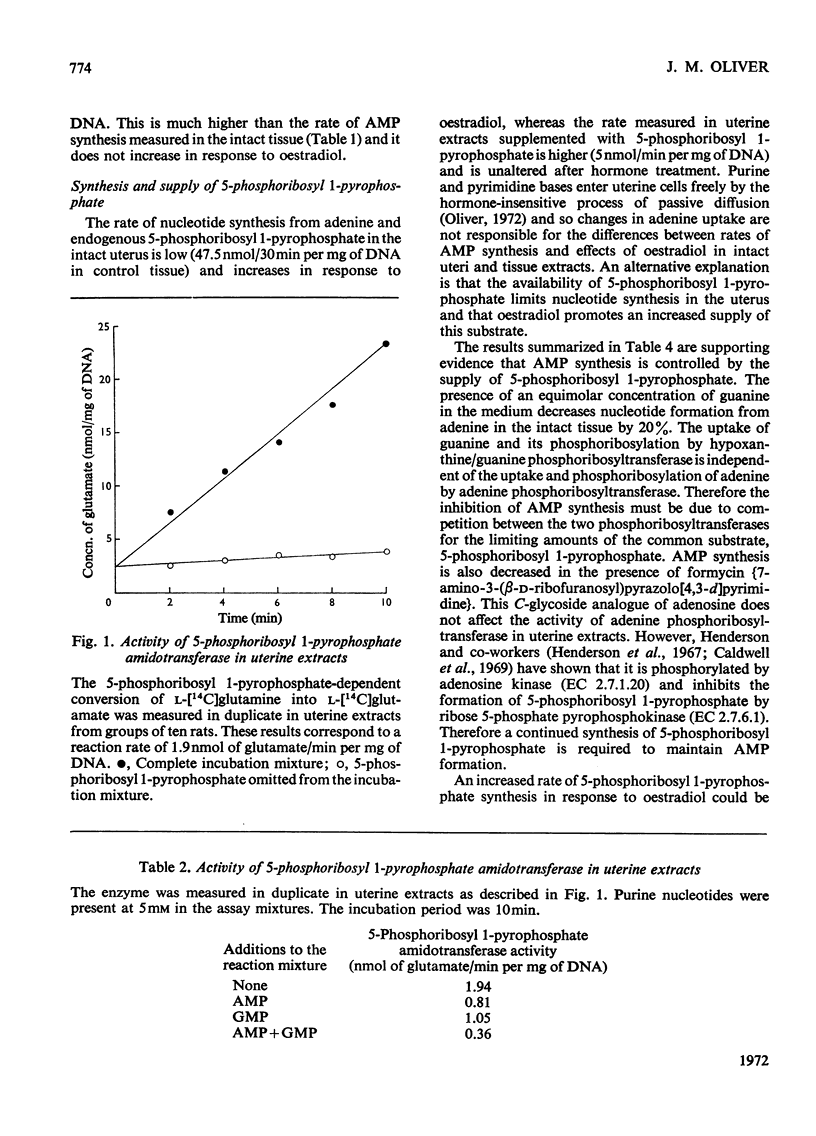
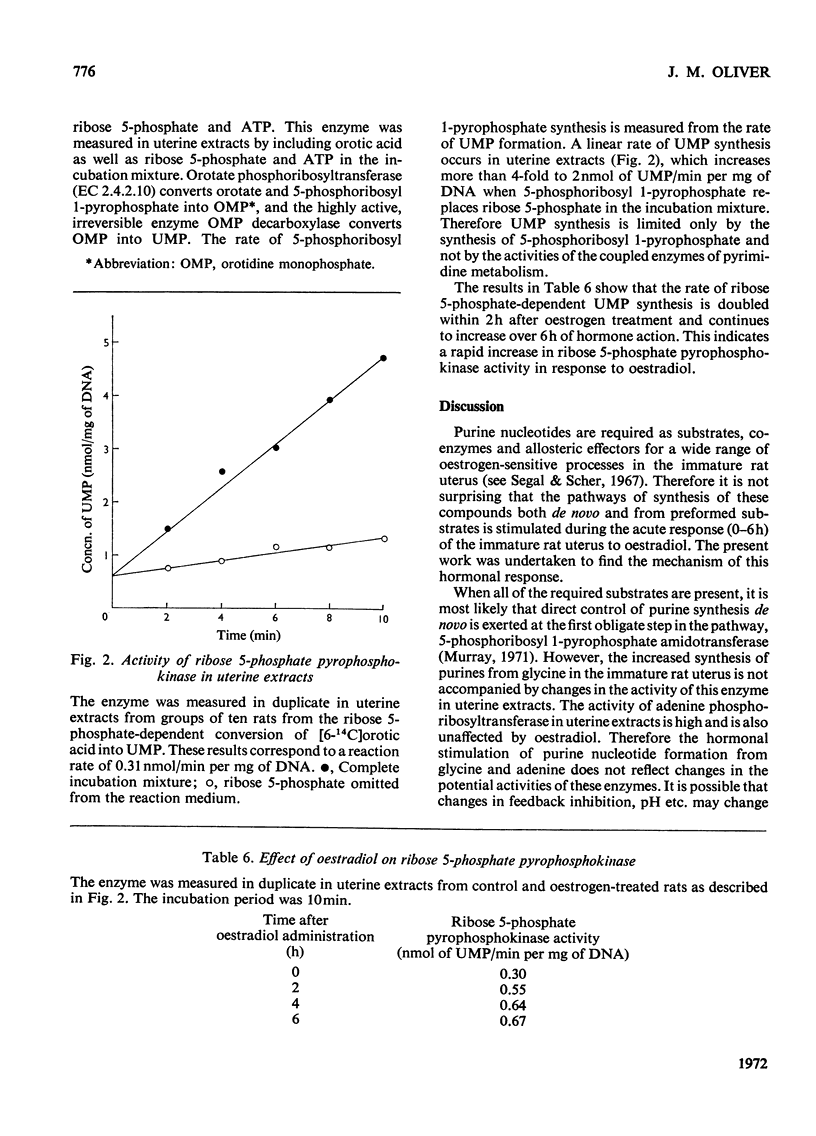
1. It has been reported that the rate of purine nucleotide synthesis de novo in the immature rat uterus is doubled at 6h after administration of oestradiol-17β. The present work confirms an increased incorporation of glycine and adenine into uterine nucleotides between 2 and 6h after hormone treatment and investigates the mechanism of this response. 2. Activation of regulatory enzymes is unlikely to promote increased nucleotide synthesis: the activities of 5-phosphoribosyl 1-pyrophosphate amidotransferase (EC 2.4.2.14) and adenine phosphoribosyltransferase (EC 2.4.2.7) are the same in uterine extracts from control and oestrogen-treated rats. 3. Therefore it was proposed that oestradiol might promote an increased supply of a rate-limiting substrate. The low oestrogen-sensitive rate of AMP synthesis from adenine and endogenous 5-phosphoribosyl 1-pyrophosphate in the intact uterus compared with the high, oestrogen-insensitive rate in uterine extracts supplemented with 5-phosphoribosyl 1-pyrophosphate is evidence that the supply of 5-phosphoribosyl 1-pyrophosphate limits purine nucleotide formation and may increase after hormone treatment. This proposal is supported by the decrease in AMP synthesis in the whole tissue in the presence of guanine and 7-amino-3-(β-d-ribofuranosyl)pyrazolo[3,4-d]pyrimidine (formycin). These compounds do not inhibit adenine uptake or adenine phosphoribosyltransferase activity, but they both decrease the availability of 5-phosphoribosyl 1-pyrophosphate, the former by promoting its utilization by hypoxanthine/guanine phosphoribosyltransferase (EC 2.4.2.8) and the latter by inhibiting its synthesis from ribose 5-phosphate and ATP by ribose 5-phosphate pyrophosphokinase (EC 2.7.6.1). 4. It is unlikely that the increased availability of 5-phosphoribosyl 1-pyrophosphate results from hormonal stimulation of ribose 5-phosphate formation. Methylene Blue and phenazine methosulphate both increase ribose 5-phosphate without altering the supply of 5-phosphoribosyl 1-pyrophosphate. 5. The activity of ribose 5-phosphate pyrophosphokinase is low in uterine extracts and increases rapidly in response to oestradiol. Therefore the hormonal activation of the routes of purine nucleotide synthesis both de novo and from preformed precursors may be due, at least in part, to an increased availability of the common rate-limiting substrate 5-phosphoribosyl 1-pyrophosphate, mediated by activation of ribose 5-phosphate pyrophosphokinase.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caldwell I. C., Henderson J. F., Paterson A. R. The metabolism of formycin, an adenosine analogue. Can J Biochem. 1969 Oct;47(10):901–908. doi: 10.1139/o69-142. [DOI] [PubMed] [Google Scholar]
- FISCHER G. A., SARTORELLI A. C. DEVELOPMENT, MAINTENANCE AND ASSAY OF DRUG RESISTANCE. Methods Med Res. 1964;10:247–262. [PubMed] [Google Scholar]
- Fausto N. The control of RNA synthesis during liver regeneration. OMP pyrophosphorylase and decarboxylase activities in normal and regenerating liver. Biochim Biophys Acta. 1969 May 20;182(1):66–75. doi: 10.1016/0005-2787(69)90521-8. [DOI] [PubMed] [Google Scholar]
- HENDERSON J. F., KHOO K. Y. ON THE MECHANISM OF FEEDBACK INHIBITION OF PURINE BIOSYNTHESIS DE NOVO IN EHRLICH ASCITES TUMOR CELLS IN VITRO. J Biol Chem. 1965 Jul;240:3104–3109. [PubMed] [Google Scholar]
- HENDERSON J. F., KHOO M. K. AVAILABILITY OF 5-PHOSPHORIBOSYL 1-PYROPHOSPHATE FOR RIBONUCLEOTIDE SYNTHESIS IN EHRLICH ASCITES TUMOR CELLS IN VITRO. J Biol Chem. 1965 Jun;240:2358–2362. [PubMed] [Google Scholar]
- HENDERSON J. F., KHOO M. K. SYNTHESIS OF 5-PHOSPHORIBOSYL 1-PYROPHOSPHATE FROM GLUCOSE IN EHRLICH ASCITES TUMOR CELLS IN VITRO. J Biol Chem. 1965 Jun;240:2349–2357. [PubMed] [Google Scholar]
- Henderson J. F., Paterson A. R., Caldwell I. C., Hori M. Biochemical effects of formycin, an adenosine analog. Cancer Res. 1967 Apr;27(4):715–719. [PubMed] [Google Scholar]
- Hershko A., Razin A., Mager J. Regulation of the synthesis of 5-phosphoribosyl-I-pyrophosphate in intact red blood cells and in cell-free preparations. Biochim Biophys Acta. 1969 Jun 17;184(1):64–76. doi: 10.1016/0304-4165(69)90099-3. [DOI] [PubMed] [Google Scholar]
- Hill D. L., Bennett L. L., Jr Purification and properties of 5-phosphoribosyl pyrophosphate amidotransferase from adenocarcinoma 755 cells. Biochemistry. 1969 Jan;8(1):122–130. doi: 10.1021/bi00829a017. [DOI] [PubMed] [Google Scholar]
- Hori M., Henderson J. F. Purification and properties of adenylate pyrophosphorylase from Ehrlich ascites tumor cells. J Biol Chem. 1966 Mar 25;241(6):1406–1411. [PubMed] [Google Scholar]
- JERVELL K. F., DINIZ C. R., MUELLER G. C. Early effects of estradiol on nucleic acid metabolism in the rat uterus. J Biol Chem. 1958 Apr;231(2):945–959. [PubMed] [Google Scholar]
- Kelley W. N., Fox I. H., Wyngaarden J. B. Regulation of purine biosynthesis in cultured human cells. I. Effects of orotic acid. Biochim Biophys Acta. 1970 Sep 22;215(3):512–516. doi: 10.1016/0304-4165(70)90101-7. [DOI] [PubMed] [Google Scholar]
- MASTER R. W. POSSIBLE SYNTHESIS OF POLYRIBONUCLEOTIDES OF KNOWN BASE-TRIPLET SEQUENCES. Nature. 1965 Apr 3;206:93–93. doi: 10.1038/206093b0. [DOI] [PubMed] [Google Scholar]
- Marko P., Gerlach E., Zimmer H. G., Pechán I., Cremer T., Trendelenburg C. Interrelationship between salvage pathway and synthesis de novo of adenine nucleotides in kidney slices. Hoppe Seylers Z Physiol Chem. 1969 Dec;350(12):1669–1674. doi: 10.1515/bchm2.1969.350.2.1669. [DOI] [PubMed] [Google Scholar]
- Murray A. W. The biological significance of purine salvage. Annu Rev Biochem. 1971;40:811–826. doi: 10.1146/annurev.bi.40.070171.004115. [DOI] [PubMed] [Google Scholar]
- Oliver J. M., Kellie A. E. The effects of oestradiol on the acid-soluble nucleotides of rat uterus. Biochem J. 1970 Sep;119(2):187–191. doi: 10.1042/bj1190187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajalakshmi S., Handschumacher R. E. Control of purine biosynthesis de novo by orotic acid in vivo and in vitro. Biochim Biophys Acta. 1968 Feb 26;155(2):317–325. doi: 10.1016/0005-2787(68)90176-7. [DOI] [PubMed] [Google Scholar]
- Reel J. R., Gorski J. Gonadotrophic regulation of precursor incorporation into ovarian RNA, protein, and acid-soluble fractions. I. Effects of pregnant mare serum gonadotrophin (PMSG), follicle-stimulating hormone (FSH), and luteinizing hormone (LH). Endocrinology. 1968 Nov;83(5):1083–1091. doi: 10.1210/endo-83-5-1083. [DOI] [PubMed] [Google Scholar]
- Rosenbloom F. M., Henderson J. F., Caldwell I. C., Kelley W. N., Seegmiller J. E. Biochemical bases of accelerated purine biosynthesis de novo in human fibroblasts lacking hypoxanthine-guanine phosphoribosyltransferase. J Biol Chem. 1968 Mar 25;243(6):1166–1173. [PubMed] [Google Scholar]
- Tay B. S., Lilley R. M., Murray A. W., Atkinson M. R. Inhibition of phosphoribosyl pyrophosphate amidotransferase from Ehrlich ascites-tumour cells by thiopurine nucleotides. Biochem Pharmacol. 1969 Apr;18(4):936–938. doi: 10.1016/0006-2952(69)90069-0. [DOI] [PubMed] [Google Scholar]


