Abstract
Ku86 plays a key role in nonhomologous end joining in mammals. Functional inactivation in rodents of either Ku86 or Ku70, which form the heterodimeric DNA end-binding subunit of the DNA-dependent protein kinase complex, is nevertheless compatible with viability. In contrast, no human patient has been described with mutations in either Ku86 or Ku70. This has led to the hypotheses that either these genes are performing an additional essential role(s) and/or redundant pathways exist that mask the phenotypic expression of these genes when they are mutated in humans. To address this issue, we describe here the construction of human somatic cell lines containing a targeted disruption of the Ku86 locus. Human HCT116 colon cancer cells heterozygous for Ku86 were haploinsufficient with an increase in polyploid cells, a reduction in cell proliferation, elevated p53 levels, and a slight hypersensitivity to ionizing radiation. Functional inactivation of the second Ku86 allele resulted in cells with a drastically reduced doubling time. These cells were capable of undergoing only a limited number of cell divisions, after which they underwent apoptosis. These experiments demonstrate that the Ku86 locus is essential in human somatic tissue culture cells.
The maintenance of chromosomal integrity is essential for cellular survival (1). Among the many forms of damage that can cause chromosomal instability, DNA double-strand breaks (DSBs) seem to be the most insidious. Improper repair of DSBs results in chromosomal translocations, inversions, and fusions; this, in turn, invariably results in cancer or cell death (1). DSBs can arise through exposure to chemotherapeutic agents or ionizing radiation (IR), occur spontaneously during DNA replication, and are formed transiently in meiosis and during V(D)J recombination in the immune system (1). Cells have evolved at least two independent pathways for repairing DSBs, homologous recombination and nonhomologous DNA end joining (NHEJ; refs. 1 and 2). Homologous recombination ensures accurate repair by using an undamaged sister chromatid or homologous chromosome as a template. NHEJ, on the other hand, uses no, or limited, sequence homology to rejoin ends in a manner that is often error prone. In mammalian cells, NHEJ is the preferred mechanism of DSB repair (2). Some of the gene products involved in this pathway include Ku70, Ku86, the DNA-dependent protein kinase catalytic subunit (DNA–PKcs), XRCC4, and DNA ligase IV (2).
Ku is a heterodimeric DNA end-binding complex composed of 70- and 86-kDa subunits (Ku70 and Ku86, respectively; ref. 3). Ku binds in a sequence nonspecific fashion to virtually all double-stranded DNA ends including 5′ and 3′ overhangs, blunt ends, and duplex DNA ending in stem-loop structures (3). One unequivocal role for Ku is as a DNA-binding subunit of the DNA-dependent protein kinase (DNA–PK) complex, which is composed of the Ku heterodimer and DNA–PKcs (3). Extensive genetic and molecular studies have identified the DNA–PK complex as an integral component of mammalian DNA NHEJ DSB repair (3). Ku is believed to bind to broken DNA ends to prevent unnecessary DNA degradation (4) and juxtapose DNA ends (5–7). The binding of Ku to free DNA ends also recruits and activates DNA–PKcs (8), DNA ligase IV (9, 10), and XRCC4, a DNA ligase IV accessory factor (11, 12), which are required for the rejoining of DNA DSBs (13–16).
Murine knockouts for each of the components of the DNA–PK and XRCC4/ligase IV complexes have been generated. Mice deficient for XRCC4 (12) and DNA ligase IV (17, 18) are not viable because of neuronal degeneration caused by p53-induced apoptosis (19, 20). Mice deficient for Ku70 (21, 22), Ku86 (23, 24), or DNA–PKcs (25–28) are viable and exhibit the expected immune deficiency and IR hypersensitivity. In addition, inactivation of the Ku86 gene results in cells with growth retardation (23), premature senescence (29), a marked increase in chromosomal aberrations (30–32), and elevated telomeric fusions (33–35).
Whereas DNA ligase IV is an essential gene in rodents, human somatic cells lacking DNA ligase IV are viable (15), and mutations in DNA ligase IV have been described in patients with clinical radiosensitivity and abnormal V(D)J recombination (36, 37). Moreover, functional inactivation in rodents of all three components of the DNA–PK complex has been achieved, yet no human patient has been described with a mutation in any of the subunits. These observations imply that there may be important differences in NHEJ between rodents and humans, and they further suggest that the genes making up the DNA–PK complex may be essential in humans.
To clarify the role of Ku86 in human cells, we have used gene targeting in human somatic tissue culture cells to functionally inactivate the Ku86 locus. Human Ku86 heterozygous cell lines displayed significant haploinsufficient phenotypes; they were defective in cell proliferation and DNA–PK and DNA end-binding activities, and they showed elevated levels of p53, polyploidy, and IR sensitivity. A second round of gene targeting generated homozygously null Ku86 cell lines. These cell lines showed a severe growth defect and ultimately underwent apoptosis after a limited number of cell doublings. These experiments demonstrate that the Ku86 locus is essential in human somatic cells.
Materials and Methods
Cell Culture.
Ku86 knockout cell lines were obtained from HCT116 cells after electroporation of the targeting vector followed by selection in 500 μg/ml G418. The modified heterozygous Ku86 knockout cell line 70–32, which was altered by Cre recombination, was obtained following negative selection with 10 μM gancyclovir.
Targeting Vector Construction.
The Ku86 gene-targeting vector was constructed in pUC19 by using a 2-kb genomic fragment just distal to exon 1 as the 5′ arm and cloned after adding PacI and NotI restriction sites to the 5′ and 3′ ends, respectively. An 8.5-kb genomic fragment containing part of intron 1, exon 2, and part of intron 2 was used as the 3′ arm and cloned in two steps that resulted in the addition of NotI and SalI restriction sites to the 5′ and 3′ ends, respectively, and which introduced a novel EcoRV site within intron I (see Fig. 1). The 3-kb neomycin selection cassette was cloned as a NotI to NotI fragment. The cassette contained a c-Myc splice acceptor, a LoxP site, a promoterless neomycin-resistance gene, an internal ribosome entry site for expression of the adjacent viral thymidine kinase gene, another loxP site, and then the SV40 polyadenylation signal. On the distal 5′ arm of the targeting vector, a diphtheria toxin (DT; pKOSelectDT plasmid) gene driven by the RNA polymerase II promoter was cloned into the PacI restriction site. Before transfection, the vector was linearized by SalI restriction enzyme digestion.
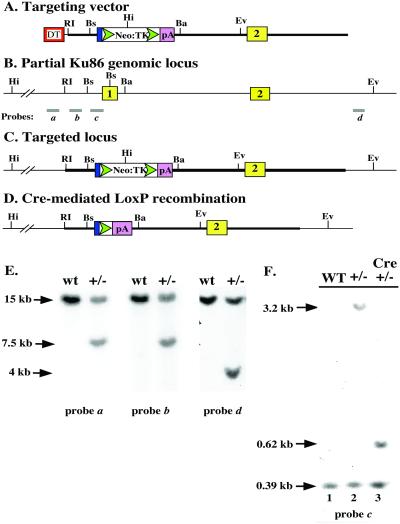
Figure 1.
Scheme for functional inactivation of the human Ku86 locus. (A) Cartoon of the targeting vector. Red rectangle designated DT, diphtheria toxin gene; blue rectangle, c-Myc splice acceptor; green triangles, loxP sites; open rectangle, neomycin resistance (Neo) and HSV thymidine kinase (TK) genes; pink rectangle, polyadenylation signal (pA); yellow rectangle, Ku86 exon 2; restriction enzyme sites: RI, EcoRI; Bs, Bsu36I; Hi, HindIII; Ba, BamHI; Ev, EcoRV. (B) Ku86 genomic locus; a and d are external probes, and b and c are internal probes used for the heterozygous knockout screen. (C) Targeted locus and (D) targeted locus following Cre-mediated loxP recombination. (E) Southern blot analysis of wild-type and Ku86 heterozygous (+/−) cells. Genomic DNA samples were doubly digested with HindIII and EcoRV and hybridized with the indicated probes. (F) Cre-mediated loxP recombination. DNA samples were doubly digested with BamHI and Bsu36I and hybridized with probe c.
Transfection.
For each transfection, 25 μg of SalI-linearized DNA was mixed with 1 × 107 HCT116 cells in 500 μl of PBS. Transfection was carried out by electroporation at 240 V, 975 μF with 0.4-cm cuvettes.
Genomic Southern Hybridization.
Chromosomal DNA was prepared, digested, subjected to electrophoresis, and transferred to nitrocellulose filters as described (38). The filters were then hybridized with the probes shown in Fig. 1. Probe a was an 802-bp StuI–SspI restriction fragment, which resides 5′ of the targeting vector sequences. Probe b was a 436-bp EagI–PstI restriction fragment, which is complementary to part of the 5′ targeting vector arm. Probe c was a 210-bp Bsu36I–SacI restriction fragment corresponding to the promoter of the Ku86 gene. Probe d was a 300-bp PCR fragment that resides 3′ of the targeting vector. This PCR fragment was amplified by using primers CCTTTATTCTGGGAATCGTACAGC and TTCTATCCGCCTCCAAACATTTC.
Genomic PCR.
Fifty nanograms of genomic DNA was amplified by using 20 pmol of each relevant primer as follows: KO4, GCGAGTTGCGACACGGCAGGTTCC; KO2, CTCTTGCCCCATTCTTTGTCTTG; 86–5, CAGGTTCAGGGGAGGTGTGGGAG; KO3, TTTCTCATAGCGCATCCCTCGGTCC.
Preparation of Extracts.
Cells were trypsinized, washed three times in PBS, and boiled in 10 mM Tris, pH 7.5/5 mM MgCl2 with a 2× Complete protease inhibitor mixture for 10 min. The samples were then digested with DNase I (0.1 unit/μl) at 37°C for 10 min. Samples obtained in this way were used as whole cell extract. For cytoplasmic and nuclear extract preparation, cells were lysed by vortexing in an equal volume of buffer A (39) supplemented with 0.1% Nonidet P-40 and 2× protease inhibitor mixture. After incubation on ice for 10 min, the cell lysate was centrifuged at 14,000 rpm for 10 min at 4°C. The supernatant was used as cytoplasmic extract. The pellet was further washed two times with ice-cold buffer A, and nuclear extract was subsequently prepared as described (39).
Immunoblotting.
For immunoblot detection, proteins were subjected to electrophoresis on a 7% SDS/PAGE, electroblotted onto a nitrocellulose filter, and detected as described (39).
Assays.
Ku DNA end-binding activity was measured by a gel mobility shift assay as described (39). Cell proliferation, x-ray survival assays, and DNA–PK kinase assays have also been described (38, 39). For immunocytochemical assays, cells were seeded in 96-well plates overnight. After rinsing with PBS, the cells were fixed and permeabilized with 2% paraformaldehyde and 0.2% Triton X-100 in PBS at 4°C for 10 min. The fixed cells were rinsed three times with PBS and probed by using the indicated primary antibody in PBS at 4°C for 30 min. After rinsing and washing with PBS, the cells were stained with secondary antibodies in PBS at 4°C for 30 min and visualized by fluorescent microscopy.
Results
Generation of Heterozygous Ku86+/− HCT116 Cells.
A targeting vector was constructed that contained 2 kb of upstream flanking genomic DNA corresponding to the promoter region of Ku86 (Fig. 1A). This was fused to the c-Myc splice acceptor, a loxP site (loxP), a promoterless neomycin-resistance gene (Neo), an internal ribosome entry site, a viral thymidine kinase gene (TK), another loxP site, the SV40 polyadenylation signal (pA), and then another 8.5 kb of downstream flanking genomic DNA, which included the first intron of Ku86 and terminated 3′ of exon 2 (Fig. 1A). Correct targeting of the endogenous genomic locus (Fig. 1B) should result in the deletion of exon 1 and the generation of a G418-resistant cell line (Fig. 1C). A promoterless neomycin-resistance targeting cassette was used because this approach is the most efficacious in human cells (40, 41). Therefore, this construct was introduced into HCT116 cells by electroporation, and G418-resistant clones were selected. HCT116 is an immortalized human colon cancer cell line that is diploid, has a stable karyotype, contains wild-type p21 and p53 genes, and responds normally to DNA-damaging agents with respect to the induction of p53 and cell cycle arrest (40, 42, 43). As importantly, the functional inactivation of p21, p53, 14–3-3σ, Bax, DNMT1, ORC2, and securin has demonstrated that gene targeting is experimentally feasible in this cell line (40, 43–48). Cell lines containing correctly targeted integration events were scored by genomic Southern blot analysis by using HindIII and EcoRV and probe a (Fig. 1B). Over 500 independent colonies were analyzed in this fashion, and no correctly targeted colonies were obtained (data not shown). Thus, the targeting vector was redesigned to contain the DT gene driven by a strong promoter on the distal 5′ arm of the targeting construct (Fig. 1A). Random integration of this construct should result in the death of the cell, as expression of DT in mammalian cells is lethal. The targeting protocol was repeated, and the overall frequency of G418-resistant clones was reduced ≈20-fold, suggesting that the DT selection was working. A total of 354 new G418-resistant colonies were screened, and two independent Ku86+/− heterozygous clones (nos. 44 and 70) were obtained. Correct targeting of the Ku86 locus in one of the clones, no. 70, was confirmed by Southern blot analysis by using 5′ external (Fig. 1E, probe a), internal (Fig. 1E, probe b), and 3′ external (Fig. 1E, probe d) probes. In each case, the appearance of a novel restriction enzyme digestion fragment, caused by the HindIII and EcoRV sites introduced on the targeting vector, permitted the unequivocal confirmation of a single, correctly targeted integration event (Fig. 1E). Clone 70 cells were expanded and transiently transfected with the expression vector, pGK-Cre. Cre mediates site-specific recombination between the loxP sites (49) and results in the excision of the bulk of the targeting construct, leaving behind a single loxP site and the pA element in the locus (Fig. 1D). Cells in which the excision event had occurred could be selected with gangcyclovir because of the loss of the TK gene. Gangcyclovir-resistant cell lines containing the correctly excised locus were identified by Southern blot analysis by using BamHI and Bsu36I digestions and hybridization with probe c (Fig. 1F). Wild-type HCT116 cells displayed only the endogenous 0.39-kb Bsu36I to Bsu36I fragment (Fig. 1F, lane 1). Clone 70 contained the endogenous 0.39-kb fragment as well as a novel 3.2-kb Bsu36I to BamHI fragment, corresponding to the loss of the Bsu36I site within exon 1 and the introduction of a BamHI site on the targeting vector (Fig. 1F, lane 2). Four of 24 Cre-treated clones analyzed, including clone 70–32, no longer contained the 3.2-kb band but now contained a 0.62-kb Bsu36I to BamHI fragment, which corresponded precisely to the loss of the Neo-TK coding sequences (Fig. 1F, lane 3).
Haploinsufficiency of Ku86+/− HCT116 Cells.
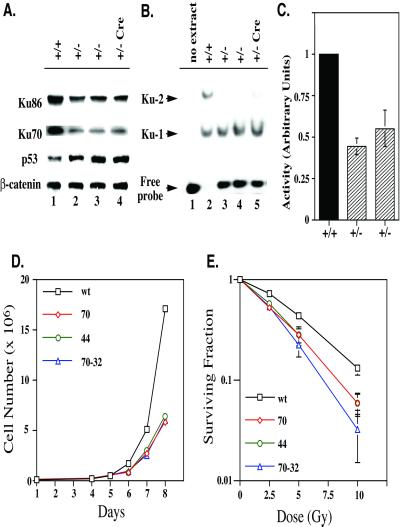
Morphologically, Ku86+/− cells seemed indistinguishable from wild-type HCT116 cells with the exception that ≈2% of the population contained giant, multinucleated, and/or polyploid cells (Fig. 2). Immunoblot analysis of Ku86+/− cells demonstrated that they only contained 20–50% as much Ku86 and Ku70 protein as the parental cells (Fig. 3A). The reduction in Ku70 levels was expected because it was known that in the absence of one subunit the other Ku subunit is unstable (3). In contrast, all of the heterozygous cell lines contained elevated (3-fold) levels of p53 (Fig. 3A). Consistent with the reduction in Ku subunits, the Ku86+/− cells contained only 50% as much Ku DNA end-binding (DEB) activity as the parental cells (Fig. 3B). Nuclear extract derived from wild-type cells was capable of binding and shifting all of the added 55-bp dsDNA probe, and a significant fraction of the probe had two Ku heterodimers bound to it (Fig. 3B, lane 2). In contrast, nuclear extract derived from the Ku86+/− cells could only shift ≈50% of the probe, and almost no multimer Ku forms were detectable (Fig. 3B, lanes 3–5). Consistent with the known dependence of DNA–PK activity on Ku DEB (5, 6), a 50% reduction in DNA–PK activity was also observed (Fig. 3C). This reduction in Ku levels and DEB and DNA–PK activities translated itself into a growth defect (Fig. 3D). The doubling time for the parental HCT116 cells was 17.7 h (±0.3), whereas Ku86+/− clones uniformly doubled once only every 20.5 h (±0.4). FACS analysis of the cell lines did not reveal any salient differences in the cell-cycle distribution of asynchronous cells (data not shown). Ku86+/− HCT116 cells were also slightly IRs with a surviving fraction at 5 Gy of only 0.25 compared with 0.42 for the wild-type cells (Fig. 3E). FACS analysis of X-irradiated cells uncovered no salient differences in the G1 and G2 cell-cycle arrests between wild-type and Ku86 heterozygous cells (data not shown). Thus, unlike Ku86+/− rodent cell lines, which are phenotypically indistinguishable from their parental cell lines (23, 24), human Ku86+/− cells were haploinsufficient and showed cell proliferation and DNA damage sensitivity defects.
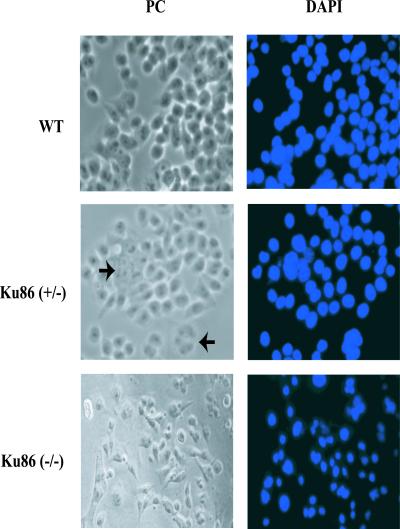
Figure 2.
Morphological alterations in Ku86-deficient cells. Wild-type (wt), Ku86 heterozygous (Ku86+/−), or Ku86 null (Ku86−/−) cells were prepared for phase contrast (PC) or fluorescent images following 4′,6-diamidino-2-phenylindole (DAPI) staining. Ku86+/− cells were indistinguishable from wild-type cells with the exception of the presence of polyploid cells (arrowheads). Ku86−/− cells appeared apoptotic under phase contrast, and this was confirmed by the DNA condensation observed in the DAPI-stained samples.
Figure 3.
Haploinsufficiency of Ku86 heterozygous cells. (A) Ku86+/− cells have diminished levels of Ku. Whole cell extract was prepared from wild-type HCT116 cells (+/+), from the two independent Ku86+/− clones (nos. 44 and 70; +/−), and from the Cre-recombined Ku86+/− clone (no. 70–32; +/− Cre) and analyzed by immunoblotting for Ku86, Ku70, p53, and β-catenin protein levels. A single blot is shown that was probed sequentially with four different antibodies. (B) Ku86+/− cells have diminished levels of Ku DEB. Nuclear extract was prepared from the indicated cell lines and incubated with a radiolabeled 55-bp dsDNA probe. DEB activity was then assessed by an electrophoretic mobility shift assay. The positions of the free probe and the probes with one (Ku-1) or two (Ku-2) Ku heterodimers bound are shown. (C) Ku86+/− cells have diminished levels of DNA–PK activity. Nuclear extracts prepared from the indicated cell lines were incubated with [γ-32P]rATP and a DNA–PK-specific substrate in the presence and absence of dsDNA. DNA–PK activity in wild-type cells was normalized to 100%. The average of two experiments, each performed in duplicate, is shown. (D) Ku86+/− cells have proliferation defects; 2 × 104 cells of the indicated cell lines (wt, wild-type; 44 and 70, Ku86 +/−; 70–32, +/− Cre) were seeded on tissue culture plates, and the increase in cell number on subsequent days was determined by cell counting by using trypan blue staining and a hematocytometer. The average of two experiments, each performed in triplicate, is shown. The error bars are too small to be seen. (E) Ku86+/− cells are IRs; 300 cells of the indicated cell lines were seeded on tissue culture plates and X-irradiated at the indicated doses. Cells surviving to form colonies (>50 cells/colony) 10 (wild type) or 14 (70, 44, and 70/32) days later were scored. The average of two experiments each performed in triplicate is shown on a semi-log scale.
Construction of Ku86-Null HCT116 Cells.
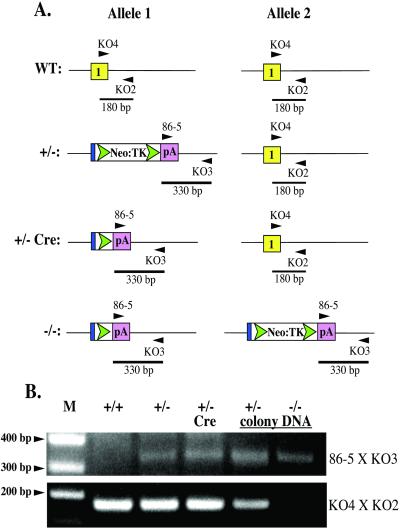
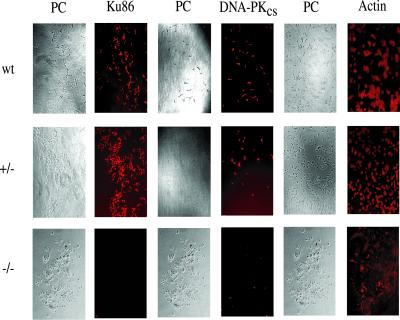
The Cre-recombined 70–32 clone was subjected to a second round of gene targeting by using the same targeting vector and selection strategies used to construct the heterozygous cell lines (Fig. 4A). A total of 487 colonies were screened, and 3 colonies, in which re-targeting of the already targeted allele had occurred, were obtained, but no homozygously targeted clones were recovered (data not shown). A small number of very slow-growing (doubling time ≈40 h) colonies were, however, observed. Some of these slow-growing clones grew to 200–1,000 cells (≈8–10 population doublings starting from a single cell) but then underwent massive apoptosis. To investigate whether any of these colonies corresponded to Ku86−/− cells, 46 slow-growing colonies were scraped off the dish at the ≈150–200 cell stage, and genomic DNA was isolated and then subjected to a diagnostic PCR analysis (Fig. 4A). As controls, faster growing colonies from the same plate were also isolated. Whereas all of the faster growing colonies produced the endogenous 180-bp and targeted 330-bp PCR products (Fig. 4B, +/− colony DNA), two of the slowest growing colonies only generated the targeted 330-bp PCR product (Fig. 4B, −/− colony). Morphologically, these two colonies were more elongated and apoptotic than the parental cells (Fig. 2). Staining of these colonies with 4′,6-diamidino-2-phenylindole revealed that the cells had highly condensed DNA and exhibited chromosomal fragmentation, both characteristics of apoptotic cells (Fig. 2). Indeed, the effect was actually more pronounced than that shown as many apoptotic cells were washed off during the fixing and staining processes. To confirm that these two colonies were null for Ku86, they were immunohistochemically stained for Ku86, DNA–PKcs, or β-actin. Whereas wild-type and heterozygous (+/−) cells stained with all three antibodies, the two slow-growing colonies tested (−/−) were totally devoid of Ku86 staining and showed reduced (≈10-fold), albeit detectable, levels of DNA–PKcs (Fig. 5). In contrast, all of the other 44 slow-growing clones analyzed stained positive for Ku86 protein, gave a positive endogenous 180-bp signal with PCR, and did not have apoptotic nuclei (data not shown). From these experiments, we concluded that the human Ku86 locus was essential. Ku86-null somatic HCT116 cells were capable of completing a limited number of cell doublings before they succumbed to apoptosis.
Figure 4.
Identification of Ku86 null cell lines by using genomic PCR. (A) A cartoon of the primers used and the expected sizes of the PCR products. (B) Ethidium bromide-stained agarose gel of the resultant PCR products. Genomic DNA was isolated from wild-type (+/+), heterozygous (+/−), Cre-treated heterozygous (+/− Cre), a random colony following the second round of targeting (+/− colony DNA), and a slow-growing colony on the same plate (−/− colony DNA). M, DNA marker.
Figure 5.
Immunohistochemical analysis of Ku86 null cells. Colonies of wild-type (wt), Ku86 heterozygous (+/−), and Ku86 null (−/−) cells were stained with antibodies to Ku86, DNA–PKcs, or actin. The cells were then counterstained with Cy3 secondary antibodies. Cells stained only with the secondary antibody exhibited no fluorescence (data not shown). The relevant phase contrast (PC) images are shown to the left of the stained images.
Discussion
In the mouse, inactivation of one allele of Ku86 results in animals that are phenotypically indistinguishable from wild-type animals, whereas inactivation of the second allele results in viable animals with cell proliferation defects and genomic instability phenotypes (23, 24, 29–33, 35). Here, we have demonstrated that inactivation of one Ku86 allele in human somatic HCT116 cells results in polyploidy (Fig. 2) and cell proliferation (Fig. 3D) and IR hypersensitivity (Fig. 3E) defects, whereas inactivation of the second allele results in cells that are extremely compromised in cell proliferation and that are ultimately nonviable because they undergo apoptosis (Figs. 4 and 5). These experiments are consistent with the demonstration that the expression of Ku86 antisense RNA in human cells leads to cellular defects in DNA DSB repair and cell proliferation (50, 51). Thus, our data suggest that species differences exist in the requirement for Ku86 and that Ku86 performs an essential function in human cells.
Human Ku86-deficient cells presumably die because they have accumulated an excess of DNA damage. First, levels of p53 are elevated 3-fold in heterozygous cells (Fig. 3A). p53 is the key downstream transcription factor in the signal transduction pathway for DNA damage in mammalian cells (52). Its elevation in Ku86+/− cells is consistent with these cells experiencing a higher level of spontaneous endogenous damage. Second, giant, polyploid, and multinucleated cells comprise 2–3% of the heterozygous cell population, whereas these cells are seen only rarely in the wild-type cell line (Fig. 2). The appearance of giant cells is often seen following X-irradiation of cells that incompletely repair the DNA damage. Polyploid and multinucleated cells are also associated with genomic instability as a result of defects in DNA repair (1). Third, Ku86+/− cells are slightly IRs (Fig. 3E), consistent with them containing reduced levels of DNA DSB repair activity. Together, these observations suggest that Ku86+/− cells have elevated levels of spontaneous endogenous damage. Thus, it is likely that Ku86−/− cells accumulate even more DNA damage, which is ultimately incompatible with survival. Importantly, however, Ku86−/− cells do not die immediately but succumb only after going 8–10 cell doublings. There are at least two explanations for this observation. First, other DNA DSB repair pathways, notably homologous recombination (1, 2), are operational in mammalian cells, and these may temporarily compensate for the loss of Ku86-dependent NHEJ. Because NHEJ is, however, the predominate pathway of DNA DSB in mammals, the homologous recombinational machinery is likely incapable of sustaining cellular viability when it is the primary defense against DNA damage. Alternatively, Ku86 is an abundant protein present at about 4 × 105 copies per cell (3), and when complexed to Ku70, it is very stable with a half-life of more than 16 h (53). Thus, even starting from a heterozygous cell where the levels of Ku86 are reduced by at least half that observed in wild-type cells (Fig. 3A), it may take multiple rounds of cell division following the second allele targeting before the levels of Ku in a Ku86−/− cell are reduced below some minimum threshold.
Alternatively, Ku may play a role in telomere homeostasis (3). In yeast, deletion of Ku results in telomere shortening, loss of telomere clustering, and deregulation of the single-strand overhang (54). In human cells, Ku is found physically associated with telomeres (55, 56), and Ku86-deficient mice have shorter telomeres (32) and show elevated telomeric fusions (33, 35). Moreover, with a strikingly similar phenotype to human Ku86−/− cells, the expression of a dominant defective telomerase in human cancer cells resulted in limited rounds of cell division followed by massive apoptosis (57). Thus, the dysregulation of telomere maintenance or function may be part, or all, of the explanation for the essential role of Ku86 in human cells.
A third possibility is that it is not the loss of Ku86 per se that is essential, but the loss of DNA–PK activity. Thus, heterozygous cells show a ≈50% reduction in DNA–PK activity (Fig. 3C), and the Ku86-null cells showed a strong reduction in the amount of DNA–PKcs protein detectable by immunohistochemical staining (Fig. 5). The reduction in DNA–PKcs protein was surprising, because although Ku mutations in rodents have been reported to reduce the level of DNA–PKcs activity, there has been no report that DNA–PKcs protein levels were also adversely affected (21–24). It should be noted, however, that DNA–PKcs, unlike Ku86, is a substrate for caspases in cells undergoing apoptosis (58), and thus it is likely that the low levels of DNA–PKcs may simply have resulted from the advanced apoptotic state of these cells (Figs. 2 and 5). Lastly, a viable human malignant glioma cell line, M059J, has been described (59) that is null or greatly reduced for DNA–PK activity, also suggesting that DNA–PK activity is not essential.
Murine cells deficient in Ku86 are viable (23, 24), whereas human cells are not (Fig. 5). One explanation for these results is the existence of a redundant pathway in mice that does not exist in humans. While the specifics of DNA DSB repair still need to be elucidated, there is evidence that in addition to the DNA–PK-dependent pathway for NHEJ, there exists a non-DNA–PK-dependent NHEJ pathway(s) (1). In mice, this alternative pathway(s) may facilitate cell survival in the absence of Ku86. In humans, if this other pathway(s) is reduced or absent, cells may totally depend on Ku86 for this type of repair. Second, as mentioned above, Ku86 plays a role in telomere function and maintenance. Because telomeres are significantly longer in mice as compared with humans (60), this physical attribute could explain why the absence of Ku86 is tolerated better in murine cells. Third, it is possible that Ku86 has evolved a novel function in human cells. It is important to note that we have described a transcript, termed KARP-1, which (through the use of an upstream promoter and exons) encodes a 9-kDa longer isoform of Ku86 that is only observed in primate cells and not in murine cells (61). Our targeting strategy (Fig. 1) removed the first exon of Ku86, which is an exon that is common to both Ku86 and KARP-1 (61). Thus, Ku86−/− cells are actually deficient in both Ku86 and KARP-1, and it is possible that it is KARP-1 which is essential.
Acknowledgments
We thank Dr. J. Sedivy (Brown University) for the gene-targeting construct and for advice on gene targeting early in the conception of this project. We thank Dr. K. Myung (University of California, San Diego) for his efforts in helping to assemble the Ku86 targeting vector. We thank Dr. A. Bielinsky (University of Minnesota) for her helpful comments on the manuscript. This work was supported in part by National Institutes of Health Grant AI35763.
Abbreviations
- NHEJ
nonhomologous DNA end joining
- PK
protein kinase
- DSB
double-strand break
- IR
ionizing radiation
- DT
diphtheria toxin
- DEB
DNA end-binding
References
- 1.Hoeijmakers J H. Nature (London) 2001;411:366–374. doi: 10.1038/35077232. [DOI] [PubMed] [Google Scholar]
- 2.Barnes D E. Curr Biol. 2001;11:R455–R457. doi: 10.1016/s0960-9822(01)00279-2. [DOI] [PubMed] [Google Scholar]
- 3.Tuteja R, Tuteja N. Crit Rev Biochem Mol Biol. 2000;35:1–33. doi: 10.1080/10409230091169177. [DOI] [PubMed] [Google Scholar]
- 4.Liang F, Jasin M. J Biol Chem. 1996;271:14405–14411. doi: 10.1074/jbc.271.24.14405. [DOI] [PubMed] [Google Scholar]
- 5.Bliss T M, Lane D P. J Biol Chem. 1997;272:5765–5773. doi: 10.1074/jbc.272.9.5765. [DOI] [PubMed] [Google Scholar]
- 6.Pang D, Yoo S, Dynan W S, Jung M, Dritschilo A. Cancer Res. 1997;57:1412–1415. [PubMed] [Google Scholar]
- 7.Walker J R, Corpina R A, Goldberg J. Nature (London) 2001;412:607–614. doi: 10.1038/35088000. [DOI] [PubMed] [Google Scholar]
- 8.Gottlieb T M, Jackson S P. Cell. 1993;72:131–142. doi: 10.1016/0092-8674(93)90057-w. [DOI] [PubMed] [Google Scholar]
- 9.McElhinny S A N, Snowden C M, McCarville J, Ramsden D A. Mol Cell Biol. 2000;20:2996–3003. doi: 10.1128/mcb.20.9.2996-3003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teo S H, Jackson S P. Curr Biol. 2000;10:165–168. doi: 10.1016/s0960-9822(00)00317-1. [DOI] [PubMed] [Google Scholar]
- 11.Li Z, Otevrei T, Gao Y, Cheng H-L, Seed B, Stamato T D, Taccioli G E, Alt F W. Cell. 1995;83:1079–1089. doi: 10.1016/0092-8674(95)90135-3. [DOI] [PubMed] [Google Scholar]
- 12.Gao Y, Frank K M, Dikkes P, Fujiwara Y, Seidl K J, Sekiguchi J M, Rathbun G A, Swat W, Wang J, Bronson R, et al. Cell. 1998;95:891–902. doi: 10.1016/s0092-8674(00)81714-6. [DOI] [PubMed] [Google Scholar]
- 13.Critchlow S E, Bowater R P, Jackson S P. Curr Biol. 1997;7:588–598. doi: 10.1016/s0960-9822(06)00258-2. [DOI] [PubMed] [Google Scholar]
- 14.Grawunder U, Wilm M, Wu X, Kulesza P, Wilson T E, Mann M, Lieber M R. Nature (London) 1997;388:492–495. doi: 10.1038/41358. [DOI] [PubMed] [Google Scholar]
- 15.Grawunder U, Zimmer D, Fugmann S, Schwarz K, Lieber M R. Mol Cell. 1998;2:477–484. doi: 10.1016/s1097-2765(00)80147-1. [DOI] [PubMed] [Google Scholar]
- 16.Wang H, Zeng Z C, Perrault A R, Cheng X, Qin W, Iliakis G. Nucleic Acids Res. 2001;29:1653–1660. doi: 10.1093/nar/29.8.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barnes D E, Stamp G, Rosewell I, Denzel A, Lindahl T. Curr Biol. 1998;8:1395–1398. doi: 10.1016/s0960-9822(98)00021-9. [DOI] [PubMed] [Google Scholar]
- 18.Frank K M, Sekiguchi J M, Seidl K J, Swat W, Rathbun G A, Cheng H L, Davidson L, Kangaloo L, Alt F W. Nature (London) 1998;396:173–177. doi: 10.1038/24172. [DOI] [PubMed] [Google Scholar]
- 19.Frank K M, Sharpless N E, Gao Y, Sekiguchi J M, Ferguson D O, Zhu C, Manis J P, Horner J, DePinho R A, Alt F W. Mol Cell. 2000;5:993–1002. doi: 10.1016/s1097-2765(00)80264-6. [DOI] [PubMed] [Google Scholar]
- 20.Gao Y, Ferguson D O, Xie W, Manis J P, Sekiguchi J, Frank K M, Chaudhuri J, Horner J, DePinho R A, Alt F W. Nature (London) 2000;404:897–900. doi: 10.1038/35009138. [DOI] [PubMed] [Google Scholar]
- 21.Ouyang H, Nussenzweig A, Kurimasa A, Soares V C, Li X, Cordon-Cardo C, Li W H, Cheong N, Nussenzweig M, Iliakis G, et al. J Exp Med. 1997;15:921–929. doi: 10.1084/jem.186.6.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li G C, Ouyang H, Li X, Nagasawa H, Little J B, Chen D J, Ling C C, Fuks Z, Cordon-Cardo C. Mol Cell. 1998;2:1–8. doi: 10.1016/s1097-2765(00)80108-2. [DOI] [PubMed] [Google Scholar]
- 23.Nussenzweig A, Chen C, da Costa Soares V, Sanchez M, Sokol K, Nussenzweig M C, Li G C. Nature (London) 1996;382:551–555. doi: 10.1038/382551a0. [DOI] [PubMed] [Google Scholar]
- 24.Zhu C, Bogue M A, Lim D-S, Hasty P, Roth D B. Cell. 1996;86:379–389. doi: 10.1016/s0092-8674(00)80111-7. [DOI] [PubMed] [Google Scholar]
- 25.Jhappan C, Morse H C, Fleischmann R D, Gottesman M M, Merlino G. Nat Genet. 1997;17:483–486. doi: 10.1038/ng1297-483. [DOI] [PubMed] [Google Scholar]
- 26.Bogue M, Jhappan C, Roth D B. Proc Natl Acad Sci USA. 1998;95:15559–15564. doi: 10.1073/pnas.95.26.15559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao Y, Chaudhuri J, Zhu C, Davidson L, Weaver D T, Alt F W. Immunity. 1998;9:367–376. doi: 10.1016/s1074-7613(00)80619-6. [DOI] [PubMed] [Google Scholar]
- 28.Taccioli G E, Amatucci A G, Beamish H J, Gell D, Xiang X H, Torres Arzayus M I, Priestley A, Jackson S P, Rothstein A M, Jeggo P A, Herrera V L M. Immunity. 1998;9:355–366. doi: 10.1016/s1074-7613(00)80618-4. [DOI] [PubMed] [Google Scholar]
- 29.Vogel H, Lim D-S, Karsenty G, Finegold M, Hasty P. Proc Natl Acad Sci USA. 1999;96:10770–10775. doi: 10.1073/pnas.96.19.10770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karanjawala Z E, Grawunder U, Hsieh C L, Lieber M R. Curr Biol. 1999;9:1501–1504. doi: 10.1016/s0960-9822(00)80123-2. [DOI] [PubMed] [Google Scholar]
- 31.Difilippantonio M J, Zhu J, Chen H T, Meffre E, Nussenzweig M C, Max E E, Ried T, Nussenzweig A. Nature (London) 2000;404:510–514. doi: 10.1038/35006670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.d'Adda di Fagagna F, Hande M P, Tong W-M, Roth D B, Lansdorp P M, Wang Z-Q, Jackson S P. Curr Biol. 2001;11:1192–1196. doi: 10.1016/s0960-9822(01)00328-1. [DOI] [PubMed] [Google Scholar]
- 33.Bailey S M, Meyne J, Chen D J, Kurimasa A, Li G C, Lehnert B E, Goodwin E H. Proc Natl Acad Sci USA. 1999;96:14899–14904. doi: 10.1073/pnas.96.26.14899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsu H L, Gilley D, Galande S A, Hande M P, Allen B, Kim S H, Li G C, Campisi J, Kohwi-Shigematsu T, Chen D J. Genes Dev. 2000;14:2807–2812. doi: 10.1101/gad.844000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Samper E, Goytisolo F A, Slijepcevic P, van Buul P P W, Blasco M A. EMBO Rep. 2000;1:244–252. doi: 10.1093/embo-reports/kvd051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riballo E, Critchlow S E, Teo S-H, Doherty A J, Priestley A, Broughton B, Kysela B, Beamish H, Plowman N, Arlett C F, et al. Curr Biol. 1999;9:699–702. doi: 10.1016/s0960-9822(99)80311-x. [DOI] [PubMed] [Google Scholar]
- 37.Riballo E, Doherty A J, Dai Y, Stiff T, Oettinger M A, Jeggo P A, Kysela B. J Biol Chem. 2001;276:31124–31132. doi: 10.1074/jbc.M103866200. [DOI] [PubMed] [Google Scholar]
- 38.Lee S E, Pulaski C R, He D M, Benjamin D M, Voss M J, Um J, Hendrickson E A. Mutat Res. 1995;336:279–291. doi: 10.1016/0921-8777(95)00002-2. [DOI] [PubMed] [Google Scholar]
- 39.Han Z, Johnston C, Reeves W H, Carter T, Wyche J H, Hendrickson E A. J Biol Chem. 1996;271:14098–14104. doi: 10.1074/jbc.271.24.14098. [DOI] [PubMed] [Google Scholar]
- 40.Waldman T, Kinzler K W, Vogelstein B. Cancer Res. 1995;55:5187–5190. [PubMed] [Google Scholar]
- 41.Sedivy J M, Vogelstein B, Liber H L, Hendrickson E A, Rosmarin A. Science. 1999;283:9a. [Google Scholar]
- 42.Parsons R, Li G-M, Longley M J, Fang W-H, Papadopoulos N, Jen J, de la Chapelle A, Kinzler K W, Vogelstein B, Modrich P. Cell. 1993;75:1227–1236. doi: 10.1016/0092-8674(93)90331-j. [DOI] [PubMed] [Google Scholar]
- 43.Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown J P, Sedivy J M, Kinzler K W, Vogelstein B. Science. 1998;282:1497–1501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- 44.Chan T A, Hermeking H, Lengauer C, Kinzler K W, Vogelstein B. Nature (London) 1999;401:616–620. doi: 10.1038/44188. [DOI] [PubMed] [Google Scholar]
- 45.Zhang L, Yu J, Park B H, Kinzler K W, Vogelstein B. Science. 2000;290:989–992. doi: 10.1126/science.290.5493.989. [DOI] [PubMed] [Google Scholar]
- 46.Rhee I, Jair K W, Yen R W, Lengauer C, Herman J G, Kinzler K W, Vogelstein B, Baylin S B, Schuebel K E. Nature (London) 2000;404:1003–1007. doi: 10.1038/35010000. [DOI] [PubMed] [Google Scholar]
- 47.Dhar S K, Yoshida K, Machida Y, Khaira P, Chaudhuri B, Wohlschlegel J A, Leffak M, Yates J, Dutta A. Cell. 2001;106:287–296. doi: 10.1016/s0092-8674(01)00458-5. [DOI] [PubMed] [Google Scholar]
- 48.Jallepalli P V, Waizenegger I C, Bunz F, Langer S, Speicher M R, Peters J M, Kinzler K W, Vogelstein B, Lengauer C. Cell. 2001;105:445–457. doi: 10.1016/s0092-8674(01)00340-3. [DOI] [PubMed] [Google Scholar]
- 49.Gu H, Marth J D, Orban P C, Mossmann H, Rajewsky K. Science. 1994;265:103–106. doi: 10.1126/science.8016642. [DOI] [PubMed] [Google Scholar]
- 50.Marangoni E, Le Romancer M, Foray N, Muller C, Douc-Rasy S, Vaganay S, Abdulkarim B, Barrois M, Calsou P, Bernier J, et al. Cancer Gene Ther. 2000;7:339–346. doi: 10.1038/sj.cgt.7700111. [DOI] [PubMed] [Google Scholar]
- 51.Sadji Z, Le Romancer M, Lewin M J, Reyl-Desmars F. Cell Signal. 2000;12:745–750. doi: 10.1016/s0898-6568(00)00126-1. [DOI] [PubMed] [Google Scholar]
- 52.Appella E, Anderson C W. Eur J Biochem. 2001;268:2764–2772. doi: 10.1046/j.1432-1327.2001.02225.x. [DOI] [PubMed] [Google Scholar]
- 53.Satoh M, Wang J, Reeves W H. Eur J Cell Biol. 1995;66:127–135. [PubMed] [Google Scholar]
- 54.Baumann P, Cech T R. Mol Biol Cell. 2000;11:3265–3275. doi: 10.1091/mbc.11.10.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bianchi A, de Lange T. J Biol Chem. 1999;274:21223–21227. doi: 10.1074/jbc.274.30.21223. [DOI] [PubMed] [Google Scholar]
- 56.Hsu H L, Gilley D, Blackburn E H, Chen D J. Proc Natl Acad Sci USA. 1999;96:12454–12458. doi: 10.1073/pnas.96.22.12454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang X, Mar V, Zhou W, Harrington L, Robinson M O. Genes Dev. 1999;13:2388–2399. doi: 10.1101/gad.13.18.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Han Z, Carter T H, Reeves W H, Wyche J H, Hendrickson E A. J Biol Chem. 1996;271:25035–25040. doi: 10.1074/jbc.271.40.25035. [DOI] [PubMed] [Google Scholar]
- 59.Lees-Miller S P, Godbout R, Chan D W, Weinfeld M, Day R S, III, Barron G M, Allalunis-Turner J. Science. 1995;267:1183–1185. doi: 10.1126/science.7855602. [DOI] [PubMed] [Google Scholar]
- 60.Greider C W. Annu Rev Biochem. 1996;65:337–365. doi: 10.1146/annurev.bi.65.070196.002005. [DOI] [PubMed] [Google Scholar]
- 61.Myung K, He D M, Lee S E, Hendrickson E A. EMBO J. 1997;16:3172–3184. doi: 10.1093/emboj/16.11.3172. [DOI] [PMC free article] [PubMed] [Google Scholar]