Abstract
Purpose
Staphylococcus aureus bacteremia (SAB) is associated with a 90-day mortality of 28–34%. Many SAB-patients (7.8–39%) have a secondary S. aureus bacteriuria (SABU) mainly without symptoms of a urinary tract infection. Due to high morbidity and mortality, there is an interest in rapid detection of S. aureus bacteremia. Here, we compared a rapid nucleic acid amplification test (NAAT) with conventional culture to detect S. aureus in urine and to identify cases with increased risk for SAB.
Methods
In a cross-sectional study, we assessed urine samples (mid-stream, clean catch and catheter urine) of patients with SAB and bacteremia other than SAB (non-SAB). Urine samples were collected ± 3 days to the collection of the positive blood culture and were cultured on a set of selective and non-selective agar plates. NAAT was performed using a commercial test (Xpert® SA Nasal Complete G3, Cepheid) from a sterile swab soaked in urine.
Results
We included samples from 100 patients (68% male, median age: 67.4 years) with SAB and 20 patients (75% male, median age: 65.84 years) with non-SAB. The sensitivity of detecting SAB from urine samples was 47% (specificity: 90%) for NAAT, when applying a Ct-value of ≤ 37.4 for positive results. Urine culture had a sensitivity of 25% and a specificity of 95%. Molecular and culture methods showed a moderate agreement (80%, Cohens kappa: 0.55).
Conclusion
NAAT from urine has a higher sensitivity than culture in patients with SAB and could potentially identify cases with increased risk for SAB. Future studies should investigate whether this characteristic could translate into a clinical benefit through rapid detection of SAB.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10096-024-04969-7.
Keywords: Staphylococcus aureus, Bacteremia, Bacteriuria, Nucleic acid amplification test, Rapid assay, Urine
Introduction
Staphylococcus aureus bacteremia (SAB) is common with an annual incidence of 17–38/100,000 residents [1–4]. Despite antibiotic therapy, SAB has a high 90-day mortality (28–34%) [5–7].
For the empirical therapy of an infection with unknown focus and/or without pathogen identification, cephalosporins with or without vancomycin are frequently chosen. Compared to the staphylococcal-specific beta-lactams (e.g. flucloxacillin, cefazolin), the empirical therapy with cephalosporins (e.g. cefuroxime, ceftriaxone/cefotaxime) or beta-lactam-beta-lactamase combinations is associated with a higher 30-day mortality of SAB in a retrospective study [8]. Several studies have described lower survival rates of SAB-patients with a methicillin-susceptible isolate under vancomycin therapy [9–11]. In addition, secondary foci may occur in 16–34% of patients as a complication of SAB [7, 12]. Treatment delay was strongly associated with the presence of metastatic foci, but not associated with mortality [13]. Since early, specific therapy is important for a favourable outcome of SAB from an antibiotic stewardship and antimicrobial resistance (AMR) view, there is a clinical need for a rapid diagnosis of SAB.
True S. aureus urinary tract infections in our setting are presumably uncommon as we detect S. aureus in urine in only 1.4–1.9% of all positive cultures from clinical samples [14]. Worldwide, many SAB patients (7.8–39%) have concomitant S. aureus bacteriuria (SABU) [15–25]. The urinary tract as the primary source of SAB is infrequent (3.2%, n = 132/4181 SAB patients) [26] and is seen almost exclusively in the context of inserted foreign bodies (e.g. indwelling catheters) or urologic interventions [15, 26]. SABU secondary to SAB is usually asymptomatic (e.g. absence of dysuria, flank or suprapubic pain, gross haematuria). The pathomechanisms of secondary SABU in patients with SAB are not fully understood and might include tissue destruction, micro-abscesses, transcytosis, paracytosis, or intracellular translocation in leukocytes and macrophages [25].
Currently, the state-of-the-art in microbiological diagnostics of bloodstream infections is species identification with matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) performed directly from positive blood cultures or from short sub-cultures on solid medium. Additionally, multiplex-polymerase chain reaction (PCR) from blood targeting the most common species can achieve a shorter turn-around time (e.g. 3.5–6 h) [27]. The species identification from blood cultures is usually available on the same day the blood culture becomes positive. The median time of blood cultures to become positive is roughly 21 h after collection of the sample including slow growing pathogens [28].
Urine is readily available, and we hypothesize that detecting secondary SABU with a nucleic acid amplification test (NAAT) may enable a more rapid detection (within one hour) than urine culture. However, the diagnostic accuracy of such an approach to identify SAB from urine has not been assessed yet. Therefore, the objective of this study was to compare the test performance of a rapid NAAT with conventional urine culture to detect S. aureus in urine and evaluate the clinical benefit through rapid detection of SAB.
Methods
Ethical consideration
Ethical approval was obtained from the institutional review board (IRB) of the University of Münster (Ethik-Kommission Westfalen-Lippe, 2020-615-f-S). The IRB granted a waiver to obtain a signed written informed consent from patients.
Patients
This cross-sectional study was carried out at a tertiary care hospital in Germany (~ 1,300 beds) between February 2020 and May 2023. Specimen of the patients were sent for clinical routine. Patients were included consecutively if they had a culture-confirmed bacteremia and a urine culture ± 3 days to the collection of the positive blood culture. Patients with a likely contamination of the blood culture (e.g. coagulase negative staphylococci in one out of four blood culture bottles without signs of infection) were excluded. For each patient, age, sex and primary focus of infection were recorded.
Microbiological culture
Mid-stream, clean catch or catheter urine of patients with bacteremia was collected in a sterile tube (Urin-Monovette®, Sarstedt, Nümbrecht, Germany). Urine samples (10 µl per plate) were cultured on Columbia Blood agar with 5% Sheep Blood (BD, Heidelberg, Germany; ambient air), MacConkey agar (BD, ambient air) and a selective agar for Gram-positive bacteria (Columbia CNA Agar with 5% Sheep Blood, BD, 5% CO2) for a maximum of 48 h at 35 ± 2 °C. We defined SABU as detection of S. aureus in urine independent of the concentration (in colony forming units (CFU)/ml) [29].
Pairs of aerobic and anaerobic blood culture bottles (BD) were incubated in a Bactec® FX device (BD) for five days (14 days in case of suspected endocarditis) and were subcultured on Columbia Blood agar (BD) and/or chocolate agar (BD, 5% CO2) when the blood culture bottles were flagged positive [30]. Species identification was done with MALDI-TOF MS (Biotyper® Sirius one, Bruker, Bremen, Germany) applying the Biotyper software IVD/Compass (version 4.2).
A screening test was used to detect substances that could inhibit the growth of bacteria in urine (e.g. antibiotics, cytostatic drugs). For that purpose, a blank cellulose disc (ThermoFisher scientific, Wesel, Germany) soaked with 10 µl urine was placed on a Mueller-Hinton II agar (BD, 35 ± 2 °C, ambient air) inoculated with a highly susceptible bacterium (ATCC 6031 Bacillus subtilis). If an inhibition zone around the disc was seen after 24 h (ambient air, 35 ± 2 °C), the test was interpreted as positive. This screening was recommended in German guidelines until 2020 to aid the interpretation of negative urine cultures in patients with a high suspicion of urinary tract infection.
Nucleic acid amplification test for S. aureus
After urine culture had been carried out urine samples were stored at -20 °C until NAAT was done to guarantee equal bacterial cell counts for culture and NAAT. For NAAT, we used the Xpert® SA Nasal Complete G3 test cartridge (Cepheid, Krefeld, Germany) which was developed and validated for rapid (within one hour) and simultaneous detection of S. aureus and methicillin-resistant S. aureus (MRSA) in nasal swab samples. The Xpert® SA Nasal Complete Assay is a qualitative test for in-vitro diagnostics. The primers and probes detect simultaneously proprietary sequences of the staphylococcal protein A (spa), mecA and SCCmec inserted into the S. aureus chromosome at the attB site.
For the current study, we soaked a sterile swab (eSwab, Copan, Brescia, Italy) with urine, washed it in the buffer solution of the test cartridge and removed it afterwards. Then, we performed the test as recommended for nasal swabs. This procedure was chosen to use the test as recommended by the manufacturer with the only variation that urine instead of nasal swabs was sampled.
Analytical sensitivity
The limit of detection (LoD) is defined as the lowest number of CFU per sample that can be distinguished from negative samples. We selected urine with negative inhibitory test to test the LoD. We calculated the mean volume of urine that can be squeezed out of the swabs into the elution buffer of the cartridge test (= sample per test). Starting from a stock concentration of 2.45 × 108/ml of ATCC 29,213 S. aureus, we prepared a dilution series in urine for NAAT. Since the calculated CFU/sample of a dilution series only gives an estimate of the real CFU/sample, we cultured the bacterial suspension of the dilution series on Columbia Blood agar (24 h, 35 ± 2 °C, ambient air) and used the cultured bacterial counts to calculate the LoD.
Statistical analysis
A specific sample size calculation was not done in the absence of any data on the performance of the NAAT in urine samples. We considered a samples size of 100 SAB and 20 non-SAB cases as appropriate for our objective applying convenience sampling. Differences between groups in categorical variables were compared using Fisher´s exact test or Chi-squared test (RStudio version 4.3.1; [31]) with a significance level of < 0.05.
To define the optimal Ct-threshold for the prediction of SAB, we performed a receiver operating characteristic (ROC) analysis using the Ct value as the predictor and SAB as the outcome. For samples with undetermined Ct values (i.e. no detection after 40 cycles), we imputed a Ct value and set a maximum value of 40. The ROC curves and optimal cut-off values (Youden-Index) were computed with the R package “cutpointr” [32].
The test performance of NAAT and culture detection to predict SAB was calculated (sensitivity, specificity) using the optimal cut-off Ct value as determined by the ROC analysis. The concordance of molecular and culture test was calculated using Cohen’s kappa coefficient.
Results
We included 100 patients (68% male, median age: 67.4 years [range 0.6–93.2]) with SAB and 20 patients (75% male, median age: 65.8 years [range 30.3–83.3]) with positive blood cultures other than S. aureus (non-SAB). The most common infective focus in patients with SAB was the central venous catheter (20%, 20/100), skin and soft tissue infections (16%, 16/100), pneumonia (10%, 10/100), endocarditis (6%, 6/100) or remained unknown (24%, 24/100).
We calculated an optimal cut-off Ct-value of 37.4 (Youden-index = 0.37) for our sample set when maximizing the sum of sensitivity and specificity (Fig. S1). The area under the curve (AUC) of the respective ROC curve was 0.69 (Fig. 1).
Fig. 1.
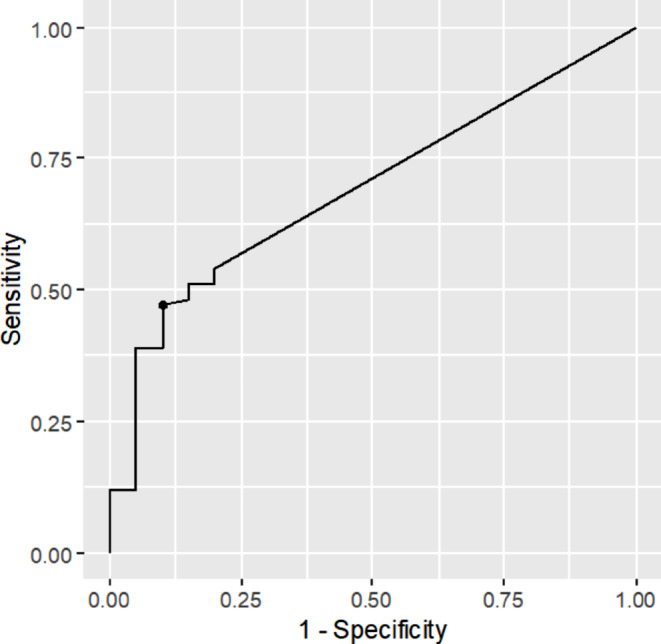
Receiver operating characteristic (ROC) analysis to predict a Staphylococcus aureus bacteremia (SAB) based on S. aureus NAAT detection in urine (Xpert® SA Nasal Complete) using an optimal cut off Ct-value of 37.4. The area under the curve (AUC) of the corresponding receiver operating characteristic (ROC) analysis was 0.69
In 25% (25/100) of SAB patients, we detected S. aureus by urine culture and in 47% (47/100) of patients (p = 0.002) by NAAT applying a Ct value of ≤ 37.4 (Table 1).
Table 1.
Results of Staphylococcus aureus detection in urine culture and NAAT from urine in patients with SAB and non-SAB
| S. aureus detection in urine | Bacteremia | |||
|---|---|---|---|---|
| SAB | Non-SAB | Total | ||
| Culture | Positive | 25 (25%) | 1 (5%) | 26 |
| Negative | 75 (75%) | 19 (95%) | 94 | |
| Total | 100 | 20 | 120 | |
| NAATa | Positive | 47 (47%) | 2 (10%) | 49 |
| Negative | 53 (53%) | 18 (90%) | 71 | |
| Total | 100 | 20 | 120 | |
aCt values ≤ 37.4 were interpreted as positive
The detection of SAB by urine culture had a sensitivity of 25% (95%CI: 18–34%) and a specificity of 95% (95% CI: 76–99%) (Table 1). The detection of SAB by NAAT had a sensitivity of 47% (95%CI: 38–57%) and a specificity of 90% (95%CI: 70–97%, Table 1). The sensitivity for the molecular detection of S. aureus in urine was 53% (95%CI: 42–64%) if only patients were considered that had urine cultures collected between three days prior and up to one day after the collection of the positive blood culture. The specificity did not change if this stringent definition was applied (Table S1).
When excluding patients with indwelling catheters, the sensitivity for the molecular detection of S. aureus in urine was 49% (95%CI: 39–59%) and the specificity was 86% (95%CI: 60–96%, Table S2). Molecular and culture methods for the detection of S. aureus in urine showed a moderate agreement (80%, Cohens kappa: 0.55, Table 2).
Table 2.
Concordance between NAAT and culture for the detection of Staphylococcus aureus in urine
| NAATa | ||||
|---|---|---|---|---|
| Positive | Negative | Total | ||
| Urine culture | Positive | 25 | 1 | 26 |
| Negative | 23 | 71 | 94 | |
| Total | 48 | 72 | 120 | |
aCt values ≤ 37.4 were interpreted as positive
Of 32 samples that were tested culture negative, but NAAT-positive (Ct value ≤ 37.4), 19 (59%) had a positive urine inhibitory test.
Median Ct values for the detection of S. aureus in urine were higher in culture negative than in culture positive urine samples (34.6 vs. 23.9, Fig. 2). One patient with MRSA-bacteremia and MRSA-positive screening of the nose, throat and axilla had no detection of S. aureus in urine by NAAT. One patient with the detection of penicillin-susceptible S. aureus in blood and urine had a positive NAAT result for MRSA.
Fig. 2.
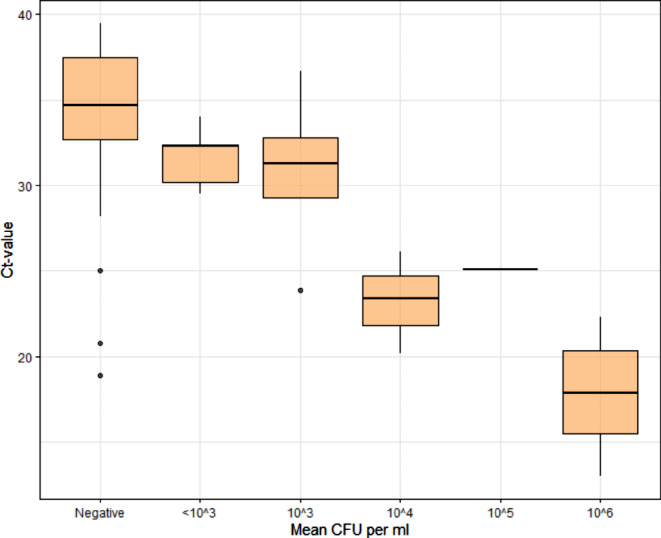
Correlation between Ct value of the NAAT detection of Staphylococcus aureus (Xpert® SA Nasal Complete) and mean colony forming units [CFU] per ml of S. aureus on Columbia Blood Agar in urine. A total of 25 samples were included for which both the culture and a positive NAAT was available. Only one specimen had a S. aureus concentration of 105 CFU/ml
In the non-SAB group, 11 different species were detected. Bacteremia in non-SAB cases was mostly caused by Escherichia coli (n = 9) followed by Staphylococcus epidermidis (n = 2) and others (n = 9). Two non-SAB patients had a S. aureus detected in urine by NAAT and one of them was also culture positive for S. aureus (106 CFU/ml). Both had nasal colonisation with S. aureus. The patient, who was also culture-positive, had primary skin disease and a superficial SSTI in the genital area.
The rate of NAAT detection of S. aureus in urine was lower in the non-SAB compared to the SAB group (5% [2/20] vs. 47% [47/100], p = 0.002).
Under the conditions of the study, the robustness/reproducibility of the test was 100% for positive and negative samples (Table S3). The LoD of the NAAT to detect SA in urine was 23 CFU/sample (Table S4). This corresponds to 354 CFU/ml of urine.
Discussion
We compared the test performance of culture and a NAAT for the detection of S. aureus in urine from patients with bacteremia (SAB and Non-SAB). Main findings were a low-moderate sensitivity (47%) and high specificity (90%) of the urine NAAT to identify patients with SAB. The sensitivity increased (53%) if only patients were considered that have a urine culture collected three days prior and up to one day after the collection of the positive blood culture. Later collections reduce sensitivity e.g. if the patient is already on empiric antibiotics. We saw that culture negative, but NAAT-positive urine samples had a positive inhibitory test in more than one-half of the cases. We conclude that antibacterial substances in urine samples (i.e. antibiotics) are frequent and might inhibit culture growth of S. aureus.
These findings support the value of detecting S. aureus in urine as early as possible after presentation/admission in the emergency department.
The relevance of bacterial detection in urine up to three days prior to detection of bacteremia remains unknown as the number of patients with a urine culture sampled on the days before the blood culture was small in our cohort (n = 6, 67% NAAT positive).
The test cartridges were used to analyse the urine samples with a rapid and easy-to-handle method. For the rapid diagnosis of invasive Legionella pneumophila and pneumococcal infections, antigen-based immunochromatographic tests from urine are integrated into routine diagnostics since many years. These tests have a good sensitivity (95–97%) and specificity (95–99%) according to the manufacturer to diagnose invasive infections (e.g. pneumonia). In contrast, the test performance of NAAT from urine to detect SAB has a much lower sensitivity in our study. When performing the NAAT, we followed the proposed method for MRSA screening from nasal swabs. We calculated a mean volume of 65 µl [49.7–83.4 µl] that can be squeezed out of the swabs and was therefore transferred into the elution buffer of the cartridge test. We suggest for further studies a higher volume of urine (e.g. 100 µl) directly transferred to the test cartridge possibly achieving a higher sensitivity. The target gene in Xpert® SA Nasal Complete Assay for the detection of S. aureus is spa, which can vary in size between different S. aureus spa types and is located as a single copy in the genome. Other (multi-copy) genes or antigens with high expression levels might therefore be alternative targets to detect S. aureus in urine of patients with SAB. Such a urine antigen test could be used as a Point-of-Care-Testing (POCT) that would enable the use of narrow spectrum antimicrobial agents in septic patients.
Taking into account the low-moderate sensitivity, further research would be needed to assess whether detection of S. aureus in urine has a clinical benefit. For that purpose, time to optimal therapy and treatment outcomes could be compared between patients (e.g. matched for age, sex, comorbidities), whose antimicrobial therapy was guided by NAAT from urine vs. blood culture, multiplex-PCR or MALDI-TOF MS from short sub-cultures on solid medium.
We had one false-positive MRSA detection in NAAT, which was not confirmed by culture of blood and/or urine. The manufacturer of the NAAT reports a specificity of 97.9% for the detection of MRSA. This minor error would lead to a less effective initial therapy (e.g. vancomycin) for susceptible isolates.
According to the manufacturer, the LoD for S. aureus in nasal swabs is 93.7 (95% CI 75.5 -137.8) CFU/sample. Compared to nasal swabs, we estimated a lower LoD (23 CFU/sample) for the detection of S. aureus from urine. One reason for this difference is most likely the difference in calculating the LoD. Since the calculated CFU/sample of a dilution series only gives an inaccurate estimate of the real CFU/sample, we decided to plate the bacterial suspension of the dilution series and use the actual bacterial counts to calculate the LoD.
Our study has limitations. First, urine samples positive for S. aureus by NAAT in patients with and without SAB might be due to perineal colonisation or contamination of the urine vial during the collection of the specimen. Second, since the study design is cross sectional, we cannot state if SAB was secondary to a urinary tract infection or vice versa. We rate the risk as low, as true S. aureus urinary tract infections in our setting are uncommon because we detect S. aureus in urine in only 1.4–1.9% of all positive cultures from clinical samples [14]. In addition, the urinary tract as the primary source of SAB is infrequent (3.2%, n = 132/4181 SAB patients) [26]. Third, the Xpert® SA Nasal Complete detects S. aureus by the amplification of spa. False negative results are possible due to deletions in spa [33]. Fourth, due to high costs of the test cartridges, the approach of this study might not be suitable for all cases in routine diagnostics. Fifth, due to the small number of non-SAB cases, the specificity could only be calculated with a low degree of accuracy.
Conclusions
Using NAAT detection of S. aureus in urine we were able to identify patients with SAB with a low-moderate sensitivity (47%) and a high specificity (90%) in our population. This warrants further evaluation of urine rapid tests for the detection of SAB.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- AMR
Antimicrobial resistance
- AUC
Area under the curve
- CFU/ml
Colony forming units per ml
- LoD
Limit of detection
- MALDI-TOF MS
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry
- MRSA
Methicillin-resistant Staphylococcus aureus
- NAAT
Nucleic acid amplification testing
- PCR
Polymerase chain reaction
- POCT
Point-of-Care-Testing
- ROC
Receiver operating characteristic
- SAB
Staphylococcus aureus bacteremia
- SABU
Staphylococcus aureus bacteriuria
Author contributions
All authors contributed to the conception and design of the study. Franziska Schuler screened patients for eligibility and took responsibility of the lab work. Franziska Schuler and Frieder Schaumburg performed the statistical analysis. All authors interpreted the findings. Franziska Schuler wrote the first draft of the manuscript and all authors reviewed subsequent versions and approved the final version. All authors had full access to all data of the study.
Funding
Financial support was received from Deutsche Forschungsgemeinschaft (Project number: 518834831). We acknowledge the support from the Open Access Publication Fund of the University of Münster.
Open Access funding enabled and organized by Projekt DEAL.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval/consent
Ethical approval was obtained from the institutional review board (IRB) of the University of Münster (Ethik-Kommission Westfalen-Lippe, 2020-615-f-S). The IRB granted a waiver to obtain signed a written informed consent from patients.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Allard C, Carignan A, Bergevin M, Boulais I, Tremblay V, Robichaud P et al (2008) Secular changes in incidence and mortality associated with Staphylococcus aureus bacteraemia in Quebec, Canada, 1991–2005. Clin Microbiol Infect 14(5):421–428 [DOI] [PubMed] [Google Scholar]
- 2.Laupland KB, Ross T, Gregson DB (2008) Staphylococcus aureus Bloodsinfectionsctrisk:factorsacoutcomestcomes, and the Influence of Methicillin Resistance in Calgary, Canada, 2000–2006. J Infect Dis 198(3):336–343 [DOI] [PubMed] [Google Scholar]
- 3.El Atrouni WI, Knoll BM, Lahr BD, Eckel-Passow JE, Sia IG, Baddour LM (2009) Temporal trends in the incidence of Staphylococcus aureus Bacteremia in Olmsted County, Minnesota, 1998 to 2005: a Population-based study. Clin Infect Dis 49(12):e130–e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thorlacius-Ussing L, Sandholdt H, Larsen AR, Petersen A, Benfield T (2019) Age-dependent increase in incidence of Staphylococcus aureus Bacteremia, Denmark, 2008–2015. Emerg Infect Dis 25(5):875–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuehl R, Morata L, Boeing C, Subirana I, Seifert H, Rieg S et al (2020) Defining persistent Staphylococcus aureus bacteraemia: secondary analysis of a prospective cohort study. Lancet Infect Dis 20(12):1409–1417 [DOI] [PubMed] [Google Scholar]
- 6.Kaasch AJ, Barlow G, Edgeworth JD, Fowler VG Jr., Hellmich M, Hopkins S et al (2014) Staphylococcus aureus bloodstream infection: a pooled analysis of five prospective, observational studies. J Infect 68(3):242–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jensen AG, Wachmann CH, Espersen F, Scheibel J, Skinhøj P, Frimodt-Møller N (2002) Treatment and outcome of Staphylococcus aureus bacteremia: a prospective study of 278 cases. Arch Intern Med 162(1):25–32 [DOI] [PubMed] [Google Scholar]
- 8.Paul M, Zemer-Wassercug N, Talker O, Lishtzinsky Y, Lev B, Samra Z et al (2011) Are all beta-lactams similarly effective in the treatment of methicillin-sensitive Staphylococcus aureus bacteraemia? Clin Microbiol Infect 17(10):1581–1586 [DOI] [PubMed] [Google Scholar]
- 9.Chan KE, Warren HS, Thadhani RI, Steele DJ, Hymes JL, Maddux FW et al (2012) Prevalence and outcomes of antimicrobial treatment for Staphylococcus aureus bacteremia in outpatients with ESRD. J Am Soc Nephrol 23(9):1551–1559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaasch AJ, Rieg S, Kuetscher J, Brodt HR, Widmann T, Herrmann M et al (2013) Delay in the administration of appropriate antimicrobial therapy in Staphylococcus aureus bloodstream infection: a prospective multicenter hospital-based cohort study. Infection 41(5):979–985 [DOI] [PubMed] [Google Scholar]
- 11.Schweizer ML, Furuno JP, Harris AD, Johnson JK, Shardell MD, McGregor JC et al (2011) Comparative effectiveness of nafcillin or cefazolin versus Vancomycin in methicillin-susceptible Staphylococcus aureus bacteremia. BMC Infect Dis 11:279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verhagen DW, van der Meer JT, Hamming T, de Jong MD, Speelman P (2003) Management of patients with Staphylococcus aureus bacteraemia in a university hospital: a retrospective study. Scand J Infect Dis 35(8):459–463 [DOI] [PubMed] [Google Scholar]
- 13.Vos FJ, Kullberg BJ, Sturm PD, Krabbe PFM, van Dijk APJ, Wanten GJA et al (2012) Metastatic infectious disease and clinical outcome in Staphylococcus aureus and Streptococcus species bacteremia. Med (Baltim) 91(2):86–94 [DOI] [PubMed] [Google Scholar]
- 14.Sandmann S, Schaumburg F, Varghese J (2023) GEFAAR: a generic framework for the analysis of antimicrobial resistance providing statistics and cluster analyses. Sci Rep 13(1):16922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asgeirsson H, Kristjansson M, Kristinsson KG, Gudlaugsson O (2012) Clinical significance of Staphylococcus aureus Bacteriuria in a nationwide study of adults with S. Aureus bacteraemia. J Infect 64(1):41–46 [DOI] [PubMed] [Google Scholar]
- 16.Chihara S, Popovich KJ, Weinstein RA, Hota B (2010) Staphylococcus aureus bacteriuria as a prognosticator for outcome of Staphylococcus aureus bacteremia: a case-control study. BMC Infect Dis 10:225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi SH, Lee SO, Choi JP, Lim SK, Chung JW, Choi SH et al (2009) The clinical significance of concurrent Staphylococcus aureus bacteriuria in patients with S. Aureus bacteremia. J Infect 59(1):37–41 [DOI] [PubMed] [Google Scholar]
- 18.Ekkelenkamp MB, Verhoef J, Bonten MJ (2007) Quantifying the relationship between Staphylococcus aureus bacteremia and S. Aureus bacteriuria: a retrospective analysis in a tertiary care hospital. Clin Infect Dis 44(11):1457–1459 [DOI] [PubMed] [Google Scholar]
- 19.Huggan PJ, Murdoch DR, Gallagher K, Chambers ST (2008) Concomitant Staphylococcus aureus bacteriuria is associated with poor clinical outcome in adults with S. Aureus bacteraemia. J Hosp Infect 69(4):345–349 [DOI] [PubMed] [Google Scholar]
- 20.Kramer TS, Schlosser B, Gruhl D, Behnke M, Schwab F, Gastmeier P et al (2020) Staphylococcus aureus Bacteriuria as a predictor of in-Hospital mortality in patients with Staphylococcus aureus Bacteremia. Results of a retrospective cohort study. J Clin Med 9(2) [DOI] [PMC free article] [PubMed]
- 21.Lee BK, Crossley K, Gerding DN (1978) The association between Staphylococcus aureus bacteremia and bacteriuria. Am J Med 65(2):303–306 [DOI] [PubMed] [Google Scholar]
- 22.Perez-Jorge EV, Burdette SD, Markert RJ, Beam WB (2010) Staphylococcus aureus bacteremia (SAB) with associated S. aureus bacteriuria (SABU) as a predictor of complications and mortality. J Hosp Med 5(4):208–211 [DOI] [PubMed] [Google Scholar]
- 23.Pulcini C, Matta M, Mondain V, Gaudart A, Girard-Pipau F, Mainardi JL et al (2009) Concomitant Staphylococcus aureus bacteriuria is associated with complicated S. aureus bacteremia. J Infect 59(4):240–246 [DOI] [PubMed] [Google Scholar]
- 24.Papadimitriou-Olivgeris M, Jacot D, Senn L, Guery B (2023) Clinical significance of concomitant bacteriuria in patients with Staphylococcus aureus bacteraemia. Eur J Clin Microbiol Infect Dis 42(3):379–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schuler F, Barth PJ, Niemann S, Schaumburg F (2021) A narrative review on the role of Staphylococcus aureus Bacteriuria in S. Aureus Bacteremia. Open Forum Infect Dis 8(6):ofab158–ofab [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grillo S, Cuervo G, Carratalà J, Grau I, Llaberia M, Aguado JM et al (eds) (2020) Characteristics and outcomes of Staphylococcus aureus bloodstream infection originating from the urinary tract: a multicenter cohort study. Oxford University Press US [DOI] [PMC free article] [PubMed]
- 27.Lamy B, Sundqvist M, Idelevich EA (2020) Bloodstream infections - standard and progress in pathogen diagnostics. Clin Microbiol Infect 26(2):142–150 [DOI] [PubMed] [Google Scholar]
- 28.Schaumburg F, Schuler F, Fobker M, Esser J (2024) Integrated decentralized blood culture incubation: a step towards a 24/7 microbiology service? J Microbiol Methods 223:106973 [DOI] [PubMed] [Google Scholar]
- 29.Schuler F, Froböse N, Schaumburg F (2020) Prevalence and risk factors for bacteremia in patients with Staphylococcus aureus bacteriuria: a retrospective cohort study. Int J Infect Dis 98:467-469 [DOI] [PubMed]
- 30.Froböse NJ, Idelevich EA, Schaumburg F (2021) Short incubation of positive blood cultures on solid media for Species identification by MALDI-TOF MS: which Agar is the fastest? Microbiol Spectr 9(1):e0003821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.RStudio IDE. Posit, Boston
- 32.Thiele C, Hirschfeld G (2021) Cutpointr: improved estimation and validation of optimal cutpoints in R. J Stat Softw 98(11):1–27 [Google Scholar]
- 33.Lee GH, Pang S, Coombs GW (2018) Misidentification of Staphylococcus aureus by the Cepheid Xpert MRSA/SA BC Assay due to deletions in the spa Gene. J Clin Microbiol 56(7) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.


