Abstract
In the management of pregnancy, ritodrine has been used to prevent preterm birth, and magnesium sulfate (MgSO4) has been used to prevent preterm labor and preeclampsia. Neonates born to mothers receiving these medications occasionally show an increase in serum potassium concentration. Recently, an elevated risk of neonatal hyperkalemia has been reported, particularly when ritodrine and MgSO4 are co-administered; however, the underlying mechanisms remain unclear. We conducted a retrospective cohort study of 142 preterm infants born between 24 and 36 weeks of gestation, categorized into groups exposed to antenatal ritodrine, MgSO4, both agents, or neither. In addition, we investigated the association between potassium levels and metabolites in the serum of umbilical cord blood from 33 infants exposed to antenatal ritodrine and MgSO4 using a metabolomic analysis. Our findings revealed a significant elevation in serum potassium concentration associated with metabolomic findings of activation of glycolysis and the derived metabolic routes in preterm neonates exposed to both ritodrine and MgSO4. Our data indicate that the concurrent administration of ritodrine and MgSO4 caused distinctive metabolic alterations, potentially leading to an additional increase in the intracellular potassium concentration in the fetus. Consequently, this mechanism may imply an elevation in serum potassium concentration postnatally through the redistribution of potassium.
Keywords: Cord blood, Hyperkalemia, Magnesium sulfate, Preterm infant, Ritodrine
Subject terms: Medical research, Risk factors
Introduction
Selective beta2-adrenergic receptor (β2-AR) agonists, such as terbutaline and ritodrine, have been historically used as uterine contraction inhibitors1,2. The U.S. Food and Drug Administration issued a warning against prolonged use (beyond 48–72 h) of selective β2-AR agonists in pregnant women for the prevention of preterm labor due to an increased risk of severe cardiovascular side effects for both the mother and the fetus in 2011. However, in Japan, the use of ritodrine (with caution with respect to its potential side effects) was widely applied as a treatment option for preterm labor threatened patients based on the guidelines published by the Japan Society of Obstetrics and Gynecology until 2017. The current Japanese guidelines published in 2020 and 2023 still include this treatment. Magnesium sulfate (MgSO4) has been widely applied to prevent seizures in women with preeclampsia or eclampsia3. MgSO4 is recommended for use in women at risk of giving birth at less than 32 weeks of gestation for neuroprotection of their infants4. In Japan, the use of MgSO4 as a tocolytic agent has been included in the guidelines since 2008. Consequently, prolonged tocolytic therapy involving the combined use of ritodrine and MgSO4 has been used in Japan when the control of uterine contractions is not achievable with either ritodrine or MgSO4 alone2,5,6.
Recent reports have highlighted an increased risk of hyperkalemia in neonates born to mothers treated with ritodrine and MgSO4 during pregnancy7,8. Potassium, the major intracellular cation, is essential for maintaining vital physiological functions9. Neonates must maintain a net positive potassium balance to support their growth, primarily by minimizing renal potassium losses9. Consequently, neonatal plasma potassium concentrations are higher than those observed in children and adults9. Preterm infants, particularly those born earlier in gestation, are especially vulnerable to elevated extracellular potassium concentrations due to additional risk factors such as acute kidney injury, hypotension, acidosis, hemorrhagic disorders, and transfusion therapy, which can lead to life-threatening complications10. The incidence of neonatal hyperkalemia is notably high in extremely low birth weight infants, with rates ranging from 25 to 50%, and nearly all infants born before 25 weeks of gestation develop hyperkalemia11–13. Although the incidence of hyperkalemia in neonates born between 32 and 36 weeks of gestation is relatively low (approximately 8%), exposure to both ritodrine and MgSO₄ significantly increases its incidence7. Severe hyperkalemia, with potassium levels exceeding 9 mEq/L, was associated with arrhythmias in 60% of cases10. Sychlowy et al. reported that only one of seven infants with hyperkalemia-induced arrhythmias survived14. In addition, hyperkalemia can lead to intracranial complications such as intraventricular hemorrhage and periventricular leukomalacia10. Given that ritodrine and MgSO4 are administered to women at risk of preterm labor, these drugs may also be independent risk factors for hyperkalemia in preterm neonates. Recognizing the risk associated with prenatal exposure to both ritodrine and MgSO4 is crucial for predicting extremely high potassium levels and the resulting fatal arrhythmias. The underlying mechanisms of neonatal hyperkalemia have scarcely been investigated due to the lack of concurrent administration of ritodrine and MgSO4 to pregnant women in the United States and Europe. The identification of markers that enable an early and accurate diagnosis of neonatal hyperkalemia resulting from exposure to ritodrine and MgSO4 could potentially assist in stratifying risk groups, thus aiding in the formulation of appropriate therapeutic strategies to improve patient management.
Metabolomics provides information on all biochemical activities, including substrates, products, and cofactors, of all enzymatic reactions in a specific biological system at a single time point15. Changes in the serum metabolome have been reported in neonatal conditions such as infections, perinatal asphyxia, cardiac diseases, and renal disorders, and metabolomics approaches are valuable for the early diagnosis and assessment of the severity of neonatal diseases16. The metabolism of fetuses may be affected by the administration of ritodrine and MgSO4 in pregnant women; hence, subtle changes in the serum metabolome of umbilical cord blood may contribute to elucidating the mechanisms of neonatal hyperkalemia that develops in association with the combined use of ritodrine and MgSO4.
In this study, we conducted a metabolomics analysis of the serum in umbilical cord blood to elucidate metabolic changes in neonates with antenatal exposure ritodrine and MgSO4. Additionally, to identify predictors of neonatal hyperkalemia, we compared the clinical symptoms and serum metabolites in the umbilical cord blood of neonates born to pregnant women who received ritodrine and MgSO4 with those of neonates born to mothers who did not receive such treatment.
Results
Perinatal characteristics
In this study, we investigated the clinical characteristics of neonatal hyperkalemia caused by antenatal exposure to ritodrine or MgSO4. This investigation targeted preterm infants at 24–36 weeks of gestational age, a period when treatment for threatened preterm labor is administered. Throughout the designated research period, 563 infants were admitted to our hospital’s neonatal intensive care unit (NICU), among whom 241 were born at 24–36 weeks of gestation (Supplementary Figure S1). To minimize the influence of confounding factors due to uncertain prior treatments, we excluded cases with uncertain details regarding the administration of specific drugs, such as tocolytic therapies, including their doses and duration. Consequently, 99 were excluded from the study for the following reasons: unknown maternal drug history (n = 71), presence of multiple malformation syndromes or chromosomal abnormalities (n = 8), hemolytic anemia or intracranial hemorrhage (n = 19), and a maternal history of anticancer drug use. Thus, a final cohort of 142 infants was included in the study. Among these infants, 68 mothers did not receive antenatal therapy, 36 mothers received antenatal ritodrine, 15 mothers received antenatal MgSO4, and 23 mothers received antenatal ritodrine and MgSO4 before delivery.
Data on the administration of ritodrine and MgSO4 in the four groups (neither ritodrine nor MgSO4, ritodrine alone, MgSO4 alone, and both ritodrine and MgSO4) are shown in Supplementary Table S1. Regarding the administration of ritodrine to mothers, the both ritodrine and MgSO4 group exhibited a longer administration duration and a higher total dosage than the ritodrine alone group. Among the cases of MgSO4 administration to mothers, the both ritodrine and MgSO4 group had a longer administration duration and a higher total dosage than the MgSO4 alone group. Maternal serum magnesium concentrations were similar between the MgSO4 alone group and the both ritodrine and MgSO4 group (Table 1).
Table 1.
Perinatal characteristics of the cases treated with antenatal ritodrine and magnesium sulfate.
| Neither ritodrine nor MgSO4 n = 68 | Ritodrine alone n = 36 | MgSO4 alone n = 15 | Both ritodrine and MgSO4 n = 23 | p | |
|---|---|---|---|---|---|
| Characteristics of infants | |||||
| Male, n (%) | 35 (51) | 16 (44) | 6 (40) | 7 (39) | < 0.001*** |
| Gestational age, mean (SD), weeks | 34.3 (2.7) | 33.7 (3.0) | 30.1 (3.7) | 32.6 (3.3) | 0.002** |
| Birth weight, mean (SD), g | 2110 (513) | 1950 (561) | 1372 (616) | 1784 (625) | < 0.001*** |
| SGA, n (%) | 4 (6) | 3 (8) | 3 (20) | 3 (13) | 0.328 |
| Twin, n (%) | 4 (6) | 10 (28) | 2 (13) | 11 (48) | < 0.001*** |
| Apgar score 1 min, mean (SD) | 7 (2) | 6 (2) | 6 (2) | 6 (2) | 0.278 |
| Apgar score 5 min, mean (SD) | 8 (1) | 8 (1) | 7 (2) | 8 (1) | 0.044* |
| Umbilical artery pH, mean (SD) | 7.32 (0.06) | 7.34 (0.06) | 7.30 (0.08) | 7.33 (0.08) | 0.475 |
| Urinary output, mean (SD), ml/kg/hr | 2.5 (0.6) | 2.6 (0.7) | 3.3 (0.6) | 2.8 (0.7) | < 0.001*** |
| Highest K level, mean (SD), mEq/L | 5.2 (0.6) | 5.4 (0.6) | 5.2 (0.5) | 6.1 (0.8) | < 0.001*** |
| Insulin/glucose perfusion, n (%) | 1 (1) | 2 (6) | 0 (0) | 5 (22) | 0.002** |
| Neonatal laboratory data immediately after birth | |||||
| Serum TP level, mean (SD), g/dL | 5.0 (0.7) | 4.9 (0.6) | 4.5 (0.9) | 4.7 (0.7) | 0.144 |
| Serum ALB level, mean (SD), g/dL | 3.3 (0.4) | 3.2 (0.4) | 3.0 (0.5) | 3.1 (0.3) | 0.033* |
| Serum T-Bil level, mean (SD), mg/dL | 2.3 (0.5) | 2.1 (0.4) | 2.3 (0.5) | 2.0 (0.4) | 0.002** |
| Serum AST level, mean (SD), IU/L | 29.7 (10.5) | 26.5 (11.4) | 41.7 (38.4) | 31.7 (31.3) | 0.186 |
| Serum ALT level, mean (SD), IU/L | 4.5 (2.4) | 4.6 (1.7) | 5.7 (5.4) | 4.4 (3.1) | 0.653 |
| Serum ALP level, mean (SD), IU/L | 229 (71) | 231 (75) | 267 (97) | 283 (67) | 0.012* |
| Serum CK level, mean (SD), IU/L | 272 (103) | 262 (122) | 337 (140) | 357 (188) | 0.023* |
| Serum LDH level, mean (SD), IU/L | 384 (85) | 358 (77) | 437 (109) | 430 (140) | 0.024* |
| Serum BUN level, mean (SD), mg/dL | 7.9 (2.4) | 7.5 (2.0) | 11.8 (4.6) | 8.0 (7.1) | 0.006** |
| Serum CRE level, mean (SD), mg/dL | 0.60 (0.09) | 0.57 (0.10) | 0.66 (0.12) | 0.63 (0.16) | 0.072 |
| Serum Na level, mean (SD), mEq/L | 138 (1) | 139 (1) | 137 (3) | 136 (2) | < 0.001*** |
| Serum K level, mean (SD), mEq/L | 4.6 (0.5) | 4.7 (0.6) | 4.6 (0.5) | 4.9 (0.6) | 0.299 |
| Serum Cl level, mean (SD), mEq/L | 107 (2) | 108 (2) | 106 (4) | 105 (3) | < 0.001*** |
| Serum Ca level, mean (SD), mg/dl | 9.6 (0.6) | 9.5 (0.6) | 8.7 (0.9) | 9.1 (0.7) | < 0.001*** |
| Serum P level, mean (SD), mg/dL | 5.5 (0.8) | 5.6 (0.6) | 6.1 (1.0) | 6.9 (0.9) | < 0.001*** |
| Serum CRP level, mean (SD), mg/dL | 0.02 (0.05) | 0.37 (1.48) | 0.01 (0.01) | 0.02 (0.07) | 0.202 |
| WBC count, mean (SD), /L | 11,835 (4,199) | 10,890 (6,027) | 8,160 (4,490) | 10,803 (4,805) | 0.150 |
| Hb level, mean (SD), g/dL | 17.5 (1.9) | 15.8 (2.1) | 16.3 (2.5) | 15.7 (2.1) | < 0.001*** |
| PLT count, mean (SD), × 103/L | 253 (67) | 288 (69) | 194 (60) | 293 (99) | < 0.001*** |
| Characteristics of mothers | |||||
| Maternal age, mean (SD), years old | 33 (5) | 33 (5) | 33 (7) | 31 (5) | 0.293 |
| Nulliparity, n (%) | 39 (57) | 19 (53) | 5 (33) | 10 (43) | 0.317 |
| Caesarean delivery, n (%) | 47 (69) | 27 (75) | 14 (93) | 18 (78) | 0.259 |
| PROM, n (%) | 26 (38) | 16 (44) | 1 (7) | 3 (13) | 0.008** |
| Gestational hypertension, n (%) | 12 (18) | 5 (14) | 12 (80) | 3 (13) | < 0.001*** |
| Gestational diabetes mellitus, n (%) | 4 (6) | 1 (3) | 2 (13) | 0 (0) | 0.268 |
| Serum Mg level, mean (SD), mg/dL | N.D | N.D | 4.8 (0.8) | 4.6 (0.9) | |
MgSO4, magnesium sulfate; SGA, small for gestational age; Urinary output, urinary output in 48 h after birth; Highest K level, highest serum potassium level within 48 h of birth; TP, serum total protein; ALB, albumin; T-Bil, total bilirubin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; CK, creatine kinase; LDH, lactate dehydrogenase; BUN, blood urea nitrogen; CRE, creatinine; Na, sodium; K, potassium; Cl, chloride; Ca, calcium; P, phosphorus; CRP, C-reactive protein; WBC, white blood cell; Hb, hemoglobin; PLT, platelet; PROM, premature rupture of membrane; Mg, magnesium; SD, standard deviation.
The perinatal characteristics of the four groups are shown in Table 1. Although no significant differences in serum K + levels were observed immediately after birth among the 4 groups, the highest serum K + level within 48 h after birth was significantly higher in the both ritodrine and MgSO4 group compared to the neither ritodrine nor MgSO4 group (Fig. 1). Regarding the characteristics of the infants, significant differences were found among the 4 groups in sex, gestational age, birth weight, rate of twin pregnancies, incidence of premature rupture of membranes (PROM), Apgar score at 5 min, urine output within 48 h post-birth, and rate of insulin/glucose therapy. Blood tests conducted immediately after birth revealed significant intergroup differences in albumin (ALB), total bilirubin (T-Bil), alkaline phosphatase (ALP), creatine kinase (CK), lactate dehydrogenase (LDH), sodium (Na), chloride (Cl), calcium (Ca), phosphorus (P), hemoglobin (Hb), and platelet counts (PLT). Regarding maternal characteristics, the incidence of gestational hypertension was significantly different between the groups. Given that MgSO4 is effective in managing preeclampsia and providing neuroprotection in extremely low birth weight infants, infants in the MgSO4 alone group had significantly lower gestational ages and birth weights, and their mothers had a significantly higher frequency of gestational hypertension. To minimize the confounding effects of prematurity due to lower gestational age and to identify parameters strongly associated with exposure to ritodrine and MgSO4, we conducted an analysis focusing solely on infants born at 32–36 weeks of gestation (Supplementary Table S2). This analysis was further confirmed by sensitivity analyses, which excluded groups with combined ritodrine and MgSO4 treatment durations of < 3 days (n = 10) and < 14 days (n = 10) (Supplementary Table S3). As a result, significant differences were consistently observed in peak serum K + levels within 48 h post-birth, the rate of twin pregnancies and PROM, urine output within 48 h post-birth, and serum levels of T-Bil, ALP, Na, Cl, P, Hb, and PLT immediately after birth. In twin pregnancies, uterine contractions were often poorly controlled, leading to a higher rate of combined use of ritodrine and MgSO4. Additionally, for cases without membrane rupture, long-term use of tocolytics up to 36 weeks resulted in a lower PROM rate in the both ritodrine and MgSO4 group compared to those in the neither ritodrine nor MgSO4 group or the ritodrine alone group.
Fig. 1.

The highest serum potassium levels within 48 h of birth. The highest serum potassium levels in neonates within 48 h of birth in the four groups prenatally exposed to magnesium sulfate and ritodrine. Error bars indicate the standard deviation. ***p < 0.001. Statistics were calculated and the figure was produced in SPSS (version 29.0) and GraphPad (version 8). MgSO4, and magnesium sulfate.
Factors associated with serum potassium levels in neonates
A multiple regression analysis was conducted to explore factors that predict the severity of neonatal hyperkalemia in neonates exposed to ritodrine and MgSO4. The highest potassium level within 48 h after birth was used as the dependent variable, and clinical characteristics that were significantly different between the four groups were used as independent variables (Table 2). The results of the analysis showed that the factor most related to the severity of neonatal hyperkalemia was the serum phosphorus level immediately after birth (β = 0.288, p < 0.001).
Table 2.
A stepwise multiple regression analysis of the clinical data variables predicting hyperkalemia within 48 h of birth.
| Variables | β | t | p | Adjusted R2 | F |
|---|---|---|---|---|---|
| P | 0.288 | 4.693 | < 0.001 | 0.133 | 22.027 |
| Excluded variables | |||||
| Twin | 0.453 | ||||
| Urinary output | 0.657 | ||||
| Serum T-Bil level | 0.415 | ||||
| Serum ALP level | 0.104 | ||||
| Serum Na level | 0.113 | ||||
| Serum Cl level | 0.380 | ||||
| Hb level | 0.384 | ||||
| PLT count | 0.995 | ||||
| PROM | 0.839 | ||||
P, phosphorus; Urinary output, urinary output in 48 h after birth; T-Bil, total bilirubin; AST, aspartate aminotransferase; ALP, alkaline phosphatase; BUN, blood urea nitrogen; Na, sodium; Cl, chloride; Hb, hemoglobin; PROM, premature rupture of membrane.
Alterations in metabolism resulting from antenatal ritodrine and MgSO4 as revealed by a multivariate analysis of metabolites
To determine the mechanism underlying the induction of neonatal hyperkalemia in association with the maternal administration of antenatal ritodrine and MgSO4, serum metabolites in umbilical cord blood collected from newborns born at 24–36 weeks of gestation were analyzed using GC-MS/MS (Supplementary Figure S2 and Supplementary Table S4). As a result, 293 metabolites were detected in all samples (Supplementary Table S5). The orthogonal partial least-squares discriminant analysis (OPLS-DA) model was applied for the multivariate analysis of metabolites. The resulting score scatter plot presented distinctive clusters among the 4 groups (neither ritodrine nor MgSO4, ritodrine alone, MgSO4 alone, and both ritodrine and MgSO4) (Fig. 2).
Fig. 2.
Overall cord blood metabolic profile of four groups. The orthogonal partial least squares discriminant analysis score scatter plot was generated from cord blood serum metabolite data of antenatal, neither MgSO4 nor ritodrine, ritodrine alone, MgSO4 alone, and both ritodrine and MgSO4 groups. The x-axis shows the intra-group variance and the y-axis shows the inter-group variance. Each point represents the results of the analysis of the data from one neonate.
Pairwise comparisons were conducted to identify potential distinctive predictors associated with neonatal hyperkalemia triggered by exposure to both ritodrine and MgSO4 within a multitude of metabolites identified in the serum of the umbilical cord blood. Specifically, comparisons were made between the neither ritodrine nor MgSO4 in conjunction with the ritodrine alone group (Fig. 3A), as well as between the neither ritodrine nor MgSO4 group in conjunction with the MgSO4 alone group (Fig. 3B), and between the neither ritodrine nor MgSO4 group in conjunction with the both ritodrine and MgSO4 group (Fig. 3C). Additionally, pairwise comparisons were performed between the ritodrine alone group and the both ritodrine and MgSO4 group (Supplementary Figure S3A), and between the MgSO4 alone group and the both ritodrine and MgSO4 group (Supplementary Figure S3B). A clustering analysis between these two sets of groups revealed substantial dissimilarities, implying significant metabolite diversity among these groups. An S-plot analysis (Fig. 3D–F and Supplementary Figure S3C and D) and variable importance in projection (VIP) values identified 16 significant metabolites in the ritodrine alone group, 4 in the MgSO4 alone group, and 18 in the both ritodrine and MgSO4 group (Table 3 and Supplementary Table S6).
Fig. 3.
Metabolic profiles associated with ritodrine and magnesium sulfate (MgSO4) in the serum of umbilical cord blood. (A) OPLS-DA score plot in the neither MgSO4 nor ritodrine group and the ritodrine alone group. (B) OPLS-DA score plot in the neither MgSO4 nor ritodrine group and the MgSO4 alone group. (C) OPLS-DA score plot in the neither MgSO4 nor ritodrine group and the both ritodrine and MgSO4 group. (D) S-plot in the neither MgSO4 nor ritodrine group and the ritodrine alone group. (E) S-plot in the neither MgSO4 nor ritodrine group and the MgSO4 alone group. (F) S-plot in the neither MgSO4 nor ritodrine group and the both ritodrine and MgSO4 group.
Table 3.
Cord blood metabolites with altered abundances induced by antenatal exposure to ritodrine and/or magnesium sulfate.
| Ritodrine alone/Neither ritodrine nor MgSO4 | MgSO4 alone/Neither ritodrine nor MgSO4 | Both ritodrine and MgSO4/Neither ritodrine nor MgSO4 | ||||||
|---|---|---|---|---|---|---|---|---|
| Metabolite | VIP | P(corr) | Metabolite | VIP | P(corr) | Metabolite | VIP | P(corr) |
| Ornithine | 3.30 | 0.95 | Dopamine | 2.32 | 0.81 | Citric acid | 2.85 | 0.87 |
| Phenylalanine | 2.01 | 0.91 | Cystamine | 1.33 | 0.78 | 2-Aminopimelic acid | 2.57 | 0.86 |
| Inositol | 3.39 | 0.89 | Spermine | 1.97 | 0.78 | Lactic acid | 3.95 | 0.83 |
| Alanine | 2.80 | 0.89 | Tryptamine | 1.26 | 0.73 | Glycerol | 3.08 | 0.83 |
| Sucrose | 3.54 | 0.88 | Hydroxylamine | 3.07 | 0.82 | |||
| 5-Oxoproline | 2.62 | 0.87 | 2-Deoxy-glucose | 1.21 | 0.81 | |||
| Leucine | 2.04 | 0.87 | Galactose | 3.54 | 0.81 | |||
| Valine | 1.77 | 0.87 | Glucose | 3.53 | 0.80 | |||
| Lysine | 1.73 | 0.87 | Inositol | 2.88 | 0.80 | |||
| Threonine | 2.84 | 0.84 | Leucine | 2.83 | 0.77 | |||
| Tyrosine | 1.95 | 0.83 | Phenylalanine | 2.85 | 0.77 | |||
| Tryptophan | 2.16 | 0.83 | Alanine | 3.79 | 0.76 | |||
| Palmitic acid | 1.99 | 0.82 | Ornithine | 3.25 | 0.76 | |||
| Glucose | 1.59 | 0.78 | Sucrose | 3.44 | 0.75 | |||
| Galactose | 1.52 | 0.78 | Valine | 3.12 | 0.74 | |||
| Hydroxylamine | 5.75 | 0.78 | Palmitic acid | 2.49 | 0.74 | |||
| Stearic acid | 2.00 | 0.73 | ||||||
| Mannose | 2.32 | 0.70 | ||||||
MgSO4, magnesium sulfate; VIP, Variable Importance in the projection; p(corr), spearman rank correlation coefficient calculated with the principle component 1 of the selected OPLS-DA model.
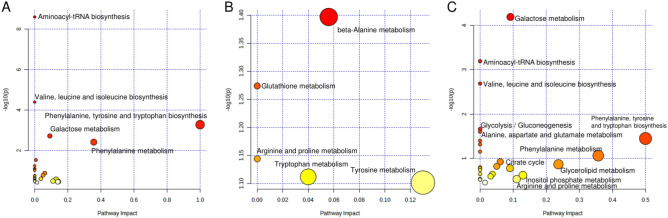
A more comprehensive analysis of the pathways and networks most closely associated with the metabolic changes induced by exposure to both ritodrine and MgSO4 was performed using a pathway enrichment analysis in MetaboAnalyst 5.0. The metabolic pathways are presented in Fig. 4, Supplementary Figure S4, and Supplementary Table S6, based on the metabolites that showed an increase in the serum of umbilical cord blood. The pathways related to amino acid biosynthesis and carbohydrate metabolism were notably influenced in the ritodrine alone group. Beta-alanine metabolism involving spermine was affected in the MgSO4 alone group. In the both ritodrine and MgSO4 group, the pathways associated with amino acid biosynthesis, carbohydrate metabolism, and glycolysis/gluconeogenesis were primarily affected. This group exhibited a trend similar to that observed in the ritodrine alone group. To further elucidate the specific metabolic pathways arising from exposure to both ritodrine and MgSO4, we assessed the metabolite pathways with significant differences between the both ritodrine and MgSO4 group and the ritodrine alone group. As a result, the citrate cycle emerged as the most affected metabolic pathway in the both ritodrine and MgSO4 group in comparison to the ritodrine alone group (Supplementary Figure S4 and Supplementary Table S7). The citrate cycle did not exhibit significant activation in the magnesium sulfate monotherapy group, suggesting a synergistic effect resulting from the combined effect of ritodrine and MgSO4.
Fig. 4.
Pathway analysis of metabolites significantly correlated with antenatal use of ritodrine and/or magnesium sulfate (MgSO4). (A) The metabolic pathway analysis of the metabolites that were significantly correlated with antenatal use ritodrine alone. (B) The metabolic pathway analysis of the metabolites that significantly correlated with antenatal use of MgSO4 alone. (C) The metabolic pathway analysis of the metabolites that were significantly correlated with antenatal use of both ritodrine and MgSO4.
Correlation between metabolite levels and postnatal peak potassium levels
To identify potential predictors of the postnatal elevation of serum potassium levels in neonates born to mothers administered both ritodrine and MgSO4, we examined 18 specific metabolites showing distinctive increases in both ritodrine and MgSO4 group compared to the neither ritodrine nor MgSO4 group, along with their correlation with peak potassium values within 48 h after birth. Employing the peak potassium level within 48 h postnatally as the dependent variable, a multiple regression analysis was conducted with these specific metabolites as independent variables. The analysis revealed that citric acid was the factor most strongly associated with the severity of neonatal hyperkalemia (Supplementary Table S8). Furthermore, we validated the correlation between peak potassium levels (which showed the most significant association in clinical tests) and phosphorus levels with citric acid. The relationships among peak potassium, phosphorus, and citric acid levels were quantified using Pearson’s correlation coefficients (Table 4). Citric acid levels exhibited the highest positive correlation with peak potassium levels (r = 0.551, p < 0.01), indicating a positive correlation. In addition, citric acid levels were positively correlated with phosphorus levels (r = 0.491, P < 0.01). Based on these findings, serum citric acid and phosphorus levels immediately after birth are considered to be potential predictors of neonatal hyperkalemia.
Table 4.
Correlation of the postnatal peak potassium levels, phosphorus levels and citric acid levels.
| Highest K level within 48 h of birth | P | Citric acid | |
|---|---|---|---|
| Highest K level within 48 h of birth | – | r = 0.544** | r = 0.551** |
| P | – | r = 0.491** | |
| Citric acid | – |
K, potassium; P, phosphorus; r, coefficient correlation; *, p < 0.05; **, p < 0.01.
To further validate citric acid and phosphate as potential biomarkers of ritodrine-induced and MgSO4-induced hyperkalemia, we performed a receiver operating characteristic (ROC) curve analysis. Supplementary Figure S5 shows the ROC curves for citric acid and phosphate in the prediction of hyperkalemia, defined as a maximum potassium level of 6 mEq/l postnatally. Citric acid demonstrated an area under the curve (AUC) of 0.782, indicating a good discriminatory capacity. A cutoff value for citric acid, set at 1.26 times the average peak area obtained from a GC/MS analysis, yielded 66.67% sensitivity and 95.83% specificity, with a PPV of 85.7 and an NPV of 88.5. Phosphate levels had an AUC of 0.755, suggesting a fair discriminatory capacity. Using a cut-off value of > 6.8 mg/dl, phosphate levels achieved 55.56% sensitivity and 91.67% specificity, with a PPV of 71.4, and an NPV of 84.6. These findings indicate that both citric acid and phosphate are promising biomarkers of hyperkalemia in the context of ritodrine and MgSO4, with citric acid having higher specificity and overall predictive accuracy.
Discussion
In this study, we demonstrated that the serum maximum potassium levels in newborns born to mothers administered antenatal ritodrine and MgSO4 for preterm labor increased significantly within 48 h of birth, although their serum potassium levels were normal immediately after birth. Furthermore, we revealed that neonates exposed to both ritodrine and MgSO4 exhibited enhanced metabolic pathways different from those observed in neonates exposed to ritodrine or MgSO4 alone, using a comprehensive analysis of serum metabolites in umbilical cord blood. Despite changes in the fetal metabolism, unaltered potassium levels immediately after birth in infants exposed to ritodrine and MgSO4 are presumably regulated by the abundance of potassium channels in the placenta17. To support this finding, no correlation was found between pH and potassium levels in umbilical cord blood immediately after birth, despite acidosis increasing the concentration of potassium ions in blood in adults18,19. Recent research has reported that the combined use of ritodrine and MgSO4 in mothers is a risk factor for hyperkalemia in preterm infants7,8. The findings of this study confirmed that the antenatal administration of ritodrine and MgSO4 to mothers induced hyperkalemia in preterm infants. Furthermore, a metabolomics analysis revealed activation not only in amino acid biosynthesis, sugar metabolism, and glycolysis/gluconeogenesis, but also in a distinctive citrate cycle in the neonatal group exposed to both ritodrine and MgSO4. Therefore, this study suggests a synergistic effect of ritodrine and MgSO4, which can induce distinctive metabolic changes and trigger neonatal hyperkalemia.
The significantly increased citric acid observed in the both ritodrine and MgSO4 group was produced in the citrate cycle through aldol condensation of oxaloacetic acid and acetyl CoA, the final products of the preceding turn of the cycle20. Acetyl-CoA originates from glucose through the glycolytic pathway20. Ritodrine functions as a β2-AR agonist. The stimulation of hepatic β2 adrenergic receptors by ritodrine activates hepatic adenylate cyclase, promoting hepatic gluconeogenesis and glycogenesis, thereby inducing hyperglycemia21,22. Prolonged glucose stimulation induces citrate synthesis in the mitochondria via the glycolysis pathway, leading to increased cytoplasmic and extracellular citrate23. The administration of MgSO4 to pregnant women results in an increased concentration of magnesium within the fetal serum and intracellular spaces, which is facilitated by the ease with which MgSO4 traverses the placenta24. The elevation in intracellular magnesium levels facilitates the uptake of glucose into the cell via Glut4, a component of the glycolytic pathway25. Additionally, magnesium serves as a cofactor for enzymes in the glycolysis pathway, including hexokinase, phosphofructokinase, phosphoglycerate kinase, and enolase26,27. In our study, neonates with antenatal exposure to both ritodrine and MgSO4 exhibited significant activation of metabolic pathways, including the citrate cycle, branched-chain amino acids, alanine, and fatty acid synthesis, derived from metabolites generated during glycolysis. Therefore, the synergistic effect of both ritodrine and MgSO4 suggests that they activate glycolysis and the derived metabolic routes.
Glycolysis regulates cell membrane ion transporters (pumps, exchangers, and channels) and modulates the intracellular distribution of potassium ions28. Adenosine triphosphate (ATP) is produced through three processes: glycolysis, the citrate cycle, and oxidative phosphorylation, starting from intracellular glucose29. The cell membrane features Na–K ATPase and ATP-sensitive K channels, with an increase in intracellular ATP levels, leading to elevated intracellular potassium concentrations28,30. Generally, stimulation of β2 adrenergic receptors by ritodrine involves the activation of Na–K ATPase pumps on the cell membrane, facilitating the intracellular transition of potassium31. The rebound following the cessation of ritodrine is known to cause the release of potassium from intracellular spaces into the serum32–35. As approximately 98% of potassium resides intracellularly, even a slight efflux of intracellular potassium results in a significant increase in serum potassium concentration31. The rebound of potassium after the injection of insulin or thiopentone has also been reported to induce the uptake of potassium into cells, indicating that rapid changes in mechanisms regulating potassium distribution in the body are risk factors for hyperkalemia36. In our study, neonates exposed to both ritodrine and MgSO4 may have experienced an enhanced influx of potassium into cells due to the activation of Na–K ATPase pumps by ritodrine coupled with increased metabolic pathways related to glycolysis. The postulated etiology in our study is shown in Fig. 5. Therefore, neonates exposed to both ritodrine and MgSO4 may have a heightened quantity of potassium redistribution from intracellular to serum compartments following the discontinuation of these medications, potentially increasing their susceptibility to postnatal hyperkalemia.
Fig. 5.
A schematic diagram of potential causes of hyperkalemia in neonates exposed to ritodrine and magnesium sulfate (MgSO4). Ritodrine crosses the placenta, inducing insulin secretion from the fetal pancreas and glucose from the fetal liver. This activates the glycolytic pathway and citric acid cycle in fetal cells, enhancing ATP synthesis. MgSO4 also crosses the placenta, increasing intracellular magnesium and promoting glucose uptake, further enhancing glycolysis. This results in elevated citric acid and ATP levels, with increased ATP facilitating extracellular potassium uptake into cells. After delivery, the discontinuation of ritodrine and MgSO4 reduces the fetal blood levels of insulin, glucose, and magnesium, causing potassium efflux from the cells. As a result, this leads to rebound hyperkalemia in the newborn.
The citric acid levels in umbilical cord blood serum were positively correlated with the highest potassium values within the first 48 h of life, suggesting its potential as a marker for the early detection of neonatal hyperkalemia (Table 4). However, citric acid is not routinely analyzed in the blood of preterm infants immediately after birth. In our investigation, serum phosphorus levels immediately after birth demonstrated a positive correlation with citric acid levels in the serum of umbilical cord blood and the highest potassium values within the first 48 h after delivery (Table 4). The presence of hypermagnesemia induced by MgSO4 administration inhibits parathyroid hormone secretion and induces hyperphosphatemia in the fetus37. Furthermore, ritodrine-induced insulin promotes the reabsorption of phosphate in the proximal tubules38. Therefore, the elevation in serum levels of phosphorus and citric acid due to the influence of ritodrine and MgSO4 can occur through different mechanisms. Consequently, serum levels of phosphorus and citric acid immediately after birth can independently serve as potential predictors of the onset of neonatal hyperkalemia. Neonatal hyperkalemia is generally recognized to occur in infants born at < 32 weeks of gestation and requires close monitoring of serum potassium levels over time9. In contrast, babies born between 32 and 36 weeks of gestation have a lower incidence of hyperkalemia; however, exposure to ritodrine and MgSO4 increases the risk of hyperkalemia7. Due to the perceived low risk of hyperkalemia in infants born between 32 and 36 weeks of gestation, there may be a delay in recognizing hyperkalemia. Hyperkalemia can appear as early as 9 h after birth and peak around 26 h postpartum39. In such cases, the evaluation of serum citric acid and phosphorus levels at birth could lead to more vigilant potassium monitoring, ensuring timely detection and treatment of hyperkalemia. Furthermore, predicting neonatal hyperkalemia could facilitate the early initiation of glucose-insulin therapy, a treatment for hyperkalemia, and assist in the decision making about the introduction of early caffeine therapy, which has recently been reported to have preventive effects against neonatal hyperkalemia40. These early interventions may mitigate the risk of fatal arrhythmias due to hyperkalemia and potentially improve neonatal outcomes.
The present study was associated with several limitations. First, it was a retrospective analysis conducted at a single institution, and was limited to the analysis of the serum of umbilical cord blood from a subset of cases. In this study, we limited our investigation to cases to minimize any bias due to treatment strategies for threatened preterm labor; however, treatment strategies that depended on individual physicians might affect the clinical and laboratory data. Additionally, pregnant women who received both ritodrine and MgSO4 had longer durations of drug administration and higher dosages than those who received ritodrine or MgSO4 alone. This discrepancy in the drug administration parameters may have influenced the outcomes of this study. Furthermore, the study did not assess neonatal blood magnesium concentrations or urinary potassium excretion rates. Additionally, the effects of diuretics and steroid hormones, which can influence the maternal, fetal, and neonatal serum potassium levels, were not considered in this study. Prospective randomized controlled trials are necessary to eliminate potential bias and elucidate the remaining issues.
The present study revealed that neonates exposed to both ritodrine and MgSO4 may experience synergistic effects of these drugs, leading to the activation of glycolysis and the derived metabolic routes, potentially inducing hyperkalemia. Citric acid levels in the serum of umbilical cord blood or serum phosphorus levels immediately after birth may serve as predictors of neonatal hyperkalemia. The early prediction of neonatal hyperkalemia would facilitate the timely introduction of preventive or therapeutic drugs for hyperkalemia. Furthermore, our findings hold the potential to contribute to future drug development aimed at preventing neonatal hyperkalemia by targeting molecules involved in glycolysis and the citrate cycle.
Methods
Ethics
This study was approved by the Institutional Review Board of Oita University Hospital, Japan (Oita University, 2413). It follows the tenets of the Declaration of Helsinki. The need to obtain informed consent from the study participants was waived by the Institutional Review Board of Oita University Hospital since this is a retrospective analysis. Patients who were eligible for this study had the opportunity to refuse to participate in the study by opting out. The research was conducted in accordance with the Ethical Guidelines for Medical and Health Research Involving Human Subjects established by the Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labour and Welfare.
Study population
This study included preterm infants born at 24–36 weeks of gestation at Oita University Hospital’s NICU between April 2017 and August 2022. Oita University Hospital is equipped to handle deliveries as early as 22 weeks of gestation. Infants with uncertain maternal drug use, multiple malformation syndrome, chromosomal abnormalities, hemolytic anemia, intracranial hemorrhage, maternal malignancy, or history of anticancer drug treatment were excluded from the study. In our study, we focused on preterm infants born at 24–36 weeks of gestation. The reasons were based on several considerations regarding gestational age-dependent management and health outcomes of preterm infants. Infants born at Oita University Hospital at ≥ 37 weeks of gestational age are managed by obstetricians after delivery. These infants do not routinely undergo electrolyte monitoring unless they present with clinical symptoms. Regardless of clinical symptoms, infants exposed to ritodrine within 24 h before birth were routinely measured for blood glucose levels after birth. Pediatricians evaluate and treat infants who develop hypoglycemia or other clinical problems. In particular, none of the term infants (born at ≥ 37 weeks of gestation) managed by pediatricians developed hyperkalemia prior to this study. Infants born at 22–23 weeks of gestation were excluded due to the variability in resuscitation practices, prognosis, and ethical considerations surrounding the initiation of intensive care, which always involves in-depth discussions with the parents. Consequently, we investigated preterm infants born at 24–36 weeks of gestation.
The umbilical cord blood serum analysis used residual samples from a previous study approved by the ethics committee due to limited sample availability. Therefore, the study included cases of infants born between April 2020 and August 2022 with gestational ages ranging from 24 to 36 weeks.
Protocol of tocolytic treatment
During the study period, the protocol of our hospital for tocolytic treatment was as follows. For threatened preterm labor, we began with the administration of ritodrine hydrochloride, with an initial dose of 50 μg/min administered via continuous intravenous infusion. The dose was increased to 100 μg/min, 150 μg/min, and up to 200 μg/min depending on the severity of the uterine contractions. If uterine contractions were not adequately controlled with ritodrine hydrochloride at 200 μg/min, MgSO4 was used either in combination with ritodrine or as a monotherapy if patients could not tolerate ritodrine due to side effects. The administration of MgSO4 began with an initial dose of 2 g administered intravenously for 20 min, followed by a continuous intravenous infusion at 1 g/h. If uterine contractions remained uncontrolled, the dosage was increased by 0.5 g/h, up to a maximum of 2 g/h. Tocolytic drugs were adjusted according to the uterine contraction status and were typically discontinued at 36 weeks of gestation. If the membranes ruptured, tocolytic therapy was stopped at 34 weeks. In cases of clinical chorioamnionitis or non-reassuring fetal status, tocolytic drugs were immediately stopped and delivery was started. Regarding the order of drug selection and administration, ritodrine hydrochloride was administered first, with magnesium sulfate added if contractions were not controlled with ritodrine alone or if ritodrine could not be used due to side effects.
Clinical observations
We retrospectively collected comprehensive clinical data from medical records of the participants, encompassing the following aspects: (a) information on the administration of ritodrine and MgSO4, which included duration of injections, final infusion rate, total dose, and maternal serum magnesium levels; (b) perinatal status of infants, encompassing factors such as gestational age, sex, weight, height, route of delivery, singleton or multiplet, Apgar scores at 1 min and 5 min, post-birth urinary output, and insulin/glucose perfusion; (c) laboratory data at birth, comprising umbilical artery pH, white blood cell (WBC) count, Hb levels, PLT count, and serum levels of total protein (TP), ALB, T-Bil, aspartate aminotransferase (AST), alanine aminotransferase (ALT), ALP, CK, LDH, blood urea nitrogen (BUN), creatinine (CRE), Na, K, Cl, Ca, P, and C-reactive protein (CRP); (d) highest serum K level in neonate within 48 h of birth; and (e) maternal data included age, parity, obstetric complications (gestational hypertension and gestational diabetes), and with or without complication of PROM during delivery.
In this study, we focused on the laboratory data obtained from infants immediately after birth, because blood tests were of all newborns were performed immediately after delivery, ensuring a consistent set of data for each infant. On the contrary, the timing of blood sampling for mothers during pregnancy varied depending on their underlying conditions and no uniform data from mothers was available around the time of delivery. Therefore, we only evaluated the infant laboratory data to ensure the consistency and reliability of the statistical analysis. Neonatal blood tests were carried out at intervals of no more than 24 h from birth to 48 h of age. If potassium levels tended to increase, blood tests were performed repeatedly every 3–6 h.
Sampling specimens and storage
Umbilical cord blood samples were collected at delivery using plastic syringes. Samples were centrifuged for 10 min at 1200×g, the serum was decanted, and serum samples were stored at − 80 °C until use in an analysis.
Metabolomic analyses
The analysis of metabolites was performed by GC-MS/MS. The GC-MS/MS analysis was performed on a GCMS-TQ8040 system (Shimadzu Corporation, Kyoto, Japan) equipped with a DB-5 capillary column (30 m × 0.25 mm inner diameter, film thickness 1 μm; Agilent, Santa Clara, CA, USA). Each 1-μm aliquot of the derivatized sample solution was automatically injected in splitless mode into a gas–liquid chromatography column using an auto-injector (AOC-20i; Shimadzu Corporation). During the GCMS-TQ8040 analysis, the injector temperature was maintained at 280 °C, and helium was used as the carrier gas at a constant flow rate of 39.0 cm/s. The GC column temperature was programmed to remain at 100 °C for 4 min, then to rise to 320 °C at a rate of 10 °C/min, holding at 320 °C for an additional 11 min. The ionization voltage was set to 70 eV. Argon was used for collision-induced dissociation. Metabolite detection was performed using the Smart Metabolite Database Ver. 3 software program (Shimadzu Corporation) using the method described in a previous study with some modifications41. The 2-isopropylmalic acid contained in the extraction solution was also used to evaluate the stability of the GC-MS/MS analysis system. Peak identification was performed automatically and then confirmed manually based on the specific precursor and product ions as well as the retention time using the method described in our previous study42.
Statistical analyses
Statistical analyses were performed using the SPSS software program version 29.0 (IBM Corporation, Armonk, NY, USA) and GraphPad Prism software version 8 (GraphPad Software, Inc., San Diego, CA, USA). Perinatal characteristics among the four groups were assessed using Fisher’s exact probability test for nominal variables and the Kruskal–Wallis test for continuous variables. The highest serum potassium concentration was observed in the Shapiro–Wilk and Brown–Forsythe tests for normal distribution and homogeneity, respectively. Since the data exhibited a normal distribution, a one-way analysis of variance (ANOVA) followed by Tukey’s pairwise comparison test was employed for the analysis. We conducted a sensitivity analysis to address the potential variability in the duration of combined therapy with ritodrine and MgSO4. Specifically, we excluded any participants with a combined treatment duration of 3 days or less, as well as those with a duration of 14 days or less, to assess the robustness of our findings. A stepwise multiple regression analysis was performed to evaluate the relationship between potassium concentration and clinical characteristics. The correlation between serum metabolites in umbilical cord blood and serum potassium concentration of phosphorus was analyzed using the Pearson correlation coefficient. A significance threshold of p < 0.05 was employed. The specificity and sensitivity were assessed by the AUC of the ROC curves.
Integral metabolomics datasets were imported into the SIMCA version 13.0.3.0 software program (Umetrics, Umea, Sweden) for multivariate statistical analyses. OPLS-DA with Pareto scaling was used to visualize the differences between the metabolomic datasets and extract significant metabolites. The primary distinctions in metabolites between each group were identified through an S-plot analysis, which visualizes both the covariance and correlation between metabolites and the modelled class designation. Significant metabolites were selected based on compounds with a p(corr) value of > 0.7 and VIP values of > 1.0, a commonly employed metric to summarize the significance of each variable in model construction43. In the pathway analysis, to determine the pathways altered between metabolomics data sets, MetPA and MSEA with significant metabolites were performed using the MetaboAnalyst 5.0 software program (https://www.metaboanalyst.ca/, accessed on June 23, 2023), which is a free web-based tool that combines results from a potent pathway enrichment analysis pertaining to the conditions under study44.
Supplementary Information
Acknowledgements
The authors express their appreciation to the patients and parents of the participants in this study. We would also like to thank the Department of Pediatrics, Oita University Hospital, for their help with patient recruitment and sample collection, and Kai S. for their excellent technical assistance. Mr. Brian Quinn in Japan Medical Communication for assistance in editing this paper.
Author contributions
M.I. designed and proposed the study. M.I., H.T., and N.I. collected the clinical data. M.I. and K.S. collected the samples and identified metabolites. M.I., N.I., and T.M. analyzed and interpreted the data. M.I. drafted the manuscript. H.I. and K.I. reviewed and edited the manuscript. All authors read, revised, and approved the final draft of the manuscript.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-85938-8.
References
- 1.Nanda, K., Cook, L. A., Gallo, M. F. & Grimes, D. A. Terbutaline pump maintenance therapy after threatened preterm labor for preventing preterm birth. Cochrane Database Syst. Rev.10.1002/14651858.CD003933 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shinohara, S., Sunami, R., Uchida, Y., Hirata, S. & Suzuki, K. Association between total dose of ritodrine hydrochloride and pulmonary oedema in twin pregnancy: A retrospective cohort study in Japan. BMJ Open7, e018118. 10.1136/bmjopen-2017-018118 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Witlin, A. G. & Sibai, B. M. Magnesium sulfate therapy in preeclampsia and eclampsia. Obstet. Gynecol.92, 883–889. 10.1016/s0029-7844(98)00277-4 (1998). [DOI] [PubMed] [Google Scholar]
- 4.Lingam, I. & Robertson, N. J. Magnesium as a neuroprotective agent: A review of its use in the fetus, term infant with neonatal encephalopathy, and the adult stroke patient. Dev. Neurosci.40, 1–12. 10.1159/000484891 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Kawagoe, Y., Sameshima, H., Ikenoue, T., Yasuhi, I. & Kawarabayashi, T. Magnesium sulfate as a second-line tocolytic agent for preterm labor: A randomized controlled trial in Kyushu Island. J. Pregnancy2011, 965060. 10.1155/2011/965060 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakamura, M. et al. Comparison of perinatal outcomes between long-term and short-term use of tocolytic agent: A historical cohort study in a single perinatal hospital. J. Obstet. Gynaecol. Res.42, 1680–1685. 10.1111/jog.13104 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Yada, Y. et al. Synergic interaction between ritodrine and magnesium sulfate on the occurrence of critical neonatal hyperkalemia: A Japanese nationwide retrospective cohort study. Sci. Rep.10, 7804. 10.1038/s41598-020-64687-w (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hira, S. et al. Antenatal mgnesium sulfate and ritodrine increased potassium levels in preterm infants: A cohort study. Pediatr. Int.64, e15315. 10.1111/ped.15315 (2022). [DOI] [PubMed] [Google Scholar]
- 9.Bonilla-Felix, M. Potassium regulation in the neonate. Pediatr. Nephrol.32, 2037–2049. 10.1007/s00467-017-3635-2 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Shortland, D., Trounce, J. Q. & Levene, M. I. Hyperkalaemia, cardiac arrhythmias, and cerebral lesions in high risk neonates. Arch. Dis. Child62, 1139–1143. 10.1136/adc.62.11.1139 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwak, J. R., Gwon, M., Lee, J. H., Park, M. S. & Kim, S. H. Non-oliguric hyperkalemia in extremely low birth weight infants. Yonsei Med. J.54, 696–701. 10.3349/ymj.2013.54.3.696 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaffer, S. G., Kilbride, H. W., Hayen, L. K., Meade, V. M. & Warady, B. A. Hyperkalemia in very low birth weight infants. J. Pediatr.121, 275–279. 10.1016/s0022-3476(05)81203-x (1992). [DOI] [PubMed] [Google Scholar]
- 13.Kilbride, H. W., Cater, G. & Warady, B. A. Early onset hyperkalemia in extremely low birth weight infants. J. Perinatol.8, 211–214 (1988). [PubMed] [Google Scholar]
- 14.Sychlowy, A., van der Gaag, H. & Hannen-Hofheinz, I. Hyperkalemia–life-threatening early complication of asphyxia in premature infants. Monatsschr Kinderheilkd138, 62–65 (1990). [PubMed] [Google Scholar]
- 15.Bahado-Singh, R. O. et al. Metabolomic prediction of fetal congenital heart defect in the first trimester. Am. J. Obstet. Gynecol.211, 240. 10.1016/j.ajog.2014.03.056 (2014). [DOI] [PubMed] [Google Scholar]
- 16.Noto, A., Fanos, V. & Dessi, A. Metabolomics in Newborns. Adv. Clin. Chem.74, 35–61. 10.1016/bs.acc.2015.12.006 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Wareing, M. & Greenwood, S. L. Review: Potassium channels in the human fetoplacental vasculature. Placenta32(Suppl 2), S203-206. 10.1016/j.placenta.2010.12.022 (2011). [DOI] [PubMed] [Google Scholar]
- 18.Vanspranghels, R. et al. Umbilical cord arterial and venous gases, ionogram, and glucose level for predicting neonatal morbidity at term. Eur. J. Obstet. Gynecol. Reprod. Biol.252, 181–186. 10.1016/j.ejogrb.2020.06.022 (2020). [DOI] [PubMed] [Google Scholar]
- 19.Aronson, P. S. & Giebisch, G. Effects of pH on potassium: New explanations for old observations. J. Am. Soc. Nephrol.22, 1981–1989. 10.1681/ASN.2011040414 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akram, M. Citric acid cycle and role of its intermediates in metabolism. Cell Biochem. Biophys.68, 475–478. 10.1007/s12013-013-9750-1 (2014). [DOI] [PubMed] [Google Scholar]
- 21.Lenz, S. et al. The effect of ritodrine on carbohydrate and lipid metabolism in normal and diabetic pregnant women. Acta Endocrinol. (Copenh)92, 669–679. 10.1530/acta.0.0920669 (1979). [DOI] [PubMed] [Google Scholar]
- 22.Mordes, D., Kreutner, K., Metzger, W. & Colwell, J. A. Dangers of intravenous ritodrine in diabetic patients. JAMA248, 973–975 (1982). [PubMed] [Google Scholar]
- 23.Farfari, S., Schulz, V., Corkey, B. & Prentki, M. Glucose-regulated anaplerosis and cataplerosis in pancreatic beta-cells: possible implication of a pyruvate/citrate shuttle in insulin secretion. Diabetes49, 718–726. 10.2337/diabetes.49.5.718 (2000). [DOI] [PubMed] [Google Scholar]
- 24.Brookfield, K. F. et al. Pharmacokinetics and placental transfer of magnesium sulfate in pregnant women. Am. J. Obstet. Gynecol.214(737), e731-739. 10.1016/j.ajog.2015.12.060 (2016). [DOI] [PubMed] [Google Scholar]
- 25.Oost, L. J. et al. Magnesium increases insulin-dependent glucose uptake in adipocytes. Front Endocrinol. (Lausanne)13, 986616. 10.3389/fendo.2022.986616 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garfinkel, L. & Garfinkel, D. Magnesium regulation of the glycolytic pathway and the enzymes involved. Magnesium4, 60–72 (1985). [PubMed] [Google Scholar]
- 27.Hosseini Dastgerdi, A., Ghanbari Rad, M. & Soltani, N. The therapeutic effects of magnesium in insulin secretion and insulin resistance. Adv. Biomed. Res.11, 54. 10.4103/abr.abr_366_21 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dhar-Chowdhury, P., Malester, B., Rajacic, P. & Coetzee, W. A. The regulation of ion channels and transporters by glycolytically derived ATP. Cell Mol. Life Sci.64, 3069–3083. 10.1007/s00018-007-7332-3 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonora, M. et al. ATP synthesis and storage. Purinergic Signal8, 343–357. 10.1007/s11302-012-9305-8 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nichols, C. G. KATP channels as molecular sensors of cellular metabolism. Nature440, 470–476. 10.1038/nature04711 (2006). [DOI] [PubMed] [Google Scholar]
- 31.Palmer, B. F. & Clegg, D. J. Diagnosis and treatment of hyperkalemia. Cleve Clin. J. Med.84, 934–942. 10.3949/ccjm.84a.17056 (2017). [DOI] [PubMed] [Google Scholar]
- 32.Kinoshita, H. et al. Acute hypokalemia induced by ritodrine and rebound hyperkalemia in a parturient undergoing a cesarean section. J. Clin. Anesth.28, 78–79. 10.1016/j.jclinane.2015.05.019 (2016). [DOI] [PubMed] [Google Scholar]
- 33.Takekawa, D., Jinushi, K., Kitayama, M. & Hirota, K. Rebound hyperkalemia after cessation of ritodrine in a parturient undergoing cesarean section. JA Clin. Rep.3, 3. 10.1186/s40981-016-0071-4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kotani, N. et al. Rebound perioperative hyperkalemia in six patients after cessation of ritodrine for premature labor. Anesth. Analg93, 709–711. 10.1097/00000539-200109000-00034 (2001). [DOI] [PubMed] [Google Scholar]
- 35.Suga, K. et al. Neonatal hyperkalemia associated with maternal ritodrine: A retrospective study. Pediatr. Int.65, e15604. 10.1111/ped.15604 (2023). [DOI] [PubMed] [Google Scholar]
- 36.Palmer, B. F. A physiologic-based approach to the evaluation of a patient with hyperkalemia. Am. J. Kidney Dis.56, 387–393. 10.1053/j.ajkd.2010.01.020 (2010). [DOI] [PubMed] [Google Scholar]
- 37.Rantonen, T. et al. Antenatal magnesium sulphate exposure is associated with prolonged parathyroid hormone suppression in preterm neonates. Acta Paediatr.90, 278–281 (2001). [PubMed] [Google Scholar]
- 38.Tiwari, S., Riazi, S. & Ecelbarger, C. A. Insulin’s impact on renal sodium transport and blood pressure in health, obesity, and diabetes. Am. J. Physiol. Renal Physiol.293, F974-984. 10.1152/ajprenal.00149.2007 (2007). [DOI] [PubMed] [Google Scholar]
- 39.Lorenz, J. M., Kleinman, L. I. & Markarian, K. Potassium metabolism in extremely low birth weight infants in the first week of life. J. Pediatr.131, 81–86. 10.1016/s0022-3476(97)70128-8 (1997). [DOI] [PubMed] [Google Scholar]
- 40.Hayata, M. et al. Early caffeine therapy on the prevention of severe hyperkalemia in preterm infants. Pediatr. Int.65, e15526. 10.1111/ped.15526 (2023). [DOI] [PubMed] [Google Scholar]
- 41.Nishiumi, S. et al. Investigations in the possibility of early detection of colorectal cancer by gas chromatography/triple-quadrupole mass spectrometry. Oncotarget8, 17115–17126. 10.18632/oncotarget.15081 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sekiguchi, K. et al. Metabolome characteristics of liver autophagy deficiency under starvation conditions in infancy. Nutrients10.3390/nu13093026 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wheelock, A. M. & Wheelock, C. E. Trials and tribulations of ’omics data analysis: assessing quality of SIMCA-based multivariate models using examples from pulmonary medicine. Mol. Biosyst.9, 2589–2596. 10.1039/c3mb70194h (2013). [DOI] [PubMed] [Google Scholar]
- 44.Pang, Z. et al. Using MetaboAnalyst 5.0 for LC-HRMS spectra processing, multi-omics integration and covariate adjustment of global metabolomics data. Nat. Protoc.17, 1735–1761. 10.1038/s41596-022-00710-w (2022). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.