Abstract
The mainstay of antiretroviral therapy (ART) has been combination oral therapy. While oral ART is highly effective, nonadherence remains a chief concern. Addressing this concern in recent years is the emergence of long‐acting antiretrovirals for the treatment and prevention of HIV‐1 infection. The most recently approved long‐acting antiretroviral is the first‐in‐class capsid inhibitor lenacapavir (LEN) for heavily treatment‐experienced adults with multidrug‐resistant HIV‐1 infection. Due to its biannual subcutaneous dosing scheme to inhibit the HIV‐1 capsid, LEN exhibits unique pharmacokinetics and reinforces an evolving era of ART. In this review, we evaluate published and accepted research articles, conference proceedings, and clinical trial records to provide a comprehensive overview of LEN for treatment and preliminary data for the prevention of HIV‐1 infection. These data include clinical trials outcomes, in vitro and in vivo resistance profiles, and preclinical data supporting downstream indications. We also discuss the unique clinical pharmacology of LEN with the goal of serving as a resource toward subsequent physiologically based, population‐based, and other miscellaneous pharmacometric‐focused analyses. Given the dynamic nature of the HIV treatment and prevention research fields, we also discuss ongoing studies related to LEN for treatment‐naïve adults and for prevention. Lastly, we discuss important pharmacologic gaps in special populations, drug–drug interactions, and at the sites of action germane to HIV treatment and prevention. The information discussed in this review will provide knowledge and understanding of the unique pharmacologic properties of LEN to assist clinicians and researchers as they navigate the dynamic HIV research landscape.
Nearly 39 million people live with human immunodeficiency virus (HIV) globally. Treatment for HIV, termed antiretroviral therapy (ART), has significantly improved the quality of life, health, and life expectancy for people with HIV (PWH). 1 ART is recommended for all PWH and should be started rapidly following diagnosis to prevent HIV‐associated morbidity and mortality and to prevent HIV transmission by suppressing plasma HIV‐1 RNA below quantifiable limits of detection. 2 ART is highly effective in suppressing and maintaining undetectable plasma HIV‐1 RNA levels, but adherence to the prescribed ART regimen is crucial to its effectiveness. 3
Generally, high adherence rates – usually 90% or greater – are necessary for sustaining viral suppression. 4 However, a recent retrospective study demonstrated that over 40% of PWH in the United States report adherence below 80% over the previous 12 months. 5 The consequences of nonadherence include the risk of HIV transmission, treatment resistance, and the emergence of HIV‐associated negative health effects. 6 , 7
Emerging options to address nonadherence are long‐acting injectable (LAI) formulations of antiretrovirals (ARVs). 8 LAIs are administered via non‐oral extravascular routes, such as intramuscular (IM) and subcutaneous (SC) injections. 9 Accompanying these routes of administration is unique pharmacokinetic (PK) properties that permit less frequent dosing schedules on the orders of weeks or months vs. daily dosing with conventional oral ART. 9
Exemplifying this evolution of ART is lenacapavir (LEN), a first‐in‐class capsid inhibitor dosed subcutaneously every 26 weeks. 10 In this review of LEN, we discuss the preclinical and clinical studies supporting its approval, unique clinical pharmacology profile, current ongoing studies for treatment and prevention, pharmacologic research gaps, and concluding thoughts.
LITERATURE SEARCH STRATEGY
A literature search was performed in PubMed using the following terms: “lenacapavir,” “LEN,” “capsid inhibitor,” and “GS‐6207.” All original research articles, review articles, and case reports were included in this review. Furthermore, proceedings (abstracts, posters, and presentations) from prominent conferences addressing LEN or its pharmacology prior to April 2024 were included. These conferences included the American Society for Clinical Pharmacology and Therapeutics; Conference on Retroviruses and Opportunistic Infections; European AIDS Clinical Society; HIV Prevention Trials Network Annual Meeting; International Workshop on Clinical Pharmacology of HIV, Hepatitis, and Other Antiviral Drugs; HIV Glasgow; and the International AIDS Conference. Lastly, we included clinical trial data that incorporated these keywords from the ClinicalTrials.gov registry.
OVERVIEW OF LENACAPAVIR
As a first‐in‐class capsid inhibitor, LEN uniquely targets capsid proteins, which assemble into a cone‐like shape around immature viral RNA and associated proteins needed for replication upon release from an infected cell. 11 , 12 The HIV‐1 capsid core consists of approximately 1,500 capsid protein monomers which surround the reverse transcriptase complex, protecting it from degradation in the host cell's cytoplasm. 11 Although the precise timing and mechanism of capsid protein uncoating is not fully understood, studies have shown that the core assists further down the replication cycle, assisting with host cell nuclear entry. 11 Di Nunzio et al. 13 demonstrated that the HIV capsid core interaction with nuclear pore components Nup152 and Nup98 was important for nuclear import and integration, respectively. Importantly, capsid protein action at both encapsulation and uncoating make capsid proteins viable therapeutic targets at both the early and late stages of the HIV life cycle. 14
For late‐stage virus entering the host cell, LEN binds to the N74 residue of the N‐terminal domain and the N183 and K70 residues of the C‐terminal domain interface between capsid monomers 1 and 2, disrupting the controlled disassembly and inhibiting interaction with proteins integral to nuclear uptake and integration, including Nup153 and CPSF6. 15 , 16 , 17 At an earlier stage, as immature virus is being released from the host cell, LEN binds to capsid monomers and accelerates the assembly of the capsid core, creating malformations in its structure and disturbing its integrity. 16 , 18 Additionally, in vitro studies demonstrated reduced levels of Gag protein and reduced levels of processed capsid, suggesting that LEN binds to capsid monomer precursors and reduces Gag polyprotein stability overall. 18 Ultimately, by disrupting precursor stability, creating malformed capsid cores, and blocking nuclear entry, LEN effectively and potently inhibits HIV replication. 16 , 18
The structure of LEN is shown in Figure 1 , and key physicochemical properties of LEN are shown in Table 1 . Importantly, LEN has a LogP value of 6.4, indicating high lipophilicity. 19 Because LEN is highly lipophilic, it is predicted that LEN will preferentially distribute into the extravascular space, including the adipose tissue, yielding a large volume of distribution. 20 Furthermore, this degree of lipophilicity may lend itself well to increased distribution into tissue spaces, such as the gastrointestinal tract and other mucosal tissues. 21 Despite exhibiting high lipophilicity, however, LEN is unlikely to penetrate the blood–brain barrier via passive diffusion or to solubilize in the endothelial cell membrane lipid bilayer due to its large molecular mass (967 Da). 22
Figure 1.
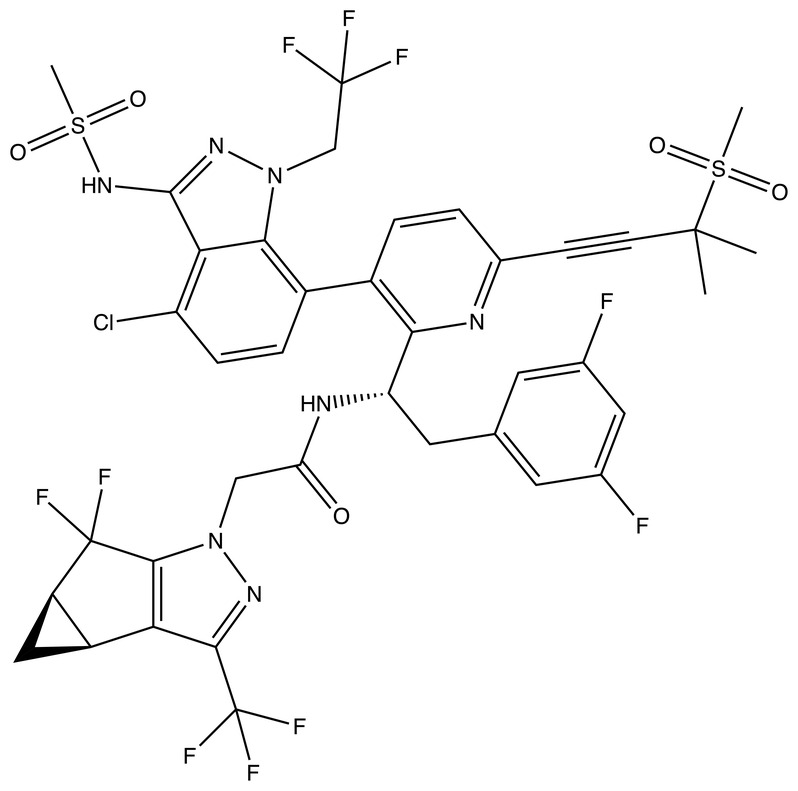
Chemical structure of lenacapavir (retrieved from PubChem).
Table 1.
Physicochemical properties of lenacapavir
| Molecular weight | 968.3 g/mol | PubChem 19 |
| XLogP3‐AA | 6.4 | PubChem 19 |
| pKa | 1.91, 6.69 | DrugBank 89 |
| Polar surface area | 157.94 Å2 | DrugBank 89 |
| HBD | 2 | PubChem 19 |
| HBA | 19 | PubChem 19 |
| Plasma protein binding | 99.8% | Package insert 10 |
| Blood‐to‐plasma ratio | 0.5–0.7 | Package insert 10 |
HBA, hydrogen bond acceptors; HBD, hydrogen bond donors; pKa, acid dissociation constant; XLogP3‐AA, log P calculated by the atom‐additive method.
PRECLINICAL STUDIES
Pharmacodynamics
Due to its unique mechanism of action of inhibiting the capsid protein, LEN possesses utility at the early and late stages of the HIV life cycle. At these stages, the potency of LEN differs for specific immune cell lineages, HIV‐1 subtypes, and between HIV‐1 and HIV‐2. Understanding these differential potencies is essential for identifying the potential benefits of LEN among different patient populations. These potencies are reviewed below.
HIV‐1
The in vitro activity of LEN has been assessed in a variety of HIV‐1‐infected immune cells. Link et al. 18 initially described the potency of LEN (then referred to as GS‐6207) by the concentrations at which half‐maximal response (EC50) is achieved. The calculated EC50 values were 105 pM in MT‐4 cells, 32 pM in primary human CD4+ cells, 56 pM in macrophages, and 20–160 pM in human peripheral blood mononuclear cells (PBMCs). 18
Additional studies by Link et al. 18 assessed the functionality of inhibiting the capsid protein at the early (MT‐2 cells) and late (HEK293T cells) stages of the viral replication cycle. In these cells, the authors demonstrated similar potency (EC50 = 23 pM in MT‐2 cells; EC50 = 439 pM in HEK393T cells). 18 Furthermore, the potency of LEN in the full‐cycle assay (EC50 = 25 pM) is more comparable to that observed in the MT‐2 cells, indicating the greater potency at the early stage of replication. 18 Lastly, the antiviral activity of LEN was observed in all major HIV‐1 subtypes, including the A, A1, AE, AG, B, BF, C, D, E, F, G, and H subtypes. 23
LEN displayed minimal cytotoxicity in human cell lines and primary cells, as indicated by the concentrations that produce half‐maximal cytotoxicity (CC50) greater than 50 μM, most notably in PBMCs. 18 When normalizing these concentrations to the respective EC50 values (CC50/EC50), the therapeutic index was large (>106‐fold), suggesting a favorable immunotoxicity profile. 18
HIV‐2
Link et al. 18 demonstrated that LEN has activity in vitro against two HIV‐2 isolates (EC50 = 885 pM). The University of Washington‐Senegal HIV‐2 Study Group recently demonstrated that following the single‐cycle assay with MAGIC‐5A indicator cells, mean half‐maximal inhibitory concentration (IC50) values were 200 pM for HIV‐1 and 2.2 nM for HIV‐2; these values represented 11‐fold less potency for HIV‐2. 24 In multicycle (e.g., 6 days) infections using CEM‐NKR‐CCR5‐Luc cells, similar relationships were observed with HIV‐1 vs. HIV‐2. 24 The EC50 values were 170 pM for HIV‐1 and 2.4 nM for HIV‐2, representing 14‐fold less potency for HIV‐2 compared to HIV‐1. 24
In vitro resistance profile
Due to its unique mechanism of action, LEN has a favorable resistance profile for PWH who have extensive resistance‐associated mutations (RAMs). LEN demonstrated potent antiviral activity in 40 viral isolates with RAMs associated with one of the four main ARV classes: nucleoside reverse transcriptase inhibitors (NRTIs), non‐nucleoside reverse transcriptase inhibitors (NNRTIs), protease inhibitors (PIs), and integrase strand transfer inhibitors (INSTIs); the fold change in the EC50 values ranges from 0.25 to 1.1. 25 This spread demonstrates little to no change in phenotypic susceptibility and suggests an absence of cross‐resistance from class‐specific RAMs to LEN. 25
Link et al. 18 noted a sequential pattern in the sequence analysis among HIV‐1‐infected MT‐2 cells: N74D substitution in capsid followed by a capsid (Q67H/N74D) variant. Despite having these RAMs, these isolates remained fully sensitive to agents from other ARV classes. 18 In human HIV‐1 PBMCs, Link et al. 18 similarly found that Q67H and N74D were the major RAMs with additional variants: L56I, M66I, K70N, Q67H/N74S, and Q67H/T107N. The presence of these RAMs decreased LEN susceptibility in the PBMCs by 6–3,200‐fold compared to wild‐type virus. 18
Taken together, these results highlighted the importance of LEN‐associated RAMs, particularly Q67H and N74D. The Q67H mutation creates a conformational change resulting in steric hindrance in the binding pocket with respect to LEN, whereas the N74D mutation results in an electrostatic repulsion between capsid and LEN. 26 In Q67H/N74D mutations, a cumulative effect is exhibited. 26
Margot et al. 27 observed high‐level resistance to LEN associated with specific mutations: M66I, M66I + Q67H double mutant, and Q67H/N74D. Interestingly, these same mutations yielded lower levels of replication capacity (1.5% for M166I and assay failure for double mutants). 27 The same group found that the highest level of LEN resistance was observed with Q67H/N74D double mutants (306‐fold above wild‐type) and Q67H/T107H double mutants (18.2‐fold above). 27
When examining the susceptibility of LEN in HIV isolates containing Gag mutations, including at the cleavage site, Margot et al. 27 demonstrated comparable antiviral activity compared to wild‐type controls (EC50 fold changes ranged 0.7–1.9) from a variety of patient isolates, suggesting efficacy regardless of treatment history.
Similar to the studies observed for HIV‐1, RAMs in the integrase, protease, and reverse transcriptase enzymes did not significantly affect the susceptibility of LEN in HIV‐2 isolates. 24 Future studies are warranted for the susceptibility of LEN in HIV‐2 isolates with RAMs for the capsid.
CLINICAL STUDIES
En route to the expedited approval of LEN by the Food and Drug Administration (FDA) in December 2022 for treatment‐experienced PWH, several phase 1 single‐dose, pharmacokinetic, dose‐escalation studies were performed to establish preliminary safety and efficacy. These initial studies were critical given the long terminal half‐life exhibited by LEN and the specific treatment population being studied. In this section, we review the phase I studies, phase II/III study, and the ongoing phase II study for treatment‐naïve PWH.
Phase I studies
In a phase Ia study in healthy volunteers receiving escalating SC LEN doses, participants reported no serious adverse events when given single doses ranging from 30 to 450 mg. 28 All participants in the study had measurable LEN concentrations up to 24 weeks post‐injection, and cohorts receiving 100 mg or more had concentrations higher than the protein‐adjusted 95% effective concentration (paEC95) 12 weeks post‐injection. 28 The 450 mg dose group showed a mean area under the curve extrapolated to infinity (AUCinf), maximal concentration (C max), and half‐life (t 1/2) of 111,000 h*ng/mL, 58.4 ng/mL, and 39.9 days, respectively. 28
In a phase Ib dose‐ranging study in 32 PWH who had not received ART in the last 12 months, participants received a single SC LEN dose of 20, 50, 150, 450, or 750 mg. 29 After day 10, all five LEN groups had significantly greater reductions of HIV‐1 RNA compared to placebo. 29 From baseline, HIV‐1 RNA declined a mean of 1.4 log10 copies/mL for the 20 mg LEN group and 2.3 log10 copies/mL in the 750 mg LEN group. 29
Phase II and III studies
CALIBRATE
The CALIBRATE study is an ongoing phase II study evaluating the efficacy of LEN‐containing regimens in treatment‐naïve PWH who were randomized to one of four treatment groups (Table 2 ). 30
Table 2.
Summary of the phase II/III clinical trials for lenacapavir
| Study | Phase | Population | Treatment arms | Outcomes | Results |
|---|---|---|---|---|---|
| CALIBRATE 30 , 31 | II | Treatment‐naïve PWH with HIV‐1 RNA levels ≥200 copies/mL and CD4 cell count ≥200 cells/μL (n=182) |
Group 1: PO LEN 600 mg on days 1 and 2, PO LEN 300 mg on day 8, SC LEN 927 mg on day 15 + PO FTC 200 mg and PO TAF 25 mg daily until week 28, then SC LEN 927 mg Q6 months + PO TAF 25 mg daily (n = 52) Group 2: PO LEN 600 mg on days 1 and 2, PO LEN 300 mg on day 8, SC LEN 927 mg on day 15 + PO FTC 200 mg and PO TAF 25 mg daily until week 28, then SC LEN 927 mg Q6 months + PO BIC 75 mg daily (n = 53) Group 3: PO LEN 600 mg on days 1 and 2, then PO LEN 50 mg daily + PO FTC 200 mg and PO TAF 25 mg daily (n = 52) Group 4: PO BIC 50 mg + PO FTC 200 mg + PO TAF 25 mg daily (n=25) |
Percent of participants with VL <50 copies/mL at week 28 |
Group 1: 94% Group 2: 92% Group 3: 94% Group 4: 100% |
| Percent of participants with VL <50 copies/mL at week 54 |
Group 1: 90% Group 2: 85% Group 3: 85% Group 4: 92% |
||||
| Percent of participants with VL <50 copies/mL at week 80 |
Group 1: 87% Group 2: 75% Group 3: 87% Group 4: 92% |
||||
| Mean change in CD4 cell count from baseline to week 80 |
Group 1: +272 cells/μL Group 2: +251 cells/μL Group 3: +245 cells/μL Group 4: +260 cells/μL |
||||
| CAPELLA 33 , 34 , 35 , 36 | III |
Cohort 1: PWH with HIV RNA ≥400 copies/mL Cohort 2: PWH with HIV RNA <400 copies/mL, a decrease of at least 0.5 log10 copies/mL between screening and cohort selection visits, or cohort 1 eligible patients who joined the study after cohort 1 selection |
Cohort 1: Group 1: PO LEN 600 mg + previous ART regimen on days 1 and 2, PO LEN 300 mg + previous ART regimen on day 8, SC LEN 927 mg Q6 months + OBT starting on day 15 (n = 24) Group 2: Previous ART regimen + placebo on days 1–14, PO LEN 600 mg + OBT on days 15 and 16, PO LEN 300 mg + OBT on day 22, SC LEN 927 mg Q6 months + OBT thereafter (n = 12) Cohort 2: Group 3: PO LEN 600 mg + OBT on Days 1 and 2, PO LEN 300 mg + OBT on Day 8, SC LEN 927 mg Q6 months + OBT starting on Day 15 (n=36) |
Percent of participants with ≥0.5 log10 reduction in HIV‐1 RNA copies/mL at day 15 |
Group 1: 88% Group 2: 17% |
| Percent of participant with VL <50 copies/mL at week 26 |
Cohort 1: 81% Cohort 2: 83% |
||||
| Percent of participants with VL <50 copies/mL at week 52 |
Cohort 1: 83% Cohort 2: 72% |
||||
| Percent of participants with VL <50 copies/mL at week 104 | 81.5% (of those that continued into follow‐up) | ||||
| Mean change in CD4 cell count from baseline to week 104 | +122 cells/μL |
ART, antiretroviral therapy; BIC, bictegravir; FTC, emtricitabine; LEN, lenacapavir; OBT, optimized background therapy; PWH, people with HIV; Q6 months, every 6 months; TAF, tenofovir alafenamide; VL, viral load.
At weeks 28 and 54, LEN‐containing regimen groups achieved virologic suppression rates of 94% and 87%, respectively. 30 Of note, 13 of the 14 LEN participants who did not achieve virologic suppression at week 54 had discontinued the LEN‐containing regimens by that time point. 30 By week 54, participants in the LEN groups exhibited slightly higher increases in CD4+ cell counts than those observed in the standard‐of‐care group. 30 Also at week 54, six participants, five of whom were in the LEN‐containing groups, experienced virologic failure, defined as suboptimal virologic response, virologic rebound, or 50 copies/mL of HIV‐1 RNA or more at study discontinuation. 30 Participants were subsequently tested for resistance mutations. 30 Of those six participants experiencing virologic failure, four participants successfully achieved virologic suppression without any change in their regimen and two participants had developed RAMs to LEN; one participant also developed reverse transcriptase‐associated mutations. 30
At week 80, viral suppression rates remained high in all groups, CD4+ cell counts increased from week 54 in participants receiving LEN, and one additional participant receiving LEN in group 1 had developed treatment‐emergent resistance. 31
The results of the CALIBRATE study demonstrate that either SC LEN in combination with bictegravir (BIC) or tenofovir alafenamide (TAF) and oral LEN in combination with emtricitabine/TAF (FTC/TAF) is non‐inferior to standard‐of‐care BIC/FTC/TAF for treatment‐naïve PWH. 31 Furthermore, LEN was well tolerated with no reports of serious adverse events observed among participants. 31 These promising results should be followed‐up with further evaluation of LEN – either SC or oral – with a long‐acting (LA) partner as part of the milieu for treatment‐naïve PWH. 32
CAPELLA
The CAPELLA study was the formative phase II/III study that led to expedited FDA approval of LEN for treatment‐experienced PWH with MDR HIV, which enrolled participants who had received a failing ART regimen for at least 8 weeks. 33 Participants were also required to have documented resistance to at least two ARVs from at least three of the four main classes, NRTIs, NNRTIs, PIs, and INSTIs (Table 2 ). 33
On day 15 – the end of the oral loading period – 21/24 (88%) participants in the LEN group had an HIV‐1 RNA reduction of at least 0.5 log10 copies/mL, compared to 2/12 participants (17%) in the placebo group. 33 Thereafter, all participants received LEN 927 mg SC in addition to optimized background therapy. 33 At week 26, 81% of participants in cohort 1 and 83% of participants in cohort 2 had HIV‐1 RNA viral loads of <50 copies/mL with improvements in CD4+ cell counts. 33
At week 52, 56/72 participants achieved HIV‐1 RNA viral loads <50 copies/mL. 34 Of the 16 participants that did not achieve viral loads <50 copies/mL, five participants never achieved viral suppression. 34 By week 52, nine participants had capsid RAMs, eight of which emerged during the first injection's dosing interval. 34 These eight participants who developed RAMs prior to week 26 had an average reduction of 2.4 log10 copies/mL in the HIV‐1 RNA viral load measures. 35 Preliminary week 104 data demonstrated sustained high viral suppression rates (81%) and CD4+ cell count recoveries (70.9% remained ≥200 cells/μL).
In vivo resistance studies
Frequency and/or phenotypic susceptibility of RAMs to LEN have been evaluated in both treatment‐naïve and treatment‐experienced PWH, and PWH receiving a LEN‐containing regimen. Considering the indication of LEN for treatment‐experienced PWH, identifying the prevalence of LEN‐associated RAMs prior to initiation – as well as the development of RAMs during treatment – will be essential for vulnerable populations. Furthermore, because combining LEN with other ARVs is an active area of research, understanding the potential for the emergence of LEN‐associated RAMs is important to ensure LEN‐containing combination regimens will be effective.
Sequence analyses in PWH not receiving LEN
In a study examining 1,500 plasma samples from treatment‐naïve and treatment‐experienced PWH with detectable viremia, no samples exhibited any of the seven mutations previously found to confer LEN resistance. 37 These findings were consistent regardless of HIV‐1 subtype or treatment history, including from PWH who had failed at least one PI‐containing regimen. 37
In a similar study of 2031 treatment‐naïve PWH, Nka et al. 38 noted that three participants (0.14%) each had distinct LEN RAMs (M66I, Q67H, and T107N). Although the prevalence of documented RAMs was low, there were higher rates of polymorphic mutations found at the resistance‐associated positions: M66C (4.18%), Q67K (3.84%), K70R (0.34%), N74R (2.81%), and T107L (4.03%). 38 The polymorphisms at these sites should be monitored for potential breakthrough resistance mutations as LEN becomes increasingly utilized in clinical practice. 38
LEN‐naïve PWH receiving functional LEN monotherapy
In a study investigating the emergence of RAMs in 29 PWH receiving SC LEN monotherapy (up to 750 mg) for 10 days, one participant receiving 20 mg and one participant receiving 50 mg developed the Q67H mutation. 27 In the participant receiving the 20 mg dose, this mutation resulted in a 1.6‐fold reduction in LEN susceptibility; no phenotypic data were generated for the participant receiving 50 mg due to documented assay failure. 27
Wirden et al. published a case report detailing the rapid selection of the N74D capsid mutation at week 3 following initiation of LEN and BIC/FTC/TAF. The patient had a history of failing regimens due to treatment interruptions and suboptimal adherence. 39 Among the three plasma samples obtained from weeks 1 to 4 during this combination regimen, only one sample contained detectable trough concentrations of their optimized background regimen (OBR) components; the respective LEN concentrations were fourfold higher than the protein‐adjusted concentration at which 95% of effect is observed (IQ4; 15.5 ng/mL). 39 In this case report, poor adherence and the resultant functional LEN monotherapy underscore the need for proper adherence to oral ARVs as part of a LEN‐containing regimen. 39
Bertine et al. 40 evaluated the emergence of LEN RAMs in eight heavily treatment‐experienced and likely MDR people with HIV‐2. LEN was found to have only short‐lived efficacy in this population, with only one participant maintaining viral suppression at 6 months and all participants presenting near‐baseline viral loads within a year of LENinitiation. 40 Five of eight participants developed capsid mutations, including N73D, A76V, Q66H, R69K, and Q66H + R69L. 40 The N73D mutation of HIV‐2, which corresponds to the N74D mutation in HIV‐1, was selected in four of the five participants who developed emergent resistance, causing a 30‐fold reduction in LEN susceptibility to HIV‐2. 40
Emergent resistance profiles from CALIBRATE and CAPELLA
In the CALIBRATE study, by week 80, 3/157 (2%) of participants had treatment‐emergent LEN resistance mutations. 31 One participant developed both Q67H and K70R capsid mutations at week 10 following the development of the M184M/I reverse transcriptase mutation while receiving SC LEN and FTC/TAF. 31 The second participant was on a fully oral LEN + FTC/TAF and developed the same capsid mutations (Q75H and K70R) at week 54 after demonstrating suboptimal adherence through pill count and drug concentrations. 31 Finally, the third participant also developed Q75H and K70R mutations at week 80 while receiving SC LEN + TAF. 31
By week 104 of the landmark CAPELLA study, 19.4% of the participants developed LEN‐associated RAMs. 41 Among these 14 participants, RAMs consisted of M66I (N = 6), Q67H (N = 5), K70N/H (N = 2), and N74D (N = 1). 41 Half of these participants resuppressed viremia, five of whom did so without a change in their optimized background regimen (OBR). 41
Conclusion
Likely due to its novel mechanism of action, baseline rates of RAMs in LEN‐naïve PWH are low regardless of treatment history. Given this, the documented emergence of RAMs during functional LEN monotherapy and among the treatment‐experienced CAPELLA cohort is notable. These RAMs illustrate the importance of both establishing a patient's OBR prior to adding LEN and stress the need for proper adherence to said OBR while on LEN. It is also important to note that among the 14 participants with RAMs in the CAPELLA study, five were able to resuppress viremia without a change in their OBR, 41 suggesting a LEN‐containing regimen may remain suppressive in some PWH with LEN‐associated RAMs. The emergence of viral resistance may differ depending on the specific OBR co‐administered with LEN, and further studies are warranted.
CLINICAL PHARMACOLOGY OVERVIEW
Absorption
Based on a population PK model, the estimated absorption rate constant after oral administration is 0.0352 1/h; the SC absorption constants are 0.00205 1/h for the indirect absorption and 0.00037 1/h for the direct absorption. 42 Bioavailability after oral LEN is 6–10% of the total dose and SC bioavailability is ~100% of the administered dose. 42 The time to maximal concentration (T max) estimates after oral and SC LEN dosing are 4 hours and 77–84 hours, respectively. 42 For the phase II/III dosing regimen, the C max after the two 600 mg and one 300 mg oral doses (days 1 to 15) is 124.4 ng/mL; the C max after 927 mg SC administration is 87.3 ng/mL. 10 For both the phase II/III and simplified regimens (see “Phase 2/3 vs. simplified initiation regimens” in Pharmacokinetic Considerations), mean LEN concentrations were higher than the IQ4 within 2 hours post‐dose on day 2 following initiation. 42
Distribution
LEN is highly bound to plasma proteins (>98.5%). 10 LEN has a mean apparent volume of distribution of 11,824 L following a 300 mg oral dose, 16,411 L following a 600 mg oral dose, and 902 L following an SC dose. 42 These large volumes of distribution estimates indicate high tissue penetration and binding affinity for LEN. LEN remains primarily unchanged within the plasma, representing up to 69% of the dose in circulating plasma after intravenous (IV) dosing. 43
Metabolism
In a mass balance study, no single metabolite contributed >10% of the total radioactivity exposure up to 1,176 hours post‐dose, suggesting that LEN is not extensively eliminated via metabolism. 43 LEN is primarily a substrate of CYP3A and UGT1A1. 10 The three most abundant metabolites in feces are the glucuronide conjugate, pentose conjugate, and hexose conjugate, all of which exist as atropisomer pairs formed from phase II conjugation pathways by UGT1A1. 43 LEN is also a moderate inhibitor of CYP3A. 10
Elimination
Following oral administration, LEN has a t 1/2 of 10–12 days and an apparent clearance of 55 L/h. 10 After SC administration, LEN exhibits a much longer t 1/2 (8–12 weeks) and lower apparent clearance (4.2 L/h). 10 The major route of excretion is via feces, representing 76% of all excreted drugs, 33% of which remains unchanged. 10 Less than 1% of the drug is renally eliminated. 10
Transport
LEN is a substrate and inhibitor of P‐glycoprotein (P‐gp) and an inhibitor of breast cancer resistance protein (BCRP). 10
PHARMACOKINETIC CONSIDERATIONS
People with HIV vs. HIV‐seronegative people
A population PK analysis developed from seven studies incorporating a total of 7,053 PK observations from 384 healthy participants after oral, IV, and SC administration of LEN 42 found a 30.4% higher estimated clearance and 133% higher peripheral volume of distribution than in PWH. 42 Following oral LEN dosing, AUC to the end of the dosing interval (AUCtau), C max, and the concentration at the end of the dosing interval (C trough) were 43%, 32%, and 39% lower in healthy participants than PWH. 42 Similarly, after SC dosing, these same parameter values were 38%, 50%, and 38% lower in people without HIV compared to PWH. 42 Ongoing pre‐exposure prophylaxis (PrEP) studies in participants at risk for HIV will help elucidate whether LEN exposure remains above the IQ4 in this population throughout the 26‐week dosing interval. 44 , 45
Pharmacokinetic model overview
Due to the unique PK of oral and LA SC LEN, PK models representing LEN administration and disposition are accordingly complex. To illustrate this complexity, Subramanian et al. 46 reported a compartmental PK model incorporating the kinetics of SC dosing across several preclinical and clinical dosing schemes. This model is recapitulated in Figure 2 .
Figure 2.
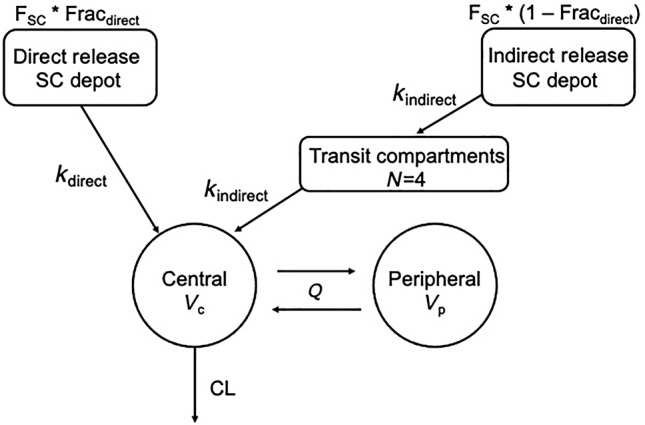
Compartmental model of SC lenacapavir injection input and exit rates. Abbreviations (in alphabetical order): CL, clearance out of body (L/h/kg); Fracdirect, fraction of dose released via direct process; Fracindirect, fraction of dose released via indirect process; F SC, bioavailability of lenacapavir following SC dose; k direct, absorption rate constant for direct release fraction (1/h); k indirect, absorption rate constant for indirect release fraction (1/h); Q, intercompartmental clearance (L/h/kg); V c, volume of central compartment (L/kg); V p, volume of peripheral compartment (L/kg). Adapted from Subramanian et al. (https://pubs.acs.org/doi/10.1021/acs.molpharmaceut.3c00626) and the FDA‐Integrated Review for NDA 215973/215974. 42
Briefly, LEN disposition is described with a two‐compartment model with linear elimination from the central compartment. Oral LEN absorption was characterized by a first‐order absorption process. For SC absorption, the model incorporates two separate and parallel first‐order absorption input rates following SC dosing: (1) direct, initial release from the soluble fraction of the formulation, and (2) indirect release of the precipitated fraction with a lag time. 46 The majority of the LEN dose is released via the indirect release from the SC depot. 46 The FDA‐integrated review of LEN noted that the delayed absorption process is accompanied with four transit compartments. 42 Model parameter estimates are outlined in Table 3 .
Table 3.
Lenacapavir population pharmacokinetic model parameter estimates 42
| Parameter | Definition | Units | Estimate | %RSE |
|---|---|---|---|---|
| k a | PO absorption rate constant | 1/h | 0.0352 | 9.7 |
| k indirect | SC transit absorption rate constant | 1/h | 0.00205 | 3.2 |
| k direct | SC direct absorption rate constant | 1/h | 0.00037 | 7.8 |
| F PO | PO bioavailability relative to IV | ‐ | 4.84% | 9.9 |
| F SC | SC bioavailability relative to IV | ‐ | 88.1% | 9.4 |
| Fracdirect | Direct fraction of SC dose | ‐ | 0.423 | 5.1 |
| V c | Volume of central compartment | L | 68.3 | 6.1 |
| Q | Intercompartmental clearance | L/h | 40.9 | 5.5 |
| V p | Volume of peripheral compartment | L | 726 | 9.8 |
| CL | Total body clearance | L/h | 3.29 | 8.7 |
CI, confidence interval; CL, total body clearance; F PO, oral bioavailability relative to IV; Fracdirect, direct fraction of SC dose; F SC, SC bioavailability relative to IV; h, hours; IV, intravenous; k a , PO absorption rate constant; k direct, SC direct absorption rate constant; k indirect, SC transit absorption rate constant; L, liters; PO, oral; Q, intercompartmental clearance; RSE, relative standard error; SC, subcutaneous; V c , volume of central compartment; V p , volume of peripheral compartment.
Phase II/III vs. simplified initiation regimens
The initiation regimen described in the CAPELLA and CALIBRATE trials (phase II/III regimen) consisted of three oral doses of LEN followed by SC injection on day 15. 30 , 33 Since those pivotal studies, a simplified dosing regimen was evaluated in a cohort of 14 HIV‐seronegative participants. 47 Specifically, the simplified regimen requires a 600 mg oral loading dose and 927 mg SC injection on day 1, followed by another 600 mg oral dose on day 2. 47 The simplified regimen yielded comparable LEN exposure over the 6‐month dosing interval. 47 Given the comparable PK profiles and the maintenance of LEN concentrations from the IQ4 threshold throughout the dosing interval, both initiation regimens are FDA‐approved. 42 The simplified regimen offers a streamlined oral loading period and single clinic visit; that said, the phase II/III regimen has a place for PWH and clinicians who aim to ensure tolerability of LEN before committing to the 6‐month dosing interval accompanying SC LEN.
In follow‐up population PK analyses, Shaik et al. 48 compared the two initiation regimens using observed data from the study discussed above. Using the previously described compartmental model (Figure 2 ), the authors examined the differences in simulated AUCtau, C max, and C trough parameters during days 1–15, day 15 to the end of 6 months, and at steady state; external validation was performed for the initial 6 months. 48 Following the phase II/III initiation regimen during the first 6‐month dosing interval, AUCtau, C max, and C trough values were 250,000 h*ng/mL, 87 ng/mL, and 32.7 ng/mL, respectively. 48 These same values following the simplified initiation regimen were 238,000 h*ng/mL, 87.1 ng/mL, and 32.7 ng/mL, respectively. 48 These values were not statistically significantly different during the initial dosing interval or at steady state (AUCtau: 300,000 ng*h/mL; C max: 97.2 ng/mL; C trough: 36.2 ng/mL for both regimens). 48 Importantly, the lower bounds of the 90% confidence intervals of mean simulated trough concentrations following both initiation regimens were above the IQ4 during the first dosing interval and at steady state. 48
Dosing window forgiveness
Providing practical administration recommendations in real‐world settings, Shaik et al. 49 performed additional population PK analyses using data from seven clinical trials. Plasma concentrations were simulated for five different scenarios for the second SC injection: 2‐week advancement (administration at Week 24), prescribed dosing interval (Week 26), 2‐week delay (Week 28), 4‐week delay (Week 30), and 6‐week delay (Week 32). 49 These concentrations were compared against the IQ4 threshold. 49
Following these simulations, it was found that a 4‐week dosing window (between 24 and 28 weeks following initiation with either regimen) was adequate to maintain trough concentrations above the IQ4. 49 Delays beyond 2 weeks (e.g., weeks 30 and 32) may yield trough concentrations below the IQ4, regardless of initiation regimen; therefore, restarting the LEN initiation regimens is recommended if delays beyond 2 weeks occur. 49
In their review of LEN, the FDA emphasized that the optimal dosing interval is 26 weeks to ensure trough concentrations consistently remain above the IQ4. 42 This is particularly important in overweight and obese PWH, in whom geometric mean trough concentrations were not consistently above the IQ4 28 weeks after the first SC injection. 42 Therefore, the dosing window forgiveness may be shorter in different body mass index (BMI) categories. Further evaluations in the dosing window forgiveness by BMI categories and age will be paramount, especially given the SC route of administration of LEN.
Drug–drug interactions
As aforementioned, LEN is primarily a substrate of CYP3A and UGT1A1 metabolizing enzymes. 10 Therefore, there are a number of clinically significant interactions with medications from numerous therapeutic classes – particularly ARVs that may be germane to patient‐specific OBR – that affect LEN exposure. 10 To determine the magnitude of these pharmacokinetic interactions, a number of in vivo drug–drug interaction studies have been conducted studying LEN with other ARVs.
LEN is not recommended in combination with efavirenz, a moderate CYP3A inducer, due to an observed 56% decrease in LEN exposure after co‐administration in a drug–drug interaction study. 42 Other ARVs that are strong CYP3A inducers (e.g., nevirapine, tipranavir/ritonavir) were predicted to significantly lower LEN exposure, reduce the therapeutic effect of LEN, and increase risk of resistance, and therefore were not included in drug–drug interaction studies. 42
Additionally, co‐administration of LEN with boosted atazanavir is not recommended; co‐administration of LEN with atazanavir/cobicistat (strong inhibitors of CYP3A, P‐gp, and UGT1A1) was shown to increase LEN exposure by four‐ to six‐fold. 42 Dose exploration studies involving co‐administration with ritonavir‐boosted protease inhibitors similarly demonstrated increases in LEN exposure (up to twofold higher compared to when LEN is administered alone). 42
LEN has also been studied in combination with broadly neutralizing antibodies (bNAbs) that target the CD4 + ‐binding site and V3 loop of gp120. In a phase Ib study looking at LEN co‐administered with the IV bNAbs teropavimab (TAB; 10 mg/kg) and zinlirvimab (ZAB; 10 or 30 mg/kg), mean LEN concentrations at week 26 were comparable to previously observed trough concentrations when administered alone. 50 The bNAb concentrations at week 26 were more than 20‐fold higher than the in vitro IC50 for both TAB and ZAB, and dose proportionality was observed between the 10 and 30 mg/kg ZAB dosing groups. 50 These data indicate no appreciable PK interactions between LEN and antibody‐based therapy.
Given the importance of potential pharmacodynamic synergy with LEN, in vitro combination studies were performed in HIV‐1IIIB‐infected MT‐2 cells. 42 While LEN was shown to be synergistic with the ARVs studied, including dolutegravir (DTG) and TAF, 42 additional in vivo studies are warranted to evaluate additional synergistic or antagonistic drug–drug interactions between other ARVs and LEN in clinical settings.
Hepatic and renal impairment
In a recent intensive PK study examining the effect of hepatic or renal impairment, participants with moderate hepatic or severe renal impairment were administered a single oral dose of LEN (300 mg) and PK parameters were compared with healthy participants. 51 The authors noted that those with hepatic impairment had AUCinf values 1.5‐fold higher (12,000 vs. 8,180 ng*h/mL in healthy participants). 51 C max values were 2.6‐fold higher (61.1 vs. 23.4 ng/mL in healthy controls). 51 T max between the two groups (6 vs. 4 hours in healthy controls) and median terminal t 1/2 estimates (12.6 vs. 13.1 days in healthy participants) were not statistically significantly different. 51
In the renal impairment study, those with renal impairment had AUCinf values 1.8‐fold higher (12,100 vs. 6,590 ng*h/mL in healthy participants) and C max values 2.6‐fold higher (51.5 vs. 19.7 ng/mL in healthy participants). 51 The T max values (8 vs. 6 hours in healthy controls) and median t 1/2 estimates (9.8 vs. 13.3 days) were not statistically significantly different. 51
Importantly, no major adverse events leading to discontinuation were observed; LEN was well tolerated in both the hepatic and renal impairment groups. 51 The authors concluded that dose adjustments based on hepatic and renal impairment are not advised as the increased exposure parameters previously described did not increase safety or tolerability risks. 51 While promising, it is important to note that this study was performed following a single LEN dose rather than the FDA‐approved initiation regimens, and larger safety and PK studies are warranted.
ONGOING STUDIES
Combination therapy
Overview
Combination therapy regimens that include LEN (either oral or SC formulations) have been wide‐ranging, including accompaniment with bNAbs and small molecules. Furthermore, these studies have examined daily and weekly regimens with other ARVs with different oral LEN doses. With the goal of expanding initial treatment options of LEN plus an ARV from a different class, these studies will provide insight regarding the efficacy and synergistic effects of LEN. Below, we describe ongoing combination therapy studies with bNAbs, INSTIs, and the still‐in‐development islatravir, a nucleoside reverse transcriptase translocation inhibitor (NRTTI).
LEN plus broadly neutralizing antibodies
As part of the same phase Ib, proof‐of‐concept study in PWH effectively treated with ART introduced in the previous section, Eron et al. 50 evaluated the efficacy and safety of twice‐yearly LEN in combination with two bNAbs, TAB and ZAB, derived from naturally occurring HIV envelope glycoprotein targeting antibodies that develop in some PWH. Participants were required to be on suppressive ART for at least 18 months and have proviral phenotypic susceptibility to both bNAbs. 50
All 21 participants received the simplified initiation LEN regimen. 50 Of the 21 participants who completed the study, all but one participant maintained undetectable viral loads through week 26. 50 The most frequently observed adverse events were injection site reactions (e.g., cellulitis and erythema), and there were no discontinuations due to adverse events. 50 This study has since progressed to phase II, in which enrollment has expanded to 83 participants. 50
A follow‐up study by this group evaluated the safety and efficacy of LEN plus bNAbs in participants who demonstrated high‐level viral sensitivity to either TAB or ZAB, but not both. 52 Similar to the phase Ib study, participants were required to have HIV‐1 RNA <50 copies/mL for at least 18 months and CD4+ count nadirs of ≥350 cells/μL. 52 Eleven participants were split into two groups: group 1 received LEN (simplified initiation regimen) + TAB 30 mg/kg IV + ZAB 10 mg/kg IV and group 2 received LEN (simplified initiation regimen) and TAB 30 mg/kg + ZAB 30 mg/kg IV. 52
Viral rebound was observed in two participants, both in group 1, yet they maintained phenotypic sensitivity to one of the bNAbs. 52 The first participant experienced viral rebound at week 20 (112 copies/mL) but suppressed viremia at week 24 (< 50 copies/mL) with no change in regimen. Viral rebound recurred at week 26 (55 copies/mL) and the participant resumed their oral ART regimen per protocol. 50 The second participant experienced viral rebound to 72 copies/mL at week 26 and resupressed after returning to their oral ART regimen. 52 All participants who received the higher dose of ZAB (30 mg/kg) maintained viral suppression through week 26. 52
Taken together, the combination of LEN and bNAbs was safe and efficacious as a complete regimen in PWH with at least 18 months of documented viral suppression and who are phenotypically susceptible to at least one of the two bNAbs studied. 50 Future studies should include larger cohorts and broader inclusion criteria to understand the generalizability of a LEN plus bNAb regimen.
LEN plus integrase strand transfer inhibitors
Combining LEN with INSTIs is an active area of research due to the ubiquity of INSTI‐based regimens. In the CALIBRATE study group 2, 53 participants received BIC 75 mg by mouth as a background regimen to LEN SC injections (Table 2 ), and 85% achieved viral suppression at week 54. 30 These results indicate that LEN and BIC taken in combination are effective at maintaining virologic suppression as a two‐drug regimen in treatment‐naïve PWH.
The ongoing ARTISTRY‐1 trial in virally suppressed PWH evaluates the efficacy of BIC + LEN and a BIC/LEN fixed‐dose combination pill in phase II and III sub‐studies, respectively (Table 4 ). 53 At week 24 of the phase II sub‐study, 96% of participants in LEN‐containing groups had maintained viral suppression. 54 The phase III component of ARTISTRY‐1 will recruit participants switching from their stable ART regimen to a BIC/LEN 75/50 mg daily single tablet regimen for at least 48 weeks. 54 Similarly, the ARTISTRY‐2 trial evaluates the effectiveness of a BIC/LEN fixed‐dose combination pill but in participants with suppressed viremia on BIC/FTC/TAF (Table 4 ). 55
Table 4.
Ongoing lenacapavir clinical trials
| Study title | Phase | Population(s) | Recruitment location(s) | Inclusion criteria | Study cohorts | Outcome | Results (as of August 5, 2024) |
|---|---|---|---|---|---|---|---|
| A study of GS‐5423 and GS‐2872 in combination with capsid inhibitor Lenacapavir in Virologically suppressed adults with HIV‐1 infection 90 | II | Virologically suppressed 18–65‐year‐old PWH | 38 locations throughout the United States of America | Stable ART consisting of no more than 2 drug classes, no documented resistance to current ART regimen, successful viral suppression for at least a year, CD4 cell counts of at least 200 cells/μL, and phenotypic susceptibility to both teropavimab and zinlirvimab |
Group 1: SC LEN 927 mg every 26 weeks, oral LEN 600 mg on days 1 and 2, teropavimab dose A and zinlirvimab dose B administered IV on the same days as SC LEN Group 2: Baseline oral ART regimen |
Percent of participants with HIV‐1 RNA counts ≥50 copies/mL at week 26 | None |
| ARTISTRY‐1 53 | II/III | Virologically suppressed PWH 18 and older | 91 locations globally | ART regimen containing ≥2 pills/day, a regimen requiring dosing more frequent than once daily, a regimen containing parenteral agent(s) alongside oral agents, or a regimen containing a boosted PI or an NNRTI with at least one other agent from a class other than NRTI; no suspected resistance to BIC; eGFR of at least 15 mL/min |
Phase II Group 1: 2‐day oral loading dose regimen of LEN 600 mg and daily oral dosing of BIC 75 mg + LEN 25 mg starting on day 1 Group 2: 2‐day oral loading dose regimen of LEN 600 mg and daily oral dosing of BIC 75 mg + LEN 50 mg starting on day 1 Group 3: Remain on stable baseline regimen Phase III Group 1: 2‐day oral loading dose regimen of LEN 600 mg and daily oral dosing of BIC 75 mg/LEN 50 mg fixed‐dose combination pill Group 2: Continue stable baseline regimen |
Phase II: Percent of participants with HIV‐1 RNA ≥50 copies/mL at week 24 Phase III: Percent of participants with HIV‐1 RNA ≥50 copies/mL at week 48 |
Phase II: Group 1: 0% Group 2: 1.9% Group 3: 0% Phase III: None |
| ARTISTRY‐2 55 | III | Virally suppressed PWH who are 18 years or older and currently on BIC/FTC/TAF | 45 locations globally | Receiving BIC/FTC/TAF for at least 6 months, no documented resistance to BIC, no documented resistance to TAF, eGFR ≥30 mL/min |
Group 1: 2‐day oral loading dose regimen of LEN 600 mg and daily oral dosing of BIC 75 mg/LEN 50 mg fixed‐dose combination pill, as well as placebo‐to‐match BIC/FTC/TAF Group 2: Continue daily oral baseline regimen of BIC/FTC/TAF (50/200/25 mg) and receive placebo‐to‐match LEN oral loading doses on days 1 and 2 |
Percent of participants with HIV‐1 RNA ≥50 copies/mL at Week 48 | None |
| Study evaluating the safety and efficacy of islatravir in combination with Lenacapavir in Virologically suppressed people with HIV 60 | II | Virally suppressed PWH who are 18 years or older and currently on BIC/FTC/TAF | 46 locations throughout the United States of America | Received BIC/FTC/TAF for at least 24 weeks prior to screening, HIV‐1 RNA <50 copies/mL for at least 24 weeks and at screening |
Group 1: LEN 600 mg oral loading dose on days 1 and 2, ISL 2 mg oral on day 1, and LEN 300 mg + ISL 2 mg oral once a week thereafter for 48 weeks Group 2: BIC/FTC/TAF (50/200/25 mg) once daily for 48 weeks |
Percent of participants with HIV‐1 RNA ≥50 copies/mL at week 24 | At week 24, both groups had 94.2% of participants maintaining viral loads <50 copies/mL |
ART, antiretroviral therapy; BIC, bictegravir; eGFR, estimated glomerular filtration rate; FTC, emtricitabine; ISL, islatravir; LEN, lenacapavir; NNRTI, non‐nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; OBT, optimized background therapy; PI, protease inhibitor; PWH, people with HIV; SC, subcutaneous; TAF, tenofovir alafenamide; VL, viral load.
A recent interesting case series examined 34 PWH with self‐reported nonadherence who transitioned to off‐label LA CAB in combination with SC LEN (simplified initiation regimen) with or without LA RPV. 56 The virologic impetus for switching to this off‐label regimen included: documented or suspected NNRTI mutations (59% of participants), integrase mutations (15%), high HIV‐1 viral load within 3 months of starting LA ART (18%; 4 of 6 had undetectable viral loads by the time of LEN initiation), or sustained viremia while receiving CAB + RPV (15%). 56 Sixteen participants (47%) achieved viral suppression prior to adding LEN (on either LA CAB + LA RPV alone or an oral regimen). 56 By week 16, 32/34 (94%) participants had achieved or maintained viral suppression (median (range): 8 (4–16 weeks)). 56 Importantly, all 21 participants with documented or suspected NNRTI mutations achieved viral suppression while taking LA CAB + LA LEN with or without LA RPV. 56 The results of this case series are promising and provide preliminary data to support further evaluation of LA CAB + LA LEN as a viable combination in those who experience persistent adherence barriers and have NNRTI RAMs.
LEN plus islatravir
Islatravir (ISL) is a novel NRTTI that demonstrated potent activity against both wild‐type and NRTI‐resistant HIV strains. 57 With potent activity, a relatively long t 1/2 (49–61 hours in plasma for the parent ISL and 118–171 hours intracellularly for the active metabolite ISL triphosphate), a favorable resistance profile, and previous studies finding no significant antagonism or synergism between ISL and LEN, the combination is actively being studied. 57 , 58 , 59 , 60
An ongoing LEN and ISL dual therapy study includes 104 virally suppressed participants (Table 4 ). 60 One participant in the LEN + ISL group who did not achieve viral suppression by week 24 had detectable HIV‐1 RNA (251 copies/mL) at study initiation; this participant achieved viral suppression by week 30 while on the LEN + ISL regimen with no detectable treatment‐emergent resistance to LEN. 61 There were no statistically significant differences in CD4+ or absolute lymphocyte counts between the two treatment groups at week 24. 61
Following week 48, participants who elect to remain in the study will remain in the ISL + LEN group if they were randomized to this group or switch to this regimen from BIC/FTC/TAF. 61 Taken together, LEN + ISL shows promise to become the first once‐weekly oral regimen for HIV‐1 treatment, and additional data (e.g., longer‐term safety and efficacy follow‐up studies and PK of LEN and ISL) are expected. 61
LEN for pre‐exposure prophylaxis
Overview
PrEP as a daily oral regimen containing FTC with tenofovir disoproxil fumarate (TDF) or TAF is highly effective in preventing HIV acquisition. 62 High adherence is needed to achieve PrEP efficacy; this high level of adherence remains a major challenge. 63 Without high adherence to PrEP, subsequent infections and the development of RAMs can occur. 64 Addressing adherence challenges is an important component of PrEP research efforts. In response, HIV prevention options have expanded to include LA CAB, approved in 2021 by the FDA for PrEP for all genders. 65 , 66 LEN, given its biannual dosing scheme, would likewise theoretically address these challenges and remains a highly active area of research.
Clinical studies
The preclinical efficacy studies of LEN for PrEP supported future clinical studies. 67 , 68 Indeed, there are five ongoing clinical studies – termed the PURPOSE studies – that are examining the safety, efficacy, PK, and acceptability of LEN for PrEP. 44 , 45 , 69 , 70 , 71 The study designs, outcomes, and results (if applicable) are summarized in Table 5 .
Table 5.
Ongoing lenacapavir PrEP studies
| Study title | Phase | Population(s) | Recruitment location(s) | Inclusion criteria | Study cohorts | Outcome | Results (as of August 5, 2024) |
|---|---|---|---|---|---|---|---|
| PURPOSE 1 72 | III | CGF | South Africa, Uganda | PrEP‐naïve, HIV‐1 antigen + antibody negative 16 to 25 years old who are sexually active (has had >1 vaginal intercourse within the past 3 months with cisgender males) |
Group 1: SC LEN 927 mg every 26 weeks, oral LEN 600 mg on days 1 and 2, and oral PTM F/TAF once daily for 52 weeks Group 2: SC LEN 927 mg every 26 weeks, oral LEN 600 mg on days 1 and 2, and oral PTM F/TDF once daily for 52 weeks Group 3: SC placebo LEN every 26 weeks, oral PTM LEN on days 1 and 2, and oral F/TAF 200/25 mg once daily for 52 weeks Group 4: SC placebo LEN every 26 weeks, oral PTM LEN on days 1 and 2, and oral F/TDF 200/300 mg once daily for 52 weeks |
Primary: HIV incidence reported per 100‐person‐years of follow‐up |
Groups 1 and 2: 0 per 100 person‐years Group 3: 2.02 per 100 person‐years Group 4: 1.69 per 100 person‐years |
| Secondary: HIV incidence among adherent participants |
None reported 69 |
||||||
| PURPOSE 2 70 | III | CGM, TGW, TGM, GNB | 95 locations across the United States of America | PrEP‐naïve, HIV‐1 antigen + antibody negative individuals 16 years or older, sexually active and have had condomless receptive anal sex with at least one partner assigned male at birth in the last 12 months |
Group 1: SC LEN 927 mg every 26 weeks, oral LEN 600 mg on days 1 and 2, and oral PTM F/TDF once daily for 52 weeks Group 2: SC placebo LEN every 26 weeks, oral PTM LEN on days 1 and 2, and oral F/TDF 200/300 mg once daily for 52 weeks |
Primary: Number of participants with HIV‐1 diagnosis | None |
| Secondary: Number of participants with HIV‐1 diagnosis among study drug‐adherent participants | None | ||||||
| PURPOSE 3 44 | II | CGF | United States of America | HIV‐seronegative females 18 years or older who reported condomless vaginal or anal sex with a cisgender male as well as at least one of the following within the last 12 months: non‐injection recreational drug use, alcohol dependence, STI history, exchange of sex for commodities, incarceration (within 5 years), two or more sexual partners assigned male at birth, or a sexual partner with all above excluding alcohol dependence and including unknown HIV status |
Group 1: SC LEN 927 mg every 26 weeks, oral LEN 600 mg on days 1 and 2 Group 2: Oral F/TDF 200/300 mg once daily for 52 weeks |
Primary: C trough for LEN at the end of week 26 and week 52 | None |
| Primary: Acceptability, satisfaction, and willingness to use LEN and F/TDF as PrEP assessed by questionnaire responses | None | ||||||
| PURPOSE 4 45 | II | All sexes | United States of America | HIV‐seronegative, long‐acting PrEP‐naïve adults actively using any drug of misuse, evidence of recent injection, and self‐reported injection paraphernalia sharing in the past 30 days |
Group 1: SC LEN 927 mg on day 1 and week 26 and oral LEN 600 mg on days 1 and 2 Group 2: Oral F/TDF 200/300 mg FDC tablets for up to 52 weeks |
Primary: C trough for LEN at the end of week 26 and week 52 | None |
| Secondary: Acceptability, satisfaction, and willingness to use LEN and F/TDF as PrEP assessed by questionnaire responses | None | ||||||
| PURPOSE 5 91 | II | CGMSM, CGW, GNB, TGW, TGM | France, United Kingdom | HIV‐seronegative, PrEP‐naïve individuals 18 years or older who may benefit from PrEP |
Group 1: SC LEN 927 mg on day 1 and week 26 and oral LEN 600 mg on days 1 and 2 Group 2: Oral F/TDF 200/300 mg FDC tablets for up to 52 weeks |
Consistent and continuous use of LEN compared with F/TDF through week 52 | None |
CGF, cisgender female; CGM, cisgender male; CGMSM, cisgender men who have sex with men; FDC, fixed‐dose combination; F/TAF, emtricitabine/tenofovir alafenamide; F/TDF, emtricitabine/tenofovir disoproxil fumarate; GNB, gender non‐binary; LEN, lenacapavir; PrEP, pre‐exposure prophylaxis; PTM, placebo‐to‐match; SC, subcutaneous; TGM, transgender man; TGW, transgender woman.
Briefly, these are phase II and III studies with global recruitment sites, including the United States, Europe, and sub‐Saharan Africa; notably, PURPOSE 1 is exclusively recruiting from South Africa and Uganda. 69 Across all studies, inclusion criteria include all genders as young as 16 years old who will be randomized to receive SC LEN every 6 months following the simplified initiation regimen or standard‐of‐care (FTC/TDF or FTC/TAF). 72
In June 2024, the data monitoring committee reviewed the interim efficacy analysis results from PURPOSE 1 and recommended stopping the randomized phase of the trial. 69 Recently, Bekker et al. reported results from the PURPOSE 1 study after week 104, which are summarized in Table 5 . 69 In the LEN group, there were 0 incident HIV infections observed (0 per 100 person‐years); LEN reduced HIV incidence by 100% compared to background HIV incidence and FTC/TDF. 69 Although retention was high among treatment arms, most participants in the FTC/TAF and FTC/TDF groups had low adherence as determined by intracellular tenofovir diphosphate concentrations in dried blood spots. 69 Importantly, LEN injections were administered on time (defined as within a ±7‐day window) for >90% of the participants at week 26 and week 52. 69 These results underscore the efficacy of LEN in cisgender women and its utility to overcome adherence challenges with PrEP. The superiority of LEN to FTC/TDF in the PURPOSE 1 study has led to early discontinuation of the blinded phase of this clinical trial and researchers will now offer open‐label LEN to all study participants. 73
The open‐label phase of the PURPOSE 1 and the PURPOSE 2 studies will continue to examine HIV incidence rates, 69 , 70 and the PURPOSE 3 and PURPOSE 4 studies will examine LEN trough concentrations at weeks 26 and 52. 44 , 45 PURPOSE 5 will examine the persistence of LEN, described as consistent and continuous use, compared to standard‐of‐care FTC/TDF. 71 The HIV prevention and pharmacology fields eagerly await preliminary data from these pivotal studies.
PHARMACOLOGIC RESEARCH GAPS
Because of the variations in dosing in the ongoing studies, the active exploration of LEN for PrEP, and the months‐long dosing interval of LEN, there are several pharmacologic research gaps and important pharmacokinetic questions to address. These questions include the pharmacokinetics in special populations, drug–drug interactions that may arise over the dosing interval, and the pharmacology at the sites of action germane to HIV infection and PrEP, such as mucosal tissues and anatomical reservoirs. Herein, we provide insight into these research gaps with calls to action for future studies to examine these unique pharmacologic scenarios.
Special populations
Pregnant and breastfeeding women
Given the multitude of PK changes during pregnancy, 74 understanding the potential changes in the pharmacology of LEN will be essential to broaden the eligible patient populations receiving LEN for treatment and/or PrEP. In preclinical rat and rabbit studies, no teratogenic effects were observed with exposures greater than 16 times that observed in humans, 10 but additional human data are necessary to understand exposure across trimesters, during the delivery and postpartum periods, within cord blood, and during infant washout.
It is not known if and to what extent LEN is present in human breast milk. In preclinical studies, following LEN administration in pregnant rats, low‐but‐detectable LEN concentrations were observed in nursing rat pups 10 days after birth. 10 Further studies are warranted to understand LEN exposure in human breast milk and the consequent infant exposure.
Pediatrics
As infrequent dosing regimens are afforded by LEN, the pediatric population would benefit through increased adherence and the decreased risk of viral resistance. 75 However, the efficacy, safety, and pharmacology of LEN in the pediatric population are unknown, which is especially important given the physiological changes that occur throughout adolescence, as reviewed elsewhere. 76 The PURPOSE‐1 study began to address these questions, demonstrating no HIV incidence in sexually active adolescent girls and adult women between 16 and 25 years old receiving SC LEN for PrEP after 104 weeks. 69 Additional studies and data are warranted to understand the pharmacology of LEN in this understudied population.
Geriatrics
Similarly, pharmacologic changes occur in the elderly populations, 77 which may unpredictably affect LEN exposure. Specifically related to the SC dosing of LEN, body fat tissue increases during aging, 78 which may potentially affect the respective direct and indirect release rates from the depot following SC administration. 46 Additional physiologic changes include decreased muscle mass and total body water, which may result in decreased plasma concentrations, lower systemic clearance, and greater accumulation of lipophilic ARVs, such as LEN. 79 Further complicating the prediction of LEN exposure is that age‐related decreases in plasma protein concentrations will likely result in higher concentrations of protein‐unbound concentrations, resulting in higher pharmacologically active LEN and a greater incidence of adverse effects. 79 Regarding the metabolism of LEN via CYP3A, the effect of aging on hepatic enzyme expression is conflicting, with studies suggesting either reduced expression of CYP3A or no difference. 79 Taken together, the effects of these changes on the pharmacokinetics of LEN are uncertain. Future studies and real‐world implementation data will hopefully elucidate the changes in the pharmacology of LEN in this growing population demographic.
Pharmacokinetic interactions across the dosing interval
Drug–drug interactions have been observed as LEN is a substrate of the common metabolizing enzymes CYP3A and UGT1A1 and the P‐gp transporter. 42 Because of these interactions, PK changes that occur during the uniquely long dosing interval will be an important consideration. For instance, the introduction of a CYP3A4 inducer – either via prescription or over‐the‐counter – may deleteriously affect LEN concentrations to unknown degrees, effects of which may vary based on time after dose as the respective contributions from direct and indirect SC depots alter throughout the dosing interval. 42 These changes would be particularly important at time points near the end of the dosing interval, at which time LEN concentrations may potentially become sub‐therapeutic. Practical questions remain on the effect of these PK changes and highlight the need to consider more personalized dosing window forgiveness recommendations depending on concomitant medications that may be prescribed or de‐prescribed during the duration of the dosing interval.
Pharmacology at the sites of action
Reproductive fluids and tissues
The pharmacology of ARVs within the male and female reproductive system is an important area of HIV research. Ensuring therapeutic concentrations at the reproductive sites is necessary to prevent HIV transmission. 80 Many factors affect ARV penetration into the genital tract and semen. 80
Drug distribution into the male genital tract is limited by the blood–testes barrier, which is an anatomical barrier that expresses active transporters, including P‐gp. 81 These transporters regulate the entry and efflux of ARVs into this immune‐privileged site. 81 Because LEN is a substrate and inhibitor of P‐gp, 10 studies examining LEN distribution into the male genital tract are warranted.
ARV penetration into the female genital tract is complex and has been reviewed elsewhere. 80 Briefly, within the female genital tract, protein binding potential and drug transporter activity are influenced by biological hormone fluctuations, potentially influencing ARV distribution throughout the menstrual cycle, during co‐administration of contraception and hormone replacement therapy, and during the aging process. 80 , 82 Similar to the male genital tract, P‐gp is expressed in the vaginal mucosa, which actively effluxes xenobiotics out of the cervicovaginal space. 82 Furthermore, vaginal tissues express major CYP enzymes, including CYP3A4. 83 The resultant PK effects of these transporter proteins and metabolizing enzymes on modulating LEN exposure are unknown. Future distributional PK studies in the female genital tract are necessary to avoid gender‐specific data gaps in HIV prevention and treatment research. 84
Lymphoid tissues
The HIV reservoir – defined as the anatomic and cellular sanctuary sites in which HIV persists despite suppressive ART – remains a critical barrier to a cure. 85 The lymphoid tissues (e.g., lymph nodes, gut‐associated lymphoid tissue, and spleen) comprise the vast majority of cellular reservoirs. 85 Compartmentalization in these anatomical compartments provides the greatest evidence of replication‐competent virus that is genomically dissimilar from other compartments, including blood plasma. 86
The distribution of LEN into secondary lymphoid tissues, such as the lymph nodes and spleen, is unknown. Importantly, because LEN is indicated for treatment‐experienced PWH, 10 the clinical pharmacology of LEN within these lymphoid tissues and the associated viral diversity is a critical pharmacologic gap. Furthermore, factors affecting drug distribution within the gastrointestinal tract (GIT) are of particular interest for oral LEN. Specifically, drug metabolizing enzymes and drug transporters germane to the PK of LEN (e.g., CYP450 enzymes and P‐gp) may modulate LEN PK across the GIT, as observed for ARVs across and within drug classes. 87 Additional studies are warranted to quantify LEN concentrations in these tissues following oral and SC LEN dosing and relate these concentrations with pharmacologic and virologic effects.
As aforementioned, LEN is unlikely to penetrate the blood–brain barrier and thus unlikely to enter the central nervous system. Additional studies are warranted to confirm this hypothesis and characterize penetration into other sanctuary sites.
CONCLUDING THOUGHTS
The landscape for HIV treatment has evolved significantly over the past four decades. To work toward the Joint United Nations Program on HIV/AIDS (UNAIDS) goal of 95% of PWH knowing their HIV status, 95% of PWH who know their HIV status starting treatment, and 95% of PWH on treatment being virally suppressed by 2025 (95‐95‐95), 88 we will need an innovative array of ARVs representing novel classes, viral targets, formulations, and dosing schemes. 71 With its novel mechanism and infrequent dosing (by both mouth and SC), LEN may contribute towards achieving this aspirational UNAIDS goal. Furthermore, the promise of LEN for PrEP represents a continuing paradigm shift of improving PrEP adherence, reducing HIV transmission, and decreasing HIV diagnoses toward ending the HIV epidemic.
Understanding the unique pharmacology of LEN – particularly in different populations – will be of utmost importance to ensure therapeutic exposure over the long dosing interval for all. Clinical and observational trials and computational approaches should be employed to address these pharmacologic research gaps and to ensure equitable access for all populations.
FUNDING
This work was supported by the Rustbelt Center for AIDS Research (CFAR; P30AI036219) and the National Heart, Lung, and Blood Institute (DP1HL174180). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
CONFLICT OF INTEREST
The authors declared no competing interests in this work.
References
- 1. Trickey, A. , Zhang, L. , Sabin, C.A. & Sterne, J.A.C. Life expectancy of people with HIV on long‐term antiretroviral therapy in Europe and North America: a cohort study. Lancet Healthy Longevity 3, S2 (2022). [Google Scholar]
- 2. Insight Start Study Group et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N. Engl. J. Med. 373, 795–807 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Byrd, K.K. et al. Antiretroviral adherence level necessary for HIV viral suppression using real‐world data. J. Acquir. Immune Defic. Syndr. 82, 245–251 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ortego, C. et al. Adherence to highly active antiretroviral therapy (HAART): a meta‐analysis. AIDS Behav. 15, 1381–1396 (2011). [DOI] [PubMed] [Google Scholar]
- 5. McComsey, G.A. , Lingohr‐Smith, M. , Rogers, R. , Lin, J. & Donga, P. Real‐world adherence to antiretroviral therapy among HIV‐1 patients across the United States. Adv. Ther. 38, 4961–4974 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kalichman, S.C. et al. Adherence to antiretroviral therapy and HIV transmission risks: implications for test‐and‐treat approaches to HIV prevention. AIDS Patient Care STDs 24, 271–277 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sethi, A.K. , Celentano, D.D. , Gange, S.J. , Moore, R.D. & Gallant, J.E. Association between adherence to antiretroviral therapy and human immunodeficiency virus drug resistance. Clin. Infect. Dis. 37, 1112–1118 (2003). [DOI] [PubMed] [Google Scholar]
- 8. Bauer, A. et al. Current state and opportunities with long‐acting Injectables: industry perspectives from the innovation and quality consortium “long‐acting Injectables” working group. Pharm. Res. 40, 1601–1631 (2023). [DOI] [PubMed] [Google Scholar]
- 9. Jindal, A.B. , Bhide, A.R. , Salave, S. , Rana, D. & Benival, D. Long‐acting parenteral drug delivery systems for the treatment of chronic diseases. Adv. Drug Deliv. Rev. 198, 114862 (2023). [DOI] [PubMed] [Google Scholar]
- 10. Gilead Sciences Prescribing information for lenacapavir injection (Sunleca) [Package Insert] (2022).
- 11. Campbell, E.M. & Hope, T.J. HIV‐1 capsid: the multifaceted key player in HIV‐1 infection. Nat. Rev. Microbiol. 13, 471–483 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Briggs, J.A.G. et al. The stoichiometry of gag protein in HIV‐1. Nat. Struct. Mol. Biol. 11, 672–675 (2004). [DOI] [PubMed] [Google Scholar]
- 13. Di Nunzio, F. et al. Nup153 and Nup98 bind the HIV‐1 core and contribute to the early steps of HIV‐1 replication. Virology 440, 8–18 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Blair, W.S. et al. HIV capsid is a tractable target for small molecule therapeutic intervention. PLoS Pathog. 6, e1001220 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rossi, E. , Meuser, M.E. , Cunanan, C.J. & Cocklin, S. Structure, function, and interactions of the HIV‐1 capsid protein. Life (Basel) 11, 100 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li, C. , Burdick, R.C. & Hu, W.S. Lenacapavir Disrupts HIV‐1 Core Integrity While Stabilizing the Capsid Lattice. (Abstract 215). Conference on Retroviruses and Opportunistic Infections (2023).
- 17. Schirra, R.T. , dos Santos, N. , Zadrozny, K.K. , Kucharska, I. , Ganser‐Pornillos, B.K. & Pornillos, O. A molecular switch modulates assembly and host factor binding of the HIV‐1 capsid. Nat. Struct. Mol. Biol. 30, 383–390 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Link, J.O. et al. Clinical targeting of HIV capsid protein with a long‐acting small molecule. Nature 584, 614–618 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. NIH Lenacapavir Chemical and Physical Properties. PubChem https://pubchem.ncbi.nlm.nih.gov/compound/Lenacapavir#section=Depositor‐Supplied‐Synonyms.
- 20. Siemons, M. , Schroyen, B. , Darville, N. & Goyal, N. Role of modeling and simulation in preclinical and clinical long‐acting injectable drug development. AAPS J. 25, 99 (2023). [DOI] [PubMed] [Google Scholar]
- 21. Thompson, C.G. , Cohen, M.S. & Kashuba, A.D.M. Antiretroviral pharmacology in mucosal tissues. J. Acquir. Immune Defic. Syndr. 63(Suppl 2), S240–S247 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lawther, B.K. , Kumar, S. & Krovvidi, H. Blood–brain barrier. Contin. Educ. Anaesth. Crit. Care Pain 11, 128–132 (2011). [Google Scholar]
- 23. VanderVeen, L. Activity and Resistance Characterization of the HIV Capsid Inhibitor Lenacapavir. (Abstract 128). Conference on Retroviruses and Opportunistic Infections (2021).
- 24. Smith, R.A. et al. Antiviral activity of Lenacapavir against HIV‐2 isolates and drug‐resistant HIV‐2 mutants. J. Infect. Dis. 229, 1290–1294 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Margot, N. , Ram, R. , Rhee, M. & Callebaut, C. Absence of Lenacapavir (GS‐6207) phenotypic resistance in HIV gag cleavage site mutants and in isolates with resistance to existing drug classes. Antimicrob. Agents Chemother. 65, e02057‐20 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bester, S.M. et al. Structural and mechanistic bases of viral resistance to HIV‐1 capsid inhibitor Lenacapavir. MBio 13, e0180422 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Margot, N. et al. Phenotypic resistance to lenacapavir and monotherapy efficacy in a proof‐of‐concept clinical study. J. Antimicrob. Chemother. 77, 989–995 (2022). [DOI] [PubMed] [Google Scholar]
- 28. Sager, J. Safety and PK of Subcutaneous GS‐6207, A Novel HIV‐1 Capsid Inhibitor. (Abstract 141). Conference on Retroviruses and Opportunistic Infections (2019).
- 29. Daar, E. Dose‐response Relationship of Subcutaneous Long‐acting HIV Capsid Inhibitor. (Abstract 469). Conference on Retroviruses and Opportunistic Infections (2020).
- 30. Gupta, S.K. et al. Lenacapavir administered every 26 weeks or daily in combination with oral daily antiretroviral therapy for initial treatment of HIV: a randomised, open‐label, active‐controlled, phase 2 trial. Lancet HIV 10, e15–e23 (2023). [DOI] [PubMed] [Google Scholar]
- 31. Hagins, D.P. et al. Long‐Acting Lenacapavir in a Combination Regimen for Treatment Naive PWH: Week 80. (Abstract 522). Conference on Retroviruses and Opportunistic Infections (2023).
- 32. Orkin, C. Lenacapavir in first‐line therapy. Lancet HIV 10, e2–e3 (2023). [DOI] [PubMed] [Google Scholar]
- 33. Segal‐Maurer, S. et al. Capsid inhibition with Lenacapavir in multidrug‐resistant HIV‐1 infection. N. Engl. J. Med. 386, 1793–1803 (2022). [DOI] [PubMed] [Google Scholar]
- 34. Ogbuagu, O. et al. Efficacy and safety of the novel capsid inhibitor lenacapavir to treat multidrug‐resistant HIV: week 52 results of a phase 2/3 trial. Lancet HIV 10, e497–e505 (2023). [DOI] [PubMed] [Google Scholar]
- 35. Margot, N.A. et al. Resistance analyses in highly treatment‐experienced people with human immunodeficiency virus (HIV) treated with the novel capsid HIV inhibitor Lenacapavir. J. Infect. Dis. 226, 1985–1991 (2022). [DOI] [PubMed] [Google Scholar]
- 36. Ogbuagu, O. et al. Efficacy and safety of long‐acting subcutaneous lenacapavir in heavily treatment‐experienced people with multi‐drug resistant HIV: week 104 results. Open Forum Infect. Dis. 10, ciae423 (2023). [DOI] [PubMed] [Google Scholar]
- 37. Marcelin, A.‐G. et al. Frequency of capsid substitutions associated with GS‐6207 in vitro resistance in HIV‐1 from antiretroviral‐naive and ‐experienced patients. J. Antimicrob. Chemother. 75, 1588–1590 (2020). [DOI] [PubMed] [Google Scholar]
- 38. Nka, A.D. et al. Evaluation of HIV‐1 capsid genetic variability and lenacapavir (GS‐6207) drug resistance‐associated mutations according to viral clades among drug‐naive individuals. J. Antimicrob. Chemother. 78, 272–275 (2022). [DOI] [PubMed] [Google Scholar]
- 39. Wirden, M. et al. Ultra‐rapid selection of the N74D capsid inhibitor resistance mutation after 3 weeks on lenacapavir. J. Antimicrob. Chemother. 79, 1706–1707 (2024). [DOI] [PubMed] [Google Scholar]
- 40. Hingrat, Q.L. Rapid Selection of HIV‐2 Capsid Mutations After Failure of a Lenacapavir‐Containing Regimen. (Abstract 682). Conference on Retroviruses and Opportunistic Infections (2024).
- 41. Ogbuagu, O. CAPELLA: Wk 104 results with long‐acting subcutaneous Lenacapavir in persons living with multidrug‐resistant HIV. IDWeek (2023).
- 42. NDA 215973/215974 . Sunlenca [FDA Integrated Review] (2022).
- 43. Weber, E. et al. Pharmacokinetics, disposition, and biotransformation of [14C]Lenacapavir, a novel, first‐in‐class, selective inhibitor of HIV‐1 capsid function, in healthy participants following a single intravenous infusion. Clin. Pharmacokinet. 63, 241–253 (2024). [DOI] [PubMed] [Google Scholar]
- 44. Gilead Sciences Study of Lenacapavir and Emtricitabine/Tenofovir Disoproxil Fumarate (F/TDF) in Prevention of HIV in Cisgender Women in the United States (HPTN‐102) (PURPOSE 3). ClinicalTrials.gov <https://clinicaltrials.gov/study/NCT06101329?intr=Lenacapavir&rank=4> (2024).
- 45. Gilead Sciences Study of Lenacapavir and Emtricitabine/Tenofovir Disoproxil Fumarate (F/TDF) for Prevention of HIV in People Who Inject Drugs (HPTN‐103) (PURPOSE‐4). Clinicaltrials.gov <https://clinicaltrials.gov/study/NCT06101342?intr=Lenacapavir&rank=5#publications> (2024).
- 46. Subramanian, R. et al. Lenacapavir: a novel, potent, and selective first‐in‐class inhibitor of HIV‐1 capsid function exhibits optimal pharmacokinetic properties for a long‐acting injectable antiretroviral agent. Mol. Pharm. 20, 6213–6225 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jogiraju, V. Pharmacokinetics of a Simplified Subcutaneous Lenacapavir Regimen Versus Phase 2/3 Regimen. (Poster PESUB22). International AIDS Conference (2022).
- 48. Shaik, N. et al. Comparison of Long‐Acting Lenacapavir Phase 2/3 Regimen vs Simplified Regimen Using Population‐PK Analysis and Simulation. (Poster EPB0230). International AIDS Society Conference (2023).
- 49. Shaik, N.A. et al. Population PK Analysis to Guide Dosing Window Following Lenacapavir SC Administration. (Abstract 504). Conference on Retroviruses and Opportunistic Infections (2023).
- 50. Eron, J.J. et al. Safety of teropavimab and zinlirvimab with lenacapavir once every 6 months for HIV treatment: a phase 1b, randomised, proof‐of‐concept study. Lancet HIV 11, e146–e155 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jogiraju, V. et al. Pharmacokinetics of long‐acting lenacapavir in participants with hepatic or renal impairment. Antimicrob. Agents Chemother. 68, e0134423 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Eron, J.J. Lenacapavir Plus bNAbs for People With HIV and Sensitivity to Either Teropavimab or Zinlirvimab. (Abstract 120). Conference on Retroviruses and Opportunistic Infections <https://www.croiconference.org/abstract/lenacapavir‐plus‐bnabs‐for‐people‐with‐hiv‐and‐sensitivity‐to‐either‐teropavimab‐or‐zinlirvimab/> (2024).
- 53. Gilead Sciences Study to Compare Bictegravir/Lenacapavir Versus Current Therapy in People With HIV‐1 Who Are Successfully Treated With a Complicated Regimen (ARTISTRY‐1). clinicaltrials.gov <https://clinicaltrials.gov/study/NCT05502341?intr=Lenacapavir&page=1&rank=9> (2024).
- 54. Mounzer, K. Phase 2 Study of Switch to Daily BIC + LEN in Individuals on a Complex HIV Treatment Regimen. (Abstract 642). Conference on Retroviruses and Opportunistic Infections (2024).
- 55. Gilead Sciences Study to Compare Bictegravir/Lenacapavir Versus Current Therapy in People With HIV‐1 Who Are Successfully Treated With Biktarvy (ARTISTRY‐2). clinicaltrials.gov <https://clinicaltrials.gov/study/NCT06333808?term=ARTISTRY‐2&rank=1> (2024).
- 56. Gandhi, M. et al. Case series of people with HIV on the long‐acting combination of Lenacapavir and Cabotegravir: call for a trial. Open Forum Infect. Dis. 11, ofae125 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Markowitz, M. & Sarafianos, S.G. 4′‐Ethynyl‐2‐fluoro‐2′‐deoxyadenosine, MK‐8591: a novel HIV‐1 reverse transcriptase translocation inhibitor. Curr. Opin. HIV AIDS 13, 294–299 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Matthews, R.P. et al. Safety, tolerability, and pharmacokinetics of single‐ and multiple‐dose administration of islatravir (MK‐8591) in adults without HIV. Clin. Transl. Sci. 14, 1935–1944 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Diamond, T.L. et al. No Antagonism or Cross‐Resistance Observed Between Islatravir and Lenacapavir. (Abstract 585). Conference on Retroviruses and Opportunistic Infections (2023).
- 60. Gilead Sciences Study Evaluating the Safety and Efficacy of Islatravir in Combination With Lenacapavir in Virologically Suppressed People With HIV. clinicaltrials.gov <https://clinicaltrials.gov/study/NCT05052996?intr=Lenacapavir&page=2&rank=12> (2023).
- 61. Colson, A. Efficacy and Safety of Weekly Islatravir Plus Lenacapavir in PWH at 24 Weeks: A Phase II Study. (Abstract 208). Conference on Retroviruses and Opportunistic Infections (2024).
- 62. Baeten, J.M. et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N. Engl. J. Med. 367, 399–410 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tolley, E.E. et al. Acceptability of a long‐acting injectable HIV prevention product among US and African women: findings from a phase 2 clinical trial (HPTN 076). J. Int. AIDS Soc. 22, e25408 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cobb, D.A. , Smith, N.A. , Edagwa, B.J. & McMillan, J.M. Long‐acting approaches for delivery of antiretroviral drugs for prevention and treatment of HIV: a review of recent research. Expert Opin. Drug Deliv. 17, 1227–1238 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Marzinke, M.A. et al. Characterization of human immunodeficiency virus (HIV) infection in cisgender men and transgender women who have sex with men receiving injectable cabotegravir for HIV prevention: HPTN 083. J. Infect. Dis. 224, 1581–1592 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Delany‐Moretlwe, S. et al. Cabotegravir for the prevention of HIV‐1 in women: results from HPTN 084, a phase 3, randomised clinical trial. Lancet 399, 1779–1789 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bekerman, E. et al. Long‐acting lenacapavir acts as an effective preexposure prophylaxis in a rectal SHIV challenge macaque model. J. Clin. Invest. 133, e167818 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Swanstrom, A.E. et al. Long‐acting lenacapavir protects macaques against intravenous challenge with simian‐tropic HIV. EBioMedicine 95, 104764 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gilead Sciences Pre‐Exposure Prophylaxis Study of Lenacapavir and Emtricitabine/Tenofovir Alafenamide in Adolescent Girls and Young Women at Risk of HIV Infection (PURPOSE 1). ClinicalTrials.gov <https://clinicaltrials.gov/study/NCT04994509?intr=Lenacapavir&rank=10> (2024).
- 70. Gilead Sciences Study of Lenacapavir for HIV Pre‐Exposure Prophylaxis in People Who Are at Risk for HIV Infection (PURPOSE 2). ClinicalTrials.gov <https://clinicaltrials.gov/study/NCT04925752?intr=Lenacapavir&page=2&rank=11> (2023).
- 71. Ross, J. & Smith, M. Gilead Sciences Announces New Clinical Trial in Europe to Assess Lenacapavir for HIV Prevention as Part of Landmark Purpose Program. Gilead <https://www.gilead.com/news‐and‐press/press‐room/press‐releases/2023/10/gilead‐sciences‐announces‐new‐clinical‐trial‐in‐europe‐to‐assess‐lenacapavir‐for‐hiv‐prevention‐as‐part‐of‐landmark‐purpose‐program> (2023).
- 72. Bekker, L.‐G. et al. Twice‐yearly Lenacapavir or daily F/TAF for HIV prevention in cisgender women. N. Engl. J. Med. (2024). 10.1056/NEJMoa2407001. [DOI] [PubMed] [Google Scholar]
- 73. Gilead Sciences, Inc . Gilead's Twice‐Yearly Lenacapavir Demonstrated 100% Efficacy and Superiority to Daily Truvada® for HIV Prevention. Gilead <https://www.gilead.com/news‐and‐press/press‐room/press‐releases/2024/6/gileads‐twiceyearly‐lenacapavir‐demonstrated‐100‐efficacy‐and‐superiority‐to‐daily‐truvada‐for‐hiv‐prevention> (2024).
- 74. Pinheiro, E.A. & Stika, C.S. Drugs in pregnancy: pharmacologic and physiologic changes that affect clinical care. Semin. Perinatol. 44, 151221 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. World Health Organization Considerations for ART in Adolescents . In Antiretroviral Therapy for HIV Infection in Infants and Children: Towards Universal Access: Recommendations for a Public Health Approach: 2010 Revision (World Health Organization, Geneva, 2010). [PubMed] [Google Scholar]
- 76. O'Hara, K. Pharmacokinetic changes with growth and development between birth and adulthood. J. Pharm. Pract. Res. 47, 313–318 (2017). [Google Scholar]
- 77. Klotz, U. Pharmacokinetics and drug metabolism in the elderly. Drug Metab. Rev. 41, 67–76 (2009). [DOI] [PubMed] [Google Scholar]
- 78. Mukker, J.K. , Singh, R.S.P. & Derendorf, H. Pharmacokinetic and pharmacodynamic considerations in elderly population. In Developing Drug Products in an Aging Society (Stegemann S.) 26, 139–151 (Springer International Publishing, Cham, 2016). [Google Scholar]
- 79. King, E.M. , Tkachuk, S. & Tseng, A. Aging on Antiretrovirals: reviewing the need for pharmacologic data in elderly people living with HIV. AIDS 38, 1609–1616 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Trezza, C.R. & Kashuba, A.D.M. Pharmacokinetics of antiretrovirals in genital secretions and anatomic sites of HIV transmission: implications for HIV prevention. Clin. Pharmacokinet. 53, 611–624 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Bart, J. et al. The distribution of drug‐efflux pumps, P‐gp, BCRP, MRP1 and MRP2, in the normal blood‐testis barrier and in primary testicular tumours. Eur. J. Cancer 40, 2064–2070 (2004). [DOI] [PubMed] [Google Scholar]
- 82. Nicol, M.R. , Corbino, J.A. & Cottrell, M.L. Pharmacology of antiretrovirals in the female genital tract for HIV prevention. J. Clin. Pharmacol. 58, 1381–1395 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. To, E.E. , Hendrix, C.W. & Bumpus, N.N. Dissimilarities in the metabolism of antiretroviral drugs used in HIV pre‐exposure prophylaxis in colon and vagina tissues. Biochem. Pharmacol. 86, 979–990 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Sheth, A.N. , Momplaisir, F. & Dumond, J.B. Shifting the narrative of preexposure prophylaxis adherence counseling for cisgender women. JAMA 331, 912–914 (2024). [DOI] [PubMed] [Google Scholar]
- 85. Estes, J.D. et al. Defining total‐body AIDS‐virus burden with implications for curative strategies. Nat. Med. 23, 1271–1276 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Chaillon, A. et al. HIV persists throughout deep tissues with repopulation from multiple anatomical sources. J. Clin. Invest. 130, 1699–1712 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Cottrell, M.L. , Srinivas, N. & Kashuba, A.D.M. Pharmacokinetics of antiretrovirals in mucosal tissue. Expert Opin. Drug Metab. Toxicol. 11, 893–905 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Stover, J. et al. Modeling the epidemiological impact of the UNAIDS 2025 targets to end AIDS as a public health threat by 2030. PLoS Med. 18, e1003831 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lenacapavir predicted properties. Drug Bank <https://go.drugbank.com/drugs/DB15673> (2023).
- 90. Gilead Sciences A Study of GS‐5423 and GS‐2872 in Combination With Capsid Inhibitor Lenacapavir in Virologically Suppressed Adults With HIV‐1 Infection. clinicaltrials.gov <https://clinicaltrials.gov/study/NCT05729568?intr=Lenacapavir&page=1&rank=2#publications> (2024).
- 91. Gilead Sciences Study of Lenacapavir Taken Twice a Year for HIV Pre‐Exposure Prophylaxis (PrEP) (PURPOSE 5). Clinicaltrials.gov <https://clinicaltrials.gov/study/NCT06513312?intr=Lenacapavir&term=PURPOSE&rank=1> (2024).


