Abstract
Plasminogen activator inhibitor-1 (PAI-1) is one of the primary inhibitors of the fibrinolytic system and has been implicated in a variety of thrombotic disorders. In this report, stress-induced changes in murine PAI-1 gene expression were investigated to study the role of this inhibitor in the development of stress-induced hypercoagulability. Restraint stress led to a dramatic induction of plasma PAI-1 antigen and of tissue PAI-1 mRNA with maximum induction in adipose tissues. In situ hybridization analysis of the stressed mice revealed that strong signals for PAI-1 mRNA were localized to hepatocytes, renal tubular epithelial cells, adrenomedullar chromaffin cells, neural cells in the paraaortic sympathetic ganglion, vascular smooth muscle cells, and adipocytes, but not to endothelial cells. These observations indicate that the stress induces the PAI-1 gene expression in a tissue-specific and cell type-specific manner. The induction of PAI-1 mRNA by restraint stress was greater than that observed for heat shock protein, a typical stress protein, suggesting that PAI-1 is one of the most highly induced stress proteins. Importantly, the magnitude of induction of PAI-1 mRNA by stress increased markedly with age, and this increase in PAI-1 correlated with tissue thrombosis in the older stressed mice. Moreover, much less tissue thrombosis was induced by restraint stress in young and aged PAI-1-deficient mice compared with age-matched wild-type mice. These results suggest that the large induction of PAI-1 by stress increases the risk for thrombosis in the older populations, and that the adipose tissue may be involved.
The stress response represents a universally conserved cellular defense program and is thought to be required for the maintenance of homeostasis (1). It is characterized by rapid changes in gene expression (i.e., the induction of stress proteins; ref. 2) in response to a variety of stressful conditions, including heat shock, inflammatory agents, ischemia, and oxidative stress (3). One of the best examples of how the stress response provides for increased cellular protection is illustrated by the induction of heat shock proteins (HSPs) during the phenomenon of acquired thermotolerance (4). The constitutively expressed heat shock proteins work as molecular chaperones to facilitate various aspects of protein maturation (5), and they play an important role in the protection against stress-induced apoptosis (6, 7). Thus, these proteins can function to protect organisms from a variety of insults that induce stress. The induction of stress proteins also may contribute to the development of a number of clinically relevant phenomena, including tissue and organ damage, and the immune response (8). In this context, the restraint stress model often has been used to investigate the stress response in terms of pharmacological, physiological, or pathological phenomena in vivo (9). This response is best characterized in the neuro-endocrine system (e.g., the hypothalamic-pituitary-adrenal axis; ref. 10), and in this setting, may be mediated, at least in part, by the activation of the glucocorticoid cascade (11) and of the sympathetic nervous system (12, 13).
Plasminogen activator inhibitor-1 (PAI-1) is a member of the serine protease inhibitor (serpin) family, and it regulates fibrinolysis and proteolysis by inhibiting plasminogen activation (14). Elevated levels of plasma PAI-1 are observed in a variety of thrombotic conditions (15), including myocardial infarction (16), deep vein thrombosis (17), and disseminated intravascular coagulation (18). Increased PAI-1 gene expression also was demonstrated in several specific histopathologies [e.g., lung fibrosis (19), glomerulonephritis (20, 21), and atherosclerosis (22, 23)]. In obese humans, increased plasma PAI-1 levels correlated with the amounts of visceral fat (24), suggesting that the adipose tissue is the primary source of PAI-1 in this condition (25). Basal PAI-1 activity in plasma was shown to increase in the elderly as well, and this increase was correlated with the serum triglyceride level (26). PAI-1 gene expression is up-regulated by cytokines, such as tumor necrosis factor-α (27), transforming growth factor-β (27), and interleukins (28), and by hormones [e.g., glucocorticoid (29), insulin (30), adrenaline (31), and angiotensin II (32)]. Mental and physical stress not only caused alterations of platelet function, but also decreased fibrinolytic activity (33–35). For example, hyperthermia stimulated PAI-1 expression in cultured endothelial cells in vitro (36), whereas PAI-1 was strongly induced by acute inflammatory stress (e.g., endotoxin administration; ref. 37) and hypoxic stress (38) in vivo. Chronic stress, defined as feelings of fatigue, lack of energy, increased irritability, and demoralization, also was shown to be associated with elevated plasma PAI-1 antigen in middle-aged men (39). Taken together, these observations have led us to hypothesize that the increased expression of PAI-1 in response to physiological and pathological stress may increase the prothrombotic potential, thus promoting thrombotic complications under stressful condition.
In the present study, the effect of restraint stress on the induction of PAI-1 gene was investigated in mice. The induction of PAI-1 was regulated in a tissue-specific and cell type-specific manner in the restraint model. Because aged subjects may have lower tolerance to stress (10, 40, 41) and be more susceptible to thrombosis caused by stress (42, 43), we also compared the magnitude of stress-induced PAI-1 expression and evaluated tissue thrombosis in young and aged mice in vivo.
Materials and Methods
Restraint Stress.
Male C57BL/6J mice aged 2, 12, and 24 months old were obtained from Shizuoka Laboratory Animal Center Japan and through the National Institute of Aging. Mice were placed into 50-ml conical centrifuge tubes fitted with multiple punctures so as to allow ventilation. The tubes were placed in horizontal holders, and the animals were thus maintained for a continuous period of restraint (9). During this time, the animals were provided with water only. Because food deprivation reportedly may induce immunological changes, control groups were maintained without food. After 2, 4, 8, 16, and 20 h of restraint, the mice were killed by overdose inhalation anesthesia with methoxyflurane (Pitman–Moore, Mundelein, IL). The blood was collected into 10% sodium citrate and centrifuged at 3000 × g for 5 min, and then the plasma was removed and stored at −70°C. Tissues were rapidly removed by standard dissection techniques, and either minced and immediately frozen in liquid nitrogen for preparation of total RNA or fixed in chilled 4% paraformaldehyde and embedded in paraffin for in situ hybridization and staining either with hematoxylin and eosin, or with periodic acid/Schiff reagent (PAS) staining. In separate experiments, male or female PAI-1-deficient (44) and wild-type (C57BL/6J) mice, obtained from Scripps Clinic Rodent Breeding Colony or kindly provided by P. Carmeliet and D. Collen, University of Leuven, Belgium, were also put into restraint tubes for 20 h and then killed. The kidneys were removed and analyzed for glomerular thrombosis by PAS and hematoxylin/eosin staining.
Determination of Active PAI-1 Antigen in Mouse Plasma.
Active PAI-1 antigen in plasma was determined by employing the tissue plasminogen activator (t-PA) binding assay as previously described (45). Active PAI-1 levels (ng/ml) were calculated from a standard curve constructed by using recombinant mouse PAI-1.
Quantitative Reverse Transcription (RT)-PCR Assay.
The concentration of PAI-1 mRNA in murine tissues was determined by quantitative RT-PCR assay as described in previous studies (20, 37). Total RNA was prepared from unfixed tissues by using the Ultraspec RNA Isolation System (Biotecx Laboratories, Houston), and then quantitated by measuring absorption at 260 nm. The control RNA (cRNA) standard was in vitro transcribed from the template containing the sequence of primers for mouse PAI-1 and β-actin, by using the Riboprobe Gemini II (Promega). Thereafter, 1 μg of total tissue RNA and a fixed amount of the cRNA, which can function as a competitor for the target mRNA, were combined and reverse transcribed by using a Gene Amp RNA PCR kit (Perkin–Elmer/Cetus). After PCR amplification of 30–35 cycles (95°C for 1 min, 60°C for 1 min, and 72°C for 1 min) using specific primers for PAI-1 in the presence of 32P-end-labeled sense primer (5 × 105 cpm), 20-μl aliquots of the PCR products were electrophoresed on a 2% agarose gel. The appropriate bands corresponding to the target mRNA and the cRNA product were excised from the gel, and each incorporated radioactivity was determined by using a scintillation counter. The number of molecules of PAI-1 mRNA was determined by extrapolation by using the cRNA standard curve, and the concentration was expressed as picograms of PAI-1 mRNA per microgram of total tissue RNA (37). The quantitation of the HSP 70.1 mRNA in murine tissues was achieved similarly by RT-PCR by using Competitive DNA Construction Kit and Competitive RNA Transcription Kit (Takara Shuzo, Otsu, Japan) with a pair of specific primers (sense, 5′-CGGAGGAGATCTCGTCCATGGTGC-3′; anti-sense, 5′-ACGACAGCGTCCTCTTGGCCCTCT-3′; ref. 46). Variations in sample loading were assessed by measuring β-actin mRNA.
In Situ Hybridization.
In situ hybridization was performed by using 35S-labeled antisense riboprobes, as described previously (20, 37). After hybridization, the slides were dehydrated by immersion in a graded alcohol series containing 0.3 M NH4Ac, and dried. Then, the slides were coated with NTB2 emulsion (Kodak; 1:2 in water), and exposed in the dark at 4°C for 4 to 10 weeks. The slides were developed for 2 min in D19 developer (Kodak), fixed, washed in water, and counterstained with hematoxylin and eosin. No specific hybridization signal could be detected in parallel sections by using 35S-labeled sense probes in each experiment (not shown).
Statistical Analysis.
Statistical analysis of all quantitative RT-PCR results was performed by using the Student's two-tailed unpaired t test for comparisons between groups (statview software; Abacus Concepts, Berkeley, CA).
Results
Kinetics of PAI-1 Induction by Restraint Stress.
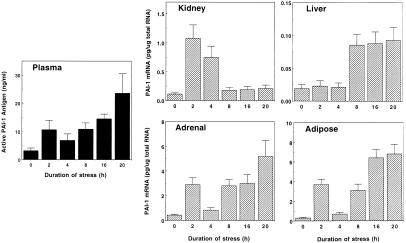
Initially, the effects of increasing exposure times to restraint stress on active PAI-1 antigen levels in plasma and PAI-1 mRNA expression in tissues were analyzed in 8-week-old male C57BL/6J mice (Fig. 1). Relatively short duration of restraint stress (i.e., 2–4 h) induced active PAI-1 antigen in plasma by 3- to 4-fold, whereas a 7-fold increase was detected after 20-h restraint stress. Unexpectedly, the kinetics of induction of PAI-1 mRNA in response to continuous stress differed considerably from tissue to tissue. For example, short stress (2–4 h) induced renal PAI-1 mRNA by 7- to 10-fold, but the response was transient and declined dramatically after that. In contrast to this relatively rapid response, PAI-1 mRNA in the liver was induced only after prolonged stress (8–20 h). Interestingly, much greater induction of PAI-1 mRNA (max. 10- to 20-fold) was detected in the adrenals and adipose tissues than in the kidney and liver, and these increases were observed both after short (2 h) and long (8–20 h) restraint stress. In both of these tissues, the induction of PAI-1 mRNA was attenuated in the case of 4-h restraint stress.
Figure 1.
Time course of stress-induced changes in PAI-1 expression in the blood and tissues. Eight-week-old mice were placed into restraint tubes for the indicated times, and then their plasma and tissues were collected. Active PAI-1 antigen levels in the plasma were determined by the tissue plasminogen activator (t-PA) binding assay. Total tissue RNA was prepared and analyzed for PAI-1 mRNA expression level by quantitative RT-PCR. The data are expressed as the mean and SD from six mice in each category.
Aged Mice Are More Sensitive to Restraint Stress in Terms of PAI-1 Induction than Young Mice.
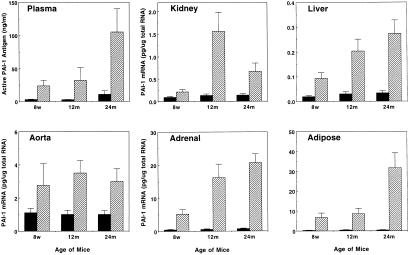
Experiments were performed to investigate the effect of aging on the induction of PAI-1 expression by restraint stress (Fig. 2). A larger increase in plasma PAI-1 antigen level was observed in aged (12 and 24 months old) mice as compared with young (8 weeks old) mice after 20-h restraint stress. Because of the possibility that increases both in systemic PAI-1 levels and in the local expression of PAI-1 by tissues may contribute to the progression of tissue thrombosis, we also compared PAI-1 mRNA levels in tissues of the restraint-stressed young and aged mice. Again, there was a much greater induction of PAI-1 mRNA in the liver (7- to 8-fold), kidney (5- to 10-fold), and adrenal (25- to 30-fold) of aged mice after 20-h restraint, as compared with young mice. Most importantly, the largest induction of PAI-1 mRNA by restraint stress among the tissues examined was observed in the adipose tissues (e.g., 16-fold in 8-week-old mice, 20-fold in 12-month-old, and 70-fold in 24-month-old). Restraint stress also caused a 3- to 4-fold increase in PAI-1 mRNA in the aorta (previously reported to contain high levels of PAI-1 mRNA; ref. 37), but there was no significant difference in the level when young and aged mice were compared. No significant induction of PAI-1 mRNA by stress was detected in the lungs, heart, and brains of the restraint-stressed young and aged mice (not shown).
Figure 2.
Comparison of PAI-1 induction by restraint stress between young and aged mice. Plasma and the indicated tissues were collected from 8-week- (8w), 12-month- (12 m), and 24-month (24 m) old mice before (filled bar) and after (hatched bar) 20-h restraint stress. Active PAI-1 antigen levels in the plasma and PAI-1 mRNA expression levels in the tissues were determined by the tissue plasminogen activator (t-PA) binding assay and by quantitative RT-PCR, respectively. The data are expressed as the mean and SD from eight mice in each category.
Table 1 shows the average amount of total RNA that was present in the indicated tissue obtained from stressed young (8 weeks old) and aged (24 months old) mice. This information was then used to calculate the specific PAI-1 mRNA content of each tissue (27). Based on this estimation, it seems likely that the liver and adipose tissue may be the major sources of the increased PAI-1 antigen observed in plasma of the stressed mice, especially in aged mice.
Table 1.
Quantitative analysis of PAI-1 mRNA expression in tissues of young (8 weeks old) and aged (24 months old) mice after 20-h restraint stress
| Tissue | pg PAI-1 mRNA/μg total RNA
|
μg total RNA/tissue
|
pg PAI-1 mRNA/tissue
|
|||
|---|---|---|---|---|---|---|
| Young | Aged | Young | Aged | Young | Aged | |
| Liver | 0.073 | 0.18 | 7,320 | 8,740 | 534 | 1,573 |
| Kidney | 0.21 | 0.67 | 1,020 | 1,160 | 214 | 777 |
| Adipose | 6.8 | 31.8 | 72 | 90 | 490 | 2,862 |
| Adrenal | 5.2 | 20.7 | 18 | 12 | 94 | 248 |
Comparison of mRNA Induction by Restraint Stress Between Heat Shock Protein (HSP70.1) and PAI-1.
The above observations suggest that PAI-1 may be one of the most highly induced stress proteins. To determine whether this is the case, we compared the magnitude of PAI-1 induction by restraint stress with that of a typical stress protein, HSP70.1, which was induced by restraint stress in rats (10). We quantitated HSP70.1 mRNA in tissues (e.g., liver, kidney, adrenal, and adipose) of the stressed young (8 weeks old) and aged (24 months old) mice by using competitive RT-PCR as described in Materials and Methods (data not shown). Two- or twenty-hour restraint stress induced HSP70.1 mRNA by no greater than 3- to 4-fold in all tissues examined in young mice. In general, the HSP70.1 response in tissues of aged mice to restraint stress was lower than that in young mice. Unexpectedly, the magnitude of induction of PAI-1 mRNA was much higher than HSP70.1 mRNA in all tissues examined of young and aged mice, especially in the adrenals (10- to 30-fold) and adipose tissues (16- to 70-fold). Again, the response of PAI-1 gene expression to restraint stress was stronger in aged mice than in young mice. These results indicate that PAI-1 is one of the most highly induced stress proteins and may be an age-related responsive gene.
Cellular Localization of Induced PAI-1 mRNA in Tissues of Restraint-Stressed Mice.
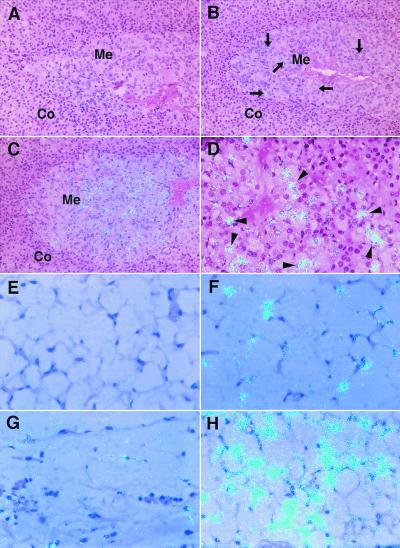
To localize the PAI-1 mRNA induced by restraint stress to specific cells in each tissue, high resolution microscopic in situ hybridization analysis was performed by using tissue sections from stressed mice. Fig. 3 shows PAI-1 mRNA in the adrenals and adipose tissues from stressed aged mice compared with young mice. PAI-1 mRNA was not detected in control adrenals (Fig. 3A) and was barely detectable in control adipose tissues from young (Fig. 3E) and aged (Fig. 3G) mice. However, in the adrenal glands from the stressed young mice, positive cells for PAI-1 mRNA increased in the medulla (Fig. 3B). In the adrenals from the stressed aged mice, medullar chromaffin cells specifically expressed abundant PAI-1 mRNA (Fig. 3 C and D). No PAI-1 mRNA was detected in the cortex (Fig. 3 B and C). In adipose tissues from stressed mice, increased expression of PAI-1 mRNA was predominantly localized to cells that appear to be adipocytes (Fig. 3 F and H). The cell type-specific induction of PAI-1 mRNA by restraint stress in both tissues was much greater in aged mice than young mice (compare Fig. 3 B and C; Fig. 3 F and H). These observations suggest that specific cell populations respond to restraint stress in terms of PAI-1 induction, and that their response increases with age in this restraint model.
Figure 3.
High resolution in situ hybridization analysis of PAI-1 mRNA in the adrenals and adipose tissues of control and restraint-stressed mice. Adrenals and adipose tissues were harvested from young (8 weeks old) and aged (24 months old) mice before and after 20-h restraint stress, and then analyzed by in situ hybridization by using 35S-labeled cRNA probes as described in Materials and Methods. The hybridization signal for PAI-1 mRNA is indicated by the light blue dots in all panels. (A–D) PAI-1 mRNA in adrenals of the unstressed young (A), stressed young (B), and stressed aged mice (C and D). Co, adrenal cortex; Me, adrenal medulla. Arrows in B denote positive cells for PAI-1 mRNA in the adrenal medulla. Arrowheads in D indicate adrenomedullar chromaffin cells. (E–H) PAI-1 mRNA in adipose tissues of the unstressed young (E), stressed young (F), unstressed aged (G), and stressed aged mice (H). Magnification: A–C, ×200; D–H, ×400. All slides were exposed for 10 weeks at 4°C.
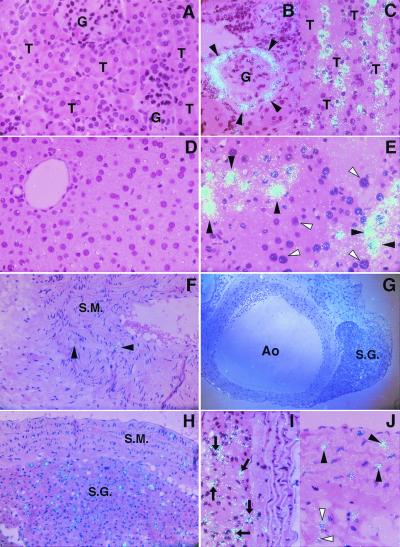
Fig. 4 shows the stress-mediated induction of PAI-1 mRNA in cells within the kidney, liver, and aorta of aged mice compared with similarly aged but unstressed mice. Although there was no detectable signal for PAI-1 mRNA in the kidneys of the unstressed aged mice (Fig. 4A), the epithelial cells of Bowman's capsule (Fig. 4B) and of proximal and distal tubules (Fig. 4C) from stressed aged mice expressed increased PAI-1 mRNA. Similarly, hepatocytes in the liver from stressed aged mice occasionally showed strong signals for PAI-1 mRNA (Fig. 4E), but there was no detectable signal in the livers of unstressed aged mice (Fig. 4D). Although some vascular smooth muscle cells in the tunica media of the aorta in the unstressed aged mice expressed PAI-1 mRNA (Fig. 4F), stress led to increased PAI-1 mRNA in these cells (Fig. 4J) and in neural cells of the paraaortic sympathetic ganglion (Fig. 4 G–I), and to adipose tissue surrounding the aorta (Fig. 4J). Little PAI-1 mRNA was detected in vascular endothelial cells in kidneys, livers, and larger vessels from any of the stressed young and aged mice (Fig. 4 B, E, and I).
Figure 4.
High resolution in situ hybridization analysis of PAI-1 mRNA in kidneys, livers, and aortas of control and stressed aged mice. Kidneys, livers, and aortas were harvested from 24-month-old mice before and after 20-h continuous restraint stress, and then analyzed by in situ hybridization as described in Materials and Methods. The hybridization signal for PAI-1 mRNA corresponds to the light blue dots in all panels. (A–C) PAI-1 mRNA in the kidneys of the unstressed (A, ×400) and stressed (B and C, ×400) mice. G, glomeruli; T, tubules. Arrowheads indicate epithelial cells in Bowman's capsule (B). (D and E) PAI-1 mRNA in the liver of the unstressed (D, ×400) and stressed (E, ×400) mice. Filled arrowheads denote hepatocytes that strongly expressed PAI-1 mRNA, and open arrowheads indicate negative hepatocytes for PAI-1 mRNA signals (E). (F–J) PAI-1 mRNA in aorta of the unstressed (F) and stressed (G–J) mice. (F) The aortic wall containing smooth muscle layer (S.M.; ×200). (G) The aorta (Ao) and paraaortic sympathetic ganglion (S.G.) at low magnification (×100). (H) The smooth muscle layer (S.M.) of aortic wall and adjacent sympathetic ganglion (S.G.) at higher magnification (×200). (I and J) The aortic wall. Arrows indicate neural cells in the sympathetic ganglion (I, ×400). Filled arrowheads indicate vascular smooth muscle cells, and open arrowheads denote adipocytes (J, ×400). All slides were exposed for 10 weeks at 4°C.
Tissue Thrombosis in Wild-Type and PAI-1 Deficient Mice After 20-h Restraint Stress.
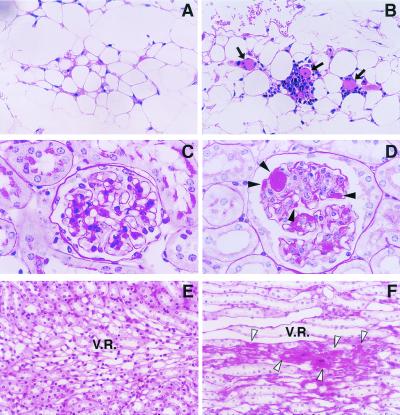
Microscopic examination of tissue sections revealed that tissue thrombosis developed in the 20-h stressed aged (24-month-old) mice, but not in the stressed young (8-week-old) mice (Fig. 5). For example, the formation of thrombi with infiltration of mononuclear cells was observed in the microvasculature between the adipocytes (Fig. 5B) in the epididymal fat of five of six aged mice. However, no thrombi were detected in adipose tissues of the stressed young mice (Fig. 5A). Similarly, renal tissues from the stressed aged mice demonstrated multiple thrombi in the glomeruli (Fig. 5D) as well as in the vasa recta (arteriolae rectae) in the medulla (Fig. 5F; in all of six mice), but again, no thrombi were detected in the same regions of the stressed young mice (Fig. 5 C and E). Thrombi were not detected by PAS and hematoxylin/eosin staining in the liver, lung, heart, brain, and adrenal from the stressed young and aged mice (not shown).
Figure 5.
Microscopic analysis on tissue thrombosis induced by restraint stress. Renal and adipose tissues were removed from young (8-week-old) and aged (24-month-old) mice after 20-h restraint stress. Tissue sections were stained with hematoxylin/eosin (A and B), or with PAS (C–F), and examined microscopically (n = 6 for each group). (A and B) Adipose tissues of the stressed young (A) and aged (B) mice. Arrows denote thrombi in the microvessels between adipocytes. (C–F) Renal tissues of the stressed young (C and E) and aged (D and F) mice. (C and D) A glomerulus. (E and F) The region of vasa recta in the medulla. Filled arrowheads indicate thrombi formation in the glomerulus (D), and open arrowheads denote thrombi in the vasa recta (F). Magnification: A and B, ×200; C and D, ×1,000; E and F, ×400.
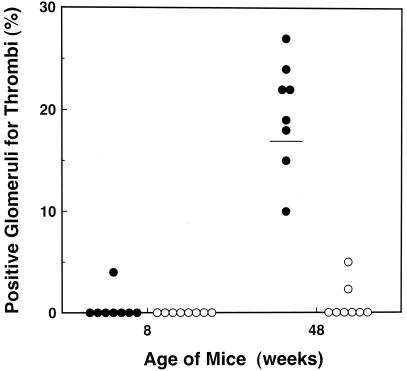
To determine whether the stress-induced PAI-1 promotes the thrombosis observed in the stressed aged mice, we performed stress experiments by using 8- and 48-week-old PAI-1-deficient and wild-type mice, and analyzed renal glomerular thrombosis by PAS and hematoxylin/eosin staining (Fig. 6). In wild-type mice, thrombi were observed in less than 5% of glomeruli in only one of eight mice of 8 weeks old, but the percentage of positive glomeruli for thrombi increased to 10–27% (mean, 17%) in 48-week-old mice. In PAI-1 deficient mice, no glomerular thrombi were detected in all of the 8-week-old mice. More importantly, less than 5% of glomeruli were positive for thrombi only in two of eight mice and others (six of eight) showed no glomerular thrombi in 48-week-old PAI-1 deficient mice. These results suggest that tissue microthrombosis induced by restraint stress is age dependent, and that PAI-1 expression should contribute to the thrombosis phenotype in this model.
Figure 6.
Renal glomerular thrombosis in PAI-1-deficient mice and wild-type after 20-h restraint stress. Mice were placed into restraint tubes for 20 h and then killed. The kidneys were removed and analyzed for glomerular thrombosis with hematoxylin/eosin or PAS staining. Stress-induced renal glomerular thrombosis was evaluated quantitatively in young (8 weeks old) wild-type (●) and PAI-1-deficient (○) mice (n = 8), or in older (48 weeks old) wild-type (●) and PAI-1 deficient (○) mice (n = 8). Quantitation was achieved by counting the positive glomeruli for thrombi out of at least 100 glomeruli in each kidney section. The figure shows the percentage of positive glomeruli for thrombi in each mouse. The horizontal bar represents the mean.
Discussion
Although hypercoagulability and thrombotic episodes appear to be induced by physical (34, 38, 39) and mental stress (33), little information is available about underlying mechanisms. In this report, we studied the expression of PAI-1 in a mouse restraint stress model and attempted to relate it to stress-induced thrombosis. Restraint stress induces the expression of the major heat shock protein, a typical stress protein, and this response may be mediated by the sympathetic nervous system (12, 13). Our study shows that PAI-1 gene expression was dramatically induced in the blood and in selective tissues by restraint stress (Figs. 1 and 2), and that the magnitude of PAI-1 mRNA induction by restraint stress was far greater than that of heat shock protein, HSP70.1 mRNA (not shown). These results indicate that PAI-1 is a major stress-induced gene. The increased signals for PAI-1 mRNA were primarily localized to epithelial cells, vascular smooth muscle cells, and neural cells, but not to vascular endothelial cells (Figs. 3 and 4). This result implies that the induction of PAI-1 by stress may depend on a cell type-specific mechanism. For example, the specific localization of the increased PAI-1 mRNA to adrenomedullar chromaffin cells (Fig. 3C) and to neural cells of the paraaortic sympathetic ganglion (Fig. 4 G–I) was demonstrated in the stressed aged mice. The adrenal medulla is embryologically derived from neural crest and is a part of the sympathetic nervous system. Restraint stress activates the hypothalamic-pituitary-adrenal axis, leading to the increased secretion of glucocorticoid (11) and adrenaline (9), both of which were shown to induce PAI-1 expression in vivo (29, 31). Taken together, the activation of the sympathetic nervous system by restraint stress may contribute to the induction of PAI-1 in neuron-derived cell populations. Thus, the adrenal gland may be one of the most responsive organs to stress stimuli, possibly because it is a primary source of hormones (e.g., glucocorticoid and mineral corticoid) necessary for maintenance of homeostasis (11).
The magnitude of PAI-1 mRNA induction by restraint stress was the highest in the adipose tissue among tissues examined (Figs. 1 and 2; Table 1), and adipocytes were responsible for this induction (Fig. 3). This observation is noteworthy because adipocyte-specific PAI-1 induction was also shown in obese mice (30) and in obese humans (24), who frequently suffer from thrombotic cardiovascular disease (47). The observation that plasma PAI-1 antigen and adipose-PAI-1 mRNA are both induced by restraint stress with similar kinetics suggests that the adipose tissue/adipocytes may be one of the principal sources of PAI-1 expression in response to stress (Fig. 1; Table 1). In the present study, we observed a dramatic increase (4- to 10-fold) in plasma levels of tumor necrosis factor-α, which was previously shown to be a strong inducer of PAI-1 expression (27), especially in the adipose tissue (25), after restraint stress (unpublished observation). The partial attenuation of the stress-induced PAI-1 mRNA in the adipose tissue by pretreatment with anti-mouse tumor necrosis factor-α antibody (unpublished observation) suggests that this cytokine may be an important mediator of the PAI-1 response to stress in adipose tissues. Meanwhile, it should be noted that the promoter region of the mouse (48) and human (49) PAI-1 gene contains the palindromic heat shock element consensus sequence (i.e., -CNNGAANNTTCNNG-; ref. 46), which is activated in the induction of stress protein. This region could directly respond to stress insults, resulting in the transcriptional activation of the PAI-1 gene.
Because aged subjects may have lower tolerance to stress (10, 40, 41) and be susceptible to thrombosis caused by a variety of stressors (42, 43), we compared the stress-induced PAI-1 expression and tissue microthrombosis between young and aged mice. The induction of PAI-1 expression by stress was shown to be much more dramatic in aged mice in most tissues examined. Aged animals showed lower responses to restraint stress in terms of the induction of heat shock protein expressed in tissues (10, 12), suggesting an impairment of protective mechanism to stress with aging. In contrast, this study shows the increased ability of the aged animals to mount the PAI-1 response to stress (Fig. 2), and this response may contribute to the increased incidence of thrombotic vascular disease in elderly individuals (50). Indeed, we detected thrombi formation in the vasculature of adipose tissues, in renal glomerular structure, and in renal small vessels in the stressed aged mice after 20-h stress (Fig. 5), and the stress-induced glomerular thrombosis was more pronounced in aged mice compared with young mice (Fig. 6). This dramatic difference in the thrombosis phenotype between young and aged mice may result from a much greater induction of regional PAI-1 mRNA in the kidneys of 12-month-old mice (Fig. 2), as well as from increases in PAI-1 antigen levels in their plasma. In contrast, no significant PAI-1 induction was observed in the lung, heart, and brain, and these tissues did not develop thrombosis (not shown). These observations seem to be inconsistent with clinical situations in patients because stress-induced thrombosis is frequently encountered in lung, heart, and brain. This discrepancy may be due to differences between species because murine models of hypercoagulability (51–53) developed thrombi or fibrin deposits at unexpected sites where thrombosis rarely occurs in humans. The development of thrombosis could depend on the vascular-bed specific hemostatic balance (54), which may explain why some organs do not develop thrombosis in this model. In any case, the aging-associated PAI-1 induction should contribute to tissue thrombosis in a restraint stress model, and this hypothesis is supported by the results of stressed PAI-1-deficient mice. Importantly, aged PAI-1-deficient mice showed much less glomerular thrombi as compared with age-matched wild-type mice after restraint stress (Fig. 6). Thus, an age-related increase in the PAI-1 response to stress may exacerbate vascular injury and subsequent tissue damage in the aging process.
In conclusion, we demonstrate here that stress causes a thrombotic tendency in an age-associated manner, and this result correlates with the large induction of PAI-1 expression. The response of PAI-1 to restraint stress was most pronounced in adipose tissue, suggesting that fat mass may contribute to the systemic and regional increases in the prothrombotic potential under stressful condition. This study presents a previously undocumented analysis of the molecular process of stress-induced hypercoagulability and suggests that stress, aging, and adipose mass together may contribute to the increased risk for thrombosis because of the induction of PAI-1.
Acknowledgments
We thank T. Thinnes, E. Yamafuji, K. Kaneko, and K. Sakakura for their expert technical assistance. This work was supported by grants-in-aid from the Ministry of Education, Science, Sports and Culture, from the Ministry of Health and Welfare, by Funds for Comprehensive Research on Aging and Health, Japan, and by National Institutes of Health Grant HL-47819 (to D.J.L.).
Abbreviations
- PAI-1
plasminogen activator inhibitor-1
- RT
reverse transcription
- cRNA
control RNA
- HSP
heat shock protein
- PAS
periodic acid/Schiff reagent
References
- 1.Selye H. J Clin Endocrinol Metab. 1946;6:117–230. doi: 10.1210/jcem-6-2-117. [DOI] [PubMed] [Google Scholar]
- 2.Anathan J, Goldberg A L, Voellmy R. Science. 1986;232:252–254. doi: 10.1126/science.3083508. [DOI] [PubMed] [Google Scholar]
- 3.Minowada G, Welch W J. J Clin Invest. 1995;95:3–12. doi: 10.1172/JCI117655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morimoto R I, Sarge K D, Abravaya K. J Biol Chem. 1992;267:21987–21990. [PubMed] [Google Scholar]
- 5.Georgopoulos C, Welch W J. Annu Rev Cell Biol. 1993;9:601–634. doi: 10.1146/annurev.cb.09.110193.003125. [DOI] [PubMed] [Google Scholar]
- 6.Buzzard K A, Giaccia A J, Killender M, Anderson R L. J Biol Chem. 1998;273:17147–17153. doi: 10.1074/jbc.273.27.17147. [DOI] [PubMed] [Google Scholar]
- 7.Mosser D D, Caron A W, Bourget L, Denis-Larose C, Massie B. Mol Cell Biol. 1997;17:5317–5327. doi: 10.1128/mcb.17.9.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonneau R H, Sheridan J F, Feng N, Glaser R. J Neuroimmunol. 1993;42:167–176. doi: 10.1016/0165-5728(93)90007-l. [DOI] [PubMed] [Google Scholar]
- 9.Glavin G B, Pare W P, Sandbak T, Bakke H K, Murison R. Neurosci Biobehav Rev. 1994;18:223–249. doi: 10.1016/0149-7634(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 10.Blake M J, Udelsman R, Feulner G J, Norton D D, Holbrook N J. Proc Natl Acad Sci USA. 1991;88:9873–9877. doi: 10.1073/pnas.88.21.9873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sapolsky R M, Krey L C, McEwen B S. Endocr Rev. 1986;7:284–301. doi: 10.1210/edrv-7-3-284. [DOI] [PubMed] [Google Scholar]
- 12.Udelsman R, Blake M J, Stagg C A, Li D, Putney D J, Holbrook N J. J Clin Invest. 1993;91:465–473. doi: 10.1172/JCI116224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chin J H, Okazaki M, Hu Z-W, Miller J W, Hoffman B B. J Clin Invest. 1996;97:2316–2323. doi: 10.1172/JCI118674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loskutoff D J, Sawdey M S, Mimuro J. In: Progress in Hemostasis and Thrombosis. Coller B, editor. Philadelphia: Saunders; 1988. pp. 87–115. [PubMed] [Google Scholar]
- 15.Yamamoto K, Saito H. Int J Hematol. 1998;68:371–385. doi: 10.1016/s0925-5710(98)00094-2. [DOI] [PubMed] [Google Scholar]
- 16.Hamsten A, Wiman B, de Faire U, Blomback M. N Engl J Med. 1985;313:1557–1563. doi: 10.1056/NEJM198512193132501. [DOI] [PubMed] [Google Scholar]
- 17.Wilman B, Hamsten A. Semin Thromb Hemost. 1990;26:207–216. doi: 10.1055/s-2007-1002671. [DOI] [PubMed] [Google Scholar]
- 18.Pralong G, Calandra T, Glauser M-P, Schellekens J, Verhoef J, Bachmann F, Kruithof E K O. Thromb Haemost. 1989;61:459–462. [PubMed] [Google Scholar]
- 19.Olman M A, Mackman N, Gladson C L, Moser K M, Loskutoff D J. J Clin Invest. 1995;96:1621–1630. doi: 10.1172/JCI118201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keeton M R, Ahn C, Eguchi Y, Burlingame R, Loskutoff D J. Kidney Int. 1995;47:148–157. doi: 10.1038/ki.1995.17. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto K, Loskutoff D J. Am J Pathol. 1997;151:725–734. [PMC free article] [PubMed] [Google Scholar]
- 22.Schneiderman J, Sawdey M S, Keeton M R, Bordin G M, Bernstein E F, Dilley R B, Loskutoff D J. Proc Natl Acad Sci USA. 1992;89:6998–7002. doi: 10.1073/pnas.89.15.6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chomiki N, Henry M, Alessi M C, Anfosso F, Juhan-Vague I. Thromb Haemost. 1994;72:44–53. [PubMed] [Google Scholar]
- 24.Shimomura I, Funahashi T, Takahashi M, Maeda K, Kotani K, Nakamura T, Yamashita S, Miura M, Fukuda Y, Takemura K, et al. Nat Med. 1996;2:800–803. doi: 10.1038/nm0796-800. [DOI] [PubMed] [Google Scholar]
- 25.Samad F, Yamamoto K, Loskutoff D J. J Clin Invest. 1996;97:37–46. doi: 10.1172/JCI118404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mehta J, Mehta B, Lawson D, Saldeen T. J Am Coll Cardiol. 1987;9:263–268. doi: 10.1016/s0735-1097(87)80373-x. [DOI] [PubMed] [Google Scholar]
- 27.Sawdey M S, Loskutoff D J. J Clin Invest. 1991;88:1346–1353. doi: 10.1172/JCI115440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schleef R R, Bevilacqua M P, Sawdey M, Gimbrone M A, Jr, Loskutoff D J. J Biol Chem. 1988;263:5797–5803. [PubMed] [Google Scholar]
- 29.Konkle B A, Schuster S J, Kelly M D, Harjes K, Hassett D E, Bohrer M, Tavassoli M. Blood. 1992;79:2636–2642. [PubMed] [Google Scholar]
- 30.Samad F, Loskutoff D J. Mol Med. 1996;2:568–582. [PMC free article] [PubMed] [Google Scholar]
- 31.Larsson P T, Wiman B, Olsson G, Angelin B, Hjemdahl P. Thromb Haemost. 1990;63:482–487. [PubMed] [Google Scholar]
- 32.Vaughan D E, Lazos S A, Tong K. J Clin Invest. 1995;95:995–1001. doi: 10.1172/JCI117809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jern C, Eriksson E, Tengborn L, Risberg B, Wadenvik H, Jern S. Thromb Haemost. 1989;62:767–771. [PubMed] [Google Scholar]
- 34.Malyszko J, Urano T, Takada Y, Takada A. Life Sci. 1994;54:1275–1280. doi: 10.1016/0024-3205(94)00855-8. [DOI] [PubMed] [Google Scholar]
- 35.Takada Y, Urano T, Takahashi H, Nagai N, Takada A. Thromb Res. 1998;89:107–114. doi: 10.1016/s0049-3848(97)00300-9. [DOI] [PubMed] [Google Scholar]
- 36.Wojta J, Holzer M, Hufnagl P, Christ G, Hoover R L, Binder B R. Am J Pathol. 1991;139:911–919. [PMC free article] [PubMed] [Google Scholar]
- 37.Yamamoto K, Loskutoff D J. J Clin Invest. 1996;97:2440–2451. doi: 10.1172/JCI118691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pinsky D J, Liao H, Lawson C A, Yan S-H, Chen J, Carmeliet P, Loskutoff D J, Stern D M. J Clin Invest. 1998;102:919–928. doi: 10.1172/JCI307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raikkonen K, Lassila R, Keltikangas-Jarvinen L, Hautanen A. Arterioscler Thromb Vasc Biol. 1996;16:363–367. doi: 10.1161/01.atv.16.3.363. [DOI] [PubMed] [Google Scholar]
- 40.Fawcett T W, Sylvester S L, Sarge K D, Morimoto I, Holbrook N J. J Biol Chem. 1994;269:32272–32278. [PubMed] [Google Scholar]
- 41.Vatassery G T, Lai J C K, Smith W E, Quach H T. Neurochem Res. 1998;23:121–125. doi: 10.1023/a:1022495804817. [DOI] [PubMed] [Google Scholar]
- 42.Ciampricotti R, el Gamal M I. Int J Cardiol. 1989;24:211–218. doi: 10.1016/0167-5273(89)90306-9. [DOI] [PubMed] [Google Scholar]
- 43.Lecomte D, Fornes P, Nicolas G. Forensic Sci Int. 1996;79:1–10. doi: 10.1016/0379-0738(95)01873-5. [DOI] [PubMed] [Google Scholar]
- 44.Carmeliet P, Stassen J M, Schoonjans L, Ream B, van den Oord J J, De Mol M, Mulligan R C, Collen D. J Clin Invest. 1993;92:2756–2760. doi: 10.1172/JCI116893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schleef R R, Sinha M, Loskutoff D J. J Lab Clin Med. 1985;106:408–415. [PubMed] [Google Scholar]
- 46.Hunt C, Calderwood S. Gene. 1990;87:199–204. doi: 10.1016/0378-1119(90)90302-8. [DOI] [PubMed] [Google Scholar]
- 47.Larsson B. Int J Obesity. 1991;15:53–57. [PubMed] [Google Scholar]
- 48.Prendergast G C, Diamond L E, Dahl D, Cole M D. Mol Cell Biol. 1990;10:1265–1269. doi: 10.1128/mcb.10.3.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bosma P J, van den Berg E A, Kooistra T, Siemieniak D R, Slightom J L. J Biol Chem. 1988;263:9129–9141. [PubMed] [Google Scholar]
- 50.Tracy R P, Bovill E G. Arch Pathol Lab Med. 1992;116:1307–1312. [PubMed] [Google Scholar]
- 51.Erickson L A, Fici G J, Lund J E, Boyle T P, Polites H G, Marotti K R. Nature (London) 1990;346:74–76. doi: 10.1038/346074a0. [DOI] [PubMed] [Google Scholar]
- 52.Weiler-Guettler H, Christie P D, Beeler D L, Healy A M, Hancock W W, Rayburn H, Edelberg J M, Rosenberg R D. J Clin Invest. 1998;101:1983–1991. doi: 10.1172/JCI2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cui J, Eitzman D T, Westrick R J, Christie P D, Xu Z J, Yang A Y, Purkayastha A A, Yang T L, Metz A L, Gallagher K P, Tyson J A, Rosenberg R D, Ginsburg D. Blood. 2000;96:4222–4226. [PubMed] [Google Scholar]
- 54.Rosenberg R D, Aird W C. N Engl J Med. 1999;340:1555–1564. doi: 10.1056/NEJM199905203402007. [DOI] [PubMed] [Google Scholar]