Abstract
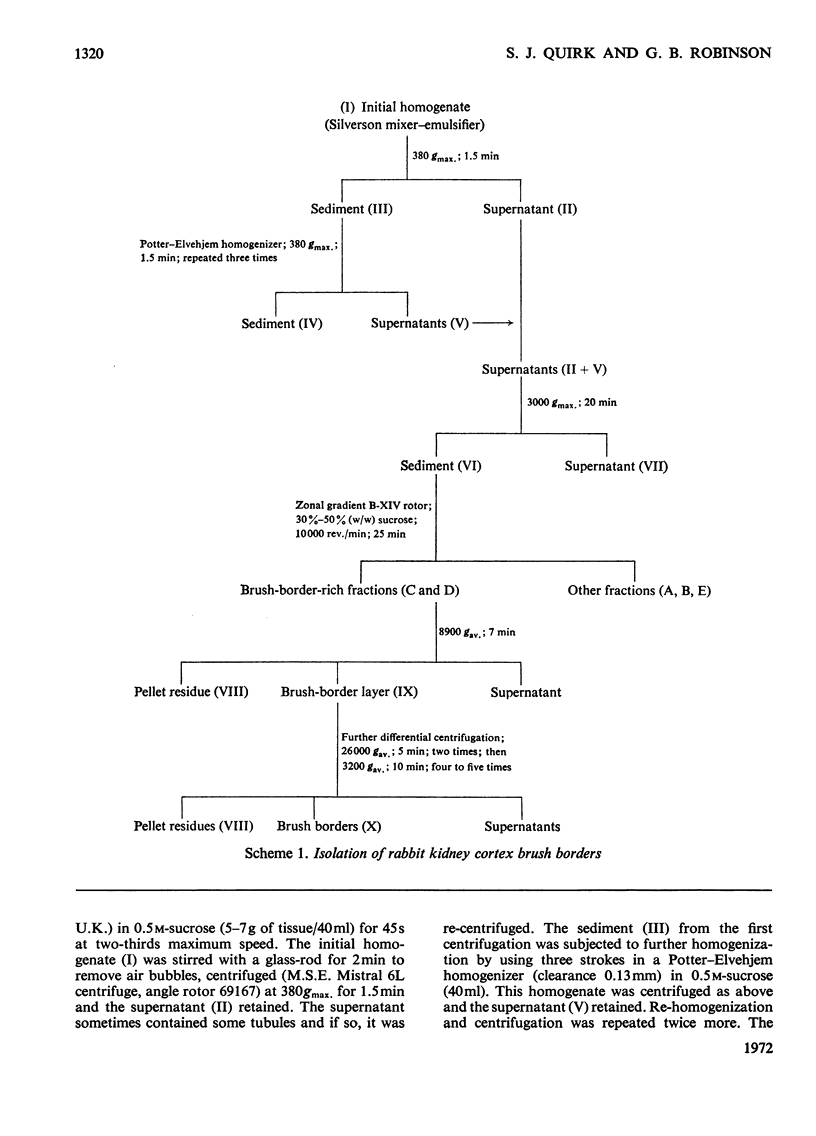
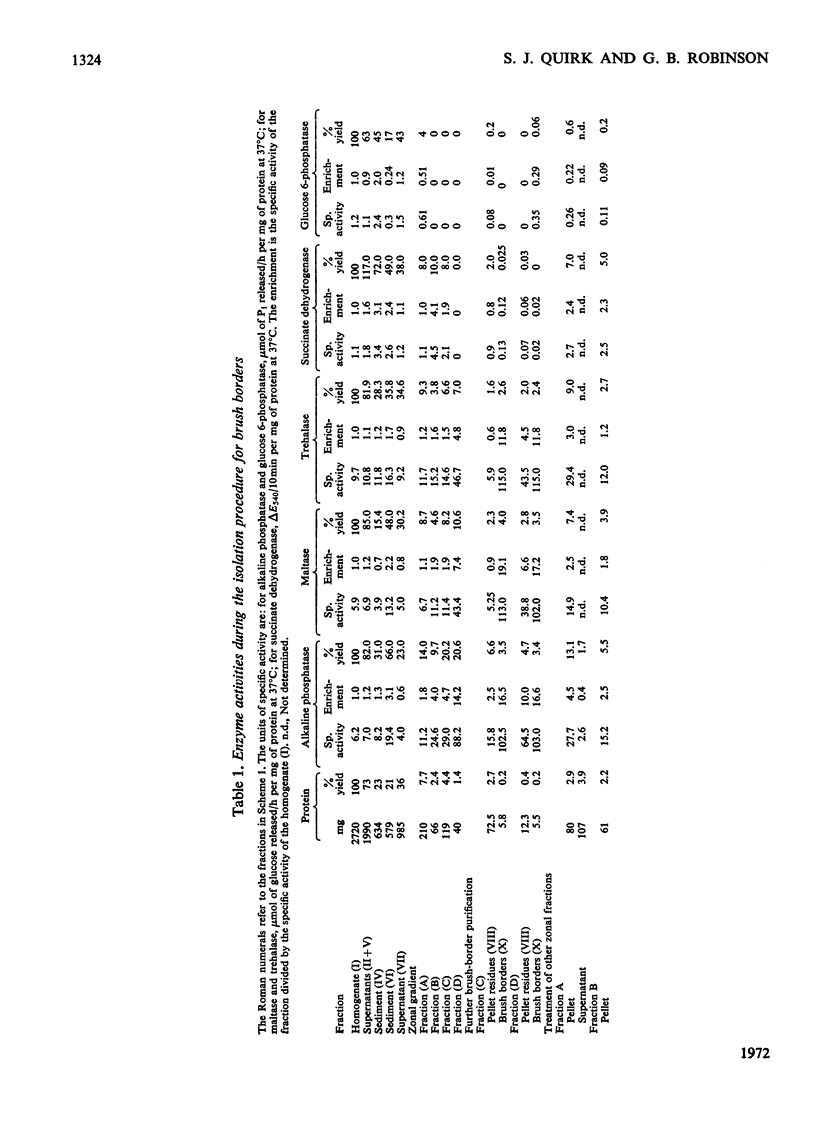
1. Brush borders were isolated from rabbit kidney-cortex homogenates by rate-zonal centrifugation through a sucrose density gradient in a B-XIV zonal rotor, followed by differential centrifugation. 2. The method of preparation gave brush borders of high purity with a reasonable yield. The morphological appearance supported the evidence from enzymic and chemical investigations, that the brush borders were only slightly contaminated with endoplasmic reticulum, mitochondria, lysosomes and nuclei. 3. The molar ratio of cholesterol to phospholipid lay within the range found in other plasma membranes, but the carbohydrate content was double that found in liver plasma membranes. 4. Alkaline phosphatase, maltase, trehalase and aminopeptidase were major enzymic constituents of the brush borders, and had an approximately equal yield and enrichment, but none of these enzymes fulfilled the criteria for marker enzymes. 5. Mg2+-dependent and Na+,K+-dependent adenosine triphosphatases, although found in brush borders, had low yields and low enrichments.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashworth L. A., Green C. Plasma membranes: phospholipid and sterol content. Science. 1966 Jan 14;151(3707):210–211. doi: 10.1126/science.151.3707.210. [DOI] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BONTING S. L., POLLAK V. E., MUEHRCKE R. C., KARK R. M. Quantitative histochemistry of the nephron. Science. 1958 Jun 6;127(3310):1342–1343. doi: 10.1126/science.127.3310.1342. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman L., Björling G., Christodoulou C. Multiple molecular forms of leucine aminopeptidase in man. Acta Genet Stat Med. 1966;16(3):223–230. doi: 10.1159/000151966. [DOI] [PubMed] [Google Scholar]
- Berger S. J., Sacktor B. Isolation and biochemical characterization of brush borders from rabbit kidney. J Cell Biol. 1970 Dec;47(3):637–645. doi: 10.1083/jcb.47.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binkley F., King N., Milikin E., Wright R. K., O'Neal C. H., Wundram I. J. Brush border particulates of renal tissue. Science. 1968 Nov 29;162(3857):1009–1011. doi: 10.1126/science.162.3857.1009. [DOI] [PubMed] [Google Scholar]
- Butterworth P. J., Moss D. W. Action of neuraminidase on human kidney alkaline phosphatase. Nature. 1966 Feb 19;209(5025):805–806. doi: 10.1038/209805a0. [DOI] [PubMed] [Google Scholar]
- Clamp J. R., Bhatti T., Chambers R. E. The determination of carbohydrate in biological materials by gas-liquid chromatography. Methods Biochem Anal. 1971;19:229–344. doi: 10.1002/9780470110386.ch3. [DOI] [PubMed] [Google Scholar]
- Coleman R. Some features of the lipid composition of rat liver surface and cytoplasmic membranes. Chem Phys Lipids. 1968 Feb;2(1):144–146. doi: 10.1016/0009-3084(68)90039-x. [DOI] [PubMed] [Google Scholar]
- Dahlqvist A. Assay of intestinal disaccharidases. Anal Biochem. 1968 Jan;22(1):99–107. doi: 10.1016/0003-2697(68)90263-7. [DOI] [PubMed] [Google Scholar]
- Eichholz A. Structural and functional organization of the brush border of intestinal epithelial cells. 3. Enzymic activities and chemical composition of various fractions of tris-disrupted brush borders. Biochim Biophys Acta. 1967 Jul 3;135(3):475–482. doi: 10.1016/0005-2736(67)90037-5. [DOI] [PubMed] [Google Scholar]
- Emmelot P., Visser A. Studies on plasma membranes. 13. Co 2+ -activated aminopeptidase(s) in the globular units locally coating rat-liver plasma membranes. Biochim Biophys Acta. 1971 Aug 13;241(2):273–289. doi: 10.1016/0005-2736(71)90028-9. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Felgenhauer K., Glenner G. G. The enzymatic hydrolysis of amino acid beta-naphthylamides. II. Partial purification and properties of a particle-bound cobalt-activated rat kidney aminopeptidase. J Histochem Cytochem. 1966 May;14(5):401–413. doi: 10.1177/14.5.401. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick D. F., Davenport G. R., Forte L., Landon E. J. Characterization of plasma membrane proteins in mammalian kidney. I. Preparation of a membrane fraction and separation of the protein. J Biol Chem. 1969 Jul 10;244(13):3561–3569. [PubMed] [Google Scholar]
- Forstner G. G. (1-14C)glucosamine incorporation by subcellular fractions of small intestinal mucosa. Identification by precursor labeling of three functionally distinct glycoprotein classes. J Biol Chem. 1970 Jul 25;245(14):3584–3592. [PubMed] [Google Scholar]
- Forstner G. G., Sabesin S. M., Isselbacher K. J. Rat intestinal microvillus membranes. Purification and biochemical characterization. Biochem J. 1968 Jan;106(2):381–390. doi: 10.1042/bj1060381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstner G. G., Tanaka K., Isselbacher K. J. Lipid composition of the isolated rat intestinal microvillus membrane. Biochem J. 1968 Aug;109(1):51–59. doi: 10.1042/bj1090051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glossmann H., Neville D. M., Jr Glycoproteins of cell surfaces. A comparative study of three different cell surfaces of the rat. J Biol Chem. 1971 Oct 25;246(20):6339–6346. [PubMed] [Google Scholar]
- Groniowski J., Biczyskowa W., Walski M. Electron microscope studies on the surface coat of the nephron. J Cell Biol. 1969 Mar;40(3):585–601. doi: 10.1083/jcb.40.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman I. W., Sacktor B. Histochemical localization of renal trehalase: demonstration of a tubular site. Science. 1968 Aug 9;161(3841):571–572. doi: 10.1126/science.161.3841.571. [DOI] [PubMed] [Google Scholar]
- HUEBSCHER G., WEST G. R. SPECIFIC ASSAYS OF SOME PHOSPHATASES IN SUBCELLULAR FRACTIONS OF SMALL INTESTINAL MUCOSA. Nature. 1965 Feb 20;205:799–800. doi: 10.1038/205799a0. [DOI] [PubMed] [Google Scholar]
- Hanson H., Glässer D., Ludewig M., Mannsfeldt H. G., John M. Struktur- und Wirkungsidentität der Leucinaminopeptidase aus Schweinenieren und Rinderaugenlinsen und Vergleich mit der Partikelaminopeptidase aus Schweinenieren. Hoppe Seylers Z Physiol Chem. 1967 Jun;348(6):689–704. [PubMed] [Google Scholar]
- Hanson H., Hütter H. J., Mannsfeldt H. G., Kretschmer K., Sohr C. Zur Darstellung und Substratspezifität einer von der Leucinaminopeptidase unterscheidbaren Aminopeptidase aus Nierenpartikeln. Hoppe Seylers Z Physiol Chem. 1967 Jun;348(6):680–688. [PubMed] [Google Scholar]
- Henning R., Kaulen H. D., Stoffel W. Biochemical analysis of the pinocytotic process. I. Isolation and chemical composition of the lysosomal and the plasma membrane of the rat liver cell. Hoppe Seylers Z Physiol Chem. 1970 Oct;351(10):1191–1199. doi: 10.1515/bchm2.1970.351.2.1191. [DOI] [PubMed] [Google Scholar]
- Hugon J., Borgers M. A direct lead method for the electron microscopic visualization of alkaline phosphatase activity. J Histochem Cytochem. 1966 May;14(5):429–431. doi: 10.1177/14.5.429. [DOI] [PubMed] [Google Scholar]
- Ito S. The enteric surface coat on cat intestinal microvilli. J Cell Biol. 1965 Dec;27(3):475–491. doi: 10.1083/jcb.27.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King E. J. The colorimetric determination of phosphorus. Biochem J. 1932;26(2):292–297. doi: 10.1042/bj0260292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinne R., Kinne-Saffran E. Isolierung und enzymatische Charakterisierung einer Bürstensaumfraktion der Rattenniere. Pflugers Arch. 1969;308(1):1–15. doi: 10.1007/BF00588029. [DOI] [PubMed] [Google Scholar]
- Kopaczyk K., Perdue J., Green D. E. The relation of structural and catalytic protein in the mitochondrial electron transfer chain. Arch Biochem Biophys. 1966 Jul;115(1):215–225. doi: 10.1016/s0003-9861(66)81060-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MILLER D., CRANE R. K. The digestive function of the epithelium of the small intestine. I. An intracellular locus of disaccharide and sugar phosphate ester hydrolysis. Biochim Biophys Acta. 1961 Sep 16;52:281–293. doi: 10.1016/0006-3002(61)90677-1. [DOI] [PubMed] [Google Scholar]
- MOELBERT E. R., DUSPIVA F., von DEIMLING O. The demonstration of alkaline phosphatase in the electron microscope. J Biophys Biochem Cytol. 1960 Apr;7:387–390. [PMC free article] [PubMed] [Google Scholar]
- Manitius A., Bensch K., Epstein F. H. (Na+K+)-activated ATPase in kidney cell membranes of normal and methyprednisolone-treated rats. Biochim Biophys Acta. 1968 Jun 11;150(4):563–571. doi: 10.1016/0005-2736(68)90045-x. [DOI] [PubMed] [Google Scholar]
- Marosvári I., Tanka D. Die Arylamidase-Aktivität aus Rattennieren isolierter Glomeruli. Klin Wochenschr. 1969 Nov 1;47(21):1178–1179. doi: 10.1007/BF01483752. [DOI] [PubMed] [Google Scholar]
- Mattenheimer H. Enzymology of kidney tissue. Curr Probl Clin Biochem. 1968;2:119–145. [PubMed] [Google Scholar]
- NACHLAS M. M., GOLDSTEIN T. P., SELIGMAN A. M. An evaluation of aminopeptidase specificity with seven chromogenic substrates. Arch Biochem Biophys. 1962 May;97:223–231. doi: 10.1016/0003-9861(62)90073-5. [DOI] [PubMed] [Google Scholar]
- PENNINGTON R. J. Biochemistry of dystrophic muscle. Mitochondrial succinate-tetrazolium reductase and adenosine triphosphatase. Biochem J. 1961 Sep;80:649–654. doi: 10.1042/bj0800649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley J. P., Gotterer G. S. Distribution of (Na+-K+)-stimulated ATPase activity in rat intestinal mucosa. Biochim Biophys Acta. 1969 Apr;173(3):456–468. doi: 10.1016/0005-2736(69)90010-8. [DOI] [PubMed] [Google Scholar]
- Rambourg A., Leblond C. P. Electron microscope observations on the carbohydrate-rich cell coat present at the surface of cells in the rat. J Cell Biol. 1967 Jan;32(1):27–53. doi: 10.1083/jcb.32.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambourg A., Neutra M., Leblond C. P. Presence of a "cell coat" rich in carbohydrate at the surface of cells in the rat. Anat Rec. 1966 Jan;154(1):41–71. doi: 10.1002/ar.1091540105. [DOI] [PubMed] [Google Scholar]
- Ray T. K., Skipski V. P., Barclay M., Essner E., Archibald F. M. Lipid composition of rat liver plasma membranes. J Biol Chem. 1969 Oct 25;244(20):5528–5536. [PubMed] [Google Scholar]
- Reale E., Luciano L. Effect of fixation on the alkaline phosphatase activity of mouse proximal convuluted tubule. J Histochem Cytochem. 1967 Jul;15(7):413–416. doi: 10.1177/15.7.413. [DOI] [PubMed] [Google Scholar]
- Seligman A. M., Wasserkrug H. L., Plapinger R. E., Seito T., Hanker J. S. Membraneous ultrastructural demonstration of aminopeptidase and gamma-glutamyl transpeptidase activities with a new diazonium salt that yields a lipophobic, osmiophilic azo dye. J Histochem Cytochem. 1970 Aug;18(8):542–551. doi: 10.1177/18.8.542. [DOI] [PubMed] [Google Scholar]
- Spiro R. G. Glycoproteins. Annu Rev Biochem. 1970;39:599–638. doi: 10.1146/annurev.bi.39.070170.003123. [DOI] [PubMed] [Google Scholar]
- Taylor D. G., Price R. G., Robinson D. The distribution of some hydrolases in glomeruli and tubular fragments prepared from rat kidney by zonal centrifugation. Biochem J. 1971 May;122(5):641–645. doi: 10.1042/bj1220641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuneberg L., Rostgaard J. Isolation of brush border fragments from homogenates of rat and rabbit kidney cortex. Exp Cell Res. 1968 Jul;51(1):123–140. doi: 10.1016/0014-4827(68)90163-8. [DOI] [PubMed] [Google Scholar]
- Touster O., Aronson N. N., Jr, Dulaney J. T., Hendrickson H. Isolation of rat liver plasma membranes. Use of nucleotide pyrophosphatase and phosphodiesterase I as marker enzymes. J Cell Biol. 1970 Dec;47(3):604–618. doi: 10.1083/jcb.47.3.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilfong R. F., Neville D. M., Jr The isolation of a brush border membrane fraction from rat kidney. J Biol Chem. 1970 Nov 25;245(22):6106–6112. [PubMed] [Google Scholar]
- Winzler R. J. Carbohydrates in cell surfaces. Int Rev Cytol. 1970;29:77–125. doi: 10.1016/s0074-7696(08)60033-9. [DOI] [PubMed] [Google Scholar]