Abstract
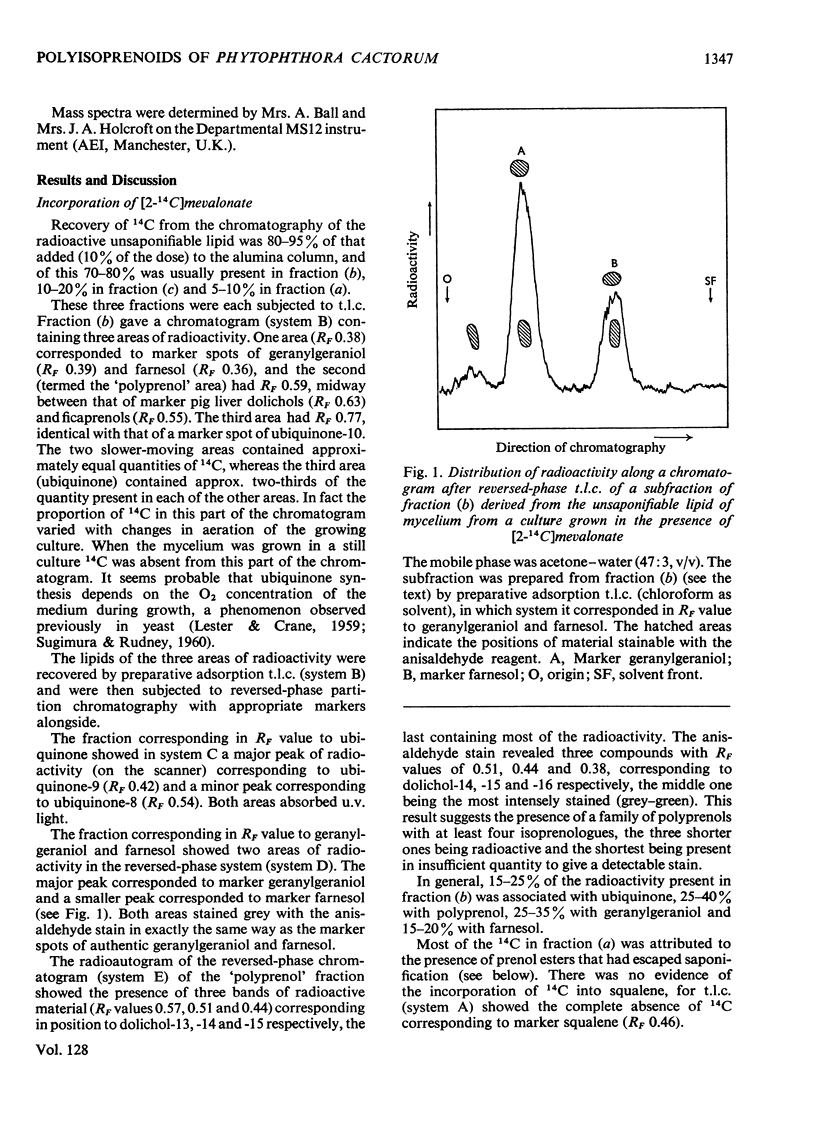
Farnesol, geranylgeraniol, dolichols and ubiquinones were the main radioactive components of the unsaponifiable lipid recovered from Phytophthora cactorum grown in aerated cultures containing [2-14C]mevalonate. The 14C recovered in each of these components was in the approximate proportion 2:4:3:5. When the culture was not aerated no radioactive ubiquinone was recovered. Most of the 14C recovered in the dolichols was found in dolichol-15 (37%), with decreasing amounts in dolichol-14 (30%) and -13 (14%) and only a little (5%) in dolichol-16, whereas the major components, by weight, of the mixture (13μg/g of damp-dry tissue) were dolichol-14, -15 and -16 in the approximate proportion of 1:3:1. Radioautography of appropriate chromatograms indicated the presence also of traces of radioactivity in dolichol-9, -10, -11, -12 and -17. Most (80%) of the 14C recovered in the ubiquinones was associated with ubiquinone-9, the rest being in ubiquinone-8. Most (80%) of the weight of ubiquinones (19μg/g of damp-dry tissue) was also ubiquinone-9. The identification of these compounds was by chromatographic methods and, for the ubiquinones and dolichols, was confirmed by mass spectrometry. In addition, the incorporation of 4R- and/or 4S-3H from [4-3H]-mevalonates showed the expected stereochemistry of biosynthesis, namely that farnesol, geranylgeraniol and ubiquinones were biogenetically all trans and the dolichols each contained three biogenetically trans isoprene residues, the remaining residues being biogenetically cis. The distribution of 14C in the components of the whole lipid of the fungus was consistent with 97% of both the farnesol and geranylgeraniol being present as the fatty acid ester. The corresponding value for dolichols was 37%. The observation by other workers, that this fungus does not form either squalene or sterol, was confirmed.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURGOS J., HEMMING F. W., PENNOCK J. F., MORTON R. A. DOLICHOL: A NATURALLY-OCCURRING C100 ISOPRENOID ALCOHOL. Biochem J. 1963 Sep;88:470–482. [PMC free article] [PubMed] [Google Scholar]
- Barr R. M., Hemming F. W. Polyprenols of Aspergillus niger. Their characterization, biosynthesis and subcellular distribution. Biochem J. 1972 Mar;126(5):1193–1202. doi: 10.1042/bj1261193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornforth J. W., Cornforth R. H., Donninger C., Popják G. Studies on the biosynthesis of cholesterol XIX. Steric course of hydrogen eliminations and of C-C bond formations in squalene biosynthesis. Proc R Soc Lond B Biol Sci. 1966 Jan 18;163(993):492–514. doi: 10.1098/rspb.1966.0004. [DOI] [PubMed] [Google Scholar]
- Dunphy P. J., Kerr J. D., Pennock J. F., Whittle K. J., Feeney J. The plurality of long chain isoprenoid alcohols (polyprenols) from natural sources. Biochim Biophys Acta. 1967 Feb 7;136(1):136–147. doi: 10.1016/0304-4165(67)90329-7. [DOI] [PubMed] [Google Scholar]
- Dunphy P. J., Whittle K. J., Pennock J. F. On the use of fluorescein and dichlorofluorescein as non-destructive stains for lipids. Chem Ind. 1965 Jul 3;27:1217–1218. [PubMed] [Google Scholar]
- Elliott C. G., Hendrie M. R., Knights B. A. The sterol requirement of Phytophthora cactorum. J Gen Microbiol. 1966 Mar;42(3):425–435. doi: 10.1099/00221287-42-3-425. [DOI] [PubMed] [Google Scholar]
- Feeney J., Hemming F. W. Nuclear magnetic resonance spectrometry of naturally occurring polyprenols. Anal Biochem. 1967 Jul;20(1):1–15. doi: 10.1016/0003-2697(67)90258-8. [DOI] [PubMed] [Google Scholar]
- Gough D. P., Hemming F. W. The characterization and stereochemistry of biosynthesis of dolichols in rat liver. Biochem J. 1970 Jun;118(1):163–166. doi: 10.1042/bj1180163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough D. P., Hemming F. W. The stereochemistry of betulaprenol biosynthesis. Biochem J. 1970 Apr;117(2):309–317. doi: 10.1042/bj1170309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough D. P., Kirby A. L., Richards J. B., Hemming F. W. The characterization of undecaprenol of Lactobacillus plantarum. Biochem J. 1970 Jun;118(1):167–170. doi: 10.1042/bj1180167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LESTER R. L., CRANE F. L. The natural occurrence of coenzyme Q and related compounds. J Biol Chem. 1959 Aug;234(8):2169–2175. [PubMed] [Google Scholar]
- Muraca R. F., Whittick J. S., Daves G. D., Jr, Friis P., Folkers K. Mass spectra of ubiquinones and ubiquinols. J Am Chem Soc. 1967 Mar 15;89(6):1505–1508. doi: 10.1021/ja00982a038. [DOI] [PubMed] [Google Scholar]
- Rothschild A. M., Gascon L. A. Sulphuric esters of polysaccharides as activators of a bradykinin-forming system in plasma. Nature. 1966 Dec 17;212(5068):1364–1364. doi: 10.1038/2121364a0. [DOI] [PubMed] [Google Scholar]
- SUGIMURA T., RUDNEY H. The adaptive formation of ubiquinone 30 (coenzyme Q6) in yeast. Biochim Biophys Acta. 1960 Jan 29;37:560–561. doi: 10.1016/0006-3002(60)90527-8. [DOI] [PubMed] [Google Scholar]
- Scher M., Lennarz W. J., Sweeley C. C. The biosynthesis of mannosyl-1-phosphoryl-polyisoprenol in Micrococcus lysodeikticus and its role in mannan synthesis. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1313–1320. doi: 10.1073/pnas.59.4.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone K. J., Butterworth P. H., Hemming F. W. Characterization of the hexahydropolyprenols of Aspergillus fumigatus Fresenius. Biochem J. 1967 Feb;102(2):443–455. doi: 10.1042/bj1020443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone K. J., Hemming F. W. The characterization and distribution of hexahydropolyprenyl esters in cultures of Aspergillus fumigatus Fresenius. Biochem J. 1968 Oct;109(5):877–882. doi: 10.1042/bj1090877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone K. J., Hemming F. W. The stereochemistry of hexahydroprenol, ubiquinone and ergosterol biosynthesis in the mycelium of Aspergillus fumigatus Fresenius. Biochem J. 1967 Jul;104(1):43–56. doi: 10.1042/bj1040043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellburn A. R., Stevenson J., Hemming F. W., Morton R. A. The characterization and properties of castaprenol-11, -12 and -13 from the leaves of Aesculus hippocastanum (horse chestnut). Biochem J. 1967 Jan;102(1):313–324. doi: 10.1042/bj1020313. [DOI] [PMC free article] [PubMed] [Google Scholar]


