Abstract
Background
Toxoplasma gondii is a ubiquitous parasite that can cause significant complications when it infects pregnant women and immunocompromised patients. These complications include miscarriage, fetal abnormalities, and fatal cerebral toxoplasmosis. Despite its significance, the true burden of toxoplasmosis in Indonesia remains underexplored. Toxoplasmosis is usually diagnosed by detecting anti-Toxoplasma antibodies, especially IgG. Therefore, we aim to assess the seroprevalence of anti-Toxoplasma IgG among the human population in Indonesia. In addition, we assessed whether the seroprevalence differed across geographical regions, populations, or population risk levels. Its correlation with annual precipitation was also assessed.
Methods
Following Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines, we conducted a systematic review and meta-analysis of data retrieved from PubMed, Portal Garuda, Neliti, and Onesearch.id. Additionally, Google Scholar, government repositories, and the reference list of studies were searched for additional data. We pooled seroprevalence data using the inverse-variance method and a random effects model. Heterogeneity was assessed using I2 statistics and Cochran’s Q test. Risk-of-Bias (RoB) was evaluated using the Joanna Briggs Institute Checklist for Prevalence Studies. Publication bias was assessed using Doi plots and the Luis Furuya-Kanamori (LFK) index. We performed subgroup analysis, meta-regression, and sensitivity analysis to explore source heterogeneity and the robustness of the pooled estimates. We used Spearman's rank correlation coefficient to assess the correlation between seroprevalence and annual precipitation.
Result
In total, 56 studies were included in this study. The adjusted seroprevalence of anti-Toxoplasma IgG was 60.06% (95% CI: 52.22–67.65%). Study location and detection method were detected as significant sources of heterogeneity by subgroup analysis but not meta-regression. However, subgroup analysis and meta-regression identified the study population and population risk level as significant sources of heterogeneity. Publication year, sample size, and RoB were identified as non-significant moderators. Seroprevalence did not correlate with annual precipitation.
Conclusion
Toxoplasmosis is highly prevalent among the human population in Indonesia; however, our study mainly relied on studies with small sample sizes. Furthermore, most of the studies were performed in Java; therefore, some high-quality population-based studies must be conducted in other regions of Indonesia to better estimate the prevalence of toxoplasmosis across the country.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12889-025-21317-2.
Keywords: Toxoplasma gondii, Systematic Review, Meta-analysis, Seroprevalence
Background
Toxoplasma gondii is an apicomplexan parasite that infects most warm-blooded animals, including humans. Humans primarily become infected by consuming water, food, or soil contaminated with Toxoplasma oocyst and eating undercooked meats infected with Toxoplasma bradyzoite cyst [1]. In fact, the consumption of several meat-producing animals, such as pigs, cattle, goats, and poultry, has been implicated in substantially increasing the risk of toxoplasmosis in humans [2–6].
In otherwise healthy adults, Toxoplasma infection, known as toxoplasmosis, typically results in asymptomatic chronic infection. However, in several populations, toxoplasmosis may lead to debilitating or even fatal illness. For example, acute toxoplasmosis in pregnant women can lead to miscarriage, preterm birth, low birth weight, and fetal abnormalities [7, 8]. In patients with HIV, acute or reactivated toxoplasmosis may result in cerebral or disseminated toxoplasmosis, with cerebral toxoplasmosis having an estimated mortality rate of approximately 30% [9–11]. Survivors of cerebral toxoplasmosis may face persistent neurological deficits [12].
Generally, toxoplasmosis in humans is diagnosed by detecting the presence of anti-Toxoplasma antibodies such as IgM and IgG. IgM antibodies can be detected as early as one week after infection and may remain detectable for approximately two years [13, 14]. On the other hand, IgG antibodies appear one to three weeks after IgM becomes detectable and remain for life [14]. However, detecting anti-Toxoplasma IgM alone is insufficient to establish the diagnosis due to its lack of specificity [15, 16]. Therefore, anti-Toxoplasma IgG detection is required to confirm the diagnosis, even in cases where acute toxoplasmosis is suspected. The Sabin-Feldman dye test is considered the gold standard for anti-Toxoplasma antibody detection [17]. However, DT is impractical. As such, other diagnostic methods have been employed to detect anti-T. gondii antibody in both clinical and research settings. These include Enzyme-linked immunosorbent assay (ELISA), Latex Agglutination Test (LAT), and Lateral-flow Immunoassay (LFA), among others.
Around two billion people are infected by this parasite globally [18]. However, the seroprevalence of toxoplasmosis in humans varies between regions and communities, depending on social, economic, and cultural factors [19, 20]. For example, Jones et al. reported an adjusted seroprevalence of 22.5% among people over 12 years old in the United States [21]. A study in Germany estimated that 55% of adults in the country are seropositive for Toxoplasma [22]. However, much lower Toxoplasma seroprevalence was observed in China, with only 12.3% of the examined samples being positive [23].
A recent meta-analysis [24] estimated that the seroprevalence of Toxoplasma infection in Indonesia is at least 60%, but this estimate was based on a single study [25]; the authors’ search strategy did not include Indonesian journal databases such as Portal Garuda, in which several articles on this subject are indexed. To address this gap, we conducted a systematic review and meta-analysis to assess the seroprevalence of anti-Toxoplasma IgG in the human population in Indonesia. In addition, we assessed whether the seroprevalence differs across geographical regions, populations, or population risk levels.
Methods
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines [26] and is registered with PROSPERO (ID CRD42023466909). The PRISMA 2020 checklist is available in Additional File 1.
Eligibility criteria
We included studies that met the following criteria:
Reported the seroprevalence of Anti-Toxoplasma IgG in the human population in Indonesia or presented data that can be used to estimate the seroprevalence
Detailed the diagnostic methods used to estimate the seroprevalence of Anti-Toxoplasma IgG in the human population in Indonesia
Were published before November 2024
We excluded studies that met the following criteria:
Did not contain primary data (e.g., systematic review and meta-analysis).
Were case reports.
Had limited access and failed to respond to follow-up emails twice.
Search strategy
We systematically searched PubMed, Portal Garuda, Neliti, and Onesearch.id for eligible records. Key search terms used in different combinations were toxoplasmosis, Toxoplasma, prevalence, epidemiology, seroprevalence, seroepidemiology, and Indonesia. Boolean operators and truncation were used to increase the sensitivity of the search strategy. No language or study type restrictions were applied. All identified records were retrieved and uploaded to Rayyan [27] for review.
Additionally, we did a manual search in the Indonesian Ministry of Health Repository, the National Research and Innovation Agency (BRIN) Repository, The National Institute of Health Research and Development (Balitbangkes) Repository, and Google Scholar for eligible records. The reference list of studies eligible for further assessment and all review articles were also screened for potentially relevant studies.
A detailed search strategy can be found in Additional File 2.
Selection process
After deduplication, two reviewers (SK and AHD) independently selected records that satisfied the eligibility criteria. Any disagreements between the reviewers were resolved through discussion. Afterwards, a third reviewer (TMP) confirmed the eligibility of the records included. The reviewers were not blinded to the journal titles, study authors, or institutions.
Data extraction
In this review, Toxoplasma infection is defined as the detection of anti-Toxoplasma IgG through serological testing of the human population. The primary outcome of this review is the pooled seroprevalence of Toxoplasma infection in the human population in Indonesia through a systematic review. Seroprevalence is the ratio of positive samples to the total number of samples. The additional outcomes of this study were the seroprevalence of Toxoplasma infection across different geographical regions, study populations, and population risk levels. The study locations were grouped based on the major geographical regions they belong to: Java, Sumatra, Kalimantan, Sulawesi and Maluku, Lesser Sunda Island, and Papua. The population risk level was stratified into high- and low-risk groups [28]. Populations that belonged to the high-risk group included women with a history of miscarriage and/or stillbirth, people who routinely have close contact with animals (e.g., animal market workers, pet shop workers), cat owners, immunocompromised patients, and psychiatric patients. Populations that did not belong to these groups were classified as low risk. Additionally, we also assessed the correlation between the toxoplasmosis seroprevalence and annual precipitation at national and local level. The national annual precipitation data were retrieved from ourworldindata website on December 24, 2024 (publicly available from https://ourworldindata.org/grapher/average-precipitation-per-year) [29]. Meanwhile, the local annual precipitation data were retrieved from publicly available data on the local Central Bureau of Statistics (BPS) website.
We extracted the following data from the studies: first author’s last name, year of publication, study location, population studied, diagnostic method, sample size, and total number of cases. If the number of cases was not presented directly, we inferred it based on the reported prevalence. The two reviewers (SK and AHD) extracted these data into a Google Sheets spreadsheet. A third author (TMP) referred to the original studies to confirm whether these data had been extracted correctly.
Quality assessment
The quality of the records that were included was assessed independently by two authors (AMTS and FPSW), using a checklist for prevalence studies developed by the Joanna Briggs Institute [30]. Any disagreements between the reviewers were resolved through discussion. When assessing the question, “Was the sample size adequate?”, we estimated the minimum sample size via the formula [n = z^2 * (p * (1-p))/e^2], where z was set at 95%, e was set at 5%, and p was set at 16.4% following the estimated prevalence of toxoplasmosis in Asia [24]. Based on these findings, we set the minimum sample size at 211 participants and used this value as our evaluation standard. A score was calculated based on the proportion of “yes” answers. Afterwards, the records were classified as “low risk of bias,” “moderate risk of bias,” or “high risk of bias” if their scores were ≥ 70%, 50–69%, or ≤ 49%, respectively [31, 32]. Studies with a high risk of bias (RoB) were excluded from further analysis.
Data analysis
Narrative and quantitative approaches were used to synthesize systematic review data. Unless otherwise stated, all data processing and analysis were conducted via Meta (version 7.0–0) and MetaSens (version 1.5–2) packages in R computational package (version 4.4.2) [33]. The prevalence estimate was calculated for each study, and we then pooled the seroprevalence data via the inverse-variance method and a random effects model. The summary measure was calculated using the Freeman-Tukey Double arcsine transformation. We also calculated the 95% confidence interval (95% CI). A summary of the results and the heterogeneity among the studies was presented via a forest plot. I2 statistics were used to determine heterogeneity, with a value of > 75% considered substantial heterogeneity [32]. Cochran’s Q test was used to test the significance of heterogeneity. Subgroup analysis and meta-regression were performed to explore potential sources of heterogeneity. In addition, sensitivity analysis was performed to assess any study that might impact the pooled prevalence estimate. Doi plots and the Luis Furuya-Kanamori (LFK) index were used to assess the presence of publication bias [34]. A p-value was considered significant if it was < 0.05.
The analysis on the correlation between toxoplasmosis seroprevalence and annual precipitation was performed using native functions in R computational package (version 4.4.2). First the distribution of seroprevalence data was tested using Shapiro–Wilk test. Next, Spearman's rank correlation coefficient was calculated to determine the correlation of toxoplasmosis seroprevalence and annual precipitation. Finally, a scatter plot with trend line was produced using ggplot2 (version 3.5.1) and smplot2 (version 0.2.4) packages in R computational package (version 4.4.2) [35, 36].
Results
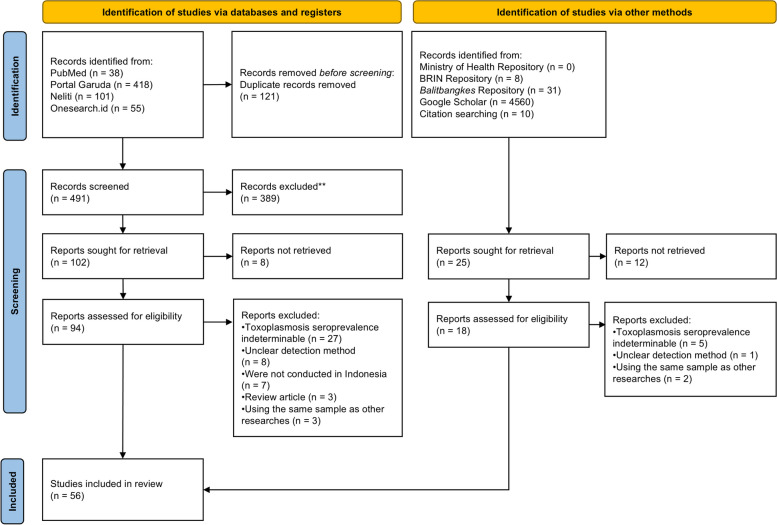
Our database search identified 612 records (Fig. 1), 102 of which passed the initial screening and were retrieved for further assessment. However, we were unable to retrieve 8 of these records. Among these, 46 were included in the subsequent analysis. Additionally, we identified 4609 records through manual search in government repositories, Google Scholar search, and manual citation searching. Through this method, we found ten records that met our inclusion criteria and were included in the downstream analysis. Taken together, 56 studies were included in this study (Table 1).
Fig. 1.
PRISMA flow diagram. Of the 5,221 identified records, 56 were included
Table 1.
List and characteristics of studies that assessed the anti-Toxoplasma IgG seroprevalence in Indonesia
| Year | Author | Location | Region | Population | Method | Case | Sample | Seroprevalence | ROB | Ref |
|---|---|---|---|---|---|---|---|---|---|---|
| 1976 | Durfee | South Kalimantan Province | Kalimantan | General Population | IHA | 330 | 1050 | 31.43% | Low | [37] |
| 1996 | Uga | Sidoarjo Regency | Java | General Population | LAT | 156 | 244 | 63.93% | Low | [38] |
| 2000 | Konishi | Surabaya City | Java | General Population | ELISA | 1021 | 1761 | 58.00% | Medium | [39] |
| 2002 | Sardjono | Malang City | Java | Women with History of Miscarriage and/or Stillbirth | ELISA | 22 | 43 | 51.16% | Low | [40] |
| Pregnant Women | ELISA | 14 | 23 | 60.87% | ||||||
| 2003 | Terazawa | Jakarta | Java | General Population | ELISA | 1185 | 1693 | 69.99% | Low | [25] |
| 2004 | Murkati | Surakarta City | Java | Women with History of Miscarriage and/or Stillbirth | ELISA | 33 | 100 | 33.00% | Medium | [41] |
| 2009 | Sanjaya | Manado City | Sulawesi and Maluku | General Population | LAT | 22 | 50 | 44.00% | Medium | [42] |
| 2011 | Lestari | National | National | General Population | ELISA | 2036 | 3067 | 66.40% | Low | [43] |
| 2012 | Tolistiawaty | Palu City | Sulawesi and Maluku | General Population | ELISA | 177 | 412 | 42.96% | Low | [44] |
| 2013 | Laksemi | Denpasar City | Lesser Sunda Islands | General Population | ELISA | 391 | 790 | 49.49% | Low | [2] |
| 2013 | Wiyarno | Surabaya City | Java | People with Close Contact with Animals | ELISA | 16 | 20 | 80.00% | Medium | [45] |
| General Population | 9 | 20 | 45.00% | |||||||
| 2014 | Prasetyo | Surakarta City | Java | General Population | ELISA | 39 | 130 | 30.00% | Medium | [46] |
| 2014 | Resnhaleksmana | West Nusa Tenggara Province | Lesser Sunda Islands | Psychiatric Patients | ELISA | 23 | 42 | 54.76% | Medium | [47] |
| 2014 | Sari | Bantul Regency | Java | Cat Owner | ELISA | 7 | 11 | 63.64% | Medium | [48] |
| General Population | 43 | 79 | 54.43% | |||||||
| 2015 | Agustin | Surabaya City | Java | Cat Owner | ELISA | 13 | 25 | 52.00% | Low | [49] |
| General Population | 12 | 25 | 48.00% | |||||||
| 2015 | Krihariyani | Surabaya City | Java | Pregnant Women | ELISA | 26 | 40 | 65.00% | High | [50] |
| 2015 | Prasetyo | Central Java | Java | HIV Patients | ELISA | 260 | 597 | 43.55% | Low | [51] |
| 2015 | Raharjo | Surakarta City | Java | HIV Patients | ELISA | 9 | 51 | 17.65% | Low | [52] |
| 2015 | Sari | Central Java | Java | General Population | ELISA | 150 | 357 | 42.02% | Low | [53] |
| 2016 | Dwinata | Badung Regency | Lesser Sunda Islands | Pregnant Women | ELISA | 36 | 330 | 10.91% | Medium | [54] |
| 2016 | Kurniawati | Surabaya City | Java | General Population | ELISA | 85 | 234 | 36.32% | Low | [55] |
| 2016 | Laksmi | Karangasem Regency | Lesser Sunda Islands | General Population | ELISA | 14 | 106 | 13.21% | Medium | [56] |
| 2016 | Lestari | Minahasa Regency | Sulawesi and Maluku | General Population | LAT | 15 | 22 | 68.18% | High | [57] |
| 2016 | Seran | Minahasa Regency | Sulawesi and Maluku | General Population | LAT | 11 | 22 | 50.00% | Low | [58] |
| 2016 | Tuda | Manado City | Sulawesi and Maluku | General Population | LAT | 72 | 135 | 53.33% | Medium | [59] |
| Pregnant Women | 47 | 109 | 43.12% | |||||||
| 2017 | Amalia | Surabaya City | Java | General Population | ELISA | 14 | 125 | 11.20% | Medium | [60] |
| 2017 | Febianingsih | Gianyar Regency | Lesser Sunda Islands | General Population | ELISA | 136 | 240 | 56.67% | Low | [61] |
| 2017 | Iskandar | Malang City | Java | General Population | ELISA | 50 | 57 | 87.72% | High | [62] |
| 2017 | Retmanasari | Central Java | Java | General Population | ELISA | 394 | 630 | 62.54% | Low | [20] |
| 2017 | Tuda | North Sulawesi, Gorontalo, and North Maluku Province | Sulawesi and Maluku | General Population | LAT | 501 | 856 | 58.53% | Low | [63] |
| 2018 | Muflikhah | Sleman Regency | Java | Psychiatric Patients | ELISA | 65 | 94 | 69.15% | Medium | [3] |
| General Population | 42 | 64 | 65.63% | |||||||
| 2019 | Alviyah | Surabaya City | Java | General Population | ELISA | 116 | 132 | 87.88% | Medium | [64] |
| 2019 | Arwie | Makassar City | Sulawesi and Maluku | People with Close Contact with Animals | LAT | 4 | 10 | 40.00% | High | [65] |
| 2019 | Darmawan | Jambi City | Sumatra | General Population | LFA | 17 | 41 | 41.46% | Medium | [66] |
| 2019 | Halleyantoro | Jakarta | Java | HIV Patients | ELISA | 34 | 88 | 38.64% | Medium | [67] |
| 2020 | Dzikriana | Surabaya City | Java | People with Close Contact with Animals | LFA | 26 | 30 | 86.67% | Medium | [68] |
| 2020 | Fihiruddin | Sleman Regency | Java | General Population | ELISA | 224 | 385 | 58.18% | High | [69] |
| 2020 | Humaryanto | Jambi City | Sumatra | General Population | LAT | 36 | 60 | 60.00% | High | [70] |
| 2020 | Marthalia | Surabaya City | Java | Cat Owner | ELFA | 11 | 19 | 57.89% | Medium | [71] |
| 2020 | Utami | Batu City | Java | People with Close Contact with Animals | MAT | 40 | 45 | 88.89% | Low | [72] |
| 2021 | Harianja | Samarinda City | Kalimantan | Cat Owner | ECLIA | 10 | 23 | 43.48% | High | [73] |
| 2021 | Polanunu | Makassar City | Sulawesi and Maluku | Pregnant Women | ELISA | 60 | 184 | 32.61% | Low | [74] |
| 2023 | Afrianti | Semarang City | Java | Cat Owner | ELFA | 6 | 25 | 24.00% | High | [75] |
| General Population | 2 | 25 | 8.00% | |||||||
| 2023 | Hanina | Jambi City | Sumatra | Women with History of Miscarriage and/or Stillbirth | ELFA | 22 | 52 | 42.31% | Medium | [76] |
| 2023 | Jayawardhana | Sidoarjo Regency | Java | HIV patients | MAT | 4 | 30 | 13.33% | High | [77] |
| 2023 | Meirawan | Jakarta | Java | HIV patients | ELISA | 38 | 56 | 67.86% | Medium | [78] |
| 2023 | Novikasari | Sampang Regency | Java | Pregnant Women | LFA | 5 | 25 | 20.00% | Medium | [79] |
| 2023 | Pratama | South Tangerang City | Java | People with Close Contact with Animals | LFA | 11 | 25 | 44.00% | Medium | [80] |
| 2023 | Riansari | Semarang City | Java | General Population | ELISA | 43 | 88 | 48.86% | Low | [81] |
| 2023 | Sari | Denpasar City | Lesser Sunda Islands | Pregnant Women | ELFA | 9 | 44 | 20.45% | Medium | [82] |
| 2023 | Setia | Malang City | Java | HIV Patients | ELISA | 87 | 87 | 10.,00% | Medium | [83] |
| 2024 | Afrianti | Semarang City | Java | General Population | ECLIA | 47 | 177 | 26.55% | Medium | [84] |
| 2024 | Rahman | Yogyakarta City | Java | General Population | LFA | 14 | 25 | 56.00% | High | [85] |
| 2024 | Widyaswara | Magelang Regency | Java | General Population | LFA | 0 | 25 | 0.00% | High | [86] |
| 2024 | Wikandari | Semarang City | Java | General Population | ELISA | 31 | 87 | 35.63% | Medium | [87] |
| 2024 | Wulandari | Yogyakarta City | Java | General Population | LFA | 13 | 69 | 18.84% | High | [88] |
IHA Indirect Hemagglutination Test, ELFA Enzyme-linked fluorescence assay, ELISA Enzyme-linked immunosorbent assay, LAT Latex Agglutination Test, MAT Modified Agglutination Test, LFA Lateral-flow Immunoassay, ECLIA Enhanced chemiluminescence immunoassay, RoB Risk-of-Bias
Quality assessment
The RoB for the included studies was assessed via a checklist for prevalence studies developed by the Joanna Briggs Institute [30]. Overall, 19 studies (33.93%) had a low RoB, 25 studies (44.64%) had a moderate RoB, and 12 studies (21.43%) had a high RoB (Additional file 3). Most studies (n = 40) had sample sizes of fewer than 211 participants. Studies that were high RoB, were excluded from subsequent analyses.
Pooled seroprevalence
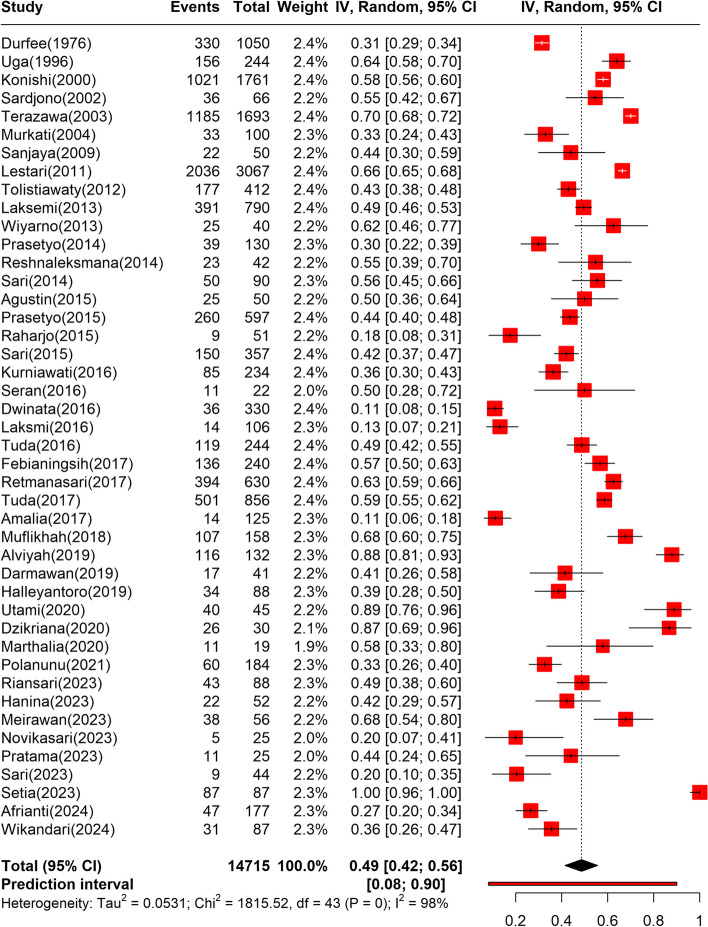
Pooled seroprevalence was derived from the data of 14,715 participants [2, 3, 20, 25, 37–39, 41–49, 52–56, 58–61, 63, 64, 66–68, 71, 72, 74, 76, 78–84, 87]. Among them, 7982 cases were identified. According to the random effects model (Fig. 2), the pooled seroprevalence of toxoplasmosis in Indonesia was 48.58% (95%CI: 41.52%–55.67%). Based on the prediction interval, future studies are expected to yield a seroprevalence rate of between 8 and 90%. Additionally, the pooling result was substantially heterogeneous (I2 = 97.6% (95% CI: 96.1%–97.1%); p < 0.01). Subgroup analysis and meta-regression were performed to investigate the source of heterogeneity.
Fig. 2.
Forest plot depicting the pooled seroprevalence of toxoplasmosis in Indonesia
Subgroup analysis
Subgroup analysis was performed based on the region where the study was conducted, the study population, the population risk level, the sample size, the detection method, and the RoB. Some of the studies derived their data from different populations. Therefore, the number of data sources for the subgroup analysis differed from the number of studies included.
By region
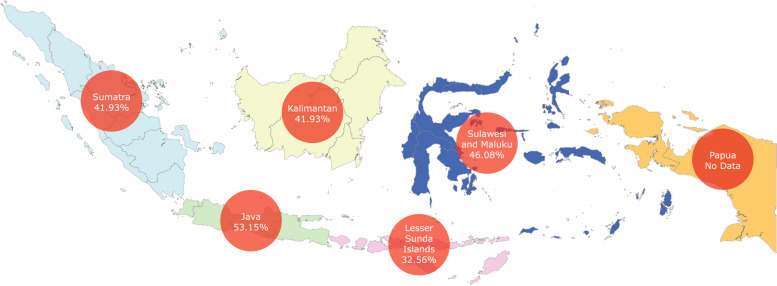
In total, 28 studies were performed in Java, two in Sumatera, one in Kalimantan, six in the Lesser Sunda Islands, and six in Sulawesi and Maluku (Table 2; Fig. 3; Additional file 4). No study that can be included in the subgroup analysis has been performed in Papua. The pooled seroprevalence rates of toxoplasmosis in Java, Sumatera, Kalimantan, Lesser Sunda Island, and Sulawesi and Maluku were 53.15% (95% CI: 43.47–62.72%), 41.93% (95% CI: 31.97%—52.22%), 31.43% (95%CI: 28.65%–34.27%), 32.56% (95% CI: 16.10%–51.56%), and 46.08% (95% CI: 38.04%–54.22%), respectively. Significant differences between subgroups were observed (p < 0.0001).
Table 2.
Subgroup analysis results
| Subgroup Name | Number of data source | Pooled Prevalence | 95%CI | I2 | Subgroup Differences |
|---|---|---|---|---|---|
| By Region | |||||
| Java | 28 | 53.15% | 43.47%–62.72% | 96.9% | P < 0.0001 |
| Sumatra | 2 | 41.93% | 31.97%–52.22% | 0.0% | |
| Kalimantan | 1 | 31.43% | 28.65%–34.27% | - | |
| Lesser Sunda Islands | 6 | 32.56% | 16.10%–51.56% | 98.1% | |
| Sulawesi and Maluku | 6 | 46.08% | 38.04%–54.22% | 91.2% | |
| By Study Population | |||||
| General Population | 27 | 47.85% | 40.72%–55.01% | 97.6% | P = 0. 0026 |
| Cat Owner | 3 | 56.40% | 42.62%–69.73% | 0.0% | |
| People with routine close contacts with animals | 4 | 76.88% | 55.51%–93.10% | 82.7% | |
| Pregnant women | 6 | 29.39% | 16.22%–44.51% | 93.6% | |
| Women with history of miscarriage and/or stillbirth | 3 | 40.85% | 30.49%–51.63% | 53.7% | |
| Immunocompromised patients | 5 | 57.89% | 23.06%–88.83% | 98.3% | |
| Psychiatric patients | 2 | 63.21% | 48.80%–76.55% | 60.9% | |
| By Population Risk Level | |||||
| Low risk | 33 | 44.57% | 37.78%–51.46% | 97.9% | P = 0.0392 |
| High risk | 17 | 59.99% | 47.01%–72.33% | 95.0% | |
| By Sample Size | |||||
| < 211 Samples | 29 | 48.30% | 38.31%–58.36% | 95.9% | P = 0.9008 |
| ≥ 211 Samples | 15 | 49.16% | 40.72%–57.62% | 98.7% | |
| By Detection Method | |||||
| IHA | 1 | 31.43% | 28.65%–34.27% | - | P < 0.0001 |
| LAT | 5 | 54.71% | 47.22%–62.10% | 75.2% | |
| ELISA | 29 | 48.64% | 39.57%–57.75% | 98.0% | |
| LFA | 4 | 48.71% | 20.78%–77.05% | 90.1% | |
| MAT | 1 | 88.89% | 77.78%–96.71% | - | |
| ELFA | 3 | 38.39% | 18.69%–60.16% | 79.0% | |
| ECLIA | 1 | 26.55% | 20.29%–33.33% | - | |
| By ROB Assessment Result | |||||
| Low risk of bias | 19 | 50.98% | 43.55%–58.39% | 97.8% | P = 0.5356 |
| Moderate risk of bias | 25 | 46.71% | 35.64%–57.94% | 97.4% | |
IHA Indirect Hemagglutination Test, ELISA Enzyme-linked immunosorbent assay, LAT Latex Agglutination Test, LFA Lateral-flow Immunoassay, MAT Modified Agglutination Test, ELFA Enzyme-linked fluorescence assay, ECLIA Enhanced chemiluminescence immunoassay, RoB Risk-of-Bias
Fig. 3.
Geographical map of seroprevalence rates of toxoplasmosis by regions
One report derived its data from a nationwide study conducted from 2007 to 2008 [43]. While this report described toxoplasmosis seroprevalence by province, the data it provided could not be pooled in the subgroup analysis. Based on their data, toxoplasmosis seroprevalence in Java, Sumatera, Kalimantan, Lesser Sunda Island, Sulawesi and Maluku, and Papua ranges between 66.2%–75.5%, 61.4%–86%, 60.2%–71.3%, 30.5%–65.0%, 57.7%–75.6%, and 50.0%–91.7%, respectively.
By study population
The data from the studies were derived from seven groups of study populations (Table 2; Additional file 4). These include the general population (n = 27), cat owners (n = 3), people who routinely had close contact with animals (n = 4), pregnant women (n = 6), women with a history of miscarriage and/or stillbirth (n = 3), immunocompromised patients (n = 5), and psychiatric patients (n = 2). Unintentionally, the immunocompromised group comprised only HIV-infected individuals. The pooled prevalence rates for each of these population groups were 47.85% (95% CI: 40.72%–55.01%) for the general population, 56.40% (95% CI: 42.62%–69.73%) for cat owners, 76.88% (95% CI: 55.51%–93.10%) for people who routinely had close contact with animals, 29.39% (95% CI: 16.22%–44.51%) for pregnant women, 40.85% (95% CI: 30.49%–51.63%) for women with a history of miscarriage and/or stillbirth, 57.89% (95% CI: 23.06%–88.83%) for immunocompromised patients, and 63.21% (95% CI: 48.80%–76.55%) for psychiatric patients. Significant differences between the subgroups were observed (p = 0. 0026).
By population risk level
We found 17 data points from the high-risk group and 33 from the low-risk group (Table 2; Additional file 4). The pooled seroprevalence of toxoplasmosis in the high-risk group was 59.99% (95% CI: 47.01%–72.33%), whereas that in the low-risk group was 44.15% (95% CI: 37.78%–51.46%). We observed no differences between the subgroups (p = 0. 0392).
By sample size
We separated the studies by whether they passed our minimum sample size (211 samples) or not. As a result, 15 studies passed the threshold, whereas 29 did not (Table 2; Additional file 4). The pooled seroprevalence of studies that passed the threshold was 49.16% (95% CI: 40.72%–57.62%), whereas studies that did not pass the threshold had a seroprevalence of 48.30% (95% CI: 38.31%–58.36%). We observed no statistically significant differences between the subgroups (p = 0. 9008).
By detection method
The studies assessed the seroprevalence of toxoplasmosis using an indirect hemagglutination test (IHA; one study), a latex agglutination test (LAT; five studies), enzyme-linked immunosorbent assay (ELISA; 29 studies), a lateral flow assay (LFA; five studies), a modified agglutination test (MAT; one studies), an enzyme-linked fluorescence assay (ELFA; three studies), and an enhanced chemiluminescence immunoassay (ECLIA; one studies). The pooled seroprevalence rates based on IHA, LAT, ELISA, LFA, MAT, ELFA, and ECLIA were 31.43% (95% CI: 28.65%–34.27%), 54.71% (95% CI: 47.22%–62.10%), 48.64% (95% CI: 39.57%–57.75%), 48.71% (95% CI: 20.78%–77.05%), 88.89% (95% CI: 77.78%–96.71%), 38.39% (95% CI: 18.69%–60.16%), and 26.55% (95% CI: 20.29%–33.33%), respectively (Table 2; Additional file 4). Substantial differences between the subgroups were observed (p < 0.0001).
By RoB
Most of the studies had a medium RoB (25), followed by a low RoB (19). The pooled seroprevalence rate based on the RoB assessment results was 50.98% (95% CI: 43.55%–58.39%) for studies with a low RoB and 46.71% (95% CI: 35.64%–57.94%) for studies with a medium RoB (Table 2; Additional file 4). No significant differences between the subgroups were observed (p = 0. 5356).
Meta-regression
To further explore potential sources of heterogeneity, we performed meta-regression. From this analysis, publication year (R2 = 0.00%; QM(df = 1) = 0.0581; p = 0.8096) and sample size (R2 = 0.00%; QM(df = 1) = 0.9339; p = 0.3339) were identified as non-significant moderators (Additional file 5).
Additionally, we performed meta-regression on subgroups with significant subgroup differences: region, study population, population risk level, and detection methods. Study population (R2 = 14.19%; QM(df = 6) = 13.5075; p = 0.0356) and population risk level (R2 = 7.54%; QM(df = 1) = 5.0388; p = 0.0248) were identified as significant moderators. Meanwhile, region (R2 = 1.66%; QM(df = 1) = 4.6536; p = 0.3247) and detection method (R2 = 0.00%; QM(df = 6) = 5.5461; p = 0.4759) were identified as non-significant moderators.
Sensitivity analysis
To determine the robustness of the pooling result, we performed a sensitivity analysis using three scenarios and a leave-one-out analysis. In the first scenario, we included eight studies [4, 89–95] that assessed the seroprevalence of toxoplasmosis but did not specify their detection methods. Therefore, these studies did not meet our inclusion criteria. In the second scenario, we included all studies, regardless of their ROB, in the meta-analysis. In the third scenario, we redid the pooling by excluding five studies that had less than 40 samples. The results of the three scenarios were 46.75% (95% CI: 40.36%–53.19%), 47.30% (95% CI: 40.65%–54.00%), and 48.58% (95% CI: 41.52%–55.67%), respectively. Furthermore, when we performed a leave-one-out analysis, the meta-analysis results remained stable after re-evaluation. Detailed sensitivity analysis results can be seen in Additional file 6.
Publication bias analysis
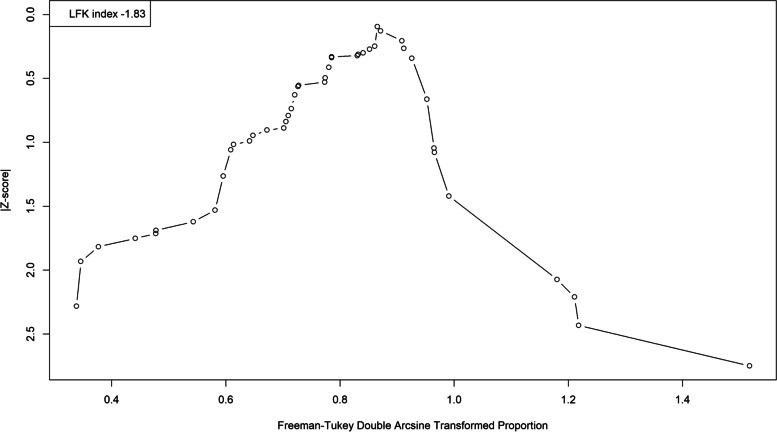
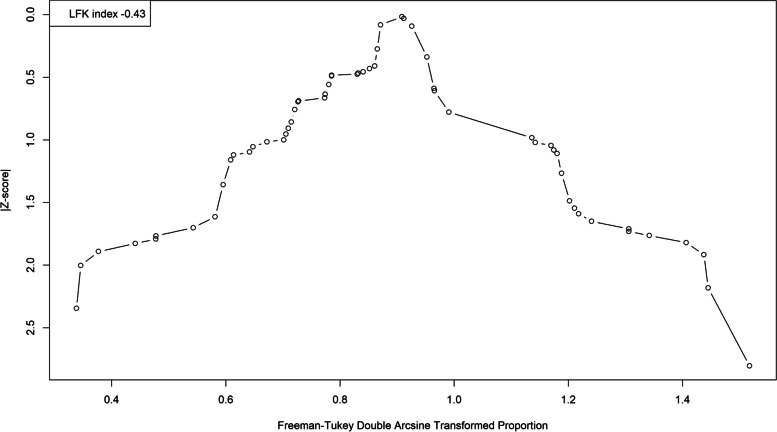
We analyzed publication bias on studies that used serological tests with the LFK index and Doi plot. We detected publication bias in favor of studies with lower seroprevalence rates (LFK index = − 1.83; Fig. 4). We then performed a trim-and-fill analysis, which assigned 13 studies (Fig. 5). This resulted in a pooled seroprevalence of 60.06% (95% CI: 52.22–67.65%; τ2: 0.0873; I2: 98.4%).
Fig. 4.
A Doi plot examining the risk of publication bias for the seroprevalence of toxoplasmosis. Note the left-side asymmetry as indicated by the LFK index
Fig. 5.
A Doi plot examining the risk of publication bias for the seroprevalence of toxoplasmosis corrected using the trim-and-fill method
Correlation between toxoplasmosis seroprevalence and annual precipitation
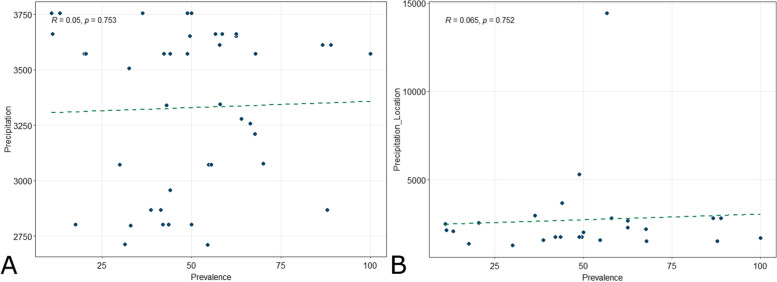
We examined toxoplasmosis seroprevalence from 42 studies for its correlation with national-level annual precipitation data [29]. On the other hand, due to the limited availability of local-level annual precipitation data [96–112], the correlation of local-level annual precipitation data with toxoplasmosis seroprevalence was examined in only 26 studies. Both national (R = 0.05, p = 0.753) and local (R = 0.065, p = 0.752) annual precipitation were not correlated with toxoplasmosis seroprevalence (Fig. 6). See Additional File 7 for the detailed annual precipitation data.
Fig. 6.
Scatterplots with trend line depicting the correlation between toxoplasmosis seroprevalence and national (A) and local (B) annual precipitation data
Discussion
Based on the available literature, this systematic review and meta-analysis were conducted to approximate the true seroprevalence of toxoplasmosis in Indonesia. Unlike other systematic reviews on similar topics, we included Portal Garuda, Neliti, and Onesearch.id, databases that index literature published in Indonesian journals and repositories. Additionally, we also searched government reports published in several repositories managed by Indonesian government bodies. Given that many of these studies were published in the Indonesian language, our attempt allowed us to minimize the occurrence of language bias in our work. In this study, we estimated the seroprevalence of anti-Toxoplasma IgG among humans in Indonesia based on data from 14,715 participants with 7,982 cases. This yields a seroprevalence of 48.58%. However, 13 hypothetical studies were added to adjust for publication bias, yielding an adjusted seroprevalence of 60.06%. Based on our prediction interval, subsequent studies on the topic will yield an anti-Toxoplasma IgG seroprevalence of between 8 and 90%. This result is similar to another systematic review that estimated that the seroprevalence of toxoplasmosis in Indonesia was at least 60% [24]. Additionally, our finding aligns with an Indonesian nationwide biomedical survey in 2007 – 2008, which stated that national toxoplasmosis seroprevalence was 66.40% [43]. Our result was also comparable to other systematic reviews conducted in Thailand and Malaysia, which reported seroprevalence rates of between 2.6% and 53.7% in Thailand and between 10.6% and 59.7% in Malaysia [113, 114]. However, other neighboring countries, namely Cambodia, Singapore, Myanmar, Laos, and Vietnam, had much lower estimated seroprevalence rates of between 5.8% and 31.7% [114]. In addition, our estimated seroprevalence was higher than the overall estimated seroprevalence in Asia, which ranges from 16.4% to 29% [24, 115, 116].
The geographical region was revealed to significantly contribute to the heterogeneity of the meta-analysis result by subgroup analysis but not meta-regression. This means that the subgroup analysis detected significant variance in toxoplasmosis prevalence across different geographical regions. However, an examination of the national biomedical survey report [43] reveals that toxoplasmosis seroprevalence across these regions is, in fact, comparable with a slightly smaller seroprevalence range in Lesser Sunda Island. Therefore, the subgroup analysis may have falsely detected a difference in toxoplasmosis seroprevalence between the regions, considering that the chance of producing a false positive result increases with the number of independent subgroups [117]. On the other hand, the opposite might also be true, as most of the studies were conducted in Java. At the same time, the other regions have at most six studies. This would make meta-regression too underpowered to detect statistically significant associations [118]. Regardless, given Indonesia's vast geographical size and extensive sociocultural diversity, it is plausible that there are actual variations in toxoplasmosis seroprevalence across the region. Different parts of Indonesia are known to have variations in living standards, cultural practices, and precipitation patterns, among others. These factors have been documented to influence the risk of toxoplasmosis both in Indonesia and other countries[56, 58, 119]. As such, it is important to investigate by conducting high-quality population-based studies outside Java to obtain a more accurate assessment of the true prevalence of toxoplasmosis in these regions.
Another factor that might affect the difference in toxoplasmosis seroprevalence between the study regions is difference in annual precipitation. Previous studies have associated the difference in annual precipitation with the incidence of congenital toxoplasmosis and reactivation of toxoplasmic retinochoroiditis [120, 121]. This is likely related to the increased survival of T. gondii oocyst in humid soil [122]. However, our analysis suggested that toxoplasmosis seroprevalence was not related to difference in annual precipitation on both national and local level. This is likely because we assessed the seroprevalence of chronic toxoplasmosis as measured by IgG seropositivity. Meanwhile, the other studies [120, 121] assessed active and reactivated toxoplasmosis. Therefore, we hypothesize that while the difference in annual precipitation may be a risk factor for congenital toxoplasmosis and reactivation of toxoplasmic retinochoroiditis, it is not a significant risk factor for chronic toxoplasmosis. Regardless, further studies assessing the association between active toxoplasmosis in Indonesia and annual precipitation is needed to clarify this finding.
Studies being analyzed in this systematic review used different diagnostic tools to detect anti-Toxoplasma IgG. These diagnostic tools were IHA, LAT, ELISA, LFA, MAT, ELFA, and ECLIA. The difference between the diagnostic tools being employed by these studies was detected to be a significant moderator by subgroup analysis but not meta-regression. One of the simplest explanations would be the difference in their sensitivity and specificity. However, several studies found that these different diagnostic tools have comparable sensitivity and specificity to detect anti-Toxoplasma IgG [13, 123]. As such, another possible explanation would be that the subgroup analysis falsely detected differences across studies using these different diagnostic tools. This suspicion is reinforced by the subgroup analysis, which included seven independent subgroups, substantially increasing the likelihood of a false positive result [117].
Study population and population risk level are two moderators that significantly contributed to the heterogeneity as assessed by subgroup analysis and meta-regression. These moderators accounted for 14.19% and 7.54% of the heterogeneity. In our study, high-risk populations comprised women with a history of miscarriage and/or stillbirth, people who routinely have close contact with animals (e.g., animal market workers, pet shop workers), cat owners, immunocompromised patients, and psychiatric patients. These population groups are people with a higher probability of contracting toxoplasmosis [28]. In line with this assumption, we found that the high-risk group had higher anti-Toxoplasma IgG seroprevalence.
We estimated that the seroprevalence of toxoplasmosis among women with a history of miscarriage and/or stillbirth was 40.85%. These data were derived from three studies that reported 195 cases in total. Our findings are similar to several other meta-analyses that investigated similar conditions. The reported seroprevalence in this population ranged from 32 to 43% [28, 124, 125]. Indeed, toxoplasmosis has long been reported as one of the risk factors for miscarriage, with IgM seropositivity resulting in a higher chance of miscarriage than IgG seropositivity alone [7, 125]. However, our study did not assess active toxoplasmosis, which is indicated by IgM.
Cats are the definitive hosts of Toxoplasma. As such, they are believed to be a significant risk factor for toxoplasmosis through close contact with them [126, 127]. In addition, given that Toxoplasma can infect virtually all warm-blooded animals, people with occupations that put them in close contact with animals are also thought to be at increased risk of contracting the disease [126]. In our study, the estimated seroprevalence of toxoplasmosis among cat owners and people who routinely have close contact with animals was higher than the general population (56.40%, 76.88%, and 47.85%, respectively). However, it should be noted that the studies that assess the toxoplasmosis seroprevalence, specifically among cat owners and people who routinely have close contact with animals in the current review, only included a small number of participants. This potentially makes them unable to assess the true prevalence accurately in the population. As such, extrapolating this finding must be performed carefully. Regardless, a large population-based study assessing this issue might be needed to estimate the seroprevalence of toxoplasmosis among cat owners in Indonesia more accurately.
Our study revealed an alarmingly high seroprevalence of toxoplasmosis among HIV-infected individuals (57.89%). This prevalence is much higher than the estimated seroprevalence in the Asia–Pacific region, only 25.1% [128]. People infected with HIV are at increased risk of suffering from severe forms of toxoplasmosis, such as cerebral toxoplasmosis. This condition has approximately a 30% mortality rate and may even leave surviving patients with lingering sequelae [9–12]. The Indonesian government recommends Toxoplasma screening for all patients newly diagnosed with HIV [129]. In addition, HIV-infected individuals with CD4 + T cells of fewer than 100 cells/µl and seropositive for Toxoplasma are required to receive primary prophylaxis via cotrimoxazole.
The connection between toxoplasmosis and psychiatric disorders in humans has been studied extensively. For example, Toxoplasma IgG seropositivity was associated with schizophrenia and bipolar disorder [130, 131]. Hence, psychiatric patients are among the populations of interest when studying toxoplasmosis. In our analysis, the estimated seroprevalence of toxoplasmosis among psychiatric patients was 63.21%. This result was higher than the estimated seroprevalence in Asia, which was 43.0% [132].
Regardless, our systematic review encountered several limitations. Most of the studies had a small sample size. Even when we set the standard to the modest 16.4% estimated prevalence in Asia overall [24], most studies did not meet our minimum sample size criteria. Studies with small-sample sizes tend to have wider margins of error, i.e., their observed prevalence is more likely to deviate from the true population prevalence [133]. In addition, most of the studies were conducted in Java. The number of studies conducted in other parts of Indonesia was scarce. Therefore, although we attempted to ensure the robustness of our findings, care should be taken when extrapolating the data to other regions. The final limitation that we encountered was our inability to access older studies. In total, we identified 20 records that could be included in our review. However, we could not find any digital or physical copies of these records.
Conclusion
Toxoplasmosis is highly prevalent among the human population in Indonesia, with an adjusted seroprevalence of 60.06%. However, our study mainly relied on studies with small sample sizes. Furthermore, most of the studies were performed in Java; therefore, some high-quality population-based studies must be conducted in other regions of Indonesia to better estimate the prevalence of toxoplasmosis across the country.
Supplementary Information
Acknowledgements
The authors would like to express their gratitude to Ahmad Watsiq Maula and Bayu Satria Wiratama from the Department of Biostatistics, Epidemiology and Population Health, Universitas Gadjah Mada for their advice during the data analysis process. We thank Enago for providing their professional English language editing service.
Abbreviations
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RoB
Risk-of-Bias
- CI
Confidence Interval
- LFK Index
Luis Furuya-Kanamori Index
- PCR
Polymerase Chain Reaction
- CSF
Cerebrospinal Fluid
- ELFA
Enzyme-linked fluorescence assay
- ELISA
Enzyme-linked immunosorbent assay
- LAT
Latex Agglutination Test
- LFA
Lateral-flow Immunoassay
- ECLIA
Enhanced chemiluminescence immunoassay
- IHA
Indirect Hemagglutination Test
- MAT
Modified Agglutination Test
Authors’ contributions
TMP wrote the systematic review protocol, confirmed the eligibility of the included studies, cross-checked the validity of data extraction, analyzed the extracted data using R, and drafted the main manuscript text. AHD screened the eligibility of identified records, extracted the data of the included studies, and prepared the figures. SK screened the eligibility of identified records, extracted the data of the included studies, and prepared the additional files. AMTS assessed the quality of the included studies and prepared the tables. FPSW assessed the quality of the included studies and checked the reference list. All authors reviewed the manuscript.
Funding
This study received no funding.
Data availability
All data that were analyzed for this study are included in both the main manuscript and the additional files.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. Int J Parasitol. 2000;30:1217–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laksemi DAAS, Tunas AW, Wijayanti MA. Seroprevalensi yang Tinggi dan Faktor-Faktor Risiko Toksoplasmosis pada Darah Donor dan Wanita di Bali. Jurnal Veteriner. 2013;14:204–12. [Google Scholar]
- 3.Muflikhah ND, Supargiyono S, Artama WT. Seroprevalence and risk factor of toxoplasmosis in schizophrenia patients referred to grhasia psychiatric hospital, Yogyakarta Indonesia. Afr J Infect Dis. 2018;12(1):76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanafiah M, Kamaruddin M, Winaruddin WN. Studi infeksi toksoplasmosis pada manusia dan hubungannya dengan hewan di Banda Aceh. J Kedokt Hewan (Banda Aceh). 2010;4:87–92. [Google Scholar]
- 5.Wulandari R, Suwandi JF, Mutiara H, Hanriko R. Seroprevalensi Toxoplasma gondii pada Hewan Ternak Sapi di Kota Bandar Lampung. J Agromedicine. 2019;6:1–5. [Google Scholar]
- 6.Insan ANM, Suwandi JF, Lisiswanti R, Mutiara H. Perbandingan Seroprevalensi Toxoplasma gondii pada Ayam Ras dan Ras di Kota Bandar Lampung. J Agromed. 2019;6:46–50. [Google Scholar]
- 7.Kalantari N, Gorgani-Firouzjaee T, Moulana Z, Chehrazi M, Ghaffari S. Toxoplasma gondii infection and spontaneous abortion: A systematic review and meta-analysis. Microb Pathog. 2021;158: 105070. [DOI] [PubMed] [Google Scholar]
- 8.Ahmed M, Sood A, Gupta J. Toxoplasmosis in pregnancy. Eur J Obstetr Gynecol Reprod Biol. 2020;255:44–50. [DOI] [PubMed] [Google Scholar]
- 9.Nissapatorn V, Lee C, Fatt Quek K, Leong CL, Mahmud R, Abdullah KA. Toxoplasmosis in HIV/AIDS patients: a current situation. Original Article Jpn J Infect Dis. 2004;57:160–5. [PubMed] [Google Scholar]
- 10.Antinori A, Larussa D, Cingolani A, Lorenzini P, Bossolasco S, Finazzi MG, et al. Prevalence, associated factors, and prognostic determinants of AIDS-related toxoplasmic encephalitis in the era of advanced highly active antiretroviral therapy. Clin Infect Dis. 2004;39:1681–91. [DOI] [PubMed] [Google Scholar]
- 11.Luma HN, Tchaleu BCN, Mapoure YN, Temfack E, Doualla MS, Halle MP, et al. Toxoplasma encephalitis in HIV/AIDS patients admitted to the Douala general hospital between 2004 and 2009: a cross sectional study. BMC Res Notes. 2013;6:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin-Iguacel R, Ahlström MG, Touma M, Engsig FN, Stærke NB, Stærkind M, et al. Incidence, presentation and outcome of toxoplasmosis in HIV infected in the combination antiretroviral therapy era. J Infect. 2017;75:263–73. [DOI] [PubMed] [Google Scholar]
- 13.Liu Q, Wang ZD, Huang SY, Zhu XQ. Diagnosis of toxoplasmosis and typing of Toxoplasma gondii. Parasites Vectors. 2015;8:292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teimouri A, Mohtasebi S, Kazemirad E, Keshavarz H. Role of Toxoplasma gondii IgG avidity testing in discriminating between acute and chronic toxoplasmosis in pregnancy. J Clin Microbiol. 2020;58:e00505. 10.1128/jcm.00505-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Toxoplasmosis. DPDx - Laboratory Identification of Parasites of Public Health Concern. 2024. https://www.cdc.gov/dpdx/toxoplasmosis/index.html. Accessed 23 Nov 2024.
- 16.Liesenfeld O, Press C, Montoya JG, Gill R, Isaac-Renton JL, Hedman K, et al. False-positive results in immunoglobulin M (IgM) Toxoplasma antibody tests and importance of confirmatory testing: the Platelia Toxo IgM test. J Clin Microbiol. 1997;35:174–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reiter-Owona I, Petersen E, Joynson D, Aspo H, Darde ML, Disko R, et al. The past and present role of the Sabin-Feldman dye test in the serodiagnosis of toxoplasmosis. Bull World Health Organ. 1999;77:929–35. [PMC free article] [PubMed] [Google Scholar]
- 18.Smith NC, Goulart C, Hayward JA, Kupz A, Miller CM, van Dooren GG. Control of human toxoplasmosis. Int J Parasitol. 2021;51:95–121. [DOI] [PubMed] [Google Scholar]
- 19.Friesema IHM, Hofhuis A, Hoek-Van Deursen D, Jansz AR, Ott A, Van Hellemond JJ, et al. Risk factors for acute toxoplasmosis in the Netherlands. Epidemiol Infect. 2023;151:e95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Retmanasari A, Widartono BS, Wijayanti MA, Artama WT. Prevalence and risk factors for toxoplasmosis in middle Java Indonesia. Ecohealth. 2017;14:162–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones JL, Kruszon-Moran D, Wilson M, McQuillan G, Navin T, McAuley JB. Toxoplasma gondii Infection in the United States: Seroprevalence and Risk Factors. Am J Epidemiol. 2001;154:357–65. [DOI] [PubMed] [Google Scholar]
- 22.Wilking H, Thamm M, Stark K, Aebischer T, Seeber F. Prevalence, incidence estimations and risk factors of Toxoplasma gondii infection in Germany: a representative, cross-sectional, serological study. Sci Rep. 2016;6:22551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiao Y, Yin J, Jiang N, Xiang M, Hao L, Lu H, et al. Seroepidemiology of human Toxoplasma gondii infection in China. BMC Infect Dis. 2010;10:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Molan A, Nosaka K, Hunter M, Wang W. Global status of Toxoplasma gondii infection: systematic review and prevalence snapshots. Trop Biomed. 2019;36:898–925. [PubMed] [Google Scholar]
- 25.Terazawa A, Muljono R, Susanto L, Margono SS, Konishi E. High Toxoplasma antibody prevalence among inhabitants in Jakarta. Indonesia Jpn J Infect Dis. 2003;56:107–9. [PubMed] [Google Scholar]
- 26.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, The PRISMA, et al. statement: An updated guideline for reporting systematic reviews. The BMJ. 2020;2021:372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de la Galvan-Ramirez ML, Troyo R, Roman S, Calvillo-Sanchez C. A systematic review and meta-analysis of Toxoplasma gondii infection among the Mexican population. Parasit Vectors. 2012;5:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ritchie H, Rosado P, Samborska V. Data Page: Annual precipitation. Climate Change. 2024. https://ourworldindata.org/grapher/average-precipitation-per-year?tab=chart&country=~IDN.
- 30.Munn Z, Lisy K, Riitano D, Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc. 2015;13:147–53. [DOI] [PubMed] [Google Scholar]
- 31.Islam MA, Alam SS, Kundu S, Hossan T, Kamal MA, Cavestro C. Prevalence of headache in patients with Coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis of 14,275 patients. Front Neurol. 2020;11:562634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hajissa K, Islam MA, Sanyang AM, Mohamed Z. Prevalence of intestinal protozoan parasites among school children in africa: A systematic review and meta-analysis. PLoS Negl Trop Dis. 2022;16:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evidence Based Mental Health. 2019;22:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Furuya-Kanamori L, Barendregt JJ, Doi SAR. A new improved graphical and quantitative method for detecting bias in meta-analysis. JBI Evid Implement. 2018;16:195. [DOI] [PubMed] [Google Scholar]
- 35.Wickham H. ggplot2: Elegant Graphics for Data Analysis. New York: Springer-Verlag, New York; 2016. [Google Scholar]
- 36.Min SH, Zhou J. smplot: an r package for easy and elegant data visualization. Front Genet. 2021;12:802894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Durfee PT, Cross JH, Rustam, Susanto. Toxoplasmosis in man and animals in South Kalimantan (Borneo), Indonesia. Am J Trop Med Hyg. 1976;25:42–7. [DOI] [PubMed]
- 38.Uga S, Ono K, Kataoka N, Hasan H. Seroepidemiology of five major zoonotic parasite infections in inhabitants of Sidoarjo, East Java, Indonesia. Southeast Asian J Trop Med Public Health. 1996;27:556–61. [PubMed] [Google Scholar]
- 39.Konishi E, Houki Y, Harano K, Mibawani RS, Marsudi D, Alibasah S, et al. High prevalence of antibody to Toxoplasma gondii among humans in Surabaya, Indonesia. Jpn J Infect Dis. 2001;53:238–41. [PubMed]
- 40.Sardjono TW, Soewarto S, Lubnah M. Kejadian Toxoplasmosis pada Kasus-kasus Abortus Spontan di RS Dr. Saiful Anwar dideteksi dengan Pemeriksaan Serologik Histopatologik dan PCR. Jurnal Kedokteran Brawijaya. 2002;18:77–82. [Google Scholar]
- 41.Murkati SN, Supargiyono TS, Hnes M, Artama WT, et al. Perbedaan Metode ELISA Sandwich A dan B dalam Deteksi Antigen Membran Toxoplasma gondii. Bioteknologi. 2004;1:54–7. [Google Scholar]
- 42.Sanjaya A, Masloman N, Wilar R, Tuda J. Toxoplasma gondii immunoglobulin G in paired infant-and-mother sera. Paediatr Indones. 2009;49:65. [Google Scholar]
- 43.Lestari CW, Pawestri HA. Laporan Akhir Penelitian Pemeriksaan Spesimen Biomedis Riskesdas Serologi ELISA & Ekstraksi DNA (Lanjutan-Tahun 2011). Jakarta: Badan Penelitian dan Pengembangan Kesehatan Departemen Kesehatan Republik Indonesia; 2011.
- 44.Tolistiawaty I, Rosmini, Suarayasa K, Gunawan, Sumolang PPF, Nelfita, et al. Studi Serologis Antibodi Spesifik Terhadap Toxoplasma gondii pada Wanita Usia Subur di Kota Palu. Donggala: Kementerian Kesehatan Republik Indonesia; 2012.
- 45.Wiyarno Y. Infeksi Toxoplasma Pada Penjual Daging Kambing Di Pasar Tradisional Surabaya. Embrio, Jurnal Kebidanan. 2013;2:18–26. [Google Scholar]
- 46.Prasetyo AA, Ariapramuda R, Kindi EA, Dirgahayu P, Sari Y, Dharmawan R, et al. Men having sex with men in Surakarta, Indonesia: demographics, behavioral characteristics and prevalence of blood borne pathogens. Southeast Asian J Trop Med Public Health. 2014;45:1032–47. [PubMed] [Google Scholar]
- 47.Resnhaleksmana E, Nursardjan N, Danuyanti IG. Studi toxoplasmosis pada penderita schizophrenia di rumah sakit Jiwa provinsi ntb Ersandhi Resnhaleksmana, Nursardjan, I Gusti Ayu Nyoman Danuyanti. Jurnal Kesehatan Prima. 2014;I:1239–45. [Google Scholar]
- 48.Sari BRY, Gugun AM. Prevalensi Seropositif IgM/IgG Toksoplasma pada Wanita Pranikah dan Tinjauan Faktor Risiko Kepemilikan Kucing. Mutiara Medika. 2014;14:1–7. [Google Scholar]
- 49.Agustin PD, Mukono J. Gambaran Keterpaparan Terhadap Kucing Dengan Kejadian Toksoplasmosis Pada Pemelihara Dan Bukan Pemelihara Kucing Di Kecamatan Mulyorejo, Surabaya. Jurnal Kesehatan Lingkungan. 2015;8:103–17. [Google Scholar]
- 50.Krihariyani D, Woelansari ED, Kurniawan E. Seroprevalensi Antibodi IgG Toxoplasma gondii Pada ibu di Rangkah 6 Surabaya. Jurnal Ilmu Dan Teknologi Kesehatan. 2015;3:29–38. [Google Scholar]
- 51.Prasetyo AA, Sariyatun R, Reviono, Sari Y, Hudiyono, Haryati S, et al. The APOBEC3B deletion polymorphism is associated with prevalence of hepatitis B virus, hepatitis C virus, Torque Teno virus, and Toxoplasma gondii co-infection among HIV-infected individuals. J Clin Virol. 2015;70:67–71. [DOI] [PubMed]
- 52.Raharjo I, Sari Y, Prasetyo AA. Koinfeksi Toxoplasma gondii pada Pasien HIV / AIDS RSUD Dr. Moewardi di Surakarta. 2015;4:149–56. [Google Scholar]
- 53.Sari Y, Haryati S, Raharjo I, Prasetyo AA. Toxoplasma and viral antibodies among HIV patients and inmates in central Java, Indonesia. Southeast Asian J Trop Med Public Health. 2015;46:977–85. [PubMed] [Google Scholar]
- 54.Dwinata M, Sutarga I, Damriyasa I. The Potential Risk Factors for Toxoplasmosis in Balinese Pregnant Women-Indonesia. Bali Medical Journal. 2016;5:130. [Google Scholar]
- 55.Kurniawati RD. Perbandingan prevalensi hasil uji positif IgG Toxoplasma pada pasien yang periksa di laboratorium klinika Surabaya Periode 2013 Dan 2014 Menggunakan Metode Elisa. Tugas Akhir D3. Surabaya: Universitas Airlangga; 2016.
- 56.Laksmi DA, Sudarmaja IM, Swastika IK, Damayanti PAAS, Diarthini NLPE. Seroprevalens serta faktor-faktor risiko toksoplasmosis pada penduduk di Desa Kubu Kabupaten Karangasem Bali. Medicina. 2016;47:82–91. [Google Scholar]
- 57.Lestari B, Kepel BJ, Budiarso F. Seroepidemiologi toksoplasmosis pada masyarakat di Desa Rumengkor Dua Kabupaten Minahasa. Jurnal e-Biomedik. 2016;4:97–103. [Google Scholar]
- 58.Seran VJ, Kepel BJ, Fatiwali F. Seroepidemiologi toksoplasmosis pada masyarakat di Desa Kumu Kabupaten Minahasa tahun 2015. Jurnal e-Biomedik. 2016;4:86–90. [Google Scholar]
- 59.Tuda JSB. Hubungan Seroprevalensi Toxoplasma gondii dengan Konsumsi Daging Babi pada Perempuan di Manado. Majalah Kedokteran UKI. 2016;XXXII:120–5. [Google Scholar]
- 60.Amalia F. Perbandingan prevalensi hasil uji positif IgG Toxoplasma pada pasien wanita yang periksa di laboratorium klinika Surabaya Periode 2015 Dan 2016 Menggunakan Metode Elisa. Tugas Akhir D3: Universitas Airlangga; 2017. [Google Scholar]
- 61.Febianingsih NPE, Artama WT, Indriani C. Seroprevalensi Toksoplasmosis di Kabupaten Gianyar. Bali Berita Kedokteran Masyarakat. 2017;33:61. [Google Scholar]
- 62.Iskandar A, Sriwedari K, Wulanda IA, Indra MR, Hartojo, Firani NK, et al. The level of chemerin and adipocyte fatty acid binding protein in Toxoplasma gondii seropositive obese individuals. Asian Pac J Trop Biomed. 2017;7:107–9.
- 63.Tuda J, Adiani S, Ichikawa-Seki M, Umeda K, Nishikawa Y. Seroprevalence of Toxoplasma gondii in humans and pigs in North Sulawesi. Indonesia Parasitol Int. 2017;66:615–8. [DOI] [PubMed] [Google Scholar]
- 64.Alviyah. Prevalensi Hasil Uji IgG Toxoplasma Pada Pasien Di Laboratorium Klinika Surabaya Periode 2017 - 2018 Menggunakan Metode Elisa. Tugas Akhir D3. Surabaya: Universitas Airlangga; 2019.
- 65.Arwie D, Aryandi R. Identifikasi antibodi spesifik Toxoplasma gondii pada wanita di komunitas pecinta sugar glider Indonesia (KPSGI) KOTA MAKASSAR. Jurnal Kesehatan Panrita Husada. 2019;4:30–48. [Google Scholar]
- 66.Darmawan A, Karolina ME, Aurora WID. Skrining toxoplasmosis dengan rapid test IgG DI Puskesmas Simpang Kawat Jambi Medic: Med Dedicat. 2019;2:49–52. [Google Scholar]
- 67.Halleyantoro R, Andriyani Y, Sari IP, Kurniawan A. Nested PCR methods for detection Toxoplasma gondii B1 gene in Cerebrospinal Fluid of HIV patients. J Biomed Transl Res. 2019;5:62–6. [Google Scholar]
- 68.Dzikriana DF, Hi’mah NK, Anggraini R. Pengaruh Jenis Leukosit Dan Jumlah trombosit dengan kejadian toksoplasmosis terhadap pekerja di lingkungan hewan. Prosiding National conference for Ummah. 2020;1:123–9.
- 69.Fihiruddin F, Artama WT, Widartono BS. Spatial analysis of toxoplasmosis through EcoHealth approaches using GRA-1 recombinant: Case in Sleman. Yogyakarta Indones J Biotechnol. 2020;25:109–19. [Google Scholar]
- 70.Humaryanto H, Hanina H, Tarawifa S. Identifikasi kasus toksoplasmosis dengan UJI Aglutinasi Latek di Puskesmas Tahtul Yaman. MEDIC: Med Dedication. 2019;2:37–40. [Google Scholar]
- 71.Marthalia W, Sulistyorini L. Chronic Toxoplasmosis infection in members of cat breeding organization in Surabaya. Jurnal Kesehatan Lingkungan. 2020;12:48–58. [Google Scholar]
- 72.Utami KWL. Hubungan Antara Higienitas Perorangan dengan Seroprevalensi Anti-Toksoplasma pada Individu yang Kontak dengan Hewan Ternak di Kota Batu. Tugas Akhir: Universitas Brawijaya; 2020. [Google Scholar]
- 73.Harianja E, Aminuddin MF. Screening Toksoplasmosis pada Wanita Komunitas Pecinta Kucing di Kota Samarinda. Jurnal Teknologi Laboratorium Medik Borneo. 2021;1:51–6. [Google Scholar]
- 74.Polanunu NFA, Wahyuni S, Hamid F. Seroprevalence and associated risk factors of Toxoplasma gondii infection among pregnant mother in Makassar, Indonesia. PLoS One. 2021;16(6):e0245572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Afrianti D, Swastya Putri AP, Wahyudi W. Kejadian Toksoplasmosis pada Wanita Hamil Pemelihara Kucing dan Wanita Hamil Bukan Pemelihara Kucing di Puskesmas Tlogosari Wetan Kota Semarang. Jurnal Laboratorium Medis. 2023;5:49–52. [Google Scholar]
- 76.Hanina PA. Seroprevalensi Toksoplasmosis Pada Wanita Usia Subur Di Puskesmas Paal X Kota Jambi. Medic. 2023;6:17–21. [Google Scholar]
- 77.Jayawardhana A, Harnida H, Purwatiningsih D, Puspitasari H, Suwanti LT, et al. Seroprevalensi Kasus Toxoplasmosis sebagai Infeksi Oportunistik pada Penderita HIV/ Acquired Immune Deficiency Syndrome (AIDS) Di Kabupaten Sidoarjo. NERSMID : Jurnal Keperawatan dan Kebidanan. 2023;6:1–6. [Google Scholar]
- 78.Meirawan RF, Ramadhani NR. Perilaku Seksual Sebagai Risiko Seropositif Imunoglobulin G (IgG) Toxoplasma gondii Pada Pria Homoseksual Penderita HIV. Jurnal Health Sains. 2023;4:112–20. [Google Scholar]
- 79.Novikasari C, Suhariyadi S, Woelansari ED, Museyaroh M. Skrining Antibodi IgM & IgG Toksoplasmolisis pada Ibu Hamil di Puskesmas Kabupaten Sampang. JPP (Jurnal Kesehatan Poltekkes Palembang). 2023;18:199–204. [Google Scholar]
- 80.Pratama SS, Kumoro A. Toxoplasmosis Examination With Anti-Toxo IgM And IgG Results In Petshop Staff. Jurnal Analis Medika Biosains. 2023;10:120–4. [Google Scholar]
- 81.Riansari A, Halleyantoro R, Dewi DP, Sudaryanto S, Annisaa E, Hapsari R. Seroprevalensi toxoplasmosis wanita Di Kota Semarang. Jurnal Kesehatan Tambusai. 2023;4:921–5. [Google Scholar]
- 82.Kusuma Sari N, Sayekti FDJ. Toxoplasmosis Pada Ibu Hamil Berdasarkan Tes Serologi di Bali. Bali Medika Jurnal. 2023;10:139–49. [Google Scholar]
- 83.Setia YD, Pawestri AR, Adawiyah R, Nurhaliza A, Audinugroho A, Chilmi S, et al. Profiles of Toxoplasma gondii circulating antigen and seropositivity in HIV patients. The 4th International Conference on Life Science and Technology (ICoLiST). 2023;2634 January:20036, 1–5.
- 84.Afrianti D, Putri AP, Wikandari RJ. Screening for the Detection of IgG anti-Toxoplasma gondii Antibodies in Female Donors at Semarang Regency Poltekita: Jurnal Ilmu Kesehatan. 2024;17:1257–62. [Google Scholar]
- 85.Rahman A, Rahayu T, Zain KR, Widyaswara G, Uswatuya NV. Antibody detection of Toxoplasma gondii on blood donor at PMI Kota Yogyakarta by rapid diagnostic methods in 2022. BIO Web Conf. 2024;94:1–5. [Google Scholar]
- 86.Widyaswara G, Rahman A, Zain KR, Rahayu T, Andriyani NK. Detection of Toxoplasma gondii parasite on blood donor at PMI Kabupaten Magelang by rapid diagnostic methods in 2022. BIO Web Conf. 2024;94:10–3. [Google Scholar]
- 87.Wikandari RJ, Afriansya R, Kuncara RB, Afrianti D, Setyowatiningsih L. Evaluation of Toxoplasmosis in female college students. Poltekita: Jurnal Ilmu Kesehatan. 2024;17:1224–31. [Google Scholar]
- 88.Wulandari M, Nafilata I, Wulandari SW. Penapisan antibodi Toxoplasma gondii dan profil leukosit pada darah donor. PREPOTIF: Jurnal Kesehatan Masyarakat. 2024;8:4052–9. [Google Scholar]
- 89.Rachmawati I. Personal Hygiene and Toxoplasmosis Occurences in “Bungkul Cat Lovers” Cat Owners Community in Surabaya: An Association Study. Jurnal Kesehatan Lingkungan. 2019;11:116–22. [Google Scholar]
- 90.Sanni Hassana D, Hadisaputro S, Sofro MAU. Toxoplasmosis and Cerebral Toxoplasmosis in HIV/AIDS Patients in Kariadi Hospital. Semarang Jurnal Epidemiologi Kesehatan Komunitas. 2021;6:213–7. [Google Scholar]
- 91.Purnami N, Etika R, Utomo MT, Wardhani P. Seropositivity of anti-rubella antibodies as a marker for rubella infection in infants at high risk of congenital deafness. Indonesian J Clin Pathol Med Lab. 2020;26:182–6. [Google Scholar]
- 92.Jasmine AP, Mutiara H, Suwandi JF, Putri GT, Kedokteran F, Lampung U, et al. Hubungan determinan sosial kesehatan dengan infeksi toksoplasma gondii relationship between social determinant of health with the incident of Toxoplasma gondii infection. MedULA. 2024;14:205–12. [Google Scholar]
- 93.Amtarina R, Andriza A, Pratiwi N, Udayana S. Pengaruh Infeksi Laten Toksoplasmosis Terhadap Lama Rawatan Skizofrenia Episode Pertama. Jurnal Ilmu Kedokteran (Journal of Medical Science). 2021;15:21. [Google Scholar]
- 94.Aini ZM, Saiminm J. Hubungan Infeksi Torch Pada Kehamilan Dengan Kejadian Kelainan Kongenital Pada Bayi Baru Lahir. MedULA. 2019;4:344–53. [Google Scholar]
- 95.Simanjuntak G. Pencegahan dan Pemberantasan Penyakit-penyakit Zoonosa New, Emerging dan Re-emerging di Indonesia. Buletin Penelitian Kesehatan. 1997;25:73–8. [Google Scholar]
- 96.Badan Pusat Statistik Kabupaten Gianyar. Curah Hujan (milimeter), 2016–2018. Badan Pusat Statistik Kabupaten Gianyar. 2024. https://gianyarkab.bps.go.id/id/statistics-table/2/NzkjMg==/curah-hujan.html. Accessed 24 Dec 2024.
- 97.Badan Pusat Statistik Kota Malang. Jumlah Curah Hujan di Kota Malang (milimeter (mm)), 2023. Badan Pusat Statistik Kota Malang. 2024. https://malangkota.bps.go.id/id/statistics-table/2/NTA4IzI=/jumlah-curah-hujan-di-kota-malang.html.
- 98.Badan Pusat Statistik Provinsi Bali. Pengamatan Unsur Iklim di Stasiun Geofisika Denpasar, 2022–2023. Badan Pusat Statistik Provinsi Bali. 2024. https://bali.bps.go.id/id/statistics-table/1/MTgzIzE=/pengamatan-unsur-iklim-di-stasiun-geofisika-denpasar--2022-2023.html.
- 99.Badan Pusat Statistik Provinsi Banten. Curah Hujan Menurut Bulan dan Stasiun Pengamatan di Provinsi Banten (mm3), 2023. Badan Pusat Statistik Provinsi Banten. 2024. https://banten.bps.go.id/id/statistics-table/2/MzcyIzI=/curah-hujan-menurut-bulan-dan-stasiun-pengamatan-di-provinsi-banten.html.
- 100.Badan Pusat Statistik Provinsi DKI Jakarta. Curah Hujan di Stasiun Tanjung Priuk Menurut Bulan 2021–2023. Badan Pusat Statistik Provinsi DKI Jakarta. 2024. https://jakarta.bps.go.id/id/statistics-table/2/NzYyIzI=/curah-hujan-di-stasiun-tanjung-priuk-menurut-bulan.html.
- 101.Badan Pusat Statistik Provinsi Jawa Tengah. Banyak Curah Hujan dan Hari Hujan Menurut Bulan di Provinsi Jawa Tengah, 2022–2023. Badan Pusat Statistik Provinsi Jawa Tengah. 2024. https://jateng.bps.go.id/id/statistics-table/2/NDUwIzI=/banyak-curah-hujan-dan-hari-hujan-menurut-bulan-di-provinsi-jawa-tengah.html.
- 102.Badan Pusat Statistik Provinsi Jawa Timur. Pengamatan Unsur Iklim di Stasiun Pengamatan Badan Meteorologi Klimatologi dan Geofisika (BMKG) Stasiun Meteorologi Kelas I Juanda Sidoarjo, 2018–2020. Badan Pusat Statistik Provinsi Jawa Timur. 2021. https://jatim.bps.go.id/id/statistics-table/1/MjE0MCMx/pengamatan-unsur-iklim-di-stasiun-pengamatan-badan-meteorologi-klimatologi-dan-geofisika--bmkg--stasiun-meteorologi-kelas-i-juanda-sidoarjo--2018-2020.html.
- 103.Badan Pusat Statistik Provinsi DKI Jakarta. Curah Hujan di Stasiun Kemayoran Menurut Bulan (Mm), 2020–2021. Badan Pusat Statistik Provinsi DKI Jakarta. 2022. https://jakarta.bps.go.id/id/statistics-table/2/MzczIzI=/curah-hujan-di-stasiun-kemayoran-menurut-bulan.html.
- 104.Badan Pusat Statistik Provinsi DI Yogyakarta. Curah Hujan per Bulan (mm), 2018. Badan Pusat Statistik Provinsi DI Yogyakarta. 2024. https://yogyakarta.bps.go.id/id/statistics-table/2/MTUyIzI=/curah-hujan-per-bulan.html.
- 105.Badan Pusat Statistik Provinsi Jawa Timur. Curah Hujan (mm3), 2015–2017. Badan Pusat Statistik Provinsi Jawa Timur. 2017. https://jatim.bps.go.id/id/statistics-table/2/OTYjMg==/curah-hujan.html.
- 106.Badan Pusat Statistik Provinsi Jawa Tengah. Banyak Curah Hujan dan Hari Hujan Menurut Bulan di Provinsi Jawa Tengah, 1973–2018. Badan Pusat Statistik Provinsi Jawa Tengah. 2024. https://jateng.bps.go.id/id/statistics-table/2/NDUwIzI=/banyak-curah-hujan-dan-hari-hujan-menurut-bulan-di-provinsi-jawa-tengah.html.
- 107.Badan Pusat Statistik Kota Manado. Jumlah Curah Hujan Menurut Bulan di Kota Manado (mm3), 2014–2016. Badan Pusat Statistik Kota Manado. 2022. https://manadokota.bps.go.id/id/statistics-table/2/MTAxIzI=/jumlah-curah-hujan-menurut-bulan-di-kota-manado.html.
- 108.Badan Pusat Statistik Kabupaten Karang Asem. Jumlah Curah Hujan (milimeter), 2014–2016. Badan Pusat Statistik Kabupaten Karang Asem. 2021. https://karangasemkab.bps.go.id/id/statistics-table/2/NzAjMg==/jumlah-curah-hujan.html.
- 109.Badan Pusat Statistik Kabupaten Badung. Angka Perbandingan Keadaan Curah Hujan dengan Angka Normal Setiap Bulan di Kabupaten Badung (mm), 2014–2016. Badan Pusat Statistik Kabupaten Badung. 2021. https://badungkab.bps.go.id/id/statistics-table/2/NjcjMg==/angka-perbandingan-keadaan-curah-hujan-dengan-angka-normal-setiap-bulan-di-kabupaten-badung--mm-.html.
- 110.Badan Pusat Statistik Provinsi Nusa Tenggara Barat. Curah Hujan (mm), 2014. Badan Pusat Statistik Provinsi Nusa Tenggara Barat. 2014. https://ntb.bps.go.id/id/statistics-table/2/NDgjMg==/curah-hujan.html.
- 111.Badan Pusat Statistik Provinsi Jawa Tengah. Banyaknya Curah Hujan Menurut Stasiun di Provinsi Jawa Tengah, 2010 - 2016 (mm). Badan Pusat Statistik Provinsi Jawa Tengah. 2018. https://jateng.bps.go.id/id/statistics-table/1/MTY1MyMx/banyaknya-curah-hujan-menurut-stasiun-di-provinsi-jawa-tengah--2010---2016--mm-.html.
- 112.Badan Pusat Statistik Provinsi Jawa Timur. Curah Hujan (mm3), 2012–2014. Badan Pusat Statistik Provinsi Jawa Timur. 2014. https://jatim.bps.go.id/id/statistics-table/2/OTYjMg==/curah-hujan.html.
- 113.Wana MN, Moklas MAM, Watanabe M, Nordin N, Unyah NZ, Abdullahi SA, et al. A review on the prevalence of Toxoplasma gondii in humans and animals reported in Malaysia from 2008–2018. Int J Environ Res Public Health. 2020;17:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fuchs FE, Pauly M, Black AP, Hübschen JM. Seroprevalence of torch pathogens in southeast asia. Microorganisms. 2021;9:1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bigna JJ, Tochie JN, Tounouga DN, Bekolo AO, Ymele NS, Youda EL, et al. Global, regional, and country seroprevalence of Toxoplasma gondii in pregnant women: a systematic review, modelling and meta-analysis. Sci Rep. 2020;10:12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Foroutan-Rad M, Majidiani H, Dalvand S, Daryani A, Kooti W, Saki J, et al. Toxoplasmosis in Blood Donors: A Systematic Review and Meta-Analysis. Transfus Med Rev. 2016;30:116–22. [DOI] [PubMed] [Google Scholar]
- 117.Spineli LM, Pandis N. Problems and pitfalls in subgroup analysis and meta-regression. Am J Orthod Dentofac Orthop. 2020;158:901–4. [DOI] [PubMed] [Google Scholar]
- 118.Migliavaca CB, Stein C, Colpani V, Barker TH, Ziegelmann PK, Munn Z, et al. Meta-analysis of prevalence: statistic and how to deal with heterogeneity. Res Synth Methods. 2022;13:363–7. [DOI] [PubMed] [Google Scholar]
- 119.Cifuentes-González C, Zapata-Bravo E, Sierra-Cote MC, Boada-Robayo L, Vargas-Largo ÁP, Reyes-Guanes J, et al. Colombian Ocular Infectious Epidemiology Study (COIES): Ocular Toxoplasmosis Incidence and Sociodemographic Characterization, 2015–2019. Int J Infect Dis. 2022;117:349–55. [DOI] [PubMed] [Google Scholar]
- 120.Rudzinski M, Meyer A, Khoury M, Couto C. Is reactivation of toxoplasmic retinochoroiditis associated to increased annual rainfall? Parasite. 2013;20:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gómez-Marin JE, de-la Torre A, Angel-Muller E, Rubio J, Arenas J, Osorio E, et al. First Colombian multicentric newborn screening for congenital toxoplasmosis. PLoS Negl Trop Dis. 2011;5:e1195-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Maud L, Isabelle V, Marie-Laure D, Dominique A, Régine G, Emilie D, et al. Quantitative estimation of the viability of Toxoplasma gondii Oocysts in soil. Appl Environ Microbiol. 2012;78:5127–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Robert-Gangneux F, Guegan H. Anti-ToxoplasmaIgG assays: What performances for what purpose? A systematic review. Parasite. 2021;28:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kazemi F, Arjmand R, Dousti M, Fasihi Karami M, Barzegar G, Mohammadi A, et al. Toxoplasma and risk of spontaneous abortion: a meta-analysis in a population of Iranian women. Int J Fertil Steril. 2023;17:7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nayeri T, Sarvi S, Moosazadeh M, Amouei A, Hosseininejad Z, Daryani A. The global seroprevalence of anti-Toxoplasma gondii antibodies in women who had spontaneous abortion: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2020;14:e0008103-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wei H-X, He C, Yang P-L, Lindsay DS, Peng H-J. Relationship between cat contact and infection by Toxoplasma gondii in humans: a meta-analysis. Comp Parasitol. 2016;83:11–9. [Google Scholar]
- 127.Chiang T-Y, Kuo M-C, Chen C-H, Yang J-Y, Kao C-F, Ji D-D, et al. Risk factors for acute Toxoplasma gondii diseases in Taiwan: a population-based case-control study. PLoS One. 2014;9:e90880-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang Z-D, Wang S-C, Liu H-H, Ma H-Y, Li Z-Y, Wei F, et al. Prevalence and burden of Toxoplasma gondii infection in HIV-infected people: a systematic review and meta-analysis. Lancet HIV. 2017;4:e177–88. [DOI] [PubMed] [Google Scholar]
- 129.Indonesian Minister of Health. Keputusan Menteri Kesehatan Republik Indonesia Nomor HK.01.07/MENKES/90/2019 Tentang Pedoman Nasional Pelayanan Kedokteran Tata Laksana HIV. 2019.
- 130.Contopoulos-Ioannidis DG, Gianniki M, Ai-Nhi Truong A, Montoya JG. Toxoplasmosis and schizophrenia: a systematic review and meta-analysis of prevalence and associations and future directions. Psychiatric Res Clin Pract. 2022;4:48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.de Barros JLVM, Barbosa IG, Salem H, Rocha NP, Kummer A, Okusaga OO, et al. Is there any association between Toxoplasma gondii infection and bipolar disorder? A systematic review and meta-analysis. J Affect Disord. 2017;209:59–65. [DOI] [PubMed] [Google Scholar]
- 132.Bisetegn H, Debash H, Ebrahim H, Mahmood N, Gedefie A, Tilahun M, et al. Global seroprevalence of Toxoplasma gondii infection among patients with mental and neurological disorders: A systematic review and meta-analysis. Health Sci Rep. 2023;6: e1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Arya R, Antonisamy B, Kumar S. Sample size estimation in prevalence studies. Indian J Pediatr. 2012;79:1482–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data that were analyzed for this study are included in both the main manuscript and the additional files.