Abstract
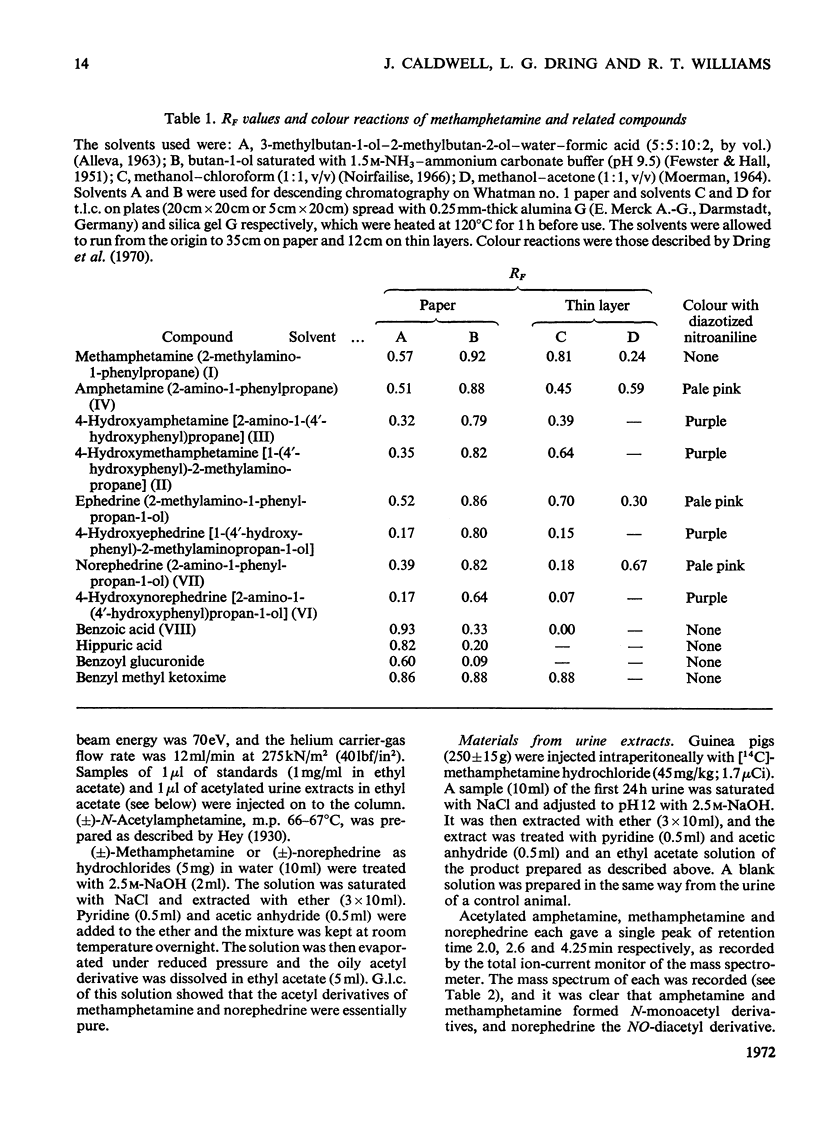
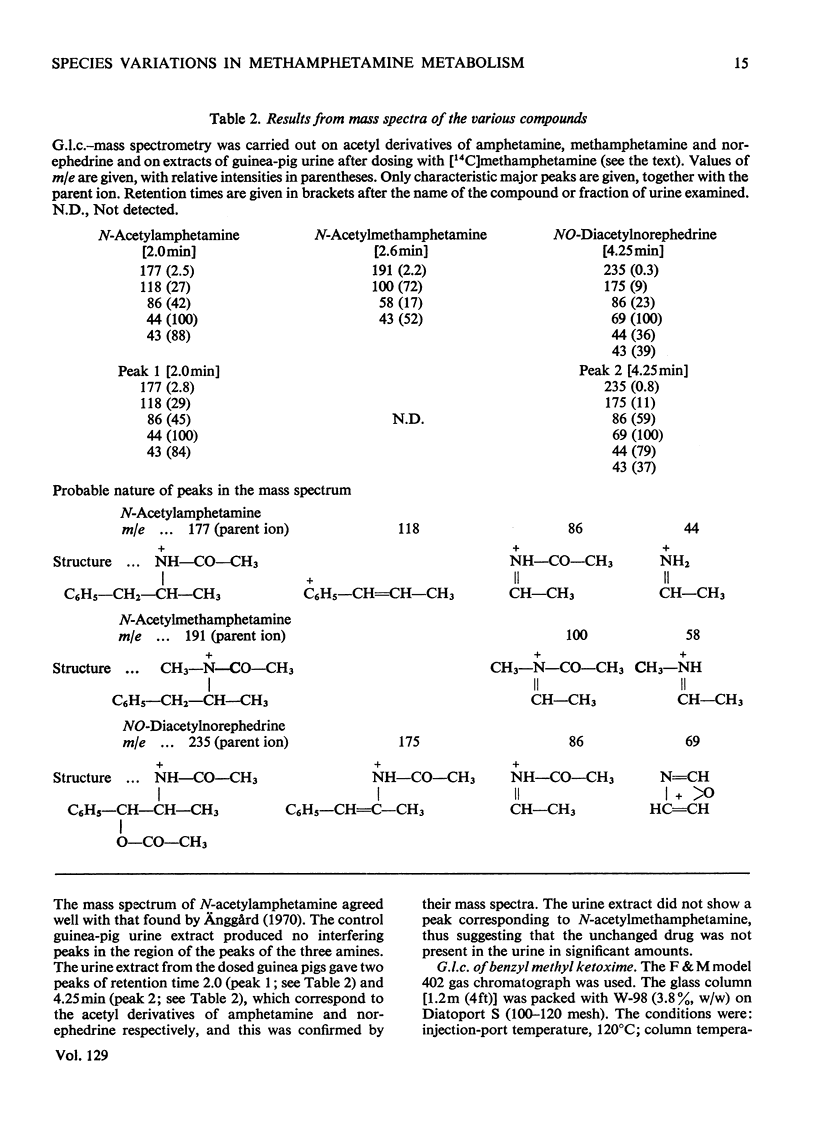
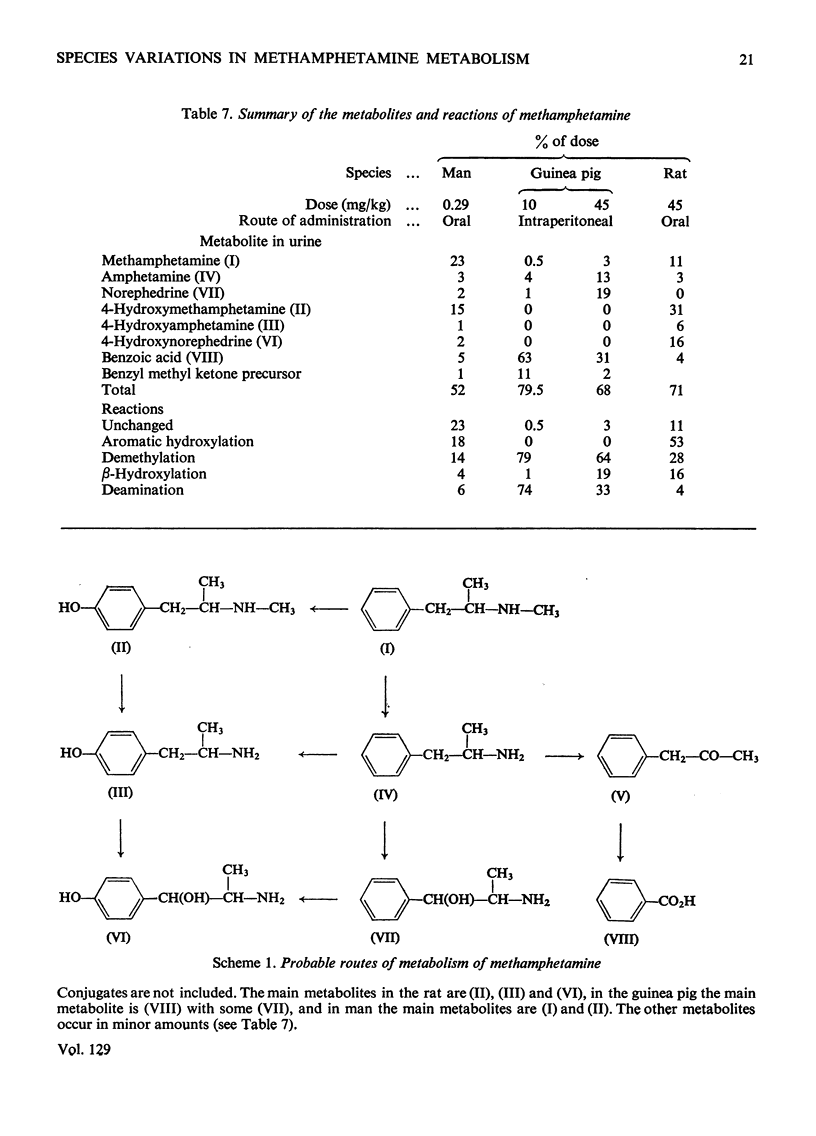
1. The metabolites of (±)-2-methylamino-1-phenyl[1-14C]propane ([14C]methamphetamine) in urine were examined in man, rat and guinea pig. 2. In two male human subjects receiving the drug orally (20mg per person) about 90% of the 14C was excreted in the urine in 4 days. The urine of the first day was examined for metabolites, and the main metabolites were the unchanged drug (22% of the dose) and 4-hydroxymethamphetamine (15%). Minor metabolites were hippuric acid, norephedrine, 4-hydroxyamphetamine, 4-hydroxynorephedrine and an acid-labile precursor of benzyl methyl ketone. 3. In the rat some 82% of the dose of 14C (45mg/kg) was excreted in the urine and 2–3% in the faeces in 3–4 days. In 2 days the main metabolites in the urine were 4-hydroxymethamphetamine (31% of dose), 4-hydroxynorephedrine (16%) and unchanged drug (11%). Minor metabolites were amphetamine, 4-hydroxyamphetamine and benzoic acid. 4. The guinea pig was injected intraperitoneally with the drug at two doses, 10 and 45mg/kg. In both cases nearly 90% of the 14C was excreted, mainly in the urine after the lower dose, but in the urine (69%) and faeces (18%) after the higher dose. The main metabolites in the guinea pig were benzoic acid and its conjugates. Minor metabolites were unchanged drug, amphetamine, norephedrine, an acid-labile precursor of benzyl methyl ketone and an unknown weakly acidic metabolite. The output of norephedrine was dose-dependent, being about 19% on the higher dose and about 1% on the lower dose. 5. Marked species differences in the metabolism of methamphetamine were observed. The main reaction in the rat was aromatic hydroxylation, in the guinea pig demethylation and deamination, whereas in man much of the drug, possibly one-half, was excreted unchanged.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEVA J. J. METABOLISM OF TRANYLEYPROMINE-C14 AND DL AMPHETAMINE-C14 IN THE RAT. J Med Chem. 1963 Nov;6:621–624. doi: 10.1021/jm00342a001. [DOI] [PubMed] [Google Scholar]
- AXELROD J. Studies on sympathomimetic amines. II. The biotransformation and physiological disposition of d-amphetamine, d-p-hydroxyamphetamine and d-methamphetamine. J Pharmacol Exp Ther. 1954 Mar;110(3):315–326. [PubMed] [Google Scholar]
- Aziz F. T., Hirom P. C., Millburn P., Smith R. L., Williams R. T. The biliary excretion of anions of molecular weight 300-800 in the rat, guinea pig and rabbit. Biochem J. 1971 Nov;125(2):25P–26P. doi: 10.1042/bj1250025p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges J. W., Davies D. S., Williams R. T. The fate of ethyltin and diethyltin derivatives in the rat. Biochem J. 1967 Dec;105(3):1261–1267. doi: 10.1042/bj1051261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell J., Dring L. G., Williams R. T. Norephedrines as metabolites of ( 14 C)amphetamine in urine in man. Biochem J. 1972 Aug;129(1):23–24. doi: 10.1042/bj1290023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dring L. G., Smith R. L., Williams R. T. The metabolic fate of amphetamine in man and other species. Biochem J. 1970 Feb;116(3):425–435. doi: 10.1042/bj1160425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FEWSTER M. E., HALL D. A. Application of buffered solvent systems to the detection of aromatic acids by paper partition chromatography. Nature. 1951 Jul 14;168(4263):78–79. doi: 10.1038/168078a0. [DOI] [PubMed] [Google Scholar]
- Hucker H. B., Michniewicz B. M., Rhodes R. E. Phenylacetone oxime--an intermediate in the oxidative deamination of amphetamine. Biochem Pharmacol. 1971 Aug;20(8):2123–2128. doi: 10.1016/0006-2952(71)90425-4. [DOI] [PubMed] [Google Scholar]
- Parli C. J., Wang N., McMahon R. E. The mechanism of the oxidation of d-amphetamine by rabbit liver oxygenase. Oxygen-18 studies. Biochem Biophys Res Commun. 1971 Jun 4;43(5):1204–1209. doi: 10.1016/0006-291x(71)90591-2. [DOI] [PubMed] [Google Scholar]
- Richter D. Elimination of amines in man. Biochem J. 1938 Oct;32(10):1763–1769. doi: 10.1042/bj0321763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoenen H., Hürlimann A., Gey K. F., Haefely W. Liberation of p-hydroxynorephedrine from cat spleen by sympathetic nerve stimulation after pretreatment with amphetamine. Life Sci. 1966 Sep;5(18):1715–1722. doi: 10.1016/0024-3205(66)90107-x. [DOI] [PubMed] [Google Scholar]


