Abstract
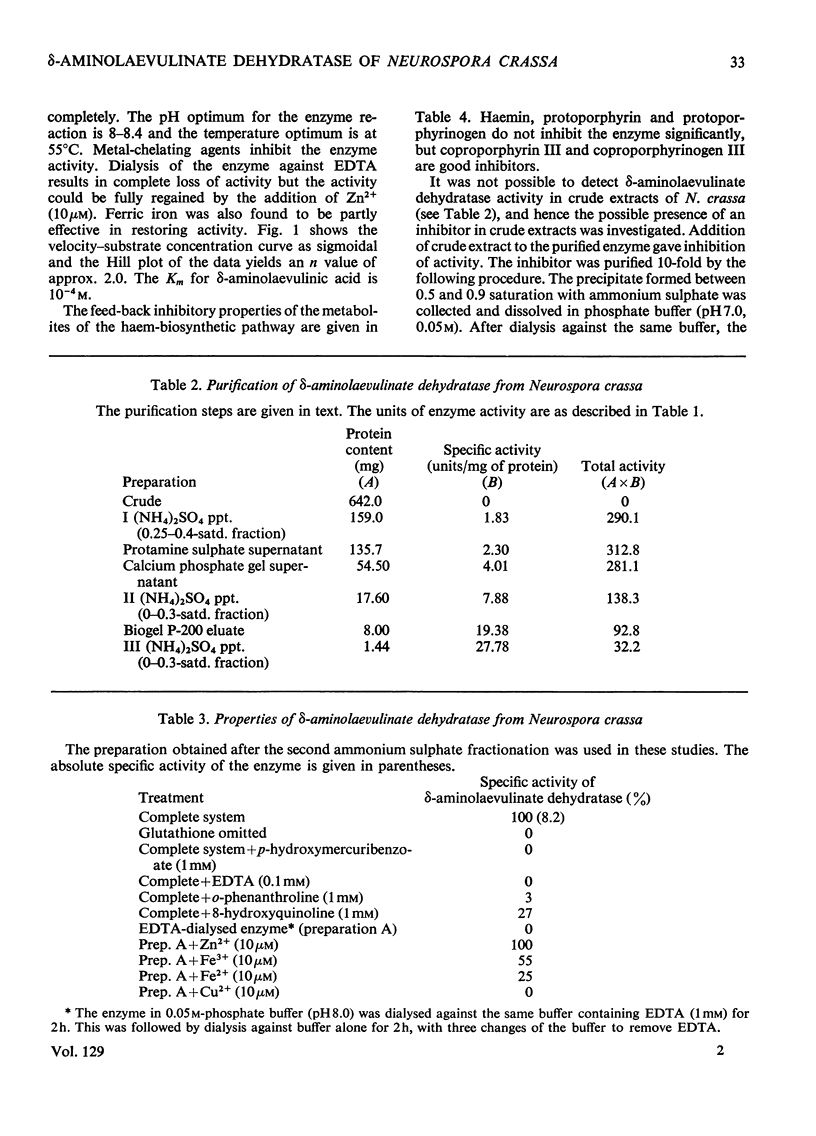
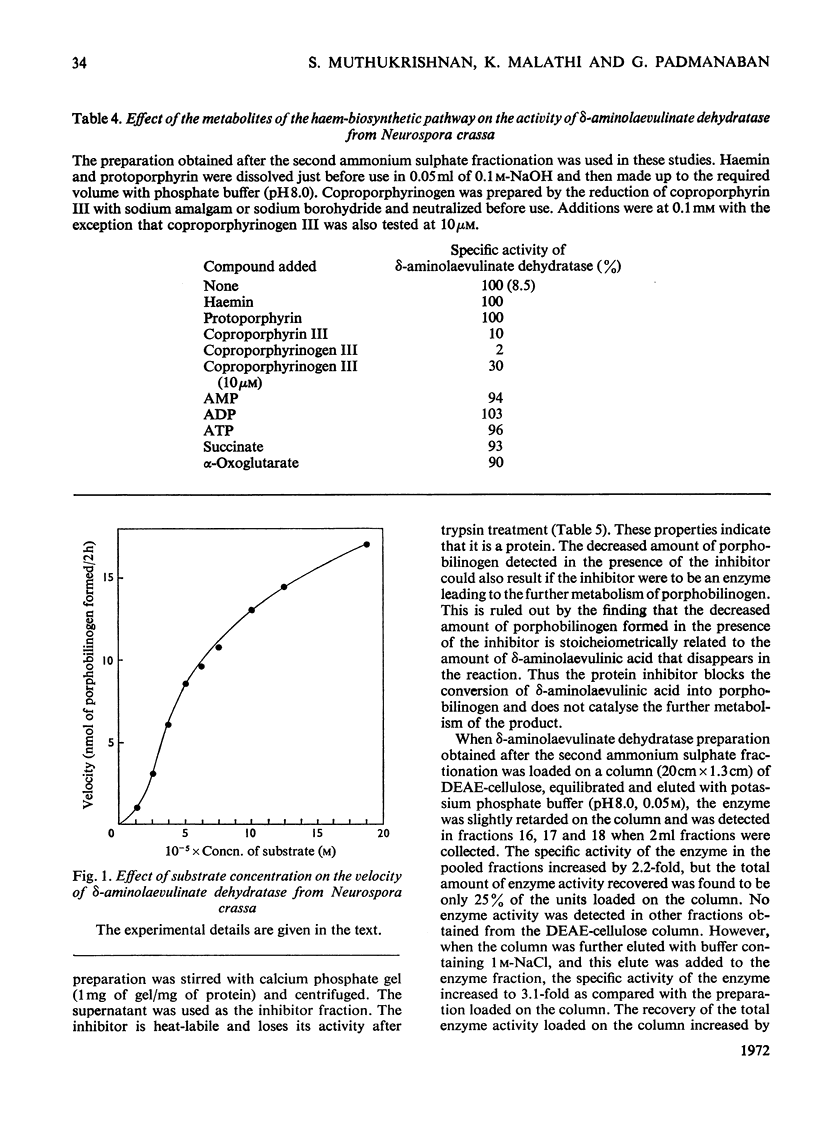
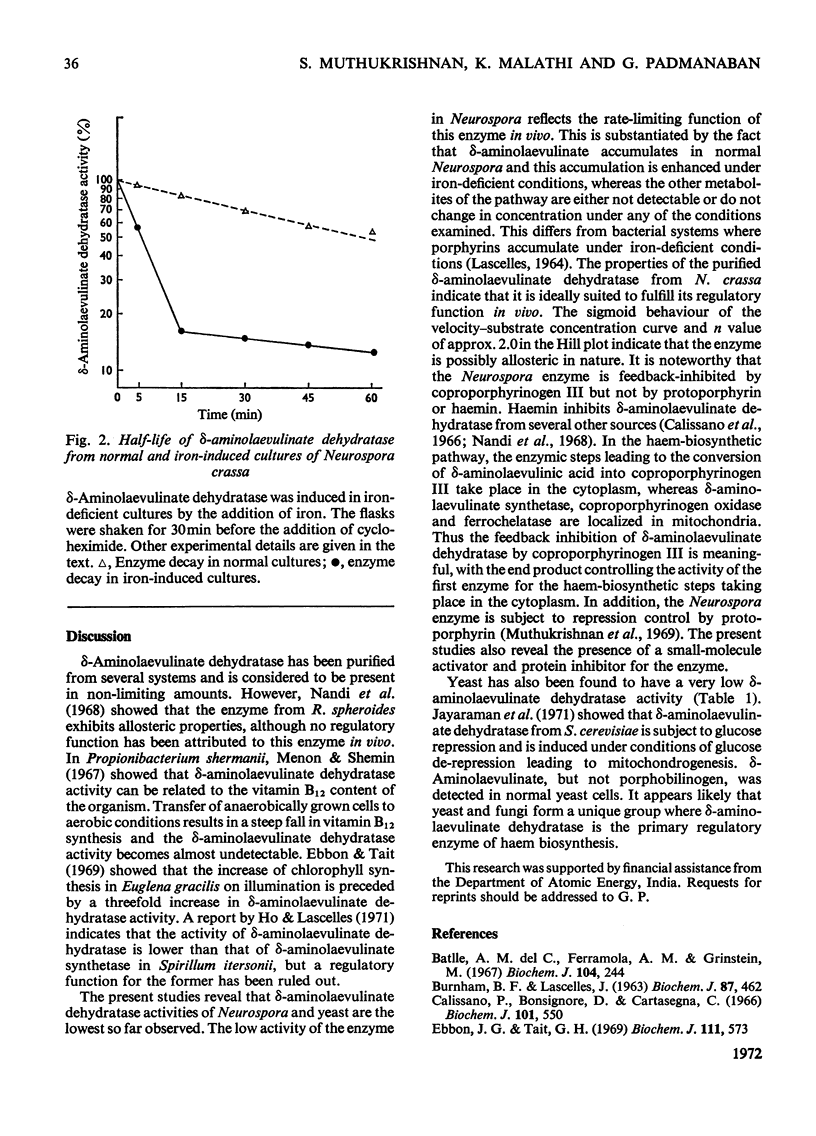
The activity of δ-aminolaevulinate dehydratase is very low in the mould Neurospora crassa compared with the activities detected in bacterial and animal systems. The enzyme is inducible in iron-deficient cultures by addition of iron and is repressed by protoporphyrin. The properties of the purified enzyme indicate its allosteric nature and susceptibility to feedback inhibition by coproporphyrinogen III. Neurospora extracts also contain a protein inhibitor of the enzyme and a small-molecule activator, which appears to be associated with the enzyme. The regulatory function of this enzyme in vivo is correlated with the accumulation of δ-aminolaevulinic acid in normal cultures of N. crassa. The decay curve of the iron-induced enzyme in vivo shows a biphasic pattern, with one of the components showing a half-life of 4–5 min.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURNHAM B. F., PIERCE W. S., WILLIAMS K. R., BOYER M. H., KIRBY C. K. delta-aminolaevulate dehydratase from Rhodopseudomonas spheroides. Biochem J. 1963 Jun;87:462–472. doi: 10.1042/bj0870462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batlle A. M., Ferramola A. M., Grinstein M. Purification and general properties of sigma-aminolaevulate dehydratase from cow liver. Biochem J. 1967 Jul;104(1):244–249. doi: 10.1042/bj1040244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calissano P., Bonsignore D., Cartasegna C. Control of haem synthesis by feedback inhibition on human-erythrocyte delta-aminolaevulate dehydratase. Biochem J. 1966 Nov;101(2):550–552. doi: 10.1042/bj1010550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebbon J. G., Tait G. H. Studies on S-adenosylmethionine-magnesium protoporphyrin methyltransferase in Euglena gracilis strain Z. Biochem J. 1969 Feb;111(4):573–582. doi: 10.1042/bj1110573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON K. D., LAVER W. G., NEUBERGER A. Initial stages in the biosynthesis of porphyrins. 2. The formation of delta-aminolaevulic acid from glycine and succinyl-coenzyme A by particles from chicken erythrocytes. Biochem J. 1958 Sep;70(1):71–81. doi: 10.1042/bj0700071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANICK S., MAUZERALL D. Pbrphyrin biosynthesis in erythrocytes. II. Enzymes converting gamma-aminolevulinic acid to coproporphyrinogen. J Biol Chem. 1958 Jun;232(2):1119–1140. [PubMed] [Google Scholar]
- Ho Y. K., Lascelles J. -aminolevulinic acid dehydratase of Spirillum itersonii and the regulation of tetrapyrrole synthesis. Arch Biochem Biophys. 1971 Jun;144(2):734–740. doi: 10.1016/0003-9861(71)90381-x. [DOI] [PubMed] [Google Scholar]
- Jayaraman J., Padmanaban G., Malathi K., Sarma P. S. Haem synthesis during mitochondrogenesis in yeast. Biochem J. 1971 Feb;121(3):531–535. doi: 10.1042/bj1210531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan S. M., Padmanaban G., Sarma P. S. Role of iron in the regulation of heme biosynthesis in Neurospora crassa. Biochem Biophys Res Commun. 1968 May 10;31(3):333–339. doi: 10.1016/0006-291x(68)90480-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Menon I. A., Shemin D. Concurrent decrease of enzymic activities concerned with the synthesis of coenzyme B 12 and of propionic acid in propionibacteria. Arch Biochem Biophys. 1967 Aug;121(2):304–310. doi: 10.1016/0003-9861(67)90080-x. [DOI] [PubMed] [Google Scholar]
- Muthukrishnan S., Padmanaban G., Sarma P. S. Regulation of heme biosynthesis in Neurspora crassa. J Biol Chem. 1969 Aug 10;244(15):4241–4246. [PubMed] [Google Scholar]
- Nandi D. L., Baker-Cohen K. F., Shemin D. Delta-aminolevulinic acid dehydratase of Rhodopseudomonas spheroides. J Biol Chem. 1968 Mar 25;243(6):1224–1230. [PubMed] [Google Scholar]
- Nandi D. L., Waygood E. R. Biosynthesis of porphyrins in wheat leaves. II. 5-aminolaevulinate hydro-lyase. Can J Biochem. 1967 Feb;45(2):327–336. doi: 10.1139/o67-036. [DOI] [PubMed] [Google Scholar]
- Padmanaban G., Sarma P. S. Studies on iron metabolism in Neurospora crassa. Arch Biochem Biophys. 1965 Jul;111(1):147–152. doi: 10.1016/0003-9861(65)90333-4. [DOI] [PubMed] [Google Scholar]
- Tigier H. A., Del Batlle A. M., Locascio G. Porphyrin biosynthesis in soybean callus tissue system. Isolation, purification and general properties of delta-aminolaevulinate dehydratase. Biochim Biophys Acta. 1968 Jan 8;151(1):300–302. doi: 10.1016/0005-2744(68)90193-9. [DOI] [PubMed] [Google Scholar]


