Abstract
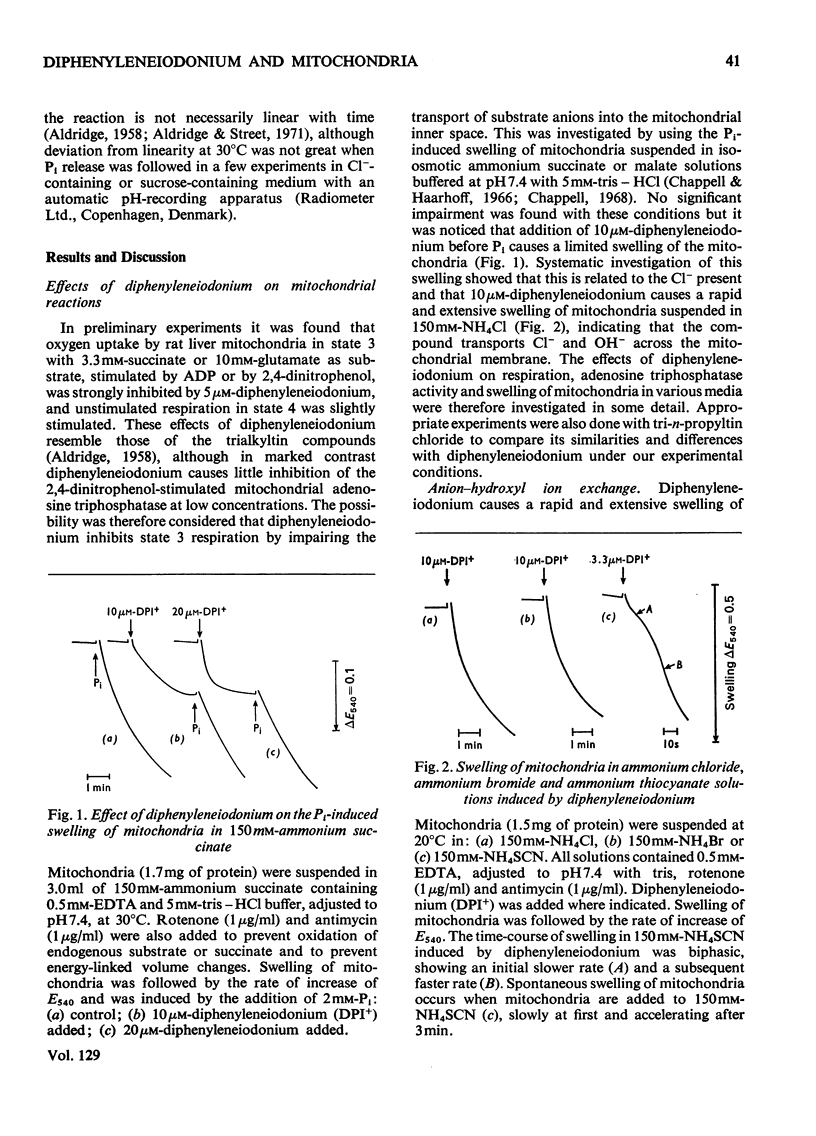
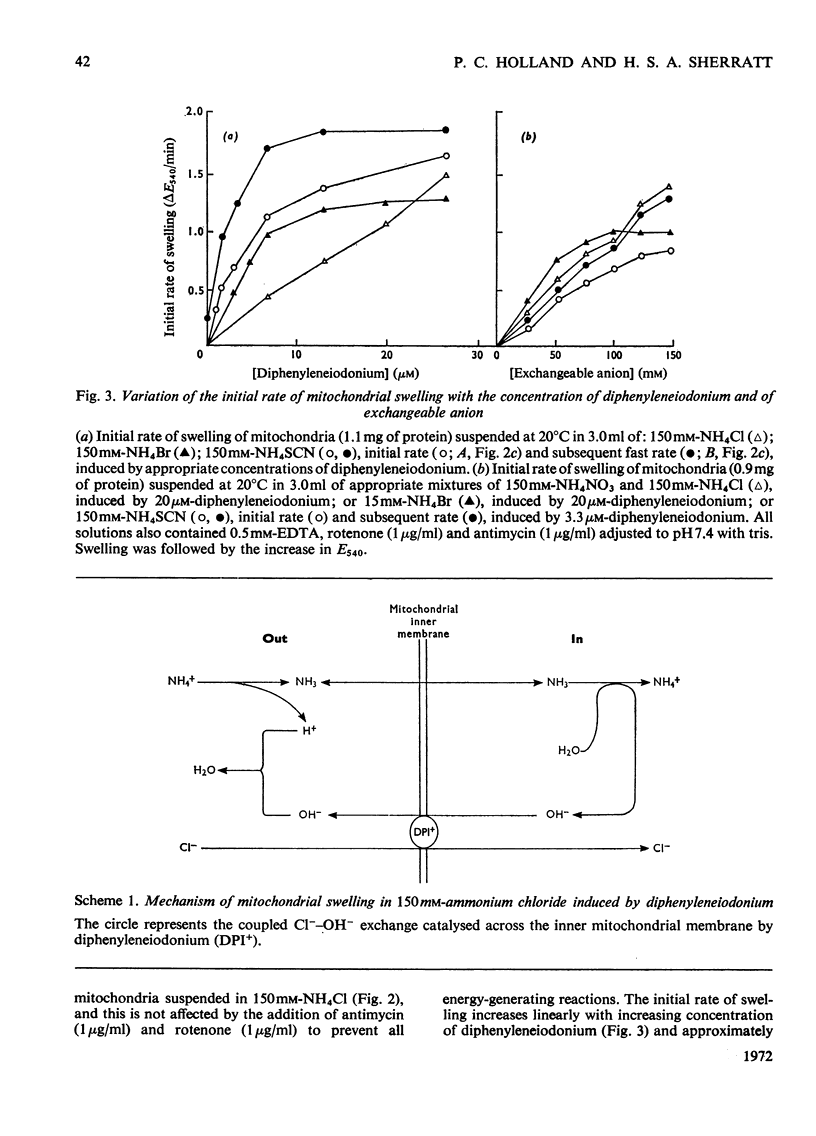
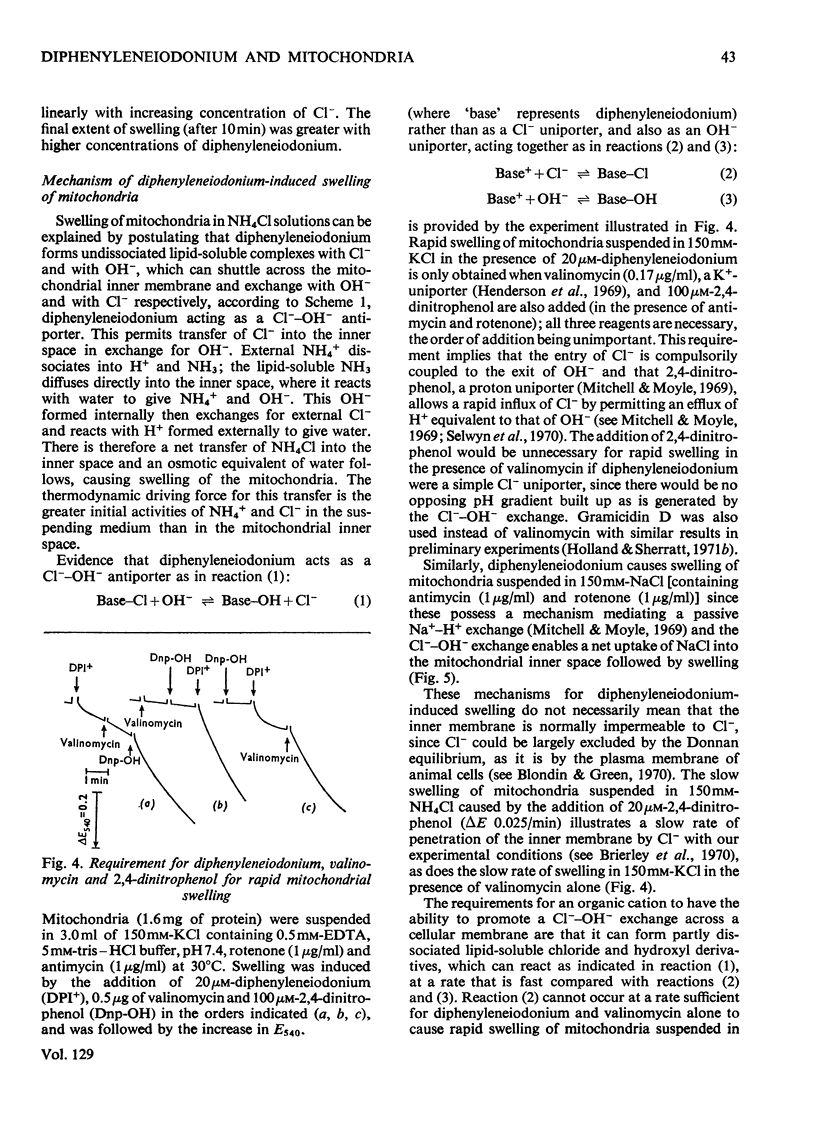
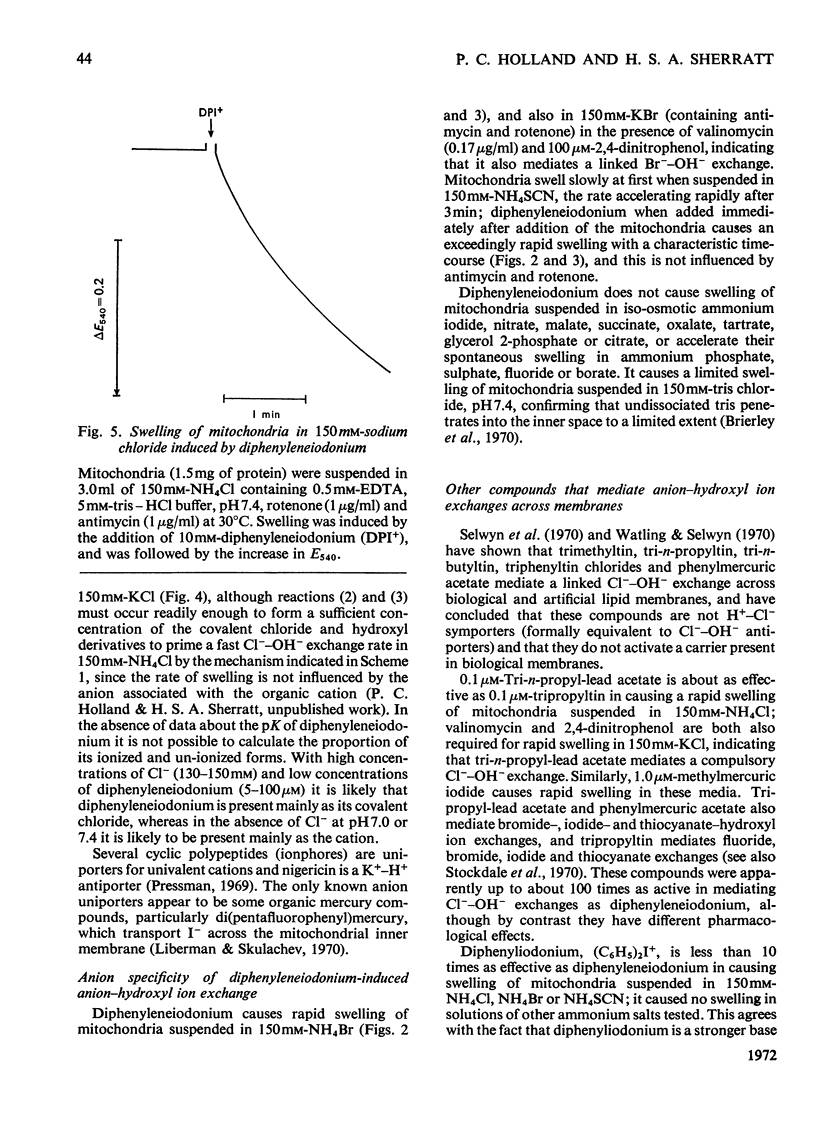
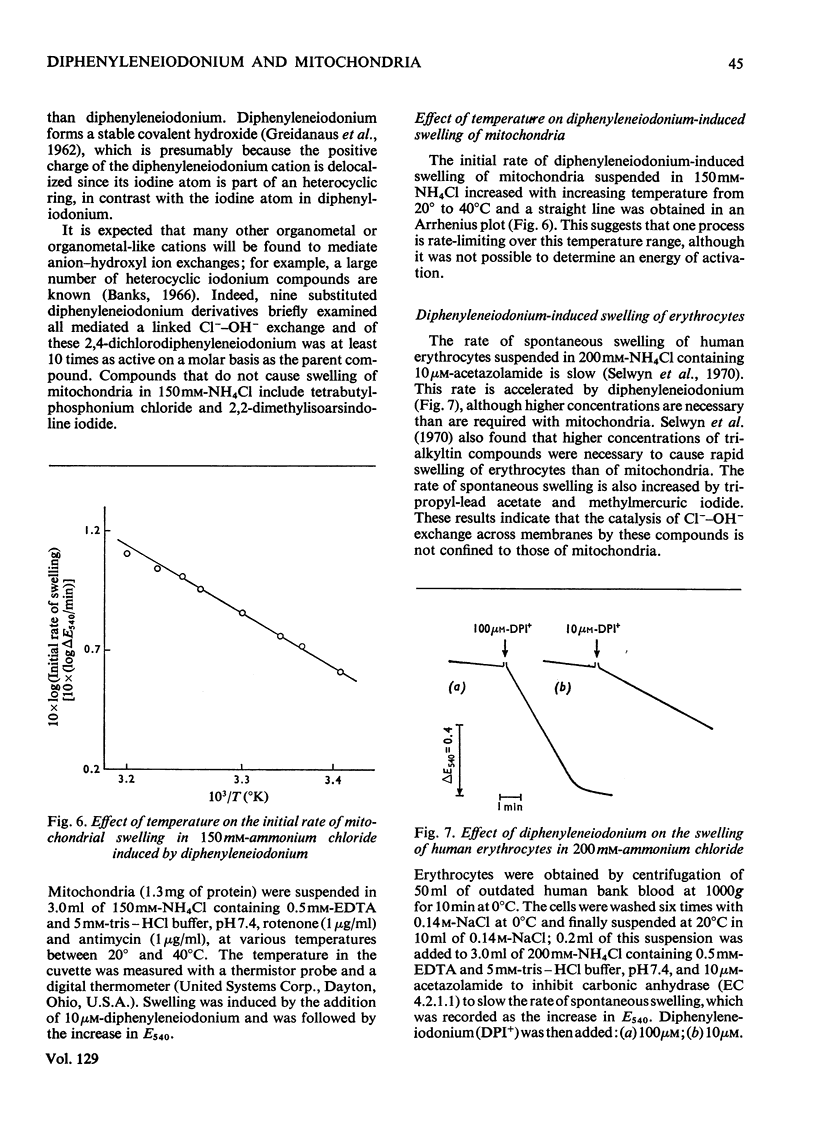
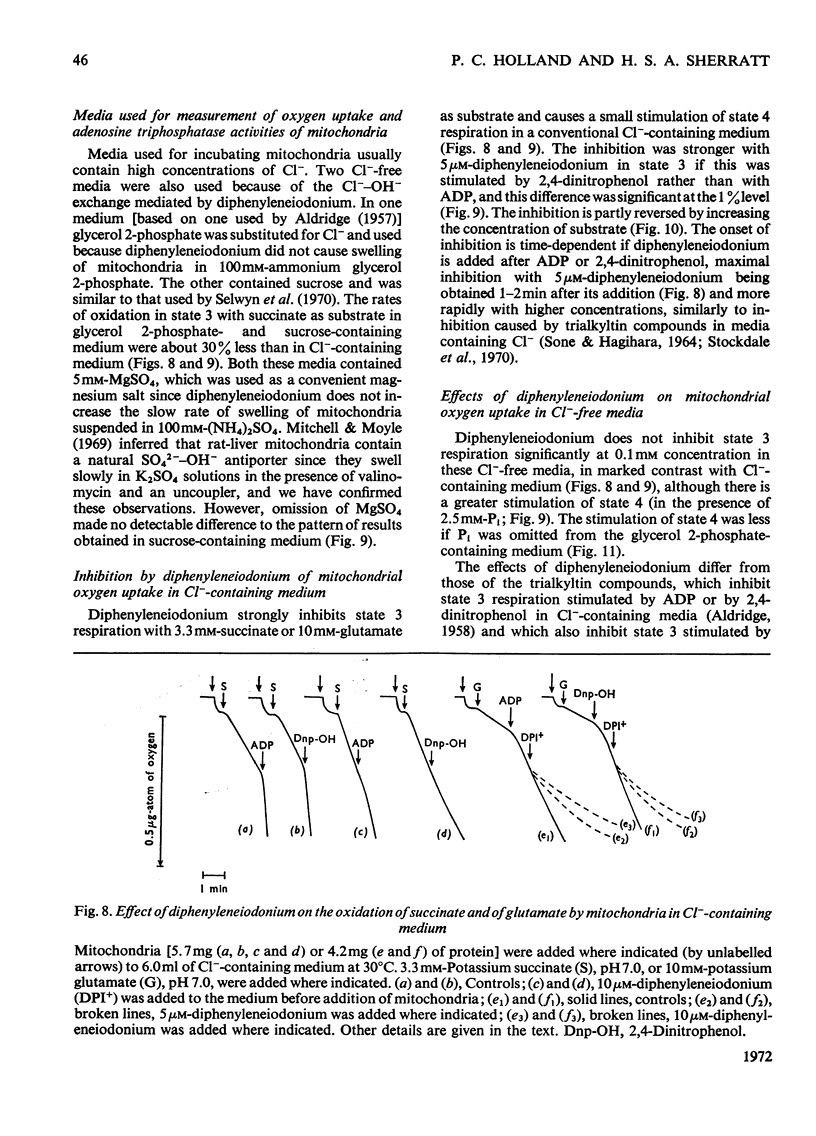
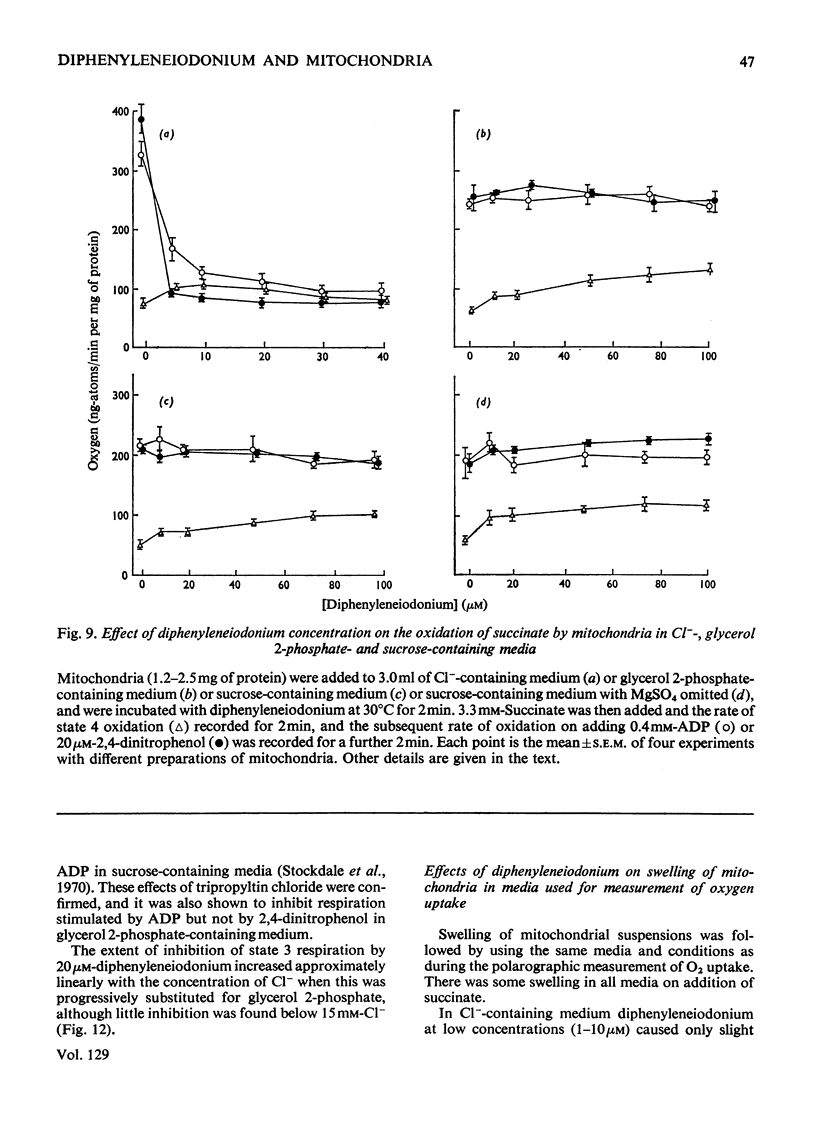
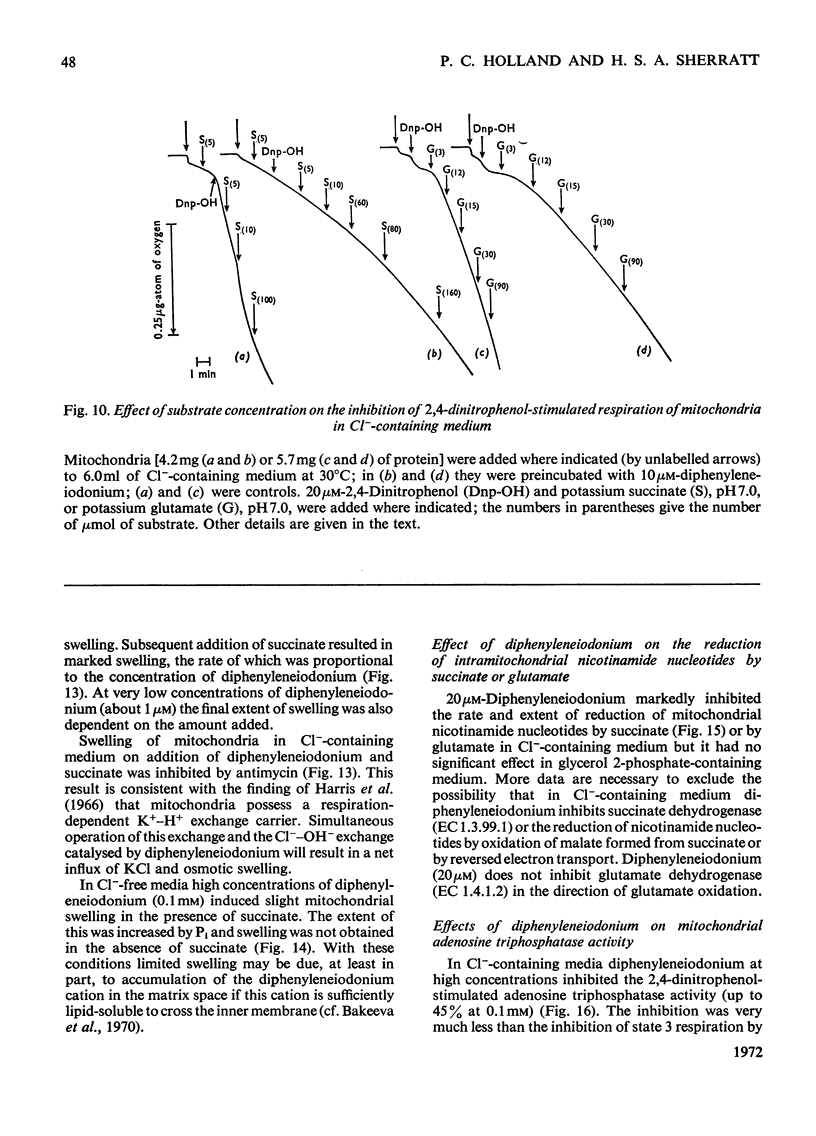
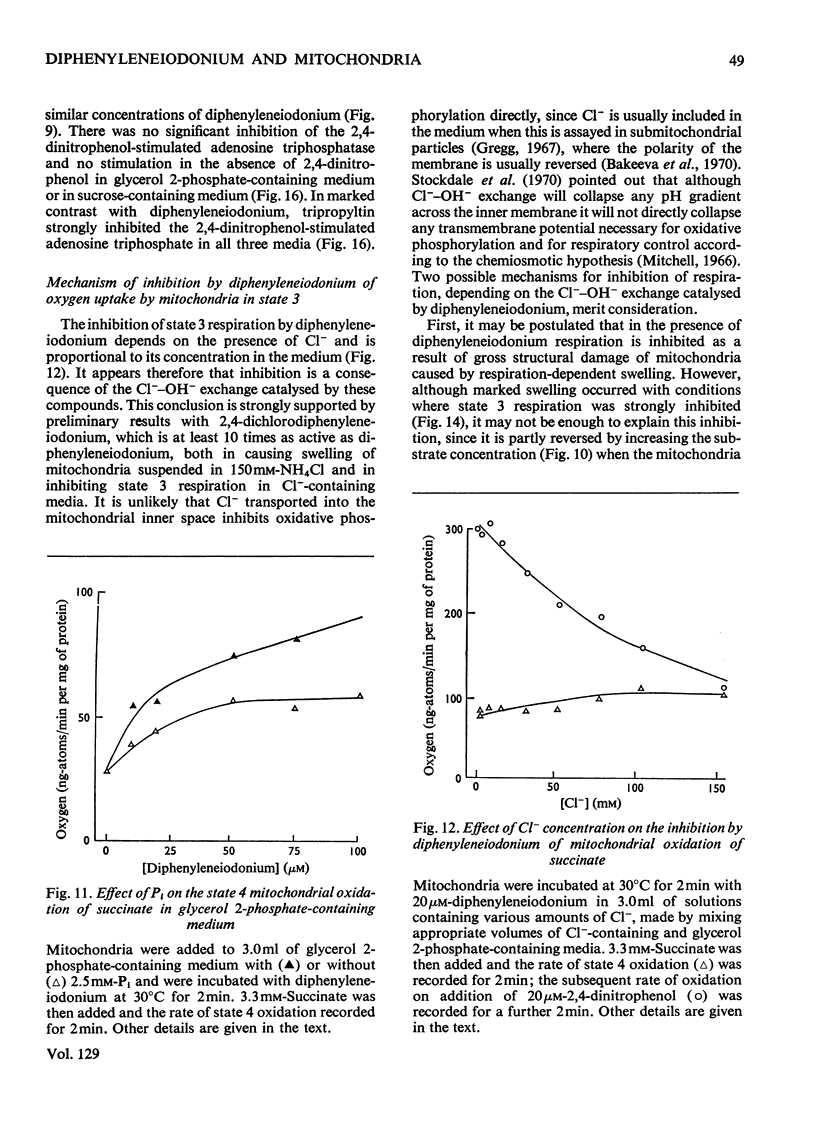
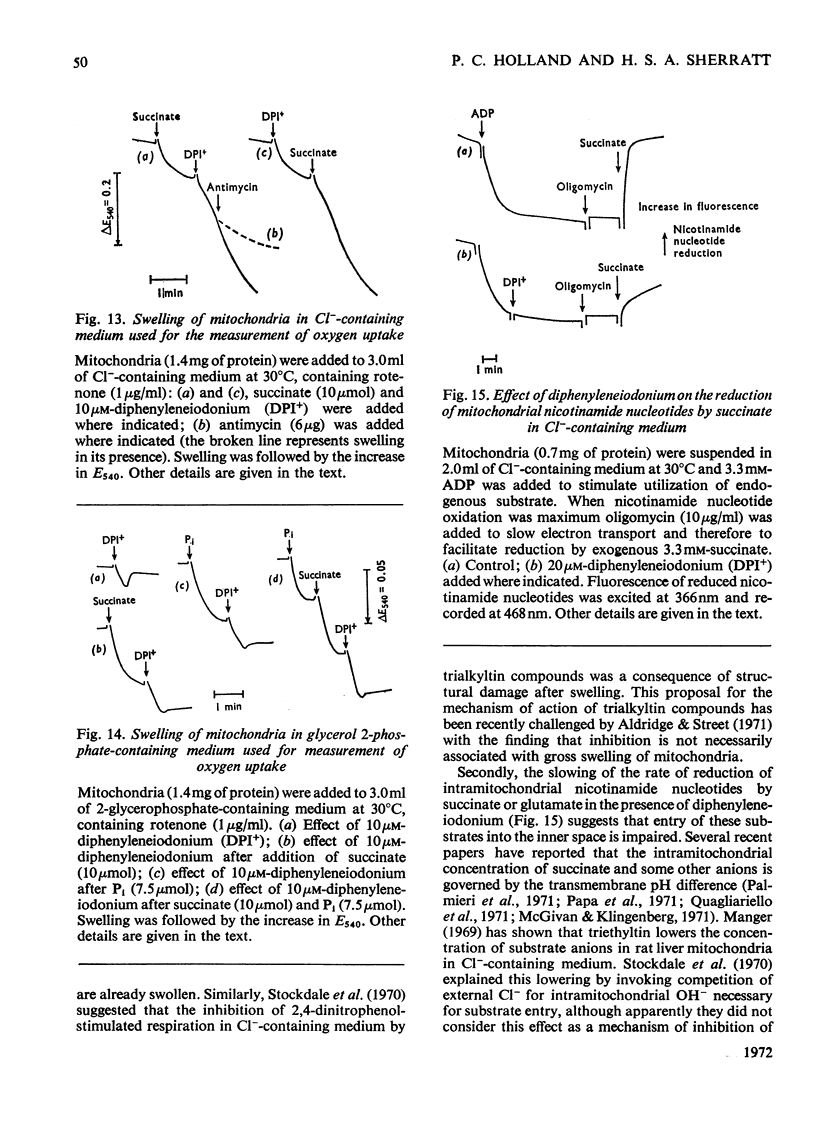
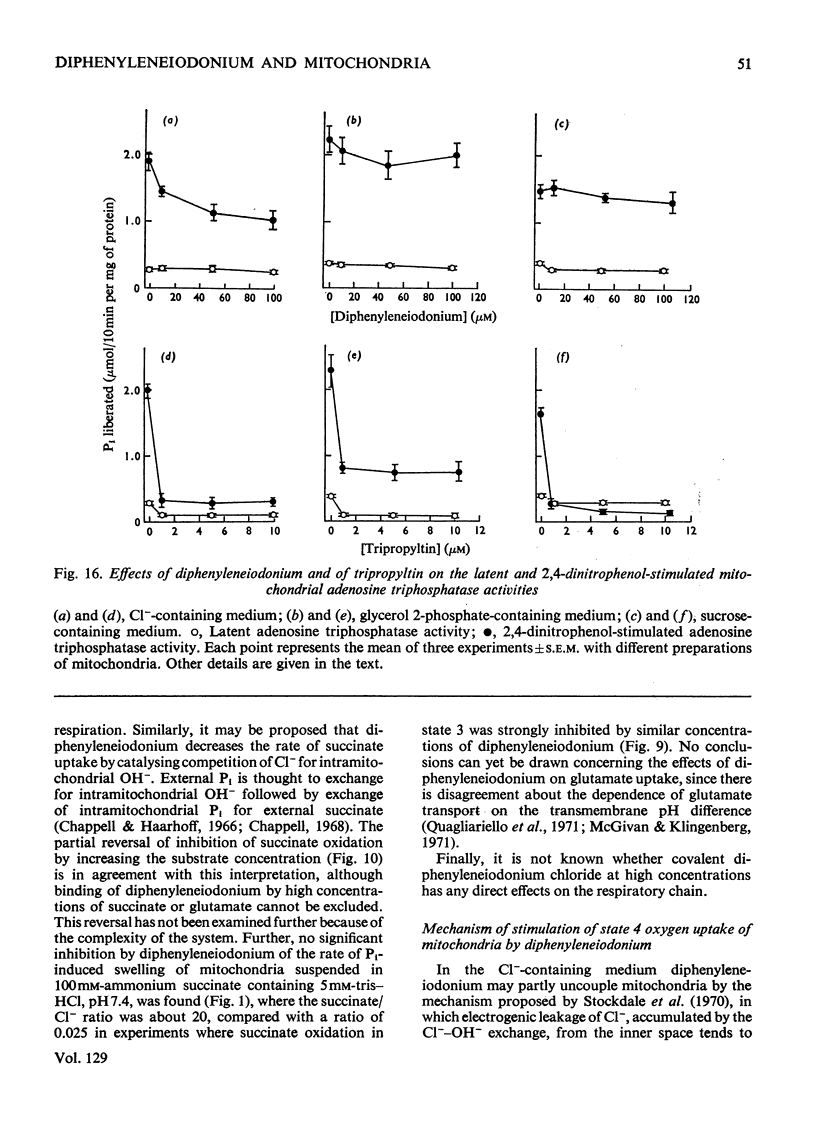
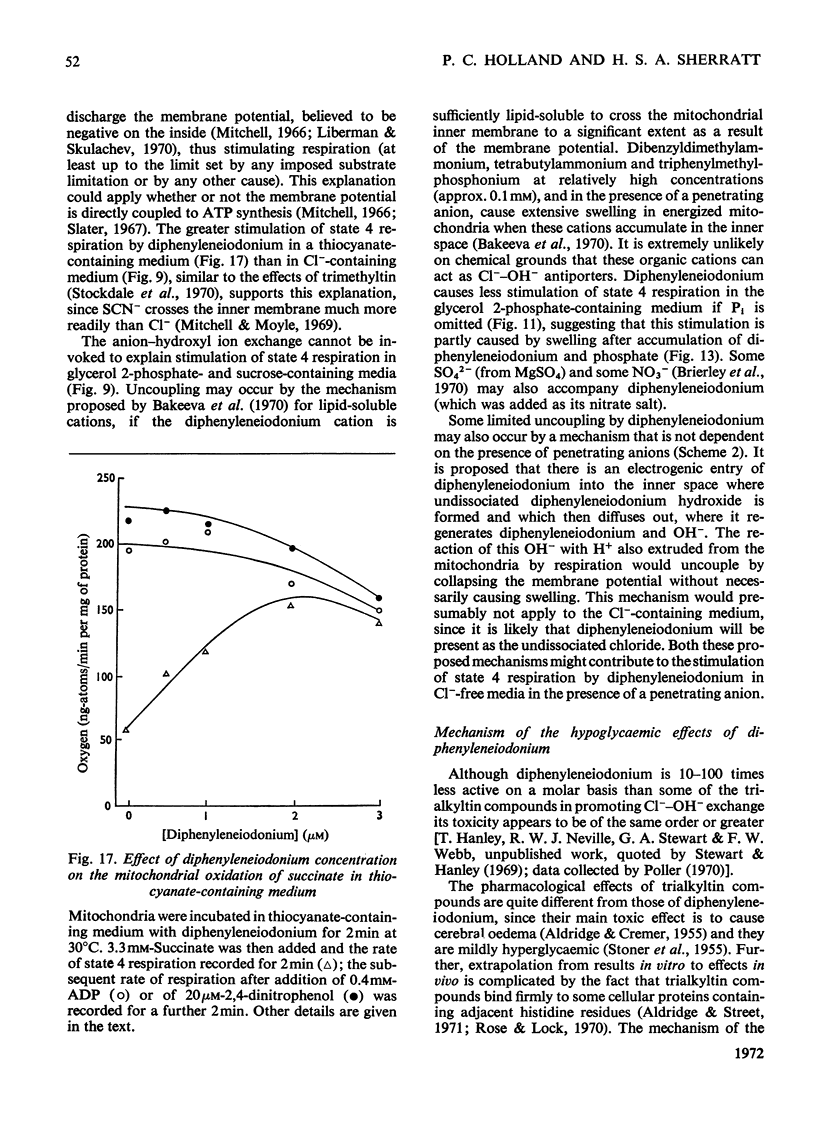
1. The hypoglycaemic compound diphenyleneiodonium causes rapid and extensive swelling of rat liver mitochondria suspended in 150mm-NH4Cl, and in 150mm-KCl in the presence of 2,4-dinitrophenol and valinomycin. This indicates that diphenyleneiodonium catalyses a compulsory exchange of OH− for Cl− across the mitochondrial inner membrane. Br− and SCN− were the only other anions found whose exchange for OH− is catalysed by diphenyleneiodonium. 2. Diphenyleneiodonium inhibited state 3 respiration of mitochondria and slightly stimulated state 4 respiration with succinate or glutamate as substrate in a standard Cl−-containing medium. 3. Diphenyleneiodonium did not inhibit state 3 respiration significantly in two Cl−-free media (based on glycerol 2-phosphate or sucrose) but caused some stimulation of state 4. 4. In Cl−-containing medium diphenyleneiodonium only slightly inhibited the 2,4-dinitrophenol-stimulated adenosine triphosphatase and it had little effect in the absence of Cl−. 5. The inhibition of respiration in the presence of Cl− is dependent on the Cl−–OH− exchange. 2,4-Dichlorodiphenyleneiodonium is ten times as active as diphenyleneiodonium both in causing swelling of mitochondria suspended in 150mm-NH4Cl and in inhibiting state 3 respiration in Cl−-containing medium. Indirect evidence suggests that the Cl−–OH− exchange impairs the rate of uptake of substrate anions. 6. It is proposed that stimulation of state 4 respiration in the absence of Cl− depends, at least in part, on an electrogenic uptake of diphenyleneiodonium cations. 7. Tripropyl-lead acetate, methylmercuric iodide and nine substituted diphenyleneiodonium derivatives also catalyse Cl−–OH− exchange across the mitochondrial membrane. 8. Diphenyleneiodonium is compared with the trialkyltin compounds, which are also known to mediate Cl−–OH− exchange and which have in addition strong oligomycin-like effects on respiration. It is concluded that diphenyleneiodonium is specific for catalysing anion–OH− exchange and will be a useful reagent for investigating membrane-dependent systems.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALDRIDGE W. N., CREMER J. E. The biochemistry of organo-tin compounds; diethyltin dichloride and triethyltin sulphate. Biochem J. 1955 Nov;61(3):406–418. doi: 10.1042/bj0610406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALDRIDGE W. N. Liver and brain mitochondria. Biochem J. 1957 Nov;67(3):423–431. doi: 10.1042/bj0670423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALDRIDGE W. N. The biochemistry of organotin compounds: trialkyltins and oxidative phosphorylation. Biochem J. 1958 Jul;69(3):367–376. doi: 10.1042/bj0690367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge W. N., Rose M. S. The mechanism of oxidative phosphorylation A hypothesis derived from studies of trimethyltin and triethyltin compounds. FEBS Lett. 1969 Jul;4(2):61–68. doi: 10.1016/0014-5793(69)80197-3. [DOI] [PubMed] [Google Scholar]
- Aldridge W. N., Street B. W. Oxidative phosphorylation. The relation between the specific binding of trimethylytin and triethyltin to mitochondria and their effects on various mitochondrial functions. Biochem J. 1971 Aug;124(1):221–234. doi: 10.1042/bj1240221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakeeva L. E., Grinius L. L., Jasaitis A. A., Kuliene V. V., Levitsky D. O., Liberman E. A., Severina I. I., Skulachev V. P. Conversion of biomembrane-produced energy into electric form. II. Intact mitochondria. Biochim Biophys Acta. 1970 Aug 4;216(1):13–21. doi: 10.1016/0005-2728(70)90154-4. [DOI] [PubMed] [Google Scholar]
- Blondin G. A., Green D. E. The mechanism of mitochondrial swelling. V. Permeability of mitochondria to alkali metal salts of strong acid anions. J Bioenerg. 1970 Jul;1(2):193–213. doi: 10.1007/BF01515981. [DOI] [PubMed] [Google Scholar]
- Brierley G. P., Jurkowitz M., Scott K. M., Merola A. J. Ion transport by heart mitochondria. XX. Factors affecting passive osmotic swelling of isolated mitochondria. J Biol Chem. 1970 Oct 25;245(20):5404–5411. [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- Chappell J. B. Systems used for the transport of substrates into mitochondria. Br Med Bull. 1968 May;24(2):150–157. doi: 10.1093/oxfordjournals.bmb.a070618. [DOI] [PubMed] [Google Scholar]
- Chappell J. B. The oxidation of citrate, isocitrate and cis-aconitate by isolated mitochondria. Biochem J. 1964 Feb;90(2):225–237. doi: 10.1042/bj0900225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson P. J., McGivan J. D., Chappell J. B. The action of certain antibiotics on mitochondrial, erythrocyte and artificial phospholipid membranes. The role of induced proton permeability. Biochem J. 1969 Feb;111(4):521–535. doi: 10.1042/bj1110521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King E. J. The colorimetric determination of phosphorus. Biochem J. 1932;26(2):292–297. doi: 10.1042/bj0260292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman E. A., Skulachev V. P. Conversion of biomembrane-produced energy into electric form. IV. General discussion. Biochim Biophys Acta. 1970 Aug 4;216(1):30–42. doi: 10.1016/0005-2728(70)90156-8. [DOI] [PubMed] [Google Scholar]
- MORGAN H. E., RANDLE P. J., REGEN D. M. Regulation of glucose uptake by muscle. 3. The effects of insulin, anoxia, salicylate and 2:4-dinitrophenol on membrane transport and intracellular phosphorylation of glucose in the isolated rat heart. Biochem J. 1959 Dec;73:573–579. doi: 10.1042/bj0730573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manger J. R. The effect of triethyltin on mitochondrial ion accumulation. FEBS Lett. 1969 Dec 30;5(5):331–334. doi: 10.1016/0014-5793(69)80349-2. [DOI] [PubMed] [Google Scholar]
- McGivan J. D., Klingenberg M. Correlation between H+ and anion movement in mitochondria and the key role of the phosphate carrier. Eur J Biochem. 1971 Jun 11;20(3):392–399. doi: 10.1111/j.1432-1033.1971.tb01405.x. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Translocation of some anions cations and acids in rat liver mitochondria. Eur J Biochem. 1969 Jun;9(2):149–155. doi: 10.1111/j.1432-1033.1969.tb00588.x. [DOI] [PubMed] [Google Scholar]
- Palmieri F., Prezioso G., Quagliariello E., Klingenberg M. Kinetic study of the dicarboxylate carrier in rat liver mitochondria. Eur J Biochem. 1971 Sep 13;22(1):66–74. doi: 10.1111/j.1432-1033.1971.tb01515.x. [DOI] [PubMed] [Google Scholar]
- Papa S., Lofrumento N. E., Kanduc D., Paradies G., Quagliariello E. The transport of citric-acid-cycle intermediates in rat-liver mitochondria. Electrical nature and coupling of the exchange-diffusion reactions with proton translocation. Eur J Biochem. 1971 Sep 13;22(1):134–143. doi: 10.1111/j.1432-1033.1971.tb01524.x. [DOI] [PubMed] [Google Scholar]
- Quagliariello E., Genchi G., Palmieri F. Respiration-dependent anion uptake by rat liver mitochondria. FEBS Lett. 1971 Mar 22;13(5):253–257. doi: 10.1016/0014-5793(71)80233-8. [DOI] [PubMed] [Google Scholar]
- RANDLE P. J., SMITH G. H. Regulation of glucose uptake by muscle. 1. The effects of insulin, anaerobiosis and cell poisons on the uptake of glucose and release of potassium by isolated rat diaphragm. Biochem J. 1958 Nov;70(3):490–500. doi: 10.1042/bj0700490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M. S., Lock E. A. The interaction of triethyltin with a component of guinea-pig liver supernatant. Evidence for histidine in the binding sites. Biochem J. 1970 Nov;120(1):151–157. doi: 10.1042/bj1200151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SONE N., HAGIHARA B. INHIBITORY ACTION OF TRIALKYLTIN COMPOUNDS ON OXIDATIVE PHOSPHORYLATION IN MITOCHONDRIA. J Biochem. 1964 Aug;56:151–156. doi: 10.1093/oxfordjournals.jbchem.a127972. [DOI] [PubMed] [Google Scholar]
- STONER H. B., BARNES J. M., DUFF J. I. Studies on the toxicity of alkyl tin compounds. Br J Pharmacol Chemother. 1955 Mar;10(1):16–25. doi: 10.1111/j.1476-5381.1955.tb00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selwyn M. J., Dawson A. P., Stockdale M., Gains N. Chloride-hydroxide exchange across mitochondrial, erythrocyte and artificial lipid membranes mediated by trialkyl- and triphenyltin compounds. Eur J Biochem. 1970 May 1;14(1):120–126. doi: 10.1111/j.1432-1033.1970.tb00268.x. [DOI] [PubMed] [Google Scholar]
- Senior A. E., Sherratt H. S. Biochemical effects of the hypoglycaemic compound pent-4-enoic acid and related non-hypoglycaemic fatty acids. Carbohydrate metabolism. Biochem J. 1968 Dec;110(3):521–527. doi: 10.1042/bj1100521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior A. E., Sherratt H. S. Biochemical effects of the hypoglycaemic compound pent-4-enoic acid and related non-hypoglycaemic fatty acids. Oxidative phosphorylation and mitochondrial oxidation of pyruvate, 3-hydroxybutyrate and tricarboxylic acid-cycle intermediates. Biochem J. 1968 Dec;110(3):499–509. doi: 10.1042/bj1100499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherratt H. S. Hypoglycin and related hypoglycaemic compounds. Br Med Bull. 1969 Sep;25(3):250–255. doi: 10.1093/oxfordjournals.bmb.a070713. [DOI] [PubMed] [Google Scholar]
- Slater E. C. An evaluation of the Mitchell hypothesis of chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Eur J Biochem. 1967 May;1(3):317–326. doi: 10.1007/978-3-662-25813-2_43. [DOI] [PubMed] [Google Scholar]
- Stockdale M., Dawson A. P., Selwyn M. J. Effects of trialkyltin and triphenyltin compounds on mitochondrial respiration. Eur J Biochem. 1970 Aug;15(2):342–351. doi: 10.1111/j.1432-1033.1970.tb01013.x. [DOI] [PubMed] [Google Scholar]
- Watling A. S., Selwyn M. J. Effect of some organometallic compounds on the permeability of chloroplast membranes. FEBS Lett. 1970 Oct 5;10(3):139–142. doi: 10.1016/0014-5793(70)80437-9. [DOI] [PubMed] [Google Scholar]


