Abstract
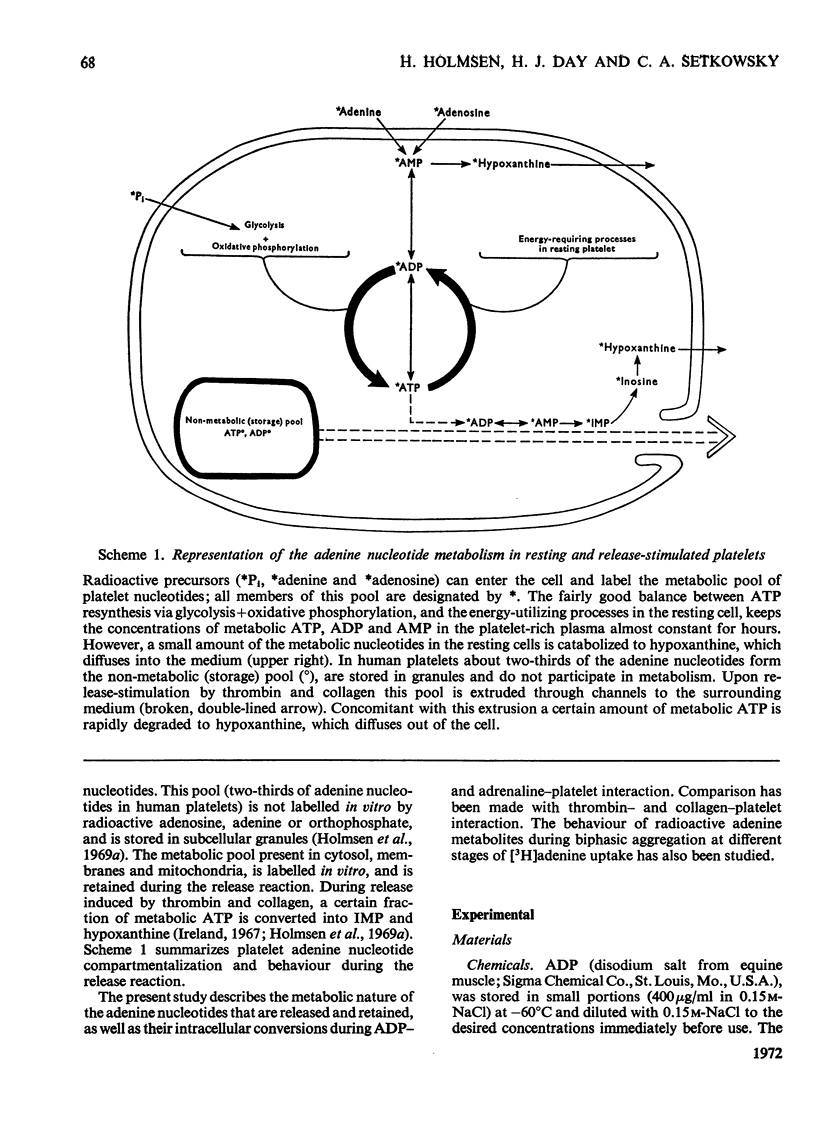
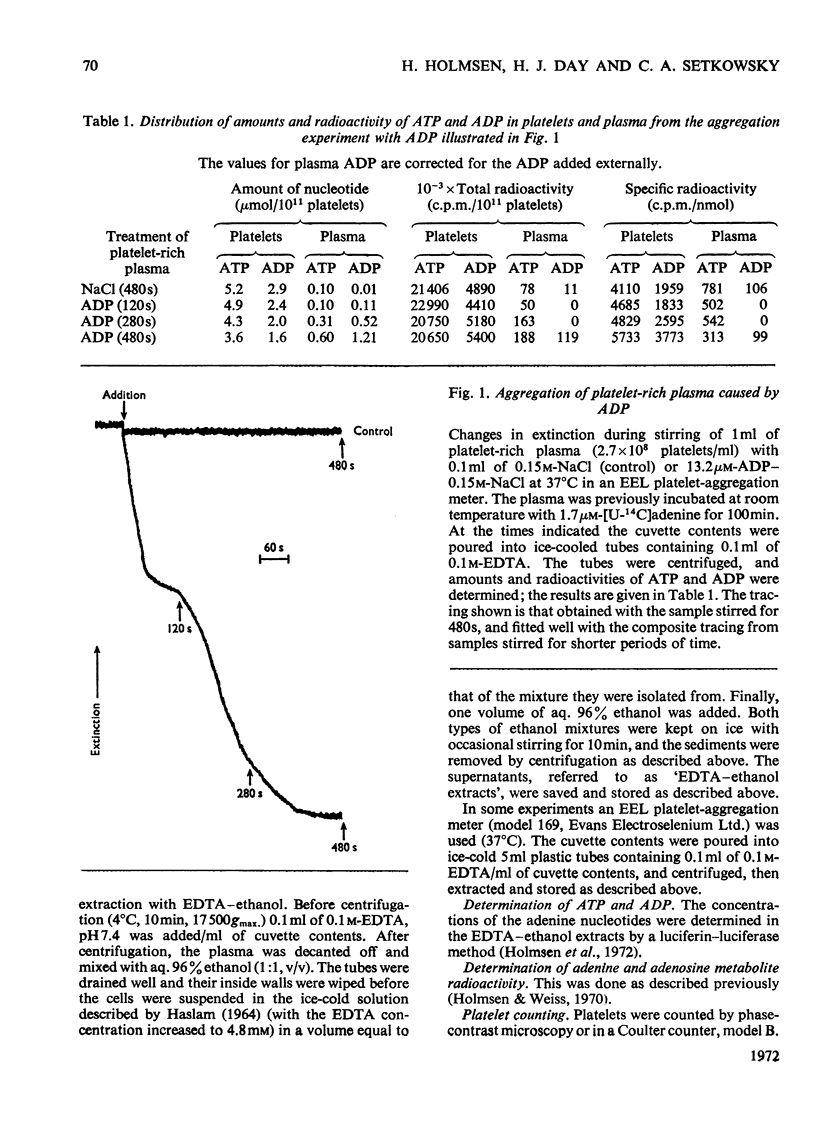
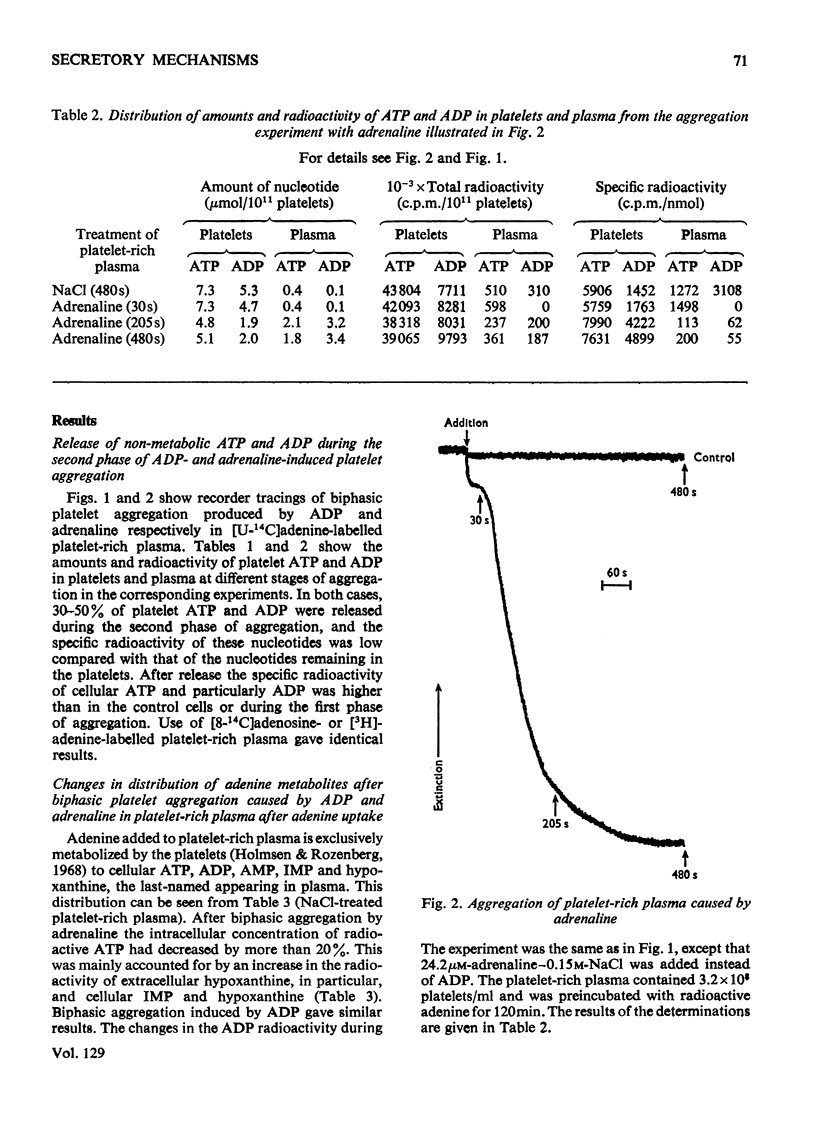
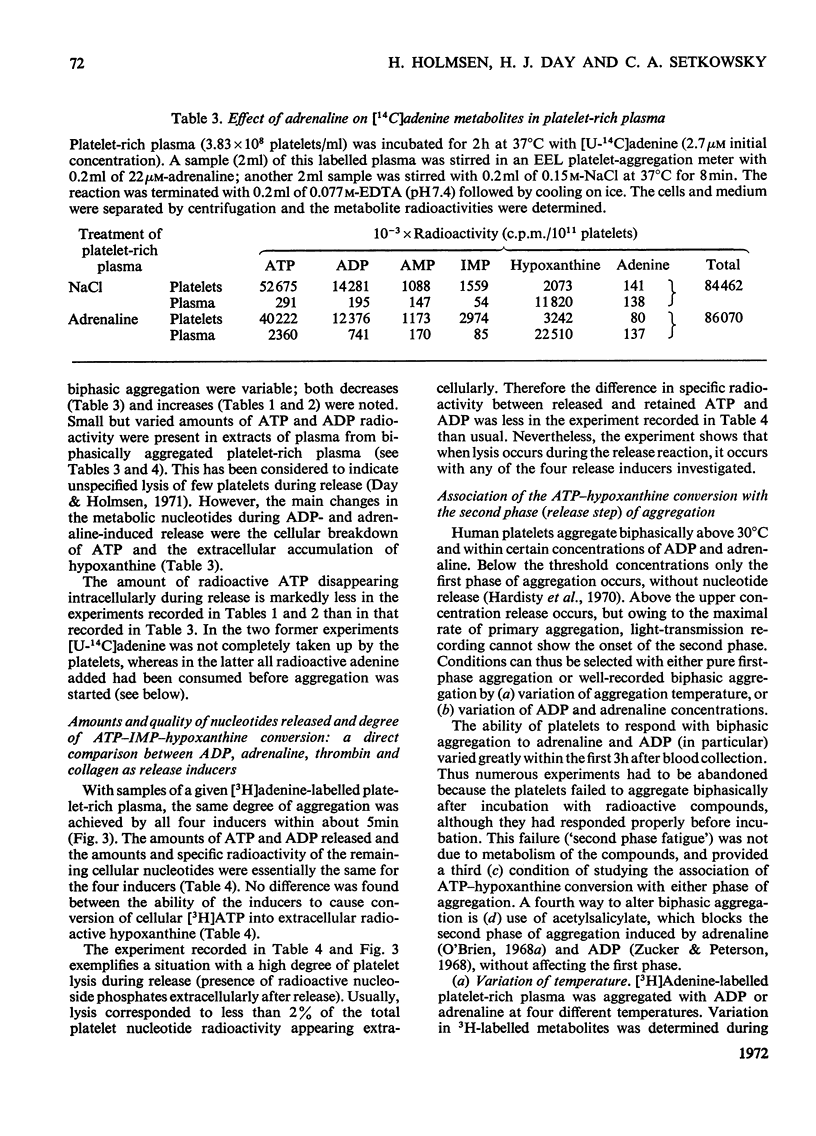
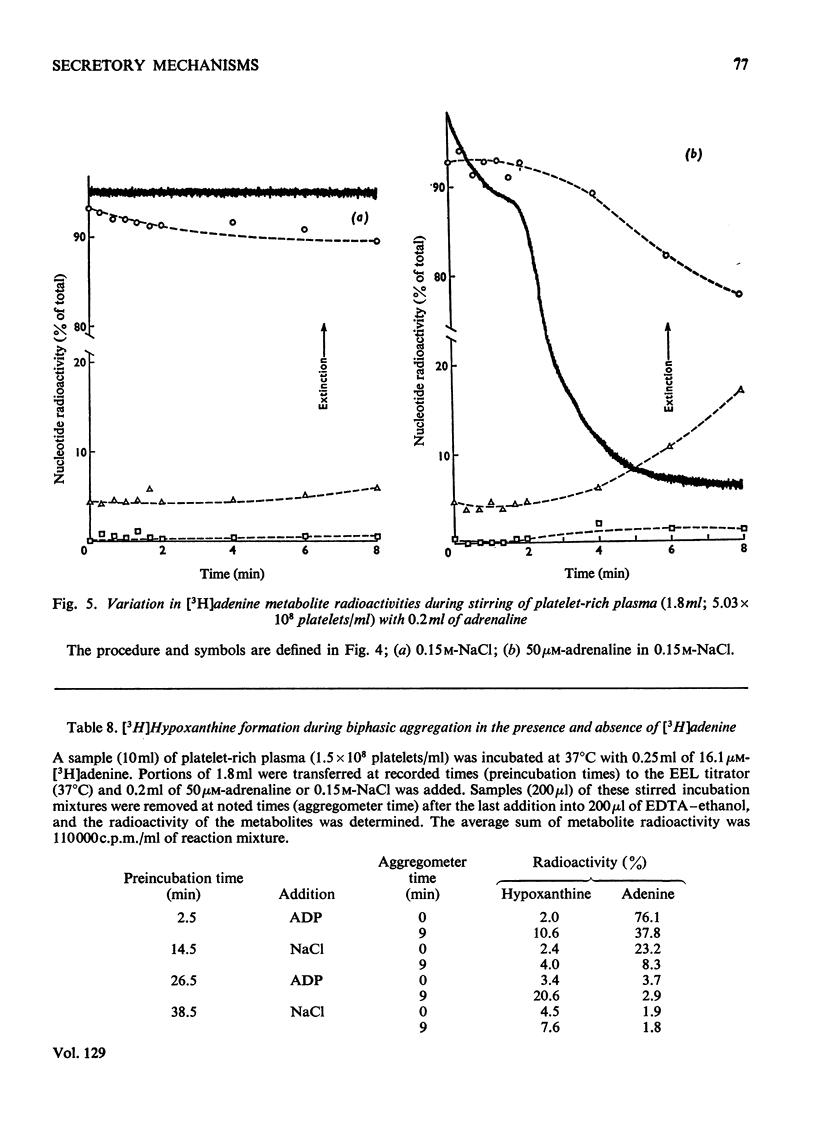
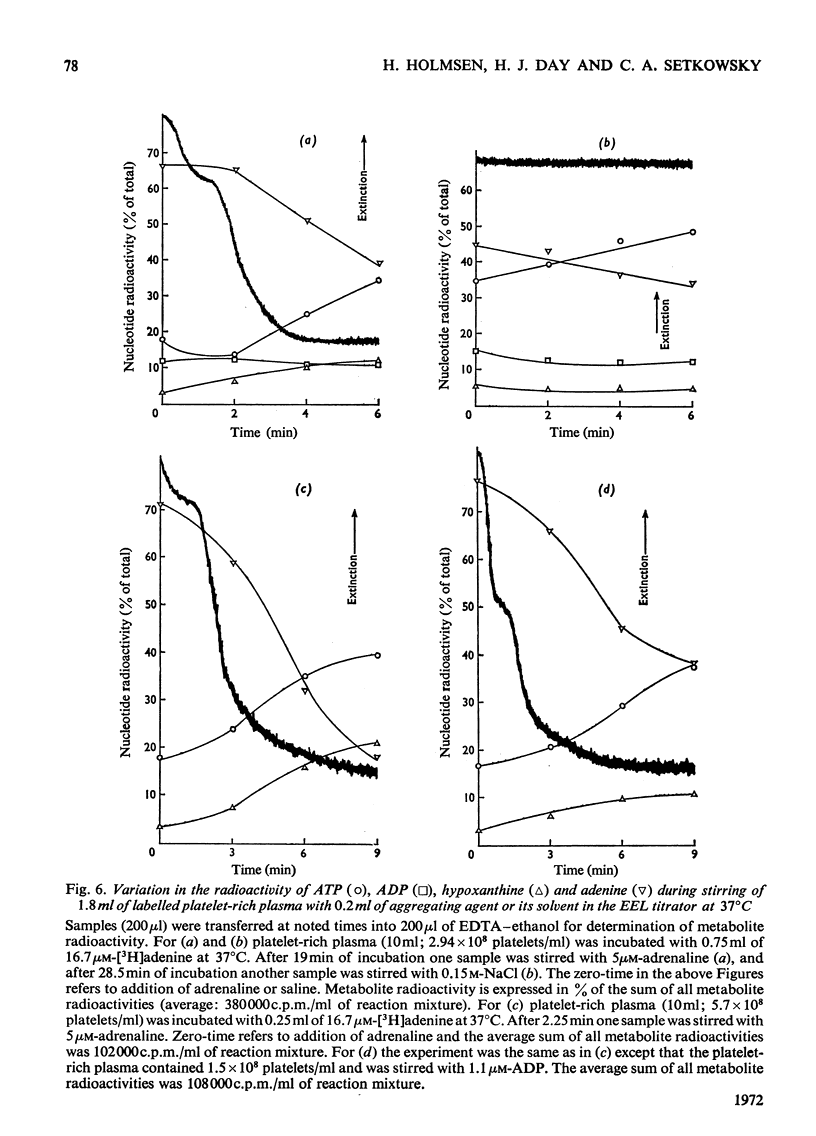
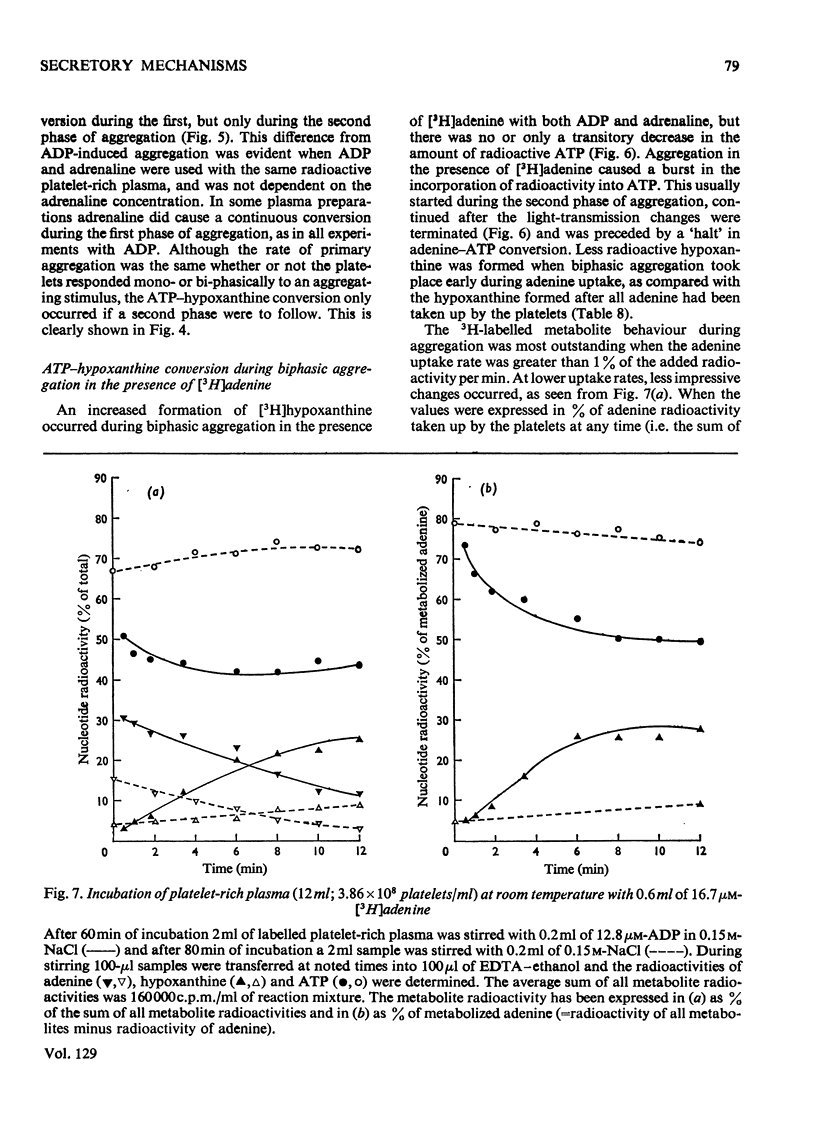
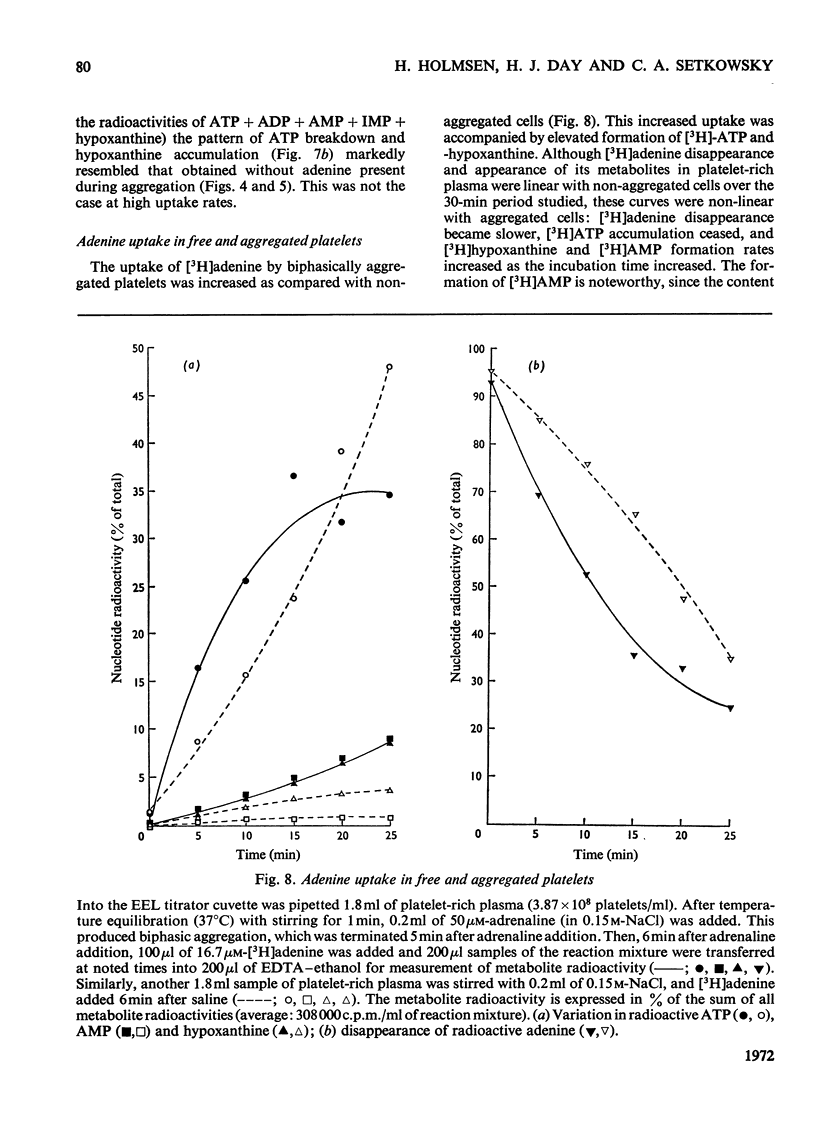
1. Platelets containing adenine nucleotides labelled with 3H and 14C in vitro were aggregated biphasically with ADP and adrenaline. Amounts of ATP and ADP as well as the radioactivity of ATP, ADP, AMP, IMP, hypoxanthine and adenine were determined in platelets and plasma at different stages of aggregation. 2. ATP and ADP were released during the second aggregation phase and had a low specific radioactivity compared with the ATP and ADP retained by the cells. The specific radioactivity of intracellular nucleotides increased during release. The parameters observed with ADP and adrenaline as release inducers were the same as for collagen and thrombin. 3. Release induced by all four inducers was accompanied by conversion of cellular [3H]ATP into extracellular [3H]-hypoxanthine. By variation of temperature, inducer concentration, time after blood withdrawal and use of acetylsalicylic acid, the aggregation pattern caused by adrenaline and ADP could be made mono- or bi-phasic. Release or second-phase aggregation was intimately connected with the ATP–hypoxanthine conversion, whereas first phase aggregation was not. 4. The [3H]ATP–hypoxanthine conversion started immediately after ADP addition. With adrenaline it usually started with the appearance of the second aggregation phase. The conversion was present during first phase of ADP-induced aggregation only if a second phase were to follow. 5. When secondary aggregation took place while radioactive adenine was being taken up by the platelets, increased formation of labelled hypoxanthine still occurred, but there was either no change or an increase in the concentration of labelled ATP. 6. Biphasically aggregated platelets converted [3H]adenine more rapidly into [3H]-ATP and -hypoxanthine than non-aggregated platelets. Addition of [3H]adenine at different stages of biphasic aggregation showed that more [3H]hypoxanthine was formed during than after the release step. 7. We conclude that ADP and adrenaline, like thrombin and collagen, cause extrusion of non-metabolic granula-located platelet adenine nucleotides. During release metabolic ATP breaks down to hypoxanthine, and this process might reflect an ATP-requiring part of the release reaction.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball G., Fulwood M., Ireland D. M., Yates P. Effect of some inhibitors of platelet aggregation on platelet nucleotides. Biochem J. 1969 Sep;114(3):669–671. doi: 10.1042/bj1140669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantine J. W. Aggregation of guinea-pig platelets by adenosine diphosphate. Nature. 1966 Apr 9;210(5032):162–164. doi: 10.1038/210162a0. [DOI] [PubMed] [Google Scholar]
- Day H. J., Holmsen H. Concepts of the blood platelet release reaction. Ser Haematol. 1971;4(1):3–27. [PubMed] [Google Scholar]
- HASLAM R. J. ROLE OF ADENOSINE DIPHOSPHATE IN THE AGGREGATION OF HUMAN BLOOD-PLATELETS BY THROMBIN AND BY FATTY ACIDS. Nature. 1964 May 23;202:765–768. doi: 10.1038/202765a0. [DOI] [PubMed] [Google Scholar]
- HOLMSEN H. COLLAGEN-INDUCED RELEASE OF ADENOSINE DIPHOSPHATE FROM BLOOD PLATELETS INCUBATED WITH RADIOACTIVE PHOSPHATE IN VITRO. Scand J Clin Lab Invest. 1965;17:239–246. [PubMed] [Google Scholar]
- Hardisty R. M., Hutton R. A., Montgomery D., Rickard S., Trebilcock H. Secondary platelet aggregation: a quantitative study. Br J Haematol. 1970 Sep;19(3):307–319. doi: 10.1111/j.1365-2141.1970.tb01628.x. [DOI] [PubMed] [Google Scholar]
- Holmsen H., Day H. J. Adenine nucleotides and platelet function. Ser Haematol. 1971;4(1):28–58. [PubMed] [Google Scholar]
- Holmsen H., Day H. J., Storm E. Adenine nucleotide metabolism of blood platelets. VI. Subcellular localization of nucleotide pools with different functions in the platelet release reaction. Biochim Biophys Acta. 1969 Aug 20;186(2):254–266. doi: 10.1016/0005-2787(69)90003-3. [DOI] [PubMed] [Google Scholar]
- Holmsen H., Day H. J. The selectivity of the thrombin-induced platelet release reaction: subcellular localization of released and retained constituents. J Lab Clin Med. 1970 May;75(5):840–855. [PubMed] [Google Scholar]
- Holmsen H., Rozenberg M. C. Adenine nucleotide metabolism of blood platelets. 3. Adenine phosphoribosyl transferase and nucleotide formation from exogenous adenine. Biochim Biophys Acta. 1968 Apr 22;157(2):266–279. [PubMed] [Google Scholar]
- Holmsen H., Storm E., Day H. J. Determination of ATP and ADP in blood platelets: a modification of the firefly luciferase assay for plasma. Anal Biochem. 1972 Apr;46(2):489–501. doi: 10.1016/0003-2697(72)90323-5. [DOI] [PubMed] [Google Scholar]
- Holmsen H., Storm E. The adenosine triphosphate inhibition of the pyruvate kinase reaction and its dependence on the total magnesium ion concentration. Biochem J. 1969 Apr;112(3):303–316. doi: 10.1042/bj1120303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmsen H., Weiss H. J. Further evidence for a deficient storage pool of adenine nucleotides in platelets from some patients with thrombocytopathia--"storage pool disease". Blood. 1972 Feb;39(2):197–209. [PubMed] [Google Scholar]
- Holmsen H., Weiss H. J. Hereditary defect in the platelet release reaction caused by a deficiency in the storage pool of platelet adenine nucleotides. Br J Haematol. 1970 Nov;19(5):643–649. doi: 10.1111/j.1365-2141.1970.tb01648.x. [DOI] [PubMed] [Google Scholar]
- Ireland D. M. Effect of thrombin on the radioactive nucleotides of human washed platelets. Biochem J. 1967 Nov;105(2):857–867. doi: 10.1042/bj1050857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macmillan D. C. Secondary clumping effect in human citrated platelet-rich plasma produced by adenosine diphosphate and adrenaline. Nature. 1966 Jul 9;211(5045):140–144. doi: 10.1038/211140a0. [DOI] [PubMed] [Google Scholar]
- Massini P., Lüscher E. F. The induction of the release reaction in human blood platelets by close cell contact. Thromb Diath Haemorrh. 1971;25(1):13–20. [PubMed] [Google Scholar]
- Mills D. C., Robb I. A., Roberts G. C. The release of nucleotides, 5-hydroxytryptamine and enzymes from human blood platelets during aggregation. J Physiol. 1968 Apr;195(3):715–729. doi: 10.1113/jphysiol.1968.sp008484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills D. C., Roberts G. C. Effects of adrenaline on human blood platelets. J Physiol. 1967 Nov;193(2):443–453. doi: 10.1113/jphysiol.1967.sp008369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mürer E. H. Clot retraction and energy metabolism of platelets. Effect and mechanism of inhibitors. Biochim Biophys Acta. 1969 Feb 25;172(2):266–276. doi: 10.1016/0005-2728(69)90069-3. [DOI] [PubMed] [Google Scholar]
- O'BRIEN J. R. SOME EFFECTS OF ADRENALINE AND ANTI-ADRENALINE COMPOUNDS ON PLATELETS IN VITRO AND IN VIVO. Nature. 1963 Nov 23;200:763–764. doi: 10.1038/200763a0. [DOI] [PubMed] [Google Scholar]
- O'Brien J. R. Effects of salicylates on human platelets. Lancet. 1968 Apr 13;1(7546):779–783. doi: 10.1016/s0140-6736(68)92228-9. [DOI] [PubMed] [Google Scholar]
- Zucker M. B., Peterson J. Inhibition of adenosine diphosphate-induced secondary aggregation and other platelet functions by acetylsalicylic acid ingestion. Proc Soc Exp Biol Med. 1968 Feb;127(2):547–551. doi: 10.3181/00379727-127-32737. [DOI] [PubMed] [Google Scholar]


