Abstract
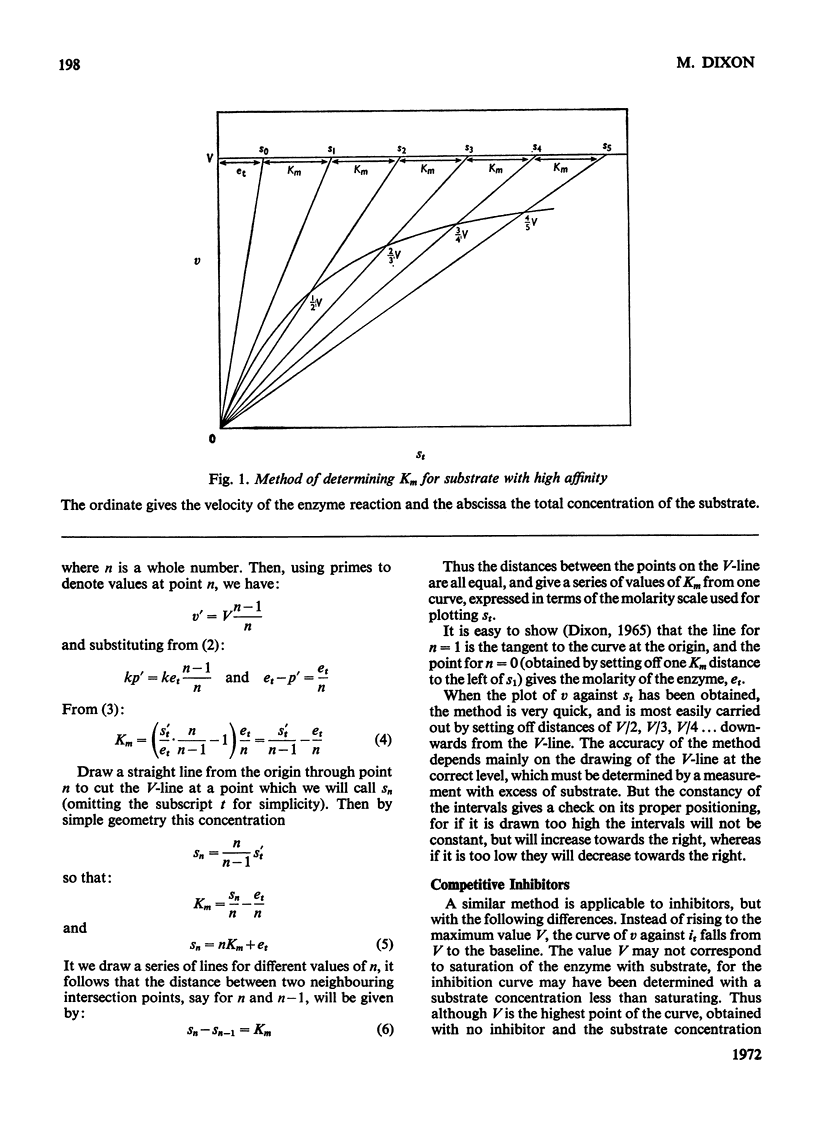
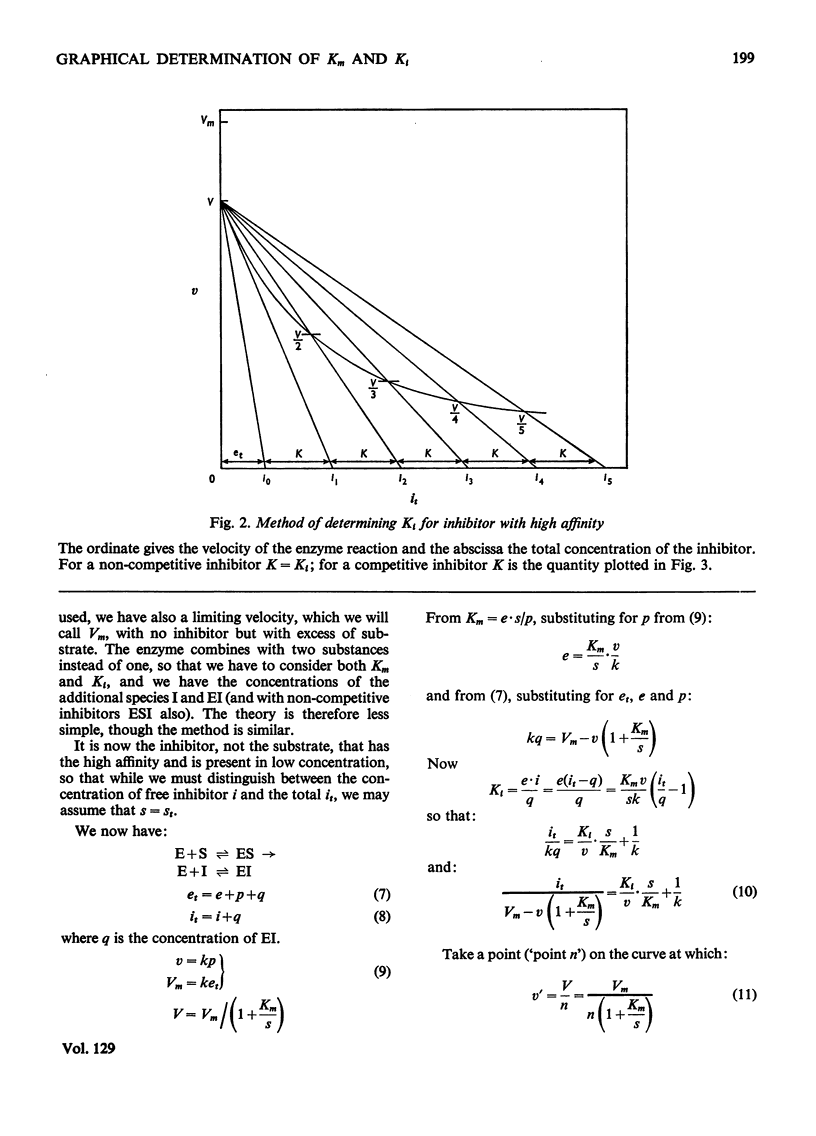
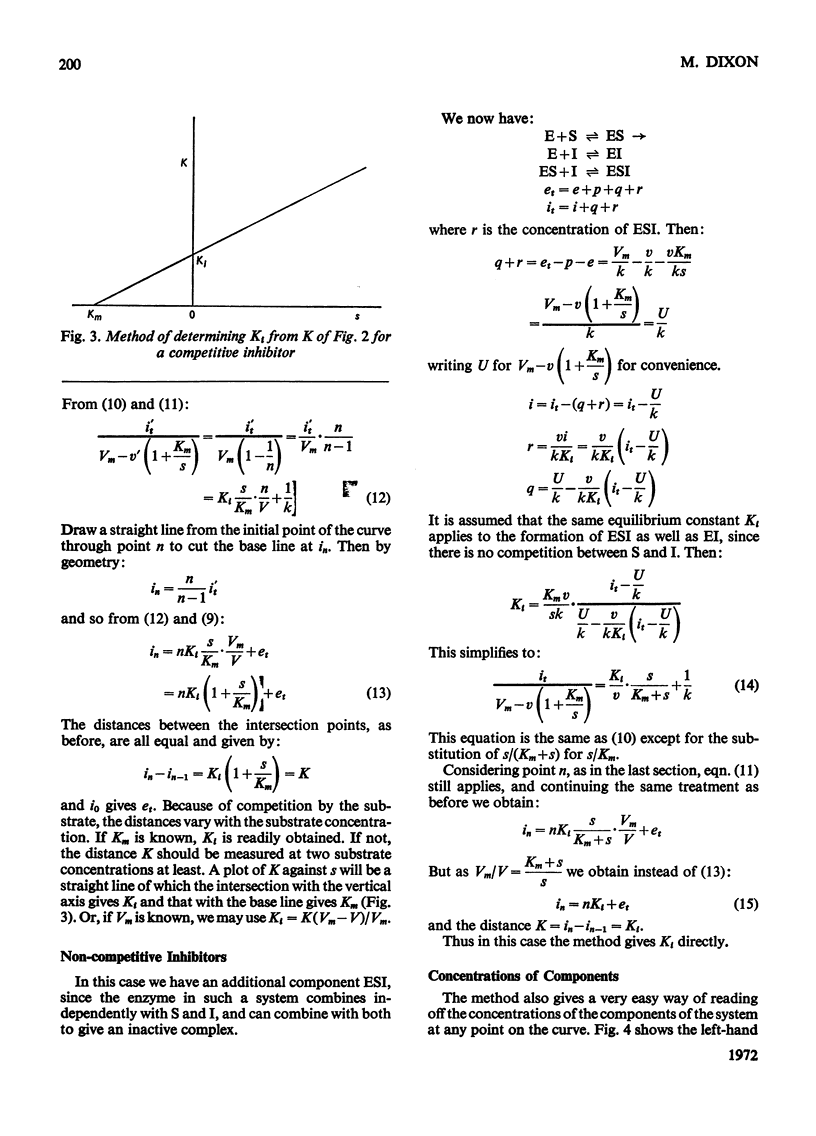
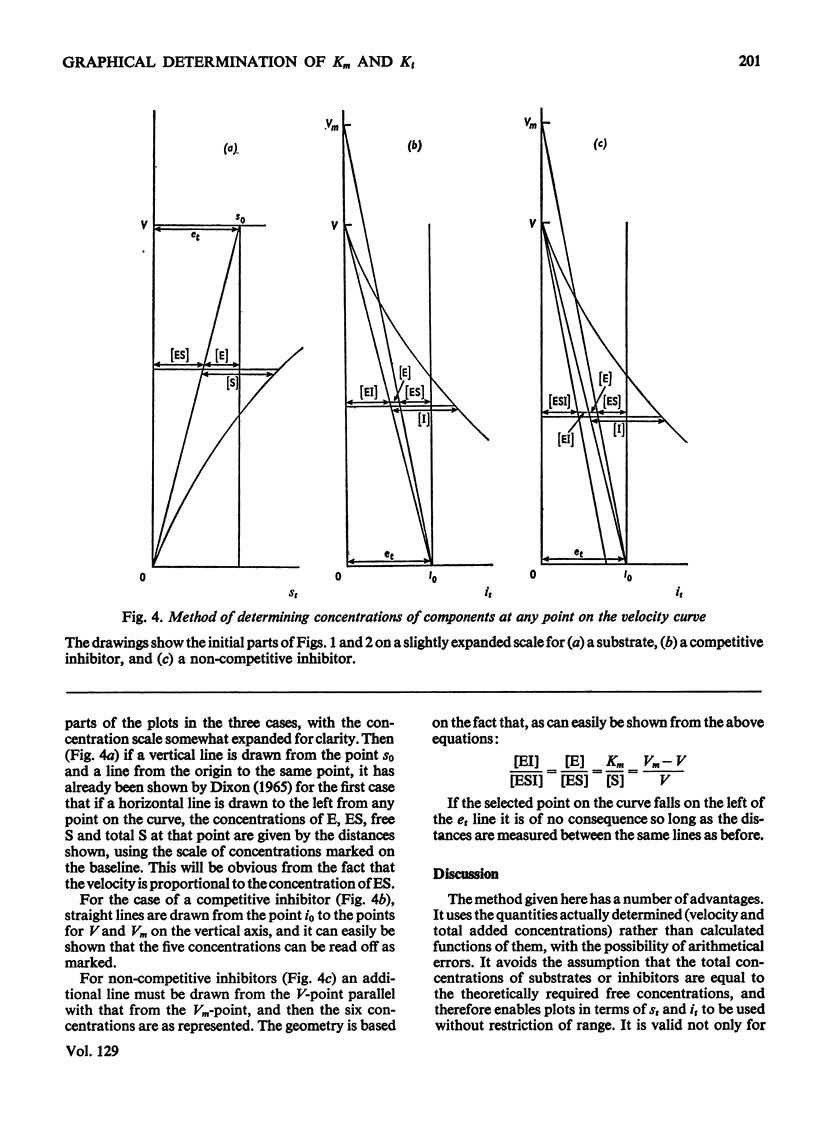
The principle of Dixon (1965) has been extended to give rapid graphical methods for determining enzyme constants for substrates (Km) and inhibitors (Ki). It does away with the sometimes questionable assumption that the amounts of substrate or inhibitor bound by the enzyme are negligible in comparison with the total amount added, and is therefore valid even for cases of high affinity, where the usual methods fail. Besides doing away with the need for calculation, it enables the concentrations of the various components of the system to be read off directly for any point of the velocity curve.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DIXON M. GRAPHICAL DETERMINATION OF EQUILIBRIUM CONSTANTS. Biochem J. 1965 Mar;94:760–762. doi: 10.1042/bj0940760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson P. J. A linear equation that describes the steady-state kinetics of enzymes and subcellular particles interacting with tightly bound inhibitors. Biochem J. 1972 Apr;127(2):321–333. doi: 10.1042/bj1270321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilroe-Smith T. A. A modified graphical method for determination of equilibrium constants. Biochem J. 1966 Aug;100(2):334–335. doi: 10.1042/bj1000334. [DOI] [PMC free article] [PubMed] [Google Scholar]


