ABSTRACT
Nuclear Factor Y (NF-Y) represents a group of transcription factors commonly present in higher eukaryotes, typically consisting of three subunits: NF-YA, NF-YB, and NF-YC. They play crucial roles in the embryonic development, photosynthesis, flowering, abiotic stress responses, and other essential processes in plants. To better understand the genome-wide NF-Y domain-containing proteins, the protein physicochemical properties, chromosomal localization, synteny, phylogenetic relationships, genomic structure, promoter cis-elements, and protein interaction network of NtNF-Ys in tobacco (Nicotiana tabacum L.) were systematically analyzed. In this study, we identified 58 NtNF-Ys in tobacco, respectively, and divided into three subfamilies corresponding to their phylogenetic relationships. Their tissue specificity and expression pattern analyses for leaf development, drought and saline-alkali stress, and ABA response were carried out using RNA-seq or qRT-PCR. These findings illuminate the role of NtNF-Ys in regulating plant leaf development, drought and saline-alkali stress tolerance, and ABA response. This study offers new insights to enhance our understanding of the roles of NtNF-Ys and identify potential genes involved in leaf development, as well as drought and saline-alkali stress tolerance of plants.
KEYWORDS: Tobacco (Nicotiana tabacum L.), NF-Y gene family, leaf development, drought stress, saline-alkali stress, ABA response
Introduction
In nature, plants are constantly affected by various non-living factors, impacting their growth and development and causing significant losses in yield and biodiversity.1,2 Abiotic stress refers to adverse environmental factors, such as drought, saline-alkali, extreme temperatures, and so on.3 In the process of long-term evolution, plants have formed complex regulatory networks, including the process of signal perception, transduction, and amplification, in order to reduce the harm of extreme environments. With the development of plant genetics and crop breeding, the in-depth analysis of the molecular network of plant perception and response to drought, saline-alkali, and high/low temperature stresses is of great significance for improving crop breeding to cope with the ever-changing natural climate.
Nuclear factor Y is also known as CCAAT-binding factor family (NF-Y; CBF), which is a ubiquitous transcription factor in higher eukaryotes.4–6 The nuclear factor Y family is mainly composed of three subunits: NF-YA (CBF-B; HAP2), NF-YB (CBF-A; HAP3), and NF-YC (CBF-C; HAP5). As that revealed in yeast and animals, the NF-YA, NF-YB, and NF-YC subunits can form a functional NF-Y TF, which can recognize and bind the CCAAT box promoter element to regulate downstream genes.7 For example, the NF-YA2/YB3/YC10 complex plays a vital role in heat shock and drought stress responses in Arabidopsis.8
Scientific studies have shown that the NF-Y TFs are important regulators of plant growth and development,9 including seed development,10 embryonic development,11 flowering time,12 root development,13 stress response,14,15 and signaling pathways of phytohormones such as IAA, GA, and ABA.16,17 Although several NF-Y proteins, such as AtNF-YB2, AtNF-YB3, ZmNF-YA3, and OsNF-YC5, have been reported to mediate abiotic stress responses in plants such as Arabidopsis, maize, and rice,18–20 the potential biological functions and regulatory mechanisms of NF-Y proteins in the drought response need to be further explored.
Tobacco (Nicotiana tabacum L.) features highly efficient genetic transformation and regeneration systems, making it a pivotal model plant in the fields of plant biology research and biotechnology applications. In recent years, tobacco is widely used to study genes functional diversity and create new germplasms. Nevertheless, the potential biological function and molecular mechanism of NtNF-Ys in tobacco remain largely unknown and need further study.
Materials and methods
Identification of NtNF-Ys
All tobacco protein sequences were extracted from the tobacco genome downloaded from http://lifenglab.hzau.edu.cn/Nicomics/.21 We used the different screening strategies to correctly identify all members of the tobacco NF-Y family members. Firstly, we obtained all AtNF-Y protein sequences (AtNF-YAs, AtNF-YBs, and AtNF-YCs) through the Arabidopsis genome database (https://www.arabidopsis.org/), which were used as decoys to retrieve the tobacco genome database at the genome-wide level using BLASTP with an E-value (≤1 × 10−5) and an identity match (≥50%) as thresholds of TBtools.22 Subsequently, we surveyed all tobacco NF-Y proteins via the Hidden Markov Model profiles (E value ≤ 1 × 10−5) of the specific NF-Y conserved domain (PF02045 and PF00808), which were extracted from the Pfam database (http://pfam.xfam.org/).23 Finally, all candidate genes were checked using SMART (http://smart.embl-heidelberg.de/) and Conserved Domains Database (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi).24
Chromosomal location and identification of homologous genes
Based on the information of chromosomal locations provided by the tobacco genome database (http://lifenglab.hzau.edu.cn/Nicomics/.), TBtools-II software was employed to generate the chromosomal locations map and synteny analysis of the NtNF-Ys.25
Property prediction of NtNF-Ys
The information about each NtNF-Ys, including the molecular weight (MW), isoelectric point (pI), and the average value of hydrophilicity (GRAVY), was calculated using the ProtProm tool on the Expasy server (https://www.expasy.org/).26 The Plant-mPLoc website was employed to predict the subcellular localization (http://www.csbio.sjtu.edu.cn/bioinf/plant-multi/).27 Using the AlphaFold 3 model (https://alphafoldserver.com/) and PyMOL software to predict the three-dimensional structure of NtNF-Ys.28
Phylogenetic analysis of NF-Ys
The alignment of the NF-Y protein sequences of different species (https://www.ncbi.nlm.nih.gov/.),29 including Nicotiana tabacum, Arabidopsis thaliana, and Oryza sativa, were carried out using the MUSCLE algorithm, and the results were imported into the MEGA 11 software for the construction of a phylogenetic tree using the Neighbor-Joining method with the Poission model with uniform rates. The phylogeny test was statistically supported by 1000 bootstrap replications to provide the probability of each branch’s formation. The phylogenetic tree was further optimized through the iTOL online tool (https://itol.embl.de/) and Adobe Illustrator software.30,31
Domain identification and conserved motifs analysis of NtNF-Ys
The conserved motifs of NtNF-Ys were analyzed using the MEME online tool (https://meme-suite.org/meme/tools/meme).32 The number of motifs was set to search for 20 motifs. In addition, using the Batch CD-Search tool, we identified the conserved domains of NtNF-Ys (https://www.ncbi.nlm.nih.gov/Structure/bwrpsb/bwrpsb.cgi).33 The visual tools are TBtools-II and Adobe Illustrator software.
Motif and structural analyses of NtNF-Ys
The exon – intron structure was generated using TBtools-II software based on the CDS and genomic sequences of NtNF-Ys.34 The cis-elements in the 2000 bp promoter regions of NtNF-Ys extracted from the tobacco genome were predicted using the online website PlantCARE and TBtools-II software (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/).35
Protein interaction network of NtNF-Ys
The protein interaction network of the NtNF-Ys was predicted using STRING (https://cn.string-db.org/) based on Nicotiana tabacum proteins.36 The network map was built using Cytoscape 3.10.0 and Adobe Illustrator software.37
Transcriptome analysis
The RNA-seq data of NtNF-Ys in different tissue of tobacco were obtained from the NCBI SRA repository (PRJNA208209). In addition, the RNA-seq data used for expression analysis of NtNF-Ys in different developmental stages in tobacco were obtained at our laboratory. Finally, the RNA-seq data of NtNF-Ys in tobacco under 200 mm mannitol (PRJNA883680), 100 mm NaCl (PRJNA532660), 100 mm NaHCO3 (PRJNA532660), and 0.1 mm ABA spraying (PRJNA684346) treatment were downloaded from the NCBI SRA repository.38–40 The process of transcriptome analysis was conducted according to previous studies.41,42
qRT-PCR analysis of NtNF-Ys
The cultivated tobacco (Nicotiana tabacum L.) cultivar ‘HongHuaDaJinYuan (HD)’ was grown in a substrate consisting of a 1:1 mixture of peat soil and vermiculite and was cultivated in a greenhouse at the Tobacco Research Institute of the Chinese Academy of Agricultural Sciences, Qingdao, China. The greenhouse conditions included a light intensity of 400 μmol m−2·s−1, a temperature regime of 25°C during the light period and 23°C during the dark period, a photoperiod of 16 h of light and 8 h of darkness, and a relative humidity of 65%. After 4 weeks of growth, healthy tobacco seedlings of the same size were grown on ½MS medium under 200 mm mannitol, 100 mm NaCl, 100 mm NaHCO3, and 0.1 mm ABA spraying treatments for 0, 0.5, 1, 1.5, 3, 6, 12, 24, 48 h (h). Three independent biological replicates were taken. The total RNA of tobacco was extracted using the TRIzol Reagent (CWBIO, Beijing, China). In order to synthesize cDNA, the RNA samples were used as templates with the M-MLV Reverse Transcriptase Kit (Takara Bio, Shiga, Japan). RT-qPCR was performed in a 20 µL reaction system containing 10 µL of 2 × UltraSYBR Mixture (CWBIO) and 0.4 µM of forward and reverse primers, to assess the expression of target genes. The reactions were incubated in a Rotor-Gene Q Machine (Qiagen) for 10 min at 95°C, followed by 40 cycles of 15 s at 95°C and 60 s at 60°C. The tobacco NtACTIN gene served as an internal control (Table S2). Relative expression levels were measured using the 2−△△Ct method.
Results
Identification and characterization of NtNF-Ys
In this study, to comprehensively and accurately identify all NF-YA genes in tobacco, three common strategies were employed based on the published genes in Arabidopsis (AtNF-YAs, AtNF-YBs, and AtNF-YCs). Then, a total of 58 NtNF-Y genes, including 23 NtNF-YAs, 25 NtNF-YBs, and 10 NtNF-YCs, were identified from the Tobacco Genome Database.21 Furtherly, we analyzed their physicochemical properties (S 1). The genomic length of the 58 NtNF-Ys showed a wide distribution, ranging from 366 bp (Nta23g00810.1) to 17,715 bp (Nta24g11400.1). Meanwhile, the coding sequences (CDS) length of the 58 NtNF-Ys exhibited a wide distribution, ranging from 366 bp (Nta23g00810.1) to 1032 bp (Nta03g16330.1), encoding 133–344 amino acids, with the molecular weight (MW) varying from 13.4 kDa to 39.42 kDa. The isoelectric point (pI) of the 58 NtNF-Ys ranged from 4.62 (Nta01g13780.1) to 10.58 (Nta24g09230.1), and the grand average of hydropathicity (GRAVY) ranged from −1.096 (Nta01g26140.1) to −0.075 (Nta23g00810.1), suggesting that they are hydrophilic proteins. The findings of subcellular localization predictions reveal that most of NtNF-Ys are localized to the nucleus except four NtNF-Ys (Nta01g24520.1, Nta05g20020.1, Nta06g18140.1, and Nta12g17940.1), which were localized to the nucleus and cytoplasm (Table 1). The diversity of three-dimensional structure of NtNF-Ys indicated the NtNF-Ys might have functional redundancy and sub/neo-functionalization in tobacco (Fig. S1).
Table 1.
Characterization of NtNF-Ys in tobacco.
| Gene ID | Chr | Chr. Location | CDS /bp |
Protein Size/aa | MW /kDa |
PI | GRAVY | Subcellular Location |
|---|---|---|---|---|---|---|---|---|
| Nta01g13780.1 | 1 | 48578034–48578417 | 384 | 128 | 14.37 | 4.62 | −0.747 | Nucleus |
| Nta01g26140.1 | 1 | 137771583–137771990 | 408 | 136 | 15.43 | 5.09 | −1.096 | Nucleus |
| Nta01g24520.1 | 1 | 122199509–122204000 | 774 | 258 | 28.39 | 6.3 | −0.465 | Cytoplasm; Nucleus |
| Nta02g01570.1 | 2 | 6651507–6657474 | 750 | 250 | 27.55 | 9.28 | −0.888 | Nucleus |
| Nta02g04480.1 | 2 | 35428509–35436959 | 903 | 301 | 33.03 | 10.11 | −0.538 | Nucleus |
| Nta02g09650.1 | 2 | 62362715–62363197 | 483 | 161 | 18.2 | 6.54 | −0.917 | Nucleus |
| Nta02g26300.1 | 2 | 132673885–132674265 | 381 | 127 | 14.32 | 4.52 | −0.76 | Nucleus |
| Nta03g05210.1 | 3 | 43429041–43433205 | 477 | 159 | 18.05 | 9.41 | −0.825 | Nucleus |
| Nta03g22790.1 | 3 | 162598544–162606246 | 630 | 210 | 23.15 | 7.84 | −0.893 | Nucleus |
| Nta03g00250.1 | 3 | 541741–542244 | 504 | 168 | 19.05 | 6.13 | −0.715 | Nucleus |
| Nta03g16330.1 | 3 | 125420496–125428054 | 1032 | 344 | 39.42 | 4.65 | −0.534 | Nucleus |
| Nta04g27850.1 | 4 | 137521912–137526592 | 531 | 177 | 19.67 | 9.62 | −0.813 | Nucleus |
| Nta04g00730.1 | 4 | 2883918–2884737 | 528 | 176 | 19.85 | 7.04 | −0.718 | Nucleus |
| Nta05g20020.1 | 5 | 174888308–174895111 | 693 | 231 | 25.19 | 4.85 | −0.44 | Cytoplasm; Nucleus |
| Nta06g18140.1 | 6 | 115546516–115551580 | 693 | 231 | 25.18 | 4.86 | −0.429 | Cytoplasm; Nucleus |
| Nta07g04020.1 | 7 | 15983378–15991132 | 498 | 166 | 17.95 | 5.33 | −0.66 | Nucleus |
| Nta09g08870.1 | 9 | 43220518–43221138 | 621 | 207 | 22.93 | 7.5 | −0.705 | Nucleus |
| Nta09g11430.1 | 9 | 68087251–68103166 | 498 | 166 | 17.79 | 5.34 | −0.603 | Nucleus |
| Nta09g18860.1 | 9 | 143250798–143251526 | 729 | 243 | 27.18 | 6.92 | −0.894 | Nucleus |
| Nta10g06850.1 | 10 | 25581369–25581980 | 612 | 204 | 22.66 | 8.21 | −0.748 | Nucleus |
| Nta10g09130.1 | 10 | 38701161–38713741 | 492 | 164 | 17.56 | 5.98 | −0.615 | Nucleus |
| Nta10g15940.1 | 10 | 105030993–105031697 | 705 | 235 | 26.23 | 6.95 | −0.833 | Nucleus |
| Nta11g28600.1 | 11 | 202222882–202227783 | 756 | 252 | 28.12 | 9.62 | −0.822 | Nucleus |
| Nta11g27510.1 | 11 | 196738748–196739420 | 651 | 217 | 22.98 | 6.63 | −0.611 | Nucleus |
| Nta11g19510.1 | 11 | 127005401–127009034 | 792 | 264 | 29.06 | 6.63 | −0.409 | Nucleus |
| Nta12g21910.1 | 12 | 124064872–124072975 | 912 | 304 | 33.79 | 9.78 | −1.031 | Nucleus |
| Nta12g31310.1 | 12 | 193904280–193911737 | 933 | 311 | 33.99 | 9.63 | −0.511 | Nucleus |
| Nta12g34600.1 | 12 | 225870865–225877437 | 738 | 246 | 27.16 | 8.77 | −0.827 | Nucleus |
| Nta12g28040.1 | 12 | 168514871–168515356 | 486 | 162 | 18.17 | 8.31 | −0.898 | Nucleus |
| Nta12g17940.1 | 12 | 81908969–81913341 | 693 | 231 | 25.25 | 5.01 | −0.464 | Cytoplasm; Nucleus |
| Nta13g03220.1 | 13 | 15625116–15633998 | 909 | 303 | 33.05 | 7.09 | −0.984 | Nucleus |
| Nta14g03090.1 | 14 | 11169915–11178474 | 909 | 303 | 33.09 | 6.68 | −1.019 | Nucleus |
| Nta15g09830.1 | 15 | 50519983–50525273 | 978 | 326 | 35.66 | 9.71 | −0.59 | Nucleus |
| Nta16g09500.1 | 16 | 46967172–46973120 | 978 | 326 | 35.86 | 8.91 | −0.629 | Nucleus |
| Nta16g22470.1 | 16 | 140231206–140238793 | 498 | 166 | 18 | 5.08 | −0.685 | Nucleus |
| Nta16g24790.1 | 16 | 148921712–148922368 | 657 | 219 | 24.66 | 5.49 | −0.525 | Nucleus |
| Nta17g24960.1 | 17 | 167407584–167413004 | 945 | 315 | 34.85 | 9.59 | −0.606 | Nucleus |
| Nta17g02850.1 | 17 | 13538629–13544670 | 543 | 181 | 19.49 | 6.79 | −0.817 | Nucleus |
| Nta17g12300.1 | 17 | 88882955–88883512 | 558 | 186 | 20.67 | 6.63 | −0.632 | Nucleus |
| Nta17g12350.1 | 17 | 89370514–89371062 | 549 | 183 | 20.2 | 5.1 | −0.714 | Nucleus |
| Nta18g23510.1 | 18 | 129281303–129287100 | 954 | 318 | 35.14 | 9.5 | −0.573 | Nucleus |
| Nta18g02800.1 | 18 | 13329935–13336252 | 543 | 181 | 19.51 | 6.79 | −0.821 | Nucleus |
| Nta18g11380.1 | 18 | 76701524–76704296 | 669 | 223 | 24.92 | 6.97 | −0.745 | Nucleus |
| Nta18g11460.1 | 18 | 77028619–77029074 | 456 | 152 | 17.16 | 5.07 | −0.668 | Nucleus |
| Nta19g05550.1 | 19 | 26763773–26772389 | 984 | 328 | 36.78 | 8.44 | −0.724 | Nucleus |
| Nta20g05760.1 | 20 | 24403505–24408392 | 813 | 271 | 30.14 | 9.91 | −0.794 | Nucleus |
| Nta21g01930.1 | 21 | 7281641–7282585 | 945 | 315 | 34.77 | 9.72 | −0.62 | Nucleus |
| Nta21g14490.1 | 21 | 96670654–96675868 | 756 | 252 | 28 | 9.88 | −0.855 | Nucleus |
| Nta22g12560.1 | 22 | 87330597–87331004 | 408 | 136 | 15.23 | 5.07 | −0.98 | Nucleus |
| Nta22g10880.1 | 22 | 78991269–78996109 | 777 | 259 | 28.44 | 6.3 | −0.462 | Nucleus |
| Nta23g11340.1 | 23 | 85686686–85701607 | 813 | 271 | 30.06 | 9.33 | −0.685 | Nucleus |
| Nta23g00810.1 | 23 | 2746202–2746567 | 366 | 122 | 13.4 | 8.22 | −0.075 | Nucleus |
| Nta23g09720.1 | 23 | 70458937–70459353 | 417 | 139 | 15.36 | 10.4 | −0.617 | Nucleus |
| Nta24g11400.1 | 24 | 87181317–87199031 | 672 | 224 | 24.93 | 10.24 | −0.838 | Nucleus |
| Nta24g17500.1 | 24 | 119563090–119569354 | 846 | 282 | 31.62 | 8.31 | −0.923 | Nucleus |
| Nta24g09230.1 | 24 | 70610850–70611431 | 462 | 154 | 16.91 | 10.58 | −0.346 | Nucleus |
| Nta00g11500.1 | 50 | 352475–355783 | 723 | 241 | 26.54 | 5.33 | −0.639 | Nucleus |
| Nta00g03900.1 | 243 | 14888–19251 | 909 | 303 | 33.55 | 9.32 | −0.968 | Nucleus |
50 and 243, scaffold50 and scaffold243; CDS, coding sequence; MW, molecular weight; pI, isoelectric point; GRAVY, grand average of hydropathicity.
Chromosome location and synteny analysis of NtNF-Ys
Among the 58 NF-Y proteins in tobacco, 56 NF-Y genes exhibited a random distribution across 23 chromosomes, with each chromosome harboring between one and five genes, and the remaining two genes were located on 2 scaffolds. Specifically, chromosome 12 had the largest number of genes (n = 5) and Chromosomes 2, 3, 17, and 18 had four genes each (Figure 1a).
Figure 1.
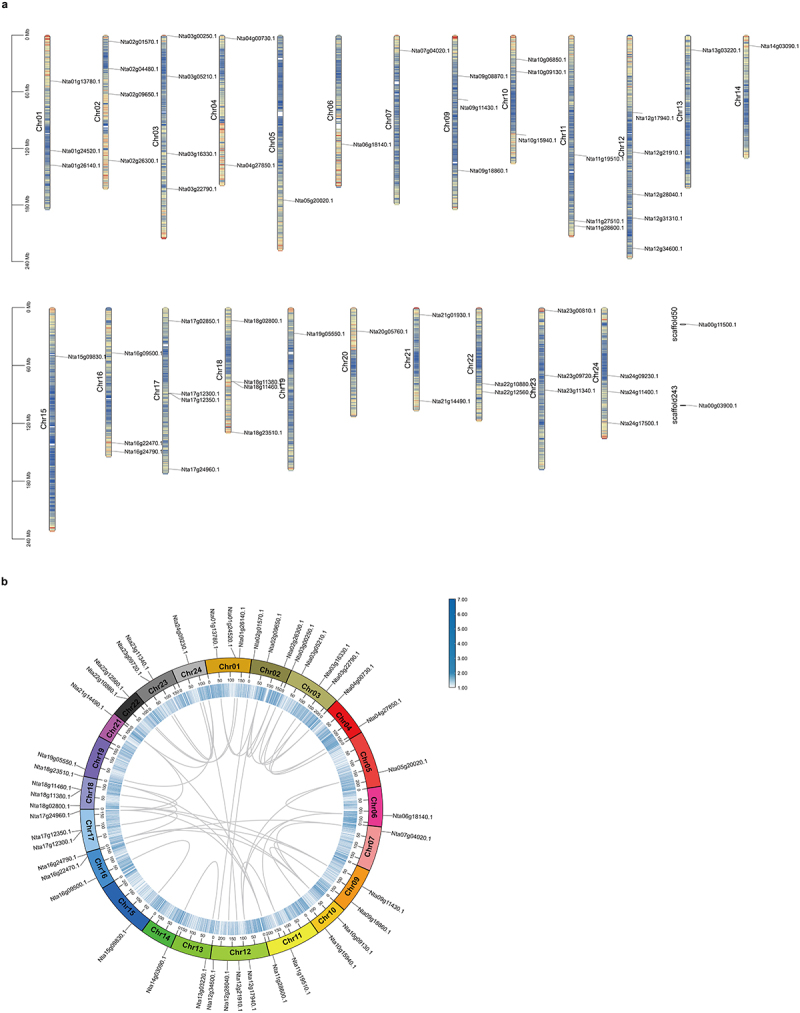
Chromosome location and synteny analysis of NtNF-Ys. (a) Chromosomal location of NtNF-Ys. The bars represent chromosome. The chromosome members are displayed on the left side, and the gene names are displayed on the right side. (b) Synteny analysis of NtNF-Ys. Different chromosomes are shown in different colors. The approximate positions of NtNF-Ys are marked with black lines on the chromosomes. Gray curves denote the syntenic relationships within nicotiana tabacum.
According to previous studies, segmental and tandem duplications are the two main ways of gene family expansion.43,44 In order to understand the expansion mode of NtNF-Ys in tobacco, the synteny analysis of NtNF-Ys was performed. Using TBtools-II, the genome duplication events within Nicotiana tabacum were investigated. A total of 43 collinear pairs in Nt-Nt were identified (Figure 1b). Furtherly, the 43 gene pairs were analyzed by calculating the non-synonymous (Ka) and synonymous (Ks). These findings show that the substitution ratios (Ka/Ks) of all gene pairs are less than 1, demonstrating that these genes are purified during evolution (Table S1).
Phylogenetic relationship of NtNF-Ys
To further study the evolutionary relationship of NtNF-Ys in Nicotiana tabacum, Arabidopsis thaliana, and Oryza sativa, we used the MEGA 11 software to generate a phylogenetic tree for 128 NF-Ys from the four species (58 in Nicotiana tabacum, 36 in Arabidopsis thaliana, and 34 in Oryza sativa) based on their protein sequences. Based on the evolutionary divergence, the phylogenetic tree was categorized into three branches corresponding to three different subfamilies (NF-YAs, NF-YBs, and NF-YCs), respectively (Figure 2). All NF-Ys were grouped together with their respective orthologs, indicating that the evolutionary relationships of the NF-Ys have been relatively conserved across various species.
Figure 2.
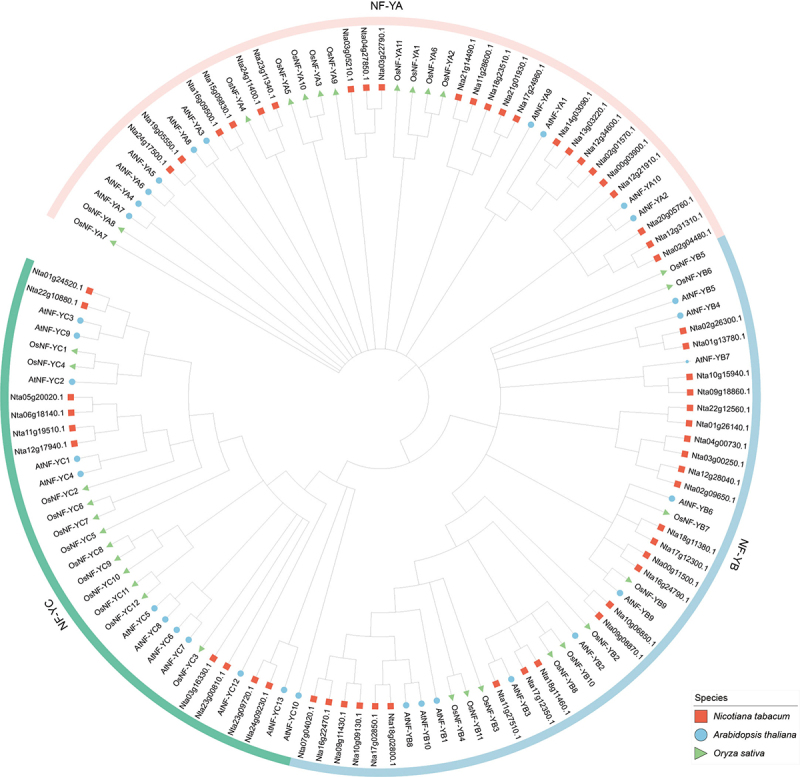
Phylogenetic analysis of the NF-Y proteins in Nicotiana tabacum, Arabidopsis thaliana, and Oryza sativa. NF-Ys were divided into three subfamilies (NF-YAs, NF-YBs, and NF-YCs) according to their subunits. The red squares, blue circles, and green triangle represent the NF-Ys in Nicotiana tabacum, Arabidopsis thaliana, and Oryza sativa, respectively.
Motif and structural analyses of NtNF-Ys
To study NtNF-Ys structure, we analyzed the conserved motif by the MEME online website, and 20 conserved motifs were identified (Figure 3a; S2). The results indicate that different subfamilies (NtNF-YAs, NtNF-YBs, and NtNF-YCs) have different motif compositions, such NtNF-YA (containing motif 11, 6, 7, 5, 2, 17, 20, 10, 15, 18, 16, and 12), NtNF-YB (containing motif 19, 3, 1, 4, and 13), and NtNF-YC (containing motif 3, 1, 8, 9, 14, and 17).
Figure 3.
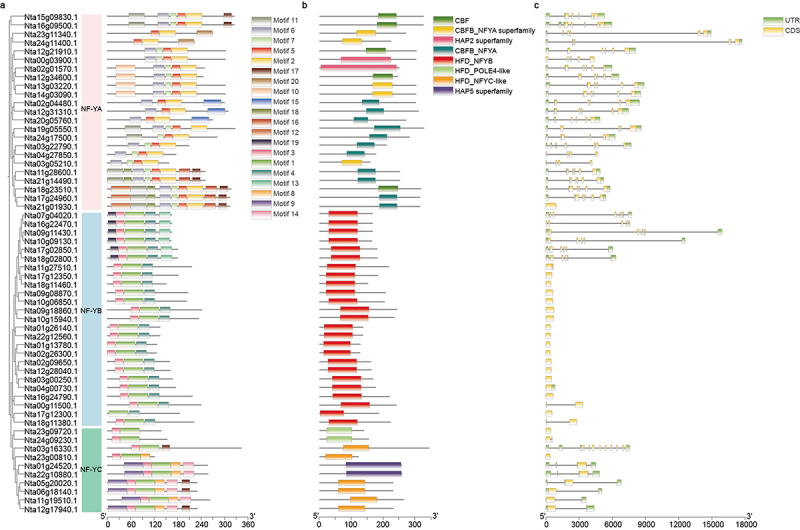
Conserved motifs and exon–intron structure analysis of NtNF-Ys. (a) The phylogenetic tree shows that the NtNF-Ys are distributed in three subfamilies (NF-YA, NF-YB, and NF-YC), which indicated by pink, blue, and green, respectively. Different colors boxes represent different motifs. (b) Conserved domain structures of NtNF-Ys. Different domains are shown using boxes with different colors. (c) Exon–intron structures of NtNF-Ys. The green and yellow boxes indicate UTR and CDS, respectively.
To analyze the conserved domains in the different NtNF-Y subfamilies, we used the Batch CD-Search tool to identify the conserved domains of NtNF-Ys. The results were similar to conserved motifs, where there were different domains in different subfamilies with different functions (Figure 3b). For example, the NtNF-YAs have one domain for DNA binding and another domain for protein interacting with NF-YB/C.
The genomic distributions of NtNF-Ys were analyzed to better understand the evolution of the NtNF-Y family. The results included that the number of exons in NtNF-YAs was the most, followed by NtNF-YCs, and most of NtNF-YBs was the least (Figure 3c). The number of exons among each member exhibited differences, indicating that the function of NtNF-Y family genes might have become more intricate during the process of evolution.
Cis-acting elements in NtNF-Ys
Generally, the distribution of gene promoter elements correlates with gene function (plant development, hormone regulation, and stress response)45,46 to explore the potential biological functions of NtNF-Ys, we analyzed the cis-element in the promoters of NtNF-Ys. Based on the PlantCARE website’s predictions, the cis-elements in NtNF-Y promoter regions were systematically segmented into six distinct components: core/binding, abiotic/biotic, development, hormone, light, and other unknown elements (Figure 4). Most of NtNF-Ys contained the core/binding elements (CAAT-box and TATA-box) except six NtNF-Y genes (Nta02g09650.1, Nta12g31310.1, Nta12g34600.1, Nta13g03220.1, Nta24g11400.1, and Nta24g09230.1). In addition, the abiotic/biotic elements MYB, MYC, and STRE existed in most of the NtNF-Ys promoters,47–49 meanwhile, a few of the NtNF-Ys promoters contained other abiotic/biotic elements (ARE, as-1, MBS, and so on). Most of the promoter regions of NtNF-Ys contained at least one development elements; however, no development-related elements were found in eight NtNF-Ys. Among the hormone-related elements, the number of abscisic acid response element (ABRE) was the most, suggesting most of the NtNF-Ys might be associated with ABA response.50,51 Moreover, light-responsive elements such as the Box 4, G-box, and GT1-motif were found in the promoters of NtNF-Ys (Figure 4). The above results reveal that NtNF-Ys play key roles in plant development, hormone signaling, abiotic stress tolerance, and other essential processes in plants.
Figure 4.
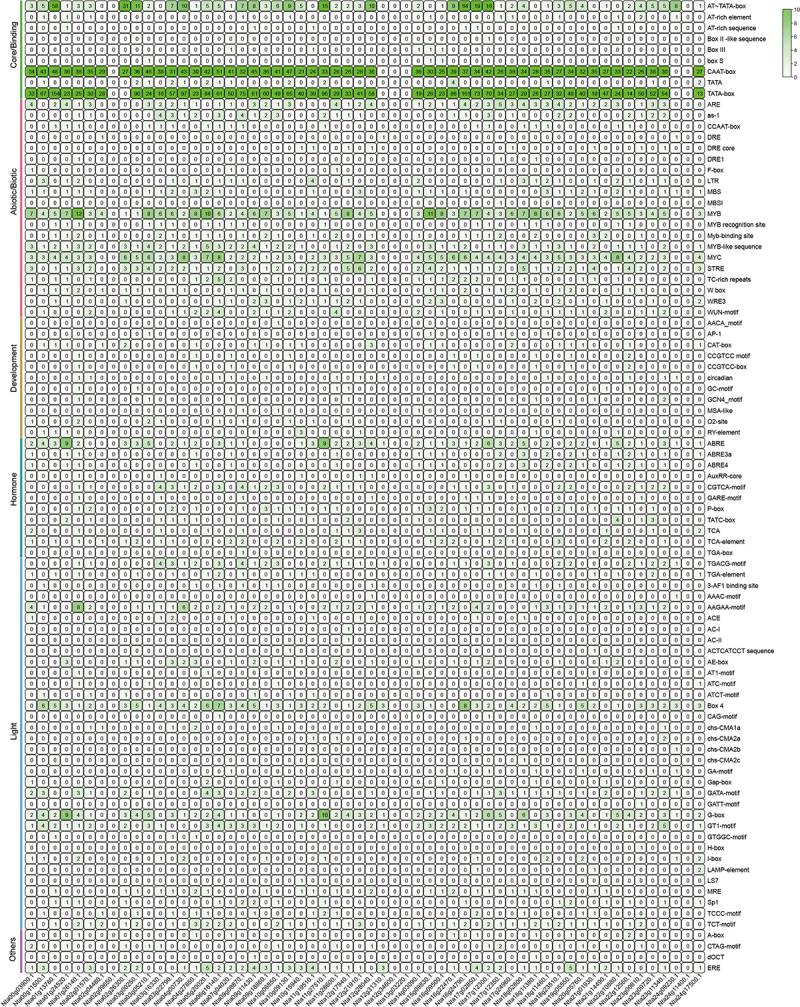
Cis-element analysis in the promoters of NtNF-Ys. The degree of green colors represents the number of cis-elements in the promoter of NtNF-Ys.
Protein interaction network of NtNF-Ys
We constructed an interaction network for NtNF-Ys using Nicotiana tabacum proteins to investigate their potential regulatory network (Figure 5). NF-YA, NF-YB, and NF-YC usually modulate the expression of downstream genes through the formation of heterotrimeric complexes.52 Obviously, NtNF-Ys could interact with other NF-Ys. Moreover, NtNF-Ys can interact with the master regulators of seed maturation (LEC2, FUS3, ABI3, and DPBF2)53,54 embryogenesis-related proteins (AGL15 and RKD4)55,56 stress response-associated proteins (PKL, bZIP28, and WRI1),57–59 flower development-related protein RGL2,60 grain quality-related protein bHLH144,61,62 and so on (Figure 5). These findings suggest that NtNF-Ys are crucial in modulating growth, development, and stress tolerance in plants.
Figure 5.
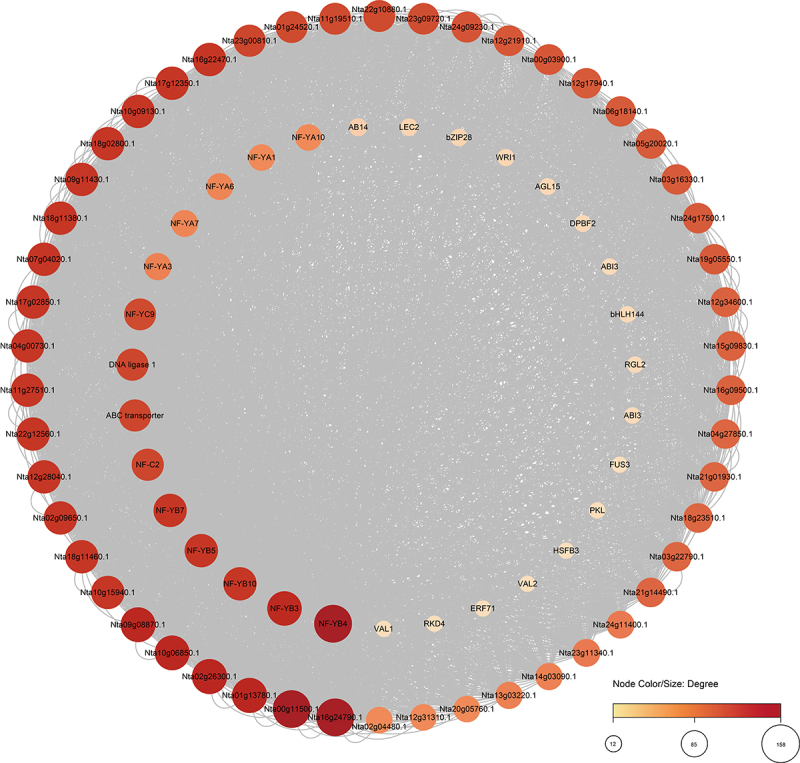
Functional interaction networks of NtNF-Ys. Network nodes represent proteins, and gray lines represent protein–protein associations. The node color/size represents the number of proteins that interact with each other.
Tissue-specific expression analysis of NtNF-Ys
For the purpose of exploring the biological functions of NtNF-Ys in tobacco plants, the expression of NtNF-Ys was examined in nine representative tobacco tissues (i.e. root, stem, young, mature, and senescent leaves, young, mature, and senescent flowers, and dry caps) using RNA-seq data, which was extracted from the NCBI SRA repository. All members of the NtNF-Ys subfamily, except Nta03g00250.1 and Nta23g00810.1, were expressed at 1 to 9 in all tissues (Figure 6). Among all the NtNF-Ys, 10 NtNF-Ys, including Nta01g24520.1, Nta11g19510.1, Nta11g27510.1, Nta12g21910.1, Nta13g03220.1, Nta14g03090.1, Nta17g02850.1, Nta18g02800.1, Nta22g10880.1, and Nta24g17500.1, were highly expressed in all tissues, especially Nta01g24520.1, which was highly expressed by more than 5 (FPKM value) in all the tissues (Figure 6). Additionally, a few of the NtNF-Ys showed tissue-specific expression, such as Nta01g24520.1 (highly expressed in root and stem), Nta11g27510.1 (highly expressed in young and senescent leaf), and Nta18g02800.1 (highly expressed in root). Interestingly, all NtNF-Ys were expressed in root except 5 NtNF-Ys (Figure 6). Above all, these findings demonstrate that NtNF-Ys exhibit distinct expression patterns and are vital to plant development.
Figure 6.
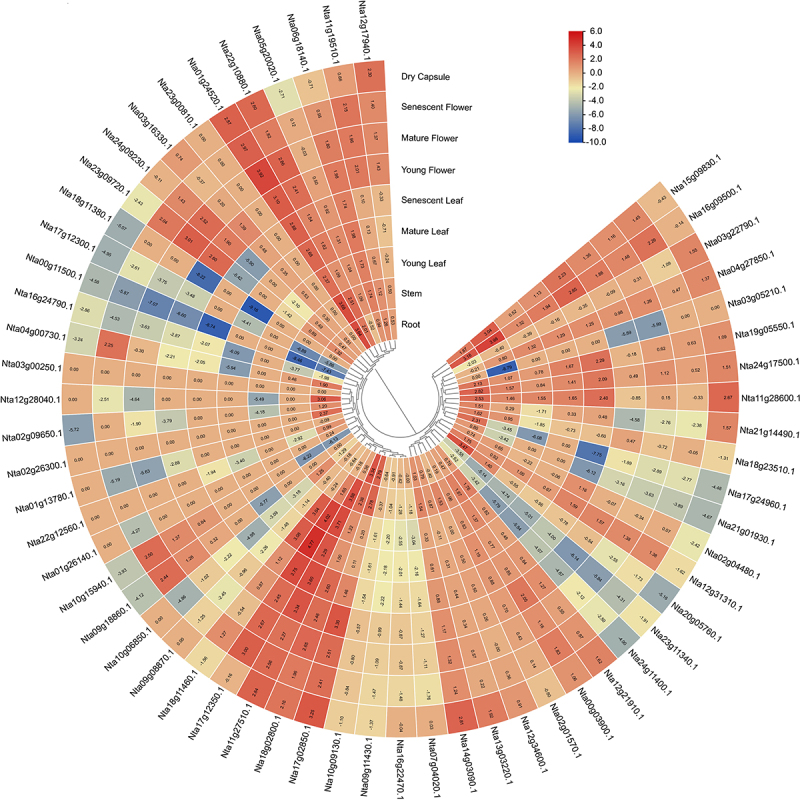
Expression analysis of NtNF-Ys in different tissues using RNA-seq. The log2 (FPKM) values are showed in boxes.
Expression analysis in different developmental stages of NtNF-Ys
In order to understand the roles of NtNF-Ys in regulating leaf development, we further analyzed the expression levels of NtNF-Ys at different developmental stages of leaves (prosperously growing, flower budding, and flower stage) using RNA-seq data. Most of NtNF-Ys were expressed in on or more stage, except few NtNF-Ys (Nta01g13780.1, Nta02g26300.1, Nta09g18860.1, Nta10g15940.1, Nta12g28040.1, Nta18g11380.1, Nta23g00810.1, and Nta00g11500.1) (Figure 7). Meanwhile, there were some NtNF-Ys (Nta01g24520.1, Nta04g27850.1, Nta11g27510.1, Nta12g21910.1, and Nta00g03900.1) highly expressed in all stage, suggesting these might play vital roles at all developmental stages of plant leaves (Figure 7).
Figure 7.
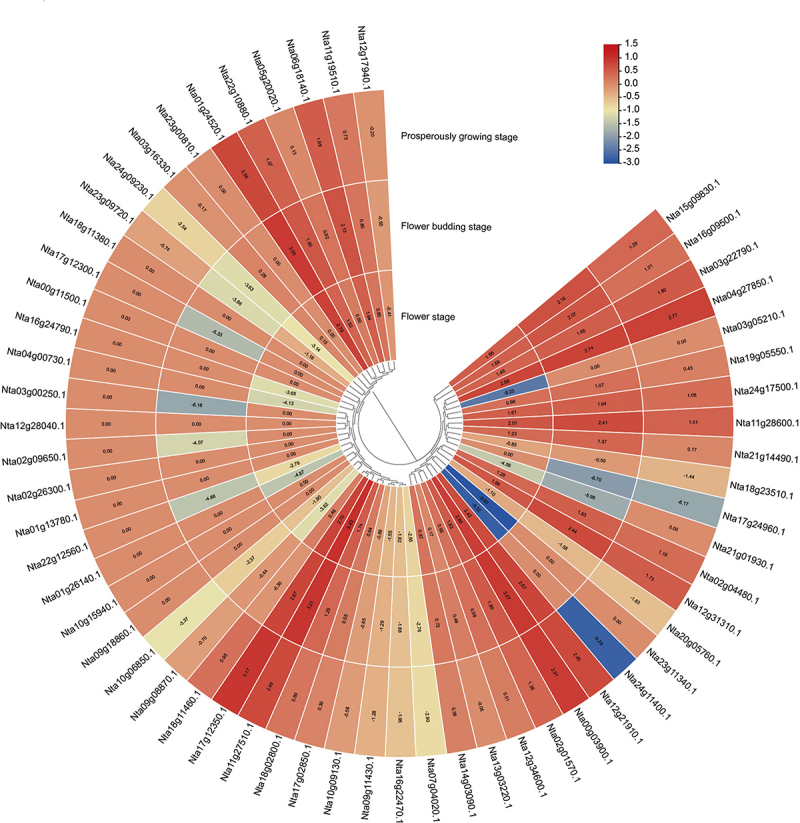
Expression analysis of NtNF-Ys in different developmental stages using RNA-seq. The log2 (FPKM) values are showed in boxes.
Expression analysis under abiotic stress treatment of NtNF-Ys
Abiotic stress, especially drought and saline-alkali stress, constrains the growth and development of plants.63,64 To study the potential regulators of NtNF-Ys in drought and saline-alkali stress responses, the expression patterns of NtNF-Ys were analyzed by using the RNA-seq data of tobacco under 200 mm mannitol, 100 mm NaCl, and 100 mm NaHCO3 stress (Figure 8). Under 200 mm mannitol treatment, the expression of many NtNF-Ys was significantly upregulated or downregulated, such as Nta00g03900.1 belonging to NtNF-YA family by almost induced by almost 9.14-fold (at 8 h), Nta09g08870.1 belonging to NtNF-YB family by almost induced by almost 0.42-fold (at 1 h), and Nta01g24520.1 belonging to NtNF-YC family by almost induced by almost 2.41-fold (at 1 h) (Figure 8a). Under 100 mm NaCl or 100 mm NaHCO3 treatment, the expression of most of the NtNF-Ys was significantly upregulated or downregulated, and there was no significant difference in the expression levels of 19 NtNF-Ys. It is interesting that the expression trends of 11 NtNF-Ys were inconsistent under NaCl or NaHCO3 treatment (Figure 8b). Abscisic acid (ABA) is a crucial phytohormone involved in plant responses to abiotic stress.65,66 To investigate the functions of NtNF-Ys in ABA-mediated responses to abiotic stress, we conducted an analysis of NtNF-Ys expression patterns through RNA-seq data from tobacco subjected to ABA treatment. Comparatively to the control (CK), the expression levels of several NtNF-Ys were significantly induced following ABA treatment (Figure 8c). For example, Nta23g11340.1 was significantly upregulated at any point in time. The above results indicated that NtNF-Ys involved in the regulation of plant stress adaption (Figure 10).
Figure 8.
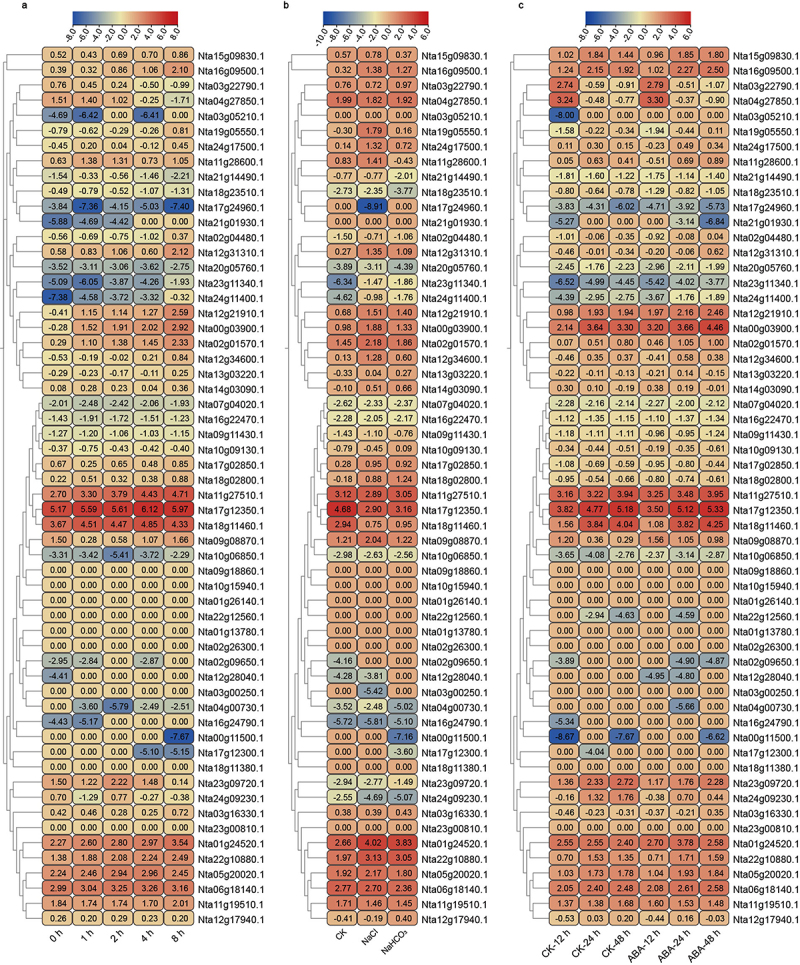
Expression analysis of NtNF-Ys under (a) mannitol, (b) NaCl, NaHCO3, and (c) ABA treatments using RNA-seq. The log2 (FPKM) values are showed in boxes.
Figure 10.
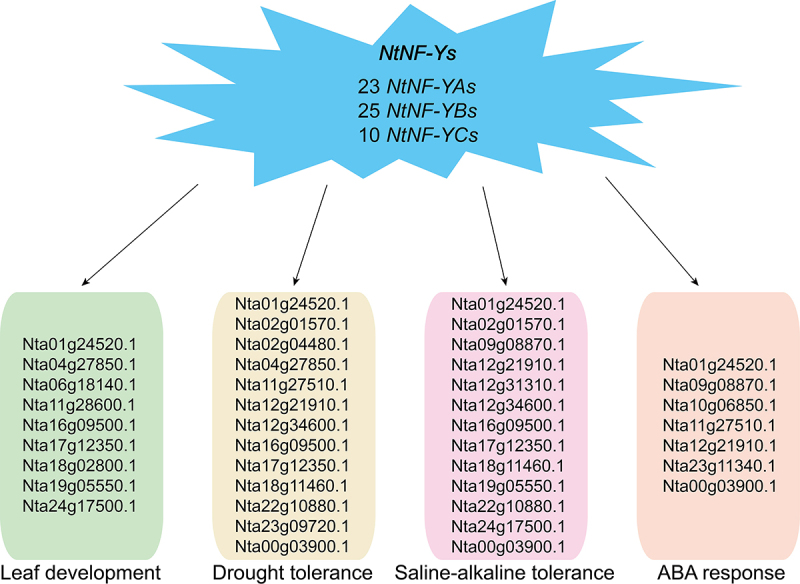
A conclusive graph of functions of NF-Ys in tobacco.
It is amazing that the expression levels of some NtNF-Ys were significantly induced under all treatment, including Nta00g03900.1 from NtNF-YA family, Nta09g08870.1 from NtNF-YB family, and Nta01g24520.1 from NtNF-YC family, suggesting these NtNF-Ys were more important. To further verify the accuracy of transcriptome data, we selected a few NtNF-Y genes with significantly differential expression for expression analysis using qRT-PCR (Quantitative reverse transcription polymerase chain reaction). As shown in Figure 9, the expression profiles of the candidate genes were consistent with RNA-Seq results (Table S2). Therefore, the RNA-seq data are authentic.
Figure 9.
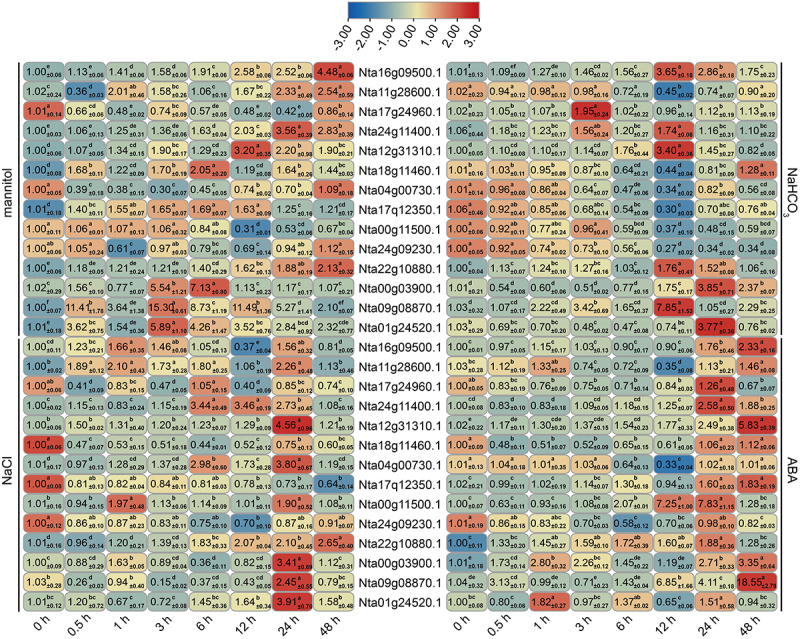
Expression analysis of NtNF-Ys under mannitol, NaCl, NaHCO3, and ABA treatments using qRT-PCR. Data are shown as mean ± SD (n = 3). Different letters indicate statistically significant differences at p < 0.05 according to one-way ANOVA followed by post-hoc Tukey’s test for each treatment. The tobacco ACTIN gene was used as a reference.
Discussion
The NF-Y transcription factor family plays vital roles in various physiological processes of plant development. Furthermore, the presence of the NF-Y family has been documented in different plant species such as Solanum tuberosum,67 Medicago sativa,29 Populus,68 Petunia hybrid,69 and others. Nevertheless, many NtNF-Ys remain largely unknown in terms of their biological functions and underlying regulatory mechanisms. Hence, it is essential to perform a comprehensive genome-wide identification and analysis of the NF-Y family genes in order to enhance our comprehension of their functions and the molecular mechanism of tobacco.
In 2024, Huazhong Agricultural University published the N. tabacum genome, which is the highest values among all published N. tabacum genome assembly.21 In this study, we identified a total of 58 NtNF-Ys based on the N. tabacum genome (Table 1). Because the N. tabacum genome is as large as 4.3 Gb, the number of NtNF-Ys is more than most of the plants. Based on the chromosomal localization of NtNF-Ys, genes were uniformly distributed among all chromosomes (Figure 1a). We speculate that segmental and tandem duplications are the main cause of the copy number of NtNF-Ys increased, which might bring about functional redundancy and differentiation (Figure 1b). According to the phylogenetic relationship of NtNF-Ys, there are similar numbers and types of NtNF-Ys distributed in each group in Arabidopsis thaliana and Oryza sativa, revealing that the plant NF-Y transcription factor family is evolutionarily conserved (Figure 2).
The genomic structure is typically conserved in the process of plant evolution.70 In our investigation, as Figure 3a shows, the NtNF-YAs, NtNF-YBs, and NtNF-YCs have their own conserved motifs, respectively (Fig. S2). Meanwhile, the domains of NtNF-YAs, NtNF-YBs, and NtNF-YCs are also relatively conserved (Figure 3b). The majority of homologous NtNF-Ys possess same number of exons and introns in NF-YAs, NF-YBs, and NF-YCs, however, a few display variations in the exon–intron structure. For example, the exon–intron structure of Nta21g01930.1 belonging to NtNF-YAs is different from other NtNF-YAs (Figure 3c). Therefore, the change in the exon–intron structures of NtNF-YAs, NtNF-YBs, and NtNF-YCs could lead to more functional diversification.
According to the previous study, NF-Ys are key players in plant development.71 In our study, there are many development-related cis-elements in the promoters of NtNF-Ys, suggesting NtNF-Ys may be involved in plant development (Figure 4). NtNF-Ys can interact with the master regulators of development-related proteins, further indicating that NtNF-Ys play crucial roles in plant development (Figure 5). We found many NtNF-Ys participate in regulation of leaf development by analyzing the leaf developmental transcriptome data of tobacco. For example, a few of NtNF-Ys (Nta01g24520.1, Nta02g01570.1, Nta02g04480.1, Nta03g22790.1, Nta04g27850.1, Nta06g18140.1, Nta11g27510.1, Nta11g28600.1, Nta12g21910.1, Nta15g09830.1, Nta16g09500.1, Nta17g12350.1, Nta18g02800.1, Nta22g10880.1, Nta24g17500.1, and Nta00g03900.1) were highly expressed at all stages of leaf development (Figure 7). Combined with results of tissue-specific expression analysis (Figure 6), we speculate Nta01g24520.1, Nta04g27850.1, Nta06g18140.1, Nta11g28600.1, Nta16g09500.1, Nta17g12350.1, Nta18g02800.1, Nta19g05550.1, and Nta24g17500.1 are vital regulators in plant leaf development (Figure 10).
The NF-Ys are not only involved in plant development but also in plant abiotic stress.29 Different NF-Ys members participate in stress responses in various plant tissues to adapt to external stress by mediating multiple signaling and plant hormone pathways, especially the ABA signaling pathway.29,71 In this research, the promoters of NtNF-Ys contained many abiotic cis-elements, revealing NtNF-Ys were involved in abiotic stress responses (Figure 4). The abiotic related proteins (PKL, bZIP28, and WRI1) can interact with NtNF-Ys, furtherly verifying the significance of NtNF-Ys (Figure 5). The RNA-seq data indicated the expression of many NtNF-Ys was significantly upregulated or downregulated, including Nta01g24520.1, Nta02g01570.1, Nta02g04480.1, Nta04g27850.1, Nta11g27510.1, Nta12g21910.1, Nta12g34600.1, Nta16g09500.1, Nta17g12350.1, Nta18g11460.1, Nta22g10880.1, Nta23g09720.1, and Nta00g03900.1 under 200 mm mannitol treatment (Figure 8a), Nta01g24520.1, Nta02g01570.1, Nta09g08870.1, Nta12g21910.1, Nta12g31310.1, Nta12g34600.1, Nta16g09500.1, Nta17g12350.1, Nta18g11460.1, Nta19g05550.1, Nta22g10880.1, Nta24g17500.1, and Nta00g03900.1 under 100 mm NaCl or NaHCO3 treatment (Figure 8b), and Nta01g24520.1, Nta09g08870.1, Nta10g06850.1, Nta11g27510.1, Nta12g21910.1, Nta23g11340.1, and Nta00g03900.1 under ABA treatment (Figure 8c). It is important that the expression levels of some NtNF-Ys were significantly induced under all treatments, such as Nta00g03900.1 from NtNF-YA family, Nta09g08870.1 from NtNF-YB family, and Nta01g24520.1 from NtNF-YC family. These results suggest that NtNF-Ys, especially Nta00g03900.1, Nta09g08870.1, and Nta01g24520.1, may underlie the response to drought, and saline-alkaline stress in plant (Figure 9).
In summary, we provide genome-wide results of the tobacco NF-Y genes, the tissue specificity and expression pattern analyses for leaf development, drought and saline-alkali stresses, and ABA responses. The information described here could contribute to further studies investigating NtNF-Y family genes in the context of abiotic stress.
Conclusion
In this study, NtNF-Ys were identified from tobacco (Nicotiana tabacum L.), and then we systematically analyzed their basic information. Their expression pattern analyses under drought, saline-alkali, and ABA treatments were performed using RNA-seq. We conducted a screening for potential NtNF-Ys that may exert a regulatory function in enhancing abiotic stress tolerance. The purpose of this study was to offer novel insights for advancing our comprehension of the functions of NtNF-Ys and identifying potential genes linked to environmental stress tolerance in plants.
Supplementary Material
Funding Statement
This work was supported by Science and Technological Project of China National Tobacco Corporation (110202103015), Science and Technological Project of China Tobacco Jiangsu Industrial Co., Ltd. (H202110), The Agricultural Science and Technology Innovation Program (ASTIP-TRIC02), and Fundamental Research Funds for Central Non-profit Scientific Institution (1610232024009).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Author contributions
S.L., Z.T., D.W., and J.C. conceived and designed the research. Z.T., L.X., J.F., and W.S. performed the experiments. Z.T., L.X., J.C., W.S., B.W., J.S., X.Y., F.C., and J.M. analyzed the data. Z.T. and L.X. wrote the paper. S.L., D.W., and J.C. revised the paper. All authors read and approved the final version of the paper.
Data availability statement
The transcriptome data were deposited at the NCBI database under accession numbers PRJNA883680, PRJNA532660, and PRJNA684346.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15592324.2025.2451700
References
- 1.Nawaz M, Sun J, Shabbir S, Khattak WA, Ren G, Nie X, Bo Y, Javed Q, Du D, Sonne C.. A review of plants strategies to resist biotic and abiotic environmental stressors. Sci Total Environ. 2023;900:165832. doi: 10.1016/j.scitotenv.2023.165832. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, Xu J, Li R, Ge Y, Li Y, Li R. Plants’ response to abiotic stress: mechanisms and strategies. Int J Mol Sci. 2023;24(13):10915. doi: 10.3390/ijms241310915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Du B, Haensch R, Alfarraj S, Rennenberg H. Strategies of plants to overcome abiotic and biotic stresses. Biol Rev. 2024;99(4):1524–16. doi: 10.1111/brv.13079. [DOI] [PubMed] [Google Scholar]
- 4.Oldfield AJ, Henriques T, Kumar D, Burkholder AB, Cinghu S, Paulet D, Bennett BD, Yang P, Scruggs BS, Lavender CA, et al. NF-Y controls fidelity of transcription initiation at gene promoters through maintenance of the nucleosome-depleted region. Nat Commun. 2019;10(1):3072. doi: 10.1038/s41467-019-10905-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pan W, Li J, Du Y, Zhao Y, Xin Y, Wang S, Liu C, Lin Z, Fang S, Yang Y, et al. Epigenetic silencing of callose synthase by VIL1 promotes bud-growth transition in lily bulbs. Nat Plants. 2023;9(9):1451–1467. doi: 10.1038/s41477-023-01492-z. [DOI] [PubMed] [Google Scholar]
- 6.Zheng L, Gao S, Bai Y, Zeng H, Shi H. NF-YC15 transcription factor activates ethylene biosynthesis and improves cassava disease resistance. Plant Biotechnol J. 2024;22(9):2424–2434. doi: 10.1111/pbi.14355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jing X, Baixiao N, Chen C. Advances on the function of NF-Y transcription factors in regulation of plant growth and development. J Plant Physiol. 2022;58(7):1191–1200. doi: 10.13592/j.cnki.ppj.300045. [DOI] [Google Scholar]
- 8.Sato H, Mizoi J, Tanaka H, Maruyama K, Qin F, Osakabe Y, Morimoto K, Ohori T, Kusakabe K, Nagata M, et al. Arabidopsis DPB3-1, a DREB2A interactor, specifically enhances heat stress-induced gene expression by forming a heat stress-specific transcriptional complex with NF-Y subunits. Plant Cell. 2014;26(12):4954–4973. doi: 10.1105/tpc.114.132928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kishor PBK, Ganie SA, Wani SH, Guddimalli R, Karumanchi AR, Edupuganti S, Naravula J, Kumar V, Polavarapu R, Suravajhala P, et al. Nuclear factor-Y (NF-Y): developmental and stress-responsive roles in the plant lineage. J Plant Growth Regul. 2022;42(5):2711–2735. doi: 10.1007/s00344-022-10739-6. [DOI] [Google Scholar]
- 10.Siriwardana CL, Kumimoto RW, Jones DS, Holt BF. 3rd. Gene family analysis of the Arabidopsis NF-YA transcription factors reveals opposing abscisic acid responses during seed germination. Plant Mol Biol Rep. 2014;32(5):971–986. doi: 10.1007/s11105-014-0704-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dai C, Miao CX, Xu XM, Liu LJ, Gu YF, Zhou D, Chen LS, Lin G, Lu GX. Transcriptional activation of human CDCA8 gene regulated by transcription factor NF-Y in embryonic stem cells and cancer cells. J Biol Chem. 2015;290(37):22423–22434. doi: 10.1074/jbc.M115.642710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siriwardana CL, Gnesutta N, Kumimoto RW, Jones DS, Myers ZA, Mantovani R, Holt BF, Muday GK. 3rd. Nuclear factor Y, subunit a (NF-YA) proteins positively regulate flowering and act through flowering locus T. PLOS Genet. 2016;12(12):e1006496. doi: 10.1371/journal.pgen.1006496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xue L, Wang Y, Fan Y, Jiang Z, Wei Z, Zhai H, He S, Zhang H, Yang Y, Zhao N, et al. IbNF-YA1 is a key factor in the storage root development of sweet potato. Plant J. 2024;118(6):1991–2002. doi: 10.1111/tpj.16723. [DOI] [PubMed] [Google Scholar]
- 14.Yu T-F, Liu Y, Fu J-D, Ma J, Fang Z-W, Chen J, Zheng L, Lu Z-W, Zhou Y-B, Chen M, et al. The NF-Y-PYR module integrates the abscisic acid signal pathway to regulate plant stress tolerance. Plant Biotechnol J. 2021;19(12):2589–2605. doi: 10.1111/pbi.13684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang H, Liu S, Ren T, Niu M, Liu X, Liu C, Wang H, Yin W, Xia X. Crucial abiotic stress regulatory network of NF-Y transcription factor in plants. Int J Mol Sci. 2023;24(5):4426. doi: 10.3390/ijms24054426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu X, Hu P, Huang M, Tang Y, Li Y, Li L, Hou X. The NF-YC–RGL2 module integrates GA and ABA signalling to regulate seed germination in Arabidopsis. Nat Commun. 2016;7(1):12768. doi: 10.1038/ncomms12768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou Y, Zhang Y, Wang X, Han X, An Y, Lin S, Shen C, Wen J, Liu C, Yin W, et al. Root-specific NF-Y family transcription factor, PdNF-YB21, positively regulates root growth and drought resistance by abscisic acid-mediated indoylacetic acid transport in Populus. New Phytol. 2020;227(2):407–426. doi: 10.1111/nph.16524. [DOI] [PubMed] [Google Scholar]
- 18.Su H, Cao Y, Ku L, Yao W, Cao Y, Ren Z, Dou D, Wang H, Ren Z, Liu H, et al. Dual functions of ZmNF-YA3 in photoperiod-dependent flowering and abiotic stress responses in maize. J Exp Bot. 2018;69(21):5177–5189. doi: 10.1093/jxb/ery299. [DOI] [PubMed] [Google Scholar]
- 19.Sato H, Suzuki T, Takahashi F, Shinozaki K, K Y-S. NF-YB2 and NF-YB3 have functionally diverged and differentially induce drought and heat stress-specific genes. Plant Physiol. 2019;180(3):1677–1690. doi: 10.1104/pp.19.00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan X, Han M, Li S, Liang Z, Ouyang J, Wang X, Liao P. A member of NF-Y family, OsNF-YC5 negatively regulates salt tolerance in rice. Gene. 2024;892:147869. doi: 10.1016/j.gene.2023.147869. [DOI] [PubMed] [Google Scholar]
- 21.Wang J, Zhang Q, Tung J, Zhang X, Liu D, Deng Y, Tian Z, Chen H, Wang T, Yin W, et al. High-quality assembled and annotated genomes of Nicotiana tabacum and Nicotiana benthamiana reveal chromosome evolution and changes in defense arsenals. Mol Plant. 2024;17(3):423–437. doi: 10.1016/j.molp.2024.01.008. [DOI] [PubMed] [Google Scholar]
- 22.Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25(17):3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mistry J, Chuguransky S, Williams L, Qureshi M, Salazar GA, Sonnhammer ELL, Tosatto SCE, Paladin L, Raj S, Richardson LJ, et al. Pfam: the protein families database in 2021. Nucleic Acids Res. 2021;49(D1):D412–d419. doi: 10.1093/nar/gkaa913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marchler-Bauer A, Lu S, Anderson JB, Chitsaz F, Derbyshire MK, DeWeese-Scott C, Fong JH, Geer LY, Geer RC, Gonzales NR, et al. CDD: a conserved Domain database issue for the functional annotation of proteins. Nucleic Acids Res. 2011;39(Database):D225–9. doi: 10.1093/nar/gkq1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen C, Wu Y, Li J, Wang X, Zeng Z, Xu J, Liu Y, Feng J, Chen H, He Y, et al. Tbtools-ii: a “one for all, all for one” bioinformatics platform for biological big-data mining. Mol Plant. 2023;16(11):1733–1742. doi: 10.1016/j.molp.2023.09.010. [DOI] [PubMed] [Google Scholar]
- 26.Wilkins MR, Gasteiger E, Bairoch A, Sanchez JC, Williams KL, Appel RD, Hochstrasser DF. Protein identification and analysis tools in the ExPASy server. Methods Mol Biol. 1999;112:531–552. doi: 10.1385/1-59259-584-7:531. [DOI] [PubMed] [Google Scholar]
- 27.Chou KC, Shen HB, Newbigin E. Plant-mPloc: a top-down strategy to augment the power for predicting plant protein subcellular localization. PLOS ONE. 2010;5(6):e11335. doi: 10.1371/journal.pone.0011335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosignoli S, Paiardini A. Boosting the full potential of PyMOL with structural biology plugins. Biomolecules. 2022;12(12):1764. doi: 10.3390/biom12121764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.An Y, Suo X, Niu Q, Yin S, Chen L. Genome-wide identification and analysis of the NF-Y transcription factor family reveal its potential roles in salt stress in alfalfa (Medicago sativa L.). Int J Mol Sci. 2022;23(12):6426. doi: 10.3390/ijms23126426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Letunic I, Bork P. Interactive tree of life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021;49(W1):W293–w296. doi: 10.1093/nar/gkab301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zou L, Su YX, Guo YT, Bai WZ, Xu DS. Application of adobe Illustrator for drawing schematic diagrams of anatomical structure of acupoints – taking five shu-points of Jueyin Meridian as an example. Zhen Ci Yan Jiu. 2021;46(8):710–716. doi: 10.13702/j.1000-0607.20210125. [DOI] [PubMed] [Google Scholar]
- 32.Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L, Ren J, Li WW, Noble WS. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res. 2009;37(Web Server issue):W202–8. doi: 10.1093/nar/gkp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gilchrist CLM, Chooi YH. Synthaser: a CD-Search enabled python toolkit for analysing domain architecture of fungal secondary metabolite megasynth(et)ases. Fungal Biol Biotechnol. 2021;8(1):13. doi: 10.1186/s40694-021-00120-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu B, Jin J, Guo AY, Zhang H, Luo J, Gao G. GSDS 2.0: an upgraded gene feature visualization server. Bioinformatics. 2015;31(8):1296–1297. doi: 10.1093/bioinformatics/btu817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lescot M, Déhais P, Thijs G, Marchal K, Moreau Y, Van de Peer Y, Rouzé P, Rombauts S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002;30(1):325–327. doi: 10.1093/nar/30.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szklarczyk D, Kirsch R, Koutrouli M, Nastou K, Mehryary F, Hachilif R, Gable AL, Fang T, Doncheva NT, Pyysalo S, et al. The STRING database in 2023: protein–protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023;51(D1):D638–d646. doi: 10.1093/nar/gkac1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doncheva NT, Morris JH, Gorodkin J, Jensen LJ. Cytoscape stringapp: network analysis and visualization of proteomics data. J Phys Chem Lett. 2019;18(2):623–632. doi: 10.1021/acs.jproteome.8b00702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu J, Chen Q, Liu P, Jia W, Chen Z, Xu Z. Integration of mRNA and miRNA analysis reveals the molecular mechanism underlying salt and alkali stress tolerance in tobacco. Int J Mol Sci. 2019;20(10):2391. doi: 10.3390/ijms20102391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu T, Li CX, Zhong J, Shu D, Luo D, Li ZM, Zhou JY, Yang J, Tan H, Ma XR. Exogenous 1‘,4’-trans-diol-aba induces stress tolerance by affecting the level of gene expression in tobacco (Nicotiana tabacum L.). Int J Mol Sci. 2021;22(5):2555. doi: 10.3390/ijms22052555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu Z, He Z, Li Y, Wang Q, Yi P, Yang J, Yang C, Borovskii G, Cheng X, Hu R, et al. Transcriptomic and metabolic regulatory network characterization of drought responses in tobacco. Front Plant Sci. 2022;13:1067076. doi: 10.3389/fpls.2022.1067076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang L, Li X, Lyu K, Wang H, Li Z, Qi W, Zhang L, Cao Y. Rosaceae phylogenomic studies provide insights into the evolution of new genes. Hortic Plant J. 2024;11(1):389–405. doi: 10.1016/j.hpj.2024.02.002. [DOI] [Google Scholar]
- 42.Cao Y, Hong J, Zhao Y, Li X, Feng X, Wang H, Zhang L, Lin M, Cai Y, Han Y. De Novo gene integration into regulatory networks via interaction with conserved genes in peach. Hortic Res. 2024;11(12). doi: 10.1093/hr/uhae252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cannon SB, Mitra A, Baumgarten A, Young ND, May G. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol. 2004;4(1):10. doi: 10.1186/1471-2229-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oren M, Barela Hudgell MA, D’Allura B, Agronin J, Gross A, Podini D, Smith LC. Short tandem repeats, segmental duplications, gene deletion, and genomic instability in a rapidly diversified immune gene family. BMC Genomics. 2016;17(1):900. doi: 10.1186/s12864-016-3241-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haberle V, Lenhard B. Promoter architectures and developmental gene regulation. Semin Cell Dev Biol. 2016;57:11–23. doi: 10.1016/j.semcdb.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 46.Haberle V, Stark A. Eukaryotic core promoters and the functional basis of transcription initiation. Nat Rev Mol Cell Biol. 2018;19(10):621–637. doi: 10.1038/s41580-018-0028-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mayordomo I, Estruch F, Sanz P. Convergence of the target of rapamycin and the Snf1 protein kinase pathways in the regulation of the subcellular localization of Msn2, a transcriptional activator of STRE (stress response element)-regulated genes. J Biol Chem. 2002;277(38):35650–35656. doi: 10.1074/jbc.M204198200. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Y, Sun T, Liu S, Dong L, Liu C, Song W, Liu J, Gai S, Wu K. MYC cis-elements in PsMPT promoter is involved in chilling response of Paeonia suffruticosa. PLOS ONE. 2016;11(5):e0155780. doi: 10.1371/journal.pone.0155780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang X, Niu Y, Zheng Y. Multiple functions of MYB transcription factors in abiotic stress responses. Int J Mol Sci. 2021;22(11):6125. doi: 10.3390/ijms22116125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hobo T, Asada M, Kowyama Y, Hattori T. Acgt-containing abscisic acid response element (ABRE) and coupling element 3 (CE3) are functionally equivalent. Plant J. 1999;19(6):679–689. doi: 10.1046/j.1365-313x.1999.00565.x. [DOI] [PubMed] [Google Scholar]
- 51.Nakashima K, Yamaguchi-Shinozaki K. ABA signaling in stress-response and seed development. Plant Cell Rep. 2013;32(7):959–970. doi: 10.1007/s00299-013-1418-1. [DOI] [PubMed] [Google Scholar]
- 52.Zhao H, Wu D, Kong F, Lin K, Zhang H, Li G. The Arabidopsis thaliana nuclear factor Y transcription factors. Front Plant Sci. 2016;7:2045. doi: 10.3389/fpls.2016.02045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim I, Lee KR, Park ME, Kim HU. The seed-specific transcription factor DPBF2 modulates the fatty acid composition in seeds. Plant Direct. 2022;6(4):e395. doi: 10.1002/pld3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Verma S, Attuluri VPS, Robert HS. Transcriptional control of Arabidopsis seed development. Planta. 2022;255(4):90. doi: 10.1007/s00425-022-03870-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Waki T, Hiki T, Watanabe R, Hashimoto T, Nakajima K. The Arabidopsis RWP-RK protein RKD4 triggers gene expression and pattern formation in early embryogenesis. Curr Biol. 2011;21(15):1277–1281. doi: 10.1016/j.cub.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 56.Joshi S, Paul P, Hartman JM, Perry SE. AGL15 promotion of somatic embryogenesis: role and molecular mechanism. Front Plant Sci. 2022;13:861556. doi: 10.3389/fpls.2022.861556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cernac A, Andre C, Hoffmann-Benning S, Benning C. WRI1 is required for seed germination and seedling establishment. Plant Physiol. 2006;141(2):745–757. doi: 10.1104/pp.106.079574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu JX, Howell SH. bZIP28 and NF-Y transcription factors are activated by ER stress and assemble into a transcriptional complex to regulate stress response genes in Arabidopsis. Plant Cell. 2010;22(3):782–796. doi: 10.1105/tpc.109.072173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jing Y, Yang Z, Yang R, Zhang Y, Qiao W, Zhou Y, Sun J. PKL is stabilized by MMS21 to negatively regulate Arabidopsis drought tolerance through directly repressing AFL1 transcription. New Phytol. 2023;239(3):920–935. doi: 10.1111/nph.18972. [DOI] [PubMed] [Google Scholar]
- 60.Gómez MD, Fuster-Almunia C, Ocaña-Cuesta J, Alonso JM, Pérez-Amador MA. RGL2 controls flower development, ovule number and fertility in Arabidopsis. Plant Sci. 2019;281:82–92. doi: 10.1016/j.plantsci.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 61.Bello BK, Hou Y, Zhao J, Jiao G, Wu Y, Li Z, Wang Y, Tong X, Wang W, Yuan W, et al. NF‐YB1‐YC12‐bHLH144 complex directly activates Wx to regulate grain quality in rice (Oryza sativa L.). Plant Biotechnol J. 2019;17(7):1222–1235. doi: 10.1111/pbi.13048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang JD, Wang J, Huang LC, Kan LJ, Wang CX, Xiong M, Zhou P, Zhou LH, Chen C, Zhao DS, et al. Aba-mediated regulation of rice grain quality and seed dormancy via the NF-YB1-SLRL2-bHLH144 module. Nat Commun. 2024;15(1):4493. doi: 10.1038/s41467-024-48760-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fang S, Hou X, Liang X. Response mechanisms of plants under saline-alkali stress. Front Plant Sci. 2021;12:667458. doi: 10.3389/fpls.2021.667458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang H, Zhu J, Gong Z, Zhu JK. Abiotic stress responses in plants. Nat Rev Genet. 2022;23(2):104–119. doi: 10.1038/s41576-021-00413-0. [DOI] [PubMed] [Google Scholar]
- 65.Dong T, Park Y, Hwang I. Abscisic acid: biosynthesis, inactivation, homoeostasis and signalling. Essays Biochem. 2015;58:29–48. doi: 10.1042/bse0580029. [DOI] [PubMed] [Google Scholar]
- 66.Xue L, Wei Z, Zhai H, Xing S, Wang Y, He S, Gao S, Zhao N, Zhang H, Liu Q. The IbPYL8-IbbHLH66-IbbHLH118 complex mediates the abscisic acid-dependent drought response in sweet potato. New Phytol. 2022;236(6):2151–2171. doi: 10.1111/nph.18502. [DOI] [PubMed] [Google Scholar]
- 67.Liu Z, Li Y, Zhu J, Ma W, Li Z, Bi Z, Sun C, Bai J, Zhang J, Liu Y. Genome-wide identification and analysis of the NF-Y gene family in potato (Solanum tuberosum L.). Front Genet. 2021;12:739989. doi: 10.3389/fgene.2021.739989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu R, Wu M, Liu HL, Gao YM, Chen J, Yan HW, Xiang Y. Genome-wide identification and expression analysis of the NF-Y transcription factor family in Populus. Physiol Plant. 2021;171(3):309–327. doi: 10.1111/ppl.13084. [DOI] [PubMed] [Google Scholar]
- 69.Wei Q, Wen S, Lan C, Yu Y, Chen G. Genome-wide identification and expression profile analysis of the NF-Y transcription factor gene family in Petunia hybrida. Plants (Basel). 2020;9(3):336. doi: 10.3390/plants9030336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bai G, Yang DH, Cao P, Yao H, Zhang Y, Chen X, Xiao B, Li F, Wang ZY, Yang J, et al. Genome-wide identification, gene structure and expression analysis of the MADS-box gene family indicate their function in the development of tobacco (Nicotiana tabacum L.). Int J Mol Sci. 2019;20(20):5043. doi: 10.3390/ijms20205043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zanetti ME, Rípodas C, Niebel A. Plant NF-Y transcription factors: key players in plant-microbe interactions, root development and adaptation to stress. BBA-Gene Regul Mech. 2017;1860(5):645–654. doi: 10.1016/j.bbagrm.2016.11.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The transcriptome data were deposited at the NCBI database under accession numbers PRJNA883680, PRJNA532660, and PRJNA684346.


