Abstract
Dendritic cells (DC) can serve to immunize the newborn immune system to foreign antigen. In a lymphopenic environment, naive T cells undergoing homeostasis-driven proliferation can acquire increased sensitivity to antigen stimulation. Here, we evaluated the capacity of DC to effectively prime the host immune system to elicit antitumor effects in the setting of early lymphoid reconstitution after bone marrow transplantation (BMT). Indeed, bone marrow-derived, cytokine-driven DC pulsed with whole tumor lysates (TP-DC) could, early on, prime a specific and long-lasting antitumor immune response, which mediated the rejection of a lethal challenge of a weakly immunogenic breast tumor. In the therapeutic setting, TP-DC could also inhibit the growth of preexisting breast tumor metastases by repetitive immunizations initiated early after BMT. Spleen T cells obtained from mice immunized with TP-DC early after BMT showed a substantial increase in tumor-specific IFN-γ production. Our findings demonstrate that it is possible to promote effective antitumor immunity in a defined lymphopenic environment through DC-based immunization.
One form of adoptive immunotherapy for cancer involves the use of bone marrow (BM) or peripheral stem cell transplants, which are currently being used for the treatment of hematopoietic and solid tumors. Well established models exist in animals of successful transplantation and hematolymphoid reconstitution with whole, unfractionated bone marrow cells (BMT) or with highly selected hematopoietic stem/progenitor cells (1). Investigators have also combined the adoptive transfer of lymphokine-activated killer cells and/or IL-2 with BMT, which has resulted in antitumor activity against residual disease both in animal models (2) and in humans (3). These promising results have engendered much enthusiasm, yet also raise the possibility of further improvement.
There are several potential advantages of combining suitable immunization approaches with BMT for overcoming tumor-induced defects in the host antitumor immune response. As examples, the preparative regimen for BMT may in some cases debulk tumor load, which, as a consequence, may reduce or eliminate tumor-induced active immune suppression (4). Lymphomyeloid reconstitution may overcome inherent defects in T cell signaling and/or in processing, presentation, and costimulatory functions of antigen-presenting cells (APC) (5, 6). Immunization may serve to educate the developing T cell repertoire to antigen(s) following BMT and thus may be more efficacious in a lymphopenic environment. In this regard, recent studies have shown that active immunization of the BM donor or the recipient before or after transplant, respectively, can result in a strategy for enhancing specific antigen reactivity of marrow grafts (7). The mechanism invokes early lymphoid progenitors being prone to “skewing” and, as a result, generates a T cell lineage of limited diversity. In lymphopenic hosts, naive T cells have been shown to masquerade as memory T cells by phenotype, by hypersensitivity to antigen stimulation, and by increased production of IFN-γ as a consequence of homeostasis-driven proliferation (8). Moreover, a very brief interaction with appropriate APC is sufficient to program naïve CD8+ T cells for their autonomous clonal expansion and development as functional effectors (9).
Currently, there is much attention being focused on the use of dendritic cells (DC) in vaccine strategies against tumors. DC are professional APC with a unique and potent capacity to induce primary and secondary immune responses (10) as well as to regulate the type of T cell-mediated immune response that is induced (11). Unlike B cells, DC can also prime newborn T cells to antigen(s) and thus render resistance to the induction of neonatal tolerance (12). Indeed, this property of DC, coupled with a state of homeostasis-driven T cell proliferation in a lymphopenic environment, may be beneficial for the induction of tumor-specific T cell responses in the BMT setting. Our group (13) and others (14) have shown the capacity of DC to prime effective antitumor immune responses as well as to induce regression of established tumors in immunocompetent animals. These results have been obtained with both local administration of DC intratumorally (15) as well as by conventional immunization with DC pulsed with distinct forms of tumor-associated antigen(s). Lastly, tumor lysate-pulsed DC (TP-DC) have been shown to specifically reactivate memory T cells from bone marrow, but not from peripheral blood, to become IFN-γ-producing and cytotoxic effector cells that mediate tumor regression in vivo (16).
Here, we investigated the capacity of bone marrow-derived, cytokine-driven TP-DC to elicit antitumor immunity in the setting of lymphopenia following BMT. We show that immunization with TP-DC during early periods of lymphoid reconstitution can prime an effective immune response against a murine breast tumor in the absence of overt signs of autoimmunity. The antitumor immunity generated is both specific and persistent in the reconstituted recipient, and it is manifested by T cell production of IFN-γ. In a therapeutic setting, TP-DC can have impact on residual tumor that remains following BMT.
Materials and Methods
Animals.
Six- to eight-week-old female BALB/c and C57BL/6 (B6) mice were purchased from The Jackson Laboratory and housed at the Animal Maintenance Facility of the University of Michigan Medical Center. The animals were used between 7 and 9 weeks of age.
Tumors.
The weakly immunogenic MT-901 was derived from an early in vivo passage of a cultured MT-7 dimethylbenzanthracene-induced mammary carcinoma cell line in the BALB/c strain. The A20 B cell lymphoma was derived from a spontaneous reticulum cell neoplasm that developed in an aged BALB/c mouse.
Generation of Tumor Lysate-Pulsed DC.
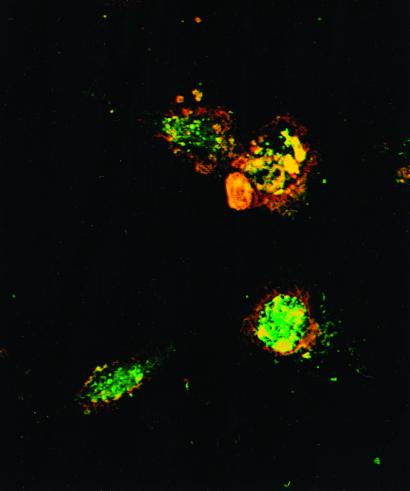
Bone marrow (BM) cells were harvested from flushed marrow cavities of femurs and tibiae of mice under aseptic conditions and were cultured in culture medium (CM) supplemented with 10 ng/ml granulocyte/macrophage colony-stimulating factor (GM-CSF) and 10 ng/ml IL-4 at 1 × 106 cells per ml (13, 15, 17). Dendritic cells were harvested from day-4 cultures, washed, and resuspended at 5 × 106 cells per ml. DC population was enriched by 14.5% (by weight) metrizamide CM density gradient separation. Following centrifugation [15 min, 4°C, 2,000 rpm, DC (>90% purity)], were collected from the low-density interface and washed twice in CM. Viable tumor cells were suspended at 1 × 107 cells per ml in CM. Following >4 cycles of rapid freeze (−160°C) and thaw (37°C), the tumor cell suspension was then centrifuged at low speed (400 rpm for 10 min). The supernatant (tumor lysate) was collected and incubated with purified DC overnight at a ratio of 3:1 tumor cells to DC. TP-DC were harvested and washed for further studies. As shown in Fig. 1, the presence of MT-901 tumor lysate (labeled with PKH-2; green fluorescence) is readily observed within the DC (labeled with PKH-26; red fluorescence) by two-color immunofluorescence and confocal microscopy. For immune priming and treatment of established tumors in vivo, TP-DC were injected s.c. as described (13).
Figure 1.
Confocal microscopy reveals efficient DC phagocytosis of MT-901 tumor cell lysates. DC and tumor cell lysates are shown labeled with red (PKH-26 dye) and green (PKH-2 dye) fluorescence, respectively. Yellow fluorescence represents overlap of both dyes. DC were analyzed 4 h after the addition of lysates.
Bone Marrow Transplantation and T Cell Reconstitution.
Six to 8-week-old female BALB/c mice received a single, lethal dose of total body irradiation (TBI; 950 rad) administered via a 137Cs γ-irradiation source (Gamma Cell 40; Nordion, Kanata, ON, Canada). Infusion of BM cells was given via tail vein injection on the following day of TBI. BM cells were harvested from femurs and tibiae of the syngeneic strain- and age-matched mice. The immune reconstitution of these mice post-BMT was determined by cell-surface staining of spleen and lymph node cells with the following fluorescein isothiocyanate-labeled specific mouse antibodies (PharMingen): CD3, CD4, CD8, and appropriate isotype-matched controls to cell surface determinants and was analyzed by flow cytometry (FACScan; Becton Dickinson). Cell number in lymphoid organs was enumerated by trypan blue exclusion and reported as mean cell yield and cellularity.
Induction of Allogeneic Mixed Lymphocyte Reactions (MLR).
Spleen cells from B6 mice were harvested, irradiated with 2,500 rad (Gamma Cell 1000; Nordion), and resuspended for further use as stimulator cells. Spleen cells from TBI/BMT BALB/c mice were harvested at 2, 4, and 6 weeks post-BMT and were incubated with stimulator cells for 3 to 5 days at 37°C in 96-well U-bottom microtiter plates. Tritiated deoxythymidine [1 μCi per well (1 Ci = 37 GBq); NEN] was added 24 h before assay completion. Responses were reported as mean cpm ± SEM from triplicate samples.
Statistical Analysis.
A one-way ANOVA (followed by a Newman–Keuls post hoc test) was performed to compare multiple treatment groups in tumor size measurement experiments. For comparison of multiple treatment groups in pulmonary metastases experiments, nonparametric ANOVA (followed by Dunn's post test) was performed. Mann–Whitney (t test) was used to compare between two treatment groups. All statistical analysis was performed using PRISM software (GraphPad, San Diego). Statistical significant was achieved at P < 0.05.
Results
We first analyzed the kinetics of T cell reconstitution in mice undergoing syngeneic BMT under our selected conditions. The total cellularity, percentages of T cell subsets, and functional responsiveness in the spleens of mice were evaluated at different time points after transplant (Table 1). Compared with the normal, non-BMT mice splenic cellularity rose from <10% at 1 week to ≈40% by 3 weeks, and gradually returned to a near normal level by 6 weeks. By FACS analysis, both CD4+ and CD8+ T cells showed similar reconstitution rates over time following BMT. We also determined the capacity of reconstituted T cells to respond to alloantigen stimulation in vitro. The level of proliferation by the responding T cells in an allogeneic mixed lymphocyte reaction was reflective of the gradual return of splenic cellularity over 6 weeks following BMT.
Table 1.
T cell reconstitution following bone marrow transplantation
| Time point post-BMT | Mean cell yield (×106) | Cellularity, % | Phenotype
|
Allogeneic MLR, CPM ± SEM | |
|---|---|---|---|---|---|
| %CD4 | %CD8 | ||||
| Normal, non-BMT | 78.5 | 100.0 | |||
| 1 week | 6.6 | 8.4 | — | — | — |
| Normal, non-BMT | 82.8 | 100.0 | 26.2 | 11.8 | 86,989 ± 1,202 |
| 2 week | 30.5 | 36.8 | 3.2 | 1.5 | 2,591 ± 147 |
| Normal, non-BMT | 79.3 | 100.0 | |||
| 3 week | 33.5 | 42.2 | 7.7 | 3.2 | — |
| Normal, non-BMT | 85.3 | 100.0 | 27.6 | 9.9 | 87,366 ± 1,971 |
| 4 week | 56.8 | 66.6 | 12.3 | 5.0 | 8,615 ± 1,813 |
| Normal, non-BMT | 85.9 | 100.0 | |||
| 5 week | 75.0 | 87.3 | 20.0 | 6.1 | — |
| Normal, non-BMT | 87.4 | 100.0 | 29.0 | 12.1 | 100,730 ± 2,627 |
| 6 week | 97.2 | 97.2 | 24.7 | 8.2 | 65,536 ± 1,487 |
Three mice were euthanized and spleen cell yield was averaged at each time point post-BMT. Cellularity was reported as percentage of normal, non-BMT spleen. Spleen cells were cocultured at a responder-to-stimulator ratio of 2:1 in triplicates in the MLR. Data are representative of three experiments.
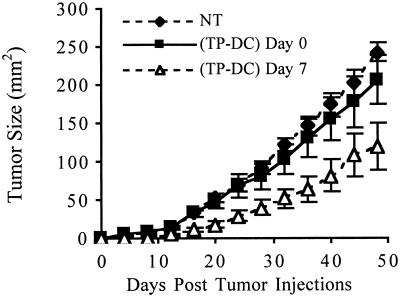
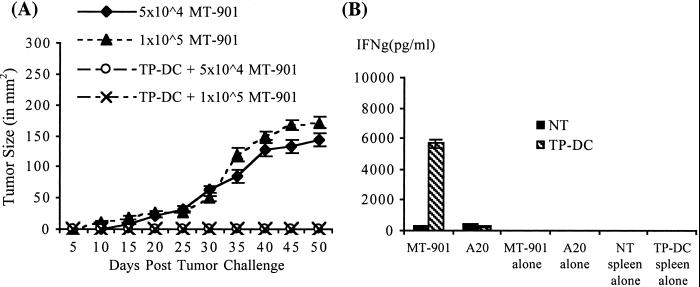
Based on the kinetics of T cell reconstitution after BMT, we next evaluated the capacity of TP-DC to elicit immune priming against the syngeneic MT-901 breast tumor during a period of lymphopenia. Our previous studies showed that TP-DC immunizations were highly effective in protecting mice against subsequent tumor challenge in normal, non-BMT mice (13). Following BMT, mice were subjected to two s.c. injections (1 week apart) of TP-DC, with the first administered on either day 0 or day 7. In a representative experiment, s.c. immunization with TP-DC at day 7 and day 14 after BMT resulted in a significant growth inhibition of a subsequent s.c. challenge with a lethal dose of viable MT-901 tumor cells compared with nonimmunized BMT mice or those receiving TP-DC on day 0 and day 7 (P < 0.01; Fig. 2). As shown in Fig. 3A, complete tumor growth inhibition could be obtained when mice received three TP-DC immunizations on days 7, 14, and 21 after BMT. Outgrowth of tumor was prevented with a challenge dose of either 5 × 104 or 1 × 105 viable MT-901 tumor cells administered s.c. (P < 0.01). In other studies performed, irradiated BALB/c mice underwent transplant with bone marrow cells from syngeneic BALB/c athymic (nu/nu) mice. In this setting, three TP-DC immunizations beginning 7 days after BMT failed to protect the recipient mice from progressive tumor outgrowth when challenged with viable MT-901 breast tumor cells (data not shown). This latter finding provided evidence of a requirement for mature T cells resident in donor bone marrow to achieve successful immune priming by TP-DC.
Figure 2.
TP-DC immunization can inhibit MT-901 breast tumor growth when initiated on day 7 post-BMT. NT mice received BMT alone. TP-DC mice were immunized twice weekly starting on day 0 or 7 post-BMT. At 4 weeks post-BMT, mice were inoculated s.c. with 3 × 105 viable MT-901 tumor cells. Data are reported as the average tumor area ± SEM (P < 0.01). Data represent the combination of three separate experiments.
Figure 3.
Repetitive TP-DC immunization can prevent MT-901 breast tumor growth and elicit specific IFN-γ-producing immune cells when initiated early post-BMT. (A) TP-DC groups were immunized thrice weekly starting on day 7 post-BMT. Immunized and control mice were challenged with two different doses of viable MT-901 tumor cells at 4 weeks post-BMT. Data are reported as the average tumor size ± SEM of five mice per group and are representative of two experiments (P < 0.01). (B) Tumor-specific IFN-γ production in BMT mice immunized with MT-901 TP-DC. Spleens were harvested 7 days after the third vaccine injection. Responder spleen cells (NT or TP-DC) were cultured with either irradiated MT-901 or A20 irrelevant tumor. After 48 h, culture supernatants were collected and the amount of IFN-γ was measured by standard ELISA method. Data are reported in pg/ml (mean ± SEM of triplicate samples) and are representative of two experiments (P < 0.03).
It was reported previously that naive T cells in a lymphopenic environment could acquire memory-like phenotype and become hypersensitive to specific antigen stimulation as measured by the production of IFN-γ (8). We next examined the level of IFN-γ production in the spleens of both TP-DC immunized and control BMT mice. One week following three injections of TP-DC, spleens of mice were harvested (three spleens per group were pooled) and were stimulated in vitro with either irradiated parental MT-901 or irrelevant A20 tumor cells. Additional control groups contained either spleen cells or tumor cells alone. As shown in Fig. 3B, there was a significant increase in the production of IFN-γ cytokine in the immunized spleen cells (5,690 ± 277 pg/ml) compared with the unimmunized spleen (331 ± 27 pg/ml; P < 0.03). This production of IFN-γ was specific because spleen cells from MT-901 TP-DC-immunized BMT mice produced a relatively low level of this cytokine (284 ± 29 pg/ml) when stimulated with A20 tumor cells. In contrast to IFN-γ secretion, harvested spleen cells from TP-DC immunized mice failed to show any detectable cytolytic T cell activity in vitro when tested against MT-901 tumor target cells in a standard 4-h 51Cr assay [<0.1 lytic unit (LU)20 per 106 cells]. We also examined the level of NK activity in the spleens of TP-DC-immunized vs. nonimmunized BMT mice. In a standard 4-h 51Cr assay against the NK-sensitive YAC-1 target cell line, there was no discernable difference in lytic activity between the two groups of fresh splenocytes [26 LU20 per 106 cells vs. 33 LU20 per 106 cells, respectively; P = not significant]. Thus, TP-DC immunizations did not appear to alter the level of endogenous splenic NK cell activity in BMT mice.
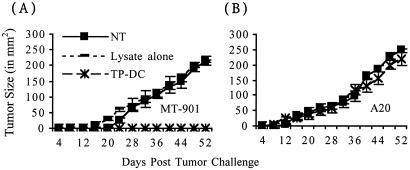
The tumor specificity of the antitumor response elicited in BMT mice by TP-DC immunization was also observed in vivo (Fig. 4). Mice immunized thrice with MT-901 TP-DC starting at day 7 following BMT could effectively reject an s.c. challenge of viable MT-901 tumor cells but not A20 B cell lymphoma cells on the opposite flank. Moreover, similar injections of MT-901 tumor lysate alone could not afford protection of BMT mice from tumor challenge, thus demonstrating the necessity of DC for effective immune priming in the setting of lymphopenia (Fig. 4).
Figure 4.
TP-DC immunization can induce tumor-specific protective immunity following BMT. BMT mice were either left untreated (NT), or were immunized thrice weekly with either MT-901 tumor lysates alone or with MT-901 TP-DC starting on day 7 post-BMT. All mice were then challenged s.c. with viable tumor cells at 4 weeks post-BMT. Mice received 3 × 105 MT-901 cells in one flank and irrelevant 1 × 105 A20 cells in the opposite flank. Data are reported as the average tumor size ± SEM of five mice per group. A representative experiment of two is shown (P < 0.01).
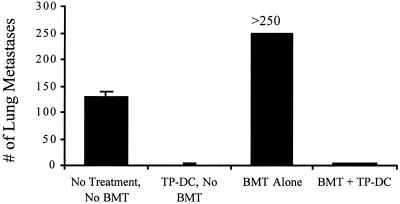
To more fully assess the antitumor efficacy afforded by TP-DC immunizations initiated early after BMT, we also challenged mice with viable MT-901 tumor cells by the i.v. route (Fig. 5). Without BMT, i.v. inoculation of 2 × 105 MT-901 tumor cells into fully immunocompetent mice resulted in an average number of 130 ± 8 (mean ± SEM) pulmonary metastases 2 weeks later. The tumor burden in the lungs of mice receiving BMT was consistently higher (>250), thus providing a more challenging environment for tumor protection by TP-DC immunization than in the non-BMT setting. Nonetheless, three TP-DC immunizations beginning on day 7 after BMT demonstrated a reduction in number of pulmonary metastases comparable to that in non-BMT mice (3.2 ± 1.2 and 1.2 ± 1.0, respectively; P < 0.01).
Figure 5.
TP-DC immunization can protect BMT mice from pulmonary metastases formation. Both BMT and normal, non-BMT mice were either left untreated or were immunized as described in the legends to Figs. 3 and 4. All mice then were challenged i.v. with 2 × 105 viable MT-901 tumor cells. Data are reported as mean number of metastases ± SEM of five mice per group at day 7 postchallenge. A representative experiment of three is shown (P < 0.01).
In a separate series of experiments, we also examined the persistence of the antitumor immunity generated by TP-DC immunizations that were conducted during the lymphopenic state. Mice received three TP-DC immunizations s.c. on days 7, 14, and 21 after BMT. Table 2 shows representative results from one of three separate experiments conducted. In this study, TP-DC-treated mice that rejected an s.c. challenge of viable MT-901 tumor cells were then rechallenged with tumor cells by the i.v. route at ≥100 days after BMT. Visually detectable MT-901 tumor nodules failed to develop in the lungs of TP-DC-immunized mice, whereas nonimmunized BMT mice became moribund and showed numerous metastases (Table 2). Thus, upon full hematolymphoid reconstitution, the antitumor immunity generated by TP-DC immunizations conducted early on after BMT could maintain a long-lasting effect in vivo.
Table 2.
Persistence of antitumor immunity elicited by TP-DC immunizations
| Treatment
|
||
|---|---|---|
| None | TP-DC | |
| Number of mice with s.c. tumor (first challenge) | 5/5 | 0/5 |
| Number of lung metastases (second challenge) | >250, >250, >250, >250, >250, >250, >250 | 0, 0, 0, 0, 0 |
Mice received TP-DC immunizations on days 7, 14, and 21 post-BMT. For the first challenge, mice were subjected to s.c. injection of 2 × 105 viable MT-901 cells. For the second challenge, mice received 1 × 106 viable MT-901 cells i.v. Lungs were harvested 7 days later. Data are representative of three experiments.
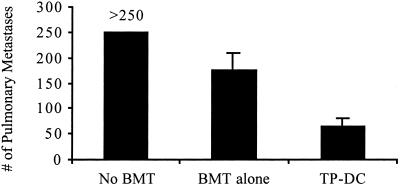
We next determined the efficacy of TP-DC immunizations in therapy against preexisting tumors. Our initial attempts to successfully treat mice receiving a BMT of bone marrow cells admixed with viable MT-901 tumor cells uniformly failed with three TP-DC immunizations starting at day 7 after the “contaminated” transplant (all >250 metastases; data not shown). We showed earlier that the tumor is more aggressive in establishing pulmonary metastases when given i.v. after TBI and BMT compared with non-BMT (Fig. 5). Because of this limitation, we evaluated the debulking impact of TBI and BMT on MT-901 tumors already established in the lungs. Indeed, tumor load could be significantly reduced by this procedure (Fig. 6). We then attempted to reduce tumor burden further by TP-DC immunizations. Mice were injected i.v. with 3 × 105 viable MT-901 tumor cells to establish pulmonary metastases and then underwent BMT alone or BMT plus three TP-DC immunizations initiated 7 days later. As shown in Fig. 6, TP-DC-treated BMT mice showed a significant decrease in the number of lung metastases (65.4 ± 15.1) compared with mice receiving BMT alone (174.2 ± 34.8; P < 0.01). The latter effect represented a significant debulking of tumor load by BMT alone compared with control tumor-bearing mice not subjected to BMT and TP-DC immunizations (>250 lung metastases; P < 0.05).
Figure 6.
Regression of established pulmonary metastases by TP-DC immunization of BMT mice. Mice were inoculated with 3 × 105 MT-901 tumor cells i.v. Seven days later, mice were left untreated or received BMT as described in Materials and Methods. TP-DC group was immunized thrice, starting 7 days post-BMT. Data are reported as mean number of metastases ± SEM of five mice per group at 7 days after three immunizations. No BMT vs. BMT alone, P < 0.05; BMT alone vs. TP-DC, P < 0.01. A representative of three experiments is shown.
Discussion
In the current study, we demonstrated that DC pulsed with whole tumor lysates could generate specific and long-lasting antitumor immune responses during early lymphoid reconstitution in the setting of BMT. Our data support the view that vaccines comprised of potent antigen(s)-pulsed DC may serve to elicit immune priming events during a state of lymphopenia and homeostasis-driven T cell proliferation (8). Evidence exists for the capacity of DC to abrogate T cell tolerance against transplant antigens (18) and tumors (19). In our study, the therapeutic antitumor efficacy of BM-derived DC to mediate regression of established tumors in the setting of BMT further adds support for its potential role in breaking immune tolerance. In other studies, however, investigators have explored the capacity of DC to induce tolerance and mediate negative selection of T cells (20). Although most studies have involved predominantly thymic (21) and liver-derived (22) DC populations, to our knowledge a formal role of DC in establishing peripheral tolerance has not yet been demonstrated particularly in the setting of BMT. Further study is needed to determine the differential roles of defined DC subsets in immune priming vs. tolerance induction in the setting of BMT, similar to studies being conducted in conventional, fully immunocompetent mice (23).
One potential concern of using whole tumor lysates in the setting of lymphopenia is the possible induction of autoimmune reactivity to self or normal tissue antigens present in the tumor lysate as a consequence of processing by potent antigen-presenting DC. It has been shown in melanoma, for example, that normal tissue antigens can be targets for cancer immunotherapy, leading in some instances to vitiligo in the treated patients. However, recent studies have demonstrated that expression of some self antigens can serve as targets for CTL-mediated destruction of tumors in the absence of any demonstrable damage to normal tissue (24). Although the potential exists for eliciting autoimmunity by immunization with tumor lysate-pulsed DC, our in vitro studies of T cell cytotoxic, proliferative, and cytokine responses, as well as in vivo immune priming and treatment of tumor in conventional, fully immunocompetent mice, have not shown patterns of autoimmunity development (13). We have also not observed the induction of autoimmunity by TP-DC immunizations in the setting of syngeneic BMT, including in cases where the treated animals were followed for at least 100 days (e.g., Table 2). Recent studies have suggested that immunization of allogeneic BMT recipients with tumor vaccines can enhance the graft vs. tumor activity without exacerbating graft vs. host disease (GVHD) in the setting of leukemia and solid tumors with and without T cell depletion (25, 26). Notwithstanding, caution is raised with the respect to the use of TP-DC in the setting of allogeneic BMT because of the known potent allostimulatory capacity of DC (10, 11) and the potential for enhancing GVHD with donor T cell reconstitution in transplanted patients with solid tumors (27).
Immunization of BMT mice with MT-901 TP-DC could elicit tumor-specific immune priming, which resulted in an antitumor effect against MT-901 but not the A20 tumor (Fig. 4). We have observed similar tumor specificity of TP-DC immunizations in vitro and in vivo in our previous studies in conventional, fully immunocompetent mice (13). Moreover, similar to the latter studies, IFN-γ production by noncytolytic splenic T cells following TP-DC immunization in the setting of BMT was found to be tumor-specific (Fig. 3B). These data are consistent with those reported for IFN-γ production by homeostasis-induced memory CD8 T cells (8). IFN-γ has not only been shown to correlate with antitumor effects of immunotherapies in vivo, but also to help prevent tumor formation in vivo (28). Although tumor lysate pulsing of the DC is required for optimal antitumor activity, in some instances we have observed that repetitive immunizations with unpulsed DC could induce a nonspecific response in the BMT setting but not in conventional, fully immunocompetent mice (data not shown). This nonspecific effect could result in some tumor growth inhibition in the setting of BMT. It is conceivable that TP-DC vaccination in a lymphopenic environment may increase the nonspecific antitumor activity of unpulsed DC, which presumably is related to FCS effects of using cultured DC and cultured tumor cells for in vivo models. Notwithstanding, splenic NK cell activity appeared to be unchanged in TP-DC immunized compared with nonimmunized BMT mice.
The systemic administration of recombinant cytokines with known activity on T cell priming, on DC differentiation, expansion, and function, as well as on thymopoiesis, may serve to augment immunization by tumor lysate-pulsed DC in the settings of lymphopenia and BMT. Our future directions will be focused on enhancing the antitumor activity by using the combination of DC vaccines with recombinant cytokines such as IL-2 and IL-7 and with the transfer of immune cells to supplement the transplant. The administration of certain recombinant cytokines has elicited potent effects on hematopoiesis and enhanced the antitumor potency of cancer vaccines. For example, we have shown that the systemic administration of recombinant IL-2 can enhance the antitumor potency of dendritic cell-based tumor vaccines in vivo (13). Moreover, IL-2 is being examined currently in the setting of human BMT for the enhancement of antitumor activity (29). Recombinant IL-7 administration to normal or tumor-bearing mice can cause a pronounced increase in B and T cells, NK cells, and macrophages in the spleen and lymph nodes. IL-7 has been shown to mobilize myeloid and lymphoid progenitor cells from the BM to the spleen and lymph nodes (30). Moreover, in vivo administration of IL-7 can enhance thymopoiesis after BMT and can prevent post-BMT immune deficiency, resulting in normalization of thymic cellularity, normal numbers of peripheral T cells, and improved antigen-specific T- and B-cell function early post-BMT (compared with the profound, general immune deficiency in the periphery and thymic hypoplasia; ref. 31).
Collectively, TP-DC vaccines appear to promote immune priming events in a setting of syngeneic BMT-induced lymphopenia that can result in antitumor effects in vivo. The approach may also allow for opportunities to enhance DC-based immunization by combination with cytokines and adoptive transfer of effector T cells. Future studies should provide additional new leads to further implement these approaches in vivo.
Acknowledgments
We thank Ms. Julie McLaughlin for production of the confocal photomicrograph. This work was supported by grants from the National Cancer Institute/National Institutes of Health (1 R01 CA71669, 1 RO1 CA87019, 5 P01 CA59327, and M01-RR00042), by grants from the Department of Defense/U.S. Army (DAMD17-96-1-6103 and DAAG55-97-1-0239), and by gifts from C. J. and E. C. Aschauer and Abbott Laboratories and the Dorothy and Herman Miller Immunology Research Endowment.
Abbreviations
- DC
dendritic cell(s)
- TP-DC
tumor lysate-pulsed dendritic cell(s)
- BMT
bone marrow transplantation
- TBI
total body irradiation
References
- 1.Uchida N, Weissman I L. J Exp Med. 1992;175:175–184. doi: 10.1084/jem.175.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katsanis E, Anderson P M, Filipovich A H, Hasz D E, Rich M L, Loeffler C M, Ochoa A C, Weisdorf D J. Blood. 1991;78:1286–1291. [PubMed] [Google Scholar]
- 3.Slavin S, Naparstek E, Nagler A, Ackerstein A, Samual S, Kapelushnik J, Brautbar C, Or R. Blood. 1996;87:2195–2204. [PubMed] [Google Scholar]
- 4.Sondak V K, Magner P D, Shu S, Chang A E. Arch Surg. 1991;126:442–446. doi: 10.1001/archsurg.1991.01410280040005. [DOI] [PubMed] [Google Scholar]
- 5.Finke J H, Zea A H, Stanley J, Longo D L, Ochoa A C. Cancer Res. 1993;53:5613–5616. [PubMed] [Google Scholar]
- 6.Restifo N P, Esquivel F, Dawakami Y, Yewdell J W, Mulé J J, Rosenberg S A, Bennink J R. J Exp Med. 1993;177:265–272. doi: 10.1084/jem.177.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mackall C L, Bare C V, Granger L A, Sharrow S O, Titus J A, Gress R E. J Immunol. 1996;156:4609–4616. [PubMed] [Google Scholar]
- 8.Cho B K, Rao V P, Ge Q, Eisen H N, Chen J. J Exp Med. 2000;192:549–556. doi: 10.1084/jem.192.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong P, Pamer E G. J Immunol. 2001;166:5864–5868. doi: 10.4049/jimmunol.166.10.5864. [DOI] [PubMed] [Google Scholar]
- 10.Steinman R M. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 11.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu Y, Pulendran B, Palucka K. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 12.Ridge J P, Fuchs E J, Matzinger P. Science. 1996;271:1723–1726. doi: 10.1126/science.271.5256.1723. [DOI] [PubMed] [Google Scholar]
- 13.Shimizu K, Fields R, Giedlin M, Mulé J J. Proc Natl Acad Sci USA. 1999;96:2268–2273. doi: 10.1073/pnas.96.5.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Porgador A, Snyder D, Gilboa E. J Immunol. 1996;156:2918–2926. [PubMed] [Google Scholar]
- 15.Candido K A, Shimizu K, McLaughlin J C, Kunkel R, Fuller J A, Redman B G, Thomas E K, Nickoloff B J, Mule J J. Cancer Res. 2001;1:228–236. [PubMed] [Google Scholar]
- 16.Feuerer M, Beckhove P, Bai L, Solomayer E F, Bastert G, Diel I J, Pedain C, Oberniedermayr M, Schirrmacher V, Umansky V. Nat Med. 2001;7:452–458. doi: 10.1038/86523. [DOI] [PubMed] [Google Scholar]
- 17.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman R. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steptoe R J, Fu F, Li W, Drakes M L, Lu L, Demetris A J, Qian S, McKenna H J, Thomson A W. J Immunol. 1997;159:5483–5491. [PubMed] [Google Scholar]
- 19.Gong J, Chen D, Kashiwaba M, Li Y, Chen L, Takeuchi H, Qu H, Rowse G J, Gendler S J, Kufe D. Proc Natl Acad Sci USA. 1998;95:6279–6283. doi: 10.1073/pnas.95.11.6279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matzinger P, Guerder S. Nature (London) 1989;338:74–76. doi: 10.1038/338074a0. [DOI] [PubMed] [Google Scholar]
- 21.Shortman K, Vremec D, Corcoran L M, Georgopoulaos K, Lucas K, Wu L. Immunol Rev. 1998;165:39–46. doi: 10.1111/j.1600-065x.1998.tb01228.x. [DOI] [PubMed] [Google Scholar]
- 23.Morelli A E, O'Connell P J, Khanna A, Logar A J, Lu L, Thomson A W. Transplant. 2000;69:2647–2657. doi: 10.1097/00007890-200006270-00027. [DOI] [PubMed] [Google Scholar]
- 23b.Pooley J L, Heath W R, Shortman K. J Immunol. 2001;166:5327–5330. doi: 10.4049/jimmunol.166.9.5327. [DOI] [PubMed] [Google Scholar]
- 24.Vierboom M P M, Nijman H W, Offringa R, van der Voort E I, van Hall T, van den Broek L, Fleuren G J, Kenemans P, Kast W M, Melief C J. J Exp Med. 1997;186:695–704. doi: 10.1084/jem.186.5.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson L D, Savary C A, Mullen C A. Blood. 2000;95:2426–2433. [PubMed] [Google Scholar]
- 26.Teshima T, Mach N, Hill G R, Pan L, Gillessen S, Dranoff G, Ferrara J L. Cancer Res. 2001;61:162–171. [PubMed] [Google Scholar]
- 27.Childs R, Chernoff A, Contentin N, Bahceci E, Schrump D, Leitman S, Read E J, Tisdale J, Dunbar C, Linehan W M, et al. N Engl J Med. 2000;343:750–758. doi: 10.1056/NEJM200009143431101. [DOI] [PubMed] [Google Scholar]
- 28.Shankaran V, Ikeda H, Bruce A T, White J M, Swanson P E, Old L J, Schreiber R D. Nature (London) 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 29.Fefer A. In: Principles and Practice of the Biologic Therapy of Cancer. Rosenberg S A, editor. Philadelphia: Lippincott; 2000. pp. 83–93. [Google Scholar]
- 30.Grzegorzewski K J, Komschlies K L, Franco J L, Ruscetti F W, Keller J R, Wiltrout R H. Blood. 1996;88:4139–4147. [PubMed] [Google Scholar]
- 31.Bolotin E, Smogorzewska M, Smith S, Widmer M, Weinberg K. Blood. 1996;88:1887–1893. [PubMed] [Google Scholar]