Abstract
Tumor necrosis factor (TNF)-induced cell death in the fibrosarcoma cell line L929 is a caspase-independent process that is characterized by increased production of reactive oxygen species (ROS) in the mitochondria. To elucidate this ROS-dependent cell death pathway, a comparative study of the phosphoproteins present in TNF-treated and control cells was performed. Here we report that TNF induces an increased phosphorylation of glyoxalase I that is mediated by protein kinase A and required for cell death. We also show that TNF induces a substantial increase in intracellular levels of methylglyoxal (MG) that leads to the formation of a specific MG-derived advanced glycation end product and that this formation occurs as a consequence of increased ROS production. These data indicate that MG modification of proteins is a targeted process and that MG may thus function as a signal molecule during the regulation of cell death. Furthermore, we provide evidence that the TNF-induced phosphorylation of glyoxalase I is not involved in detoxification of MG by means of the glyoxalase system, but that phosphorylated glyoxalase I is on the pathway leading to the formation of a specific MG-derived advanced glycation end product.
Tumor necrosis factor (TNF) is a pleiotropic cytokine that plays a role in the host defense against microorganisms and bacterial pathogens and in the pathophysiology of various diseases (1, 2). Furthermore, TNF has potent antitumor and antimalignant cell effects. Depending on the cell type, cell death by TNF can occur by apoptosis or necrosis (3, 4). TNF-induced cell death in the murine fibrosarcoma cell line L929, the cell line used in this study, is characterized by a necrosis phenotype that is caspase-independent and does not involve DNA fragmentation, but instead requires the increased production of reactive oxygen species (ROS) in the mitochondria (5–7). Much effort has been directed at elucidating the molecular mechanisms of caspase-dependent cell death, but relatively little is yet known about the TNF-induced ROS-dependent cell death pathway.
To identify signaling molecules downstream of the receptor-proximal events that are involved in this cell death pathway, protein phosphorylation changes in L929 cells were examined by comparative two-dimensional (2D) gel electrophoresis after stimulation with TNF for 1.5 h (that is, just before cells start to die). Previous work has shown that TNF-induced increase in oncoprotein 18 (Op18, stathmin) phosphorylation is responsible for TNF-induced microtubule stabilization, which in turn promotes cell death (8). Now, we demonstrate that phosphorylation of glyoxalase I (lactoylglutathione lyase, EC 4.4.1.5) is a key step in TNF-induced cell death.
The glyoxalase system, consisting of the enzymes glyoxalase I (GLO1) and glyoxalase II (GLO2), is an integral component of cellular metabolism in mammalian systems (see review in ref. 9). Although the glyoxalase pathway was reported as early as 1913 and is ubiquitous in nature, its full biological function has never been elucidated. The work of Szent-Györgyi suggested that GLO1 and methylglyoxal (MG) were involved in the regulation of cellular growth, but a direct mechanistic link has yet to be identified (reviewed in ref. 10). A major function of the glyoxalase pathway is believed to be detoxification of α-ketoaldehydes, especially MG. MG is a cytotoxic metabolite produced primarily as a by-product of glycolysis through nonenzymatic phosphate elimination from the glycolytic pathway intermediates dihydroxyacetone phosphate and glyceraldehyde 3-phosphate (for a review on MG, see ref. 11). The glyoxalase system requires reduced glutathione (GSH) as a cofactor and catalyzes the conversion of MG to D-lactate. The substrate for GLO1 is the hemithioacetal formed through the nonenzymatic conjugation of MG with GSH. The product of the GLO1-catalyzed reaction is S-D-lactoylglutathione, which is then hydrolyzed by glyoxalase II to D-lactate; GSH is regenerated by the action of GLO2. D-Lactate is further metabolized to pyruvate in some mammalian tissues.
Increased expression of GLO1 occurs in diabetic patients and in some tumors, such as colon carcinoma (12), breast cancer (13), and prostate cancer (14). Recently, it has been shown that GLO1 is involved in resistance of human leukemia cells to antitumor agent-induced apoptosis (15). Under normal physiological conditions, most MG is bound to cellular proteins as adducts formed with Lys, Arg, and Cys residues (16, 17). Although the reaction with Cys is considered reversible, elevated concentrations of MG can lead to irreversible modifications of Lys and Arg residues through formation of advanced glycation end products (AGEs) (18). AGE formation is thought to contribute to several pathophysiological conditions, such as tissue damage after ischemia/reperfusion (19), to aging (20), and to the development of complications in diabetic patients such as blindness, neuropathy, and diabetic vascular diseases (21).
Materials and Methods
Cell Lines.
L929 cells were cultured in Dulbecco's modified Eagle's medium supplemented with heat-inactivated FCS (5% vol/vol), heat-inactivated newborn calf serum (5% vol/vol), penicillin (100 units/ml), streptomycin (0.1 mg/ml), and L-glutamine (2 mM), at 37°C in a humidified incubator under a 5% CO2 atmosphere.
Reagents.
Murine TNF was obtained from Roche Diagnostics. Propidium iodide (PI), cycloheximide (CHX), and butylated hydroxyanisole (BHA) (all from Sigma) were used at concentrations of 30 μM, 50 μg/ml, and 100 μM, respectively. The PKA inhibitor H89 was obtained from Calbiochem. The glyoxalase I inhibitor S-p-bromobenzylglutathione cyclopentyl diester (BBGD) and the anti-glyoxalase I antibody were a kind gift from Dr. P. Thornalley (Department of Biological Sciences, University of Essex, United Kingdom). The monoclonal anti-MG-derived AGE antibody (mAb6B) was donated by Dr. K. Uchida (Nagoya University Graduate School of Bioagricultural Sciences, Nagoya, Japan).
Radiolabeling of Cells and Preparation of the Subcellular Protein Fractions and 2D Gel Electrophoresis.
These procedures were performed as described (8).
Western Blotting.
Proteins were separated by SDS/12.5% PAGE and transferred to a poly(vinylidene difluoride) membrane (Hybond-P, Amersham Pharmacia). The blots were incubated with a polyclonal anti-human glyoxalase I antibody, followed by enhanced chemiluminescence (ECL)-based detection (Amersham Pharmacia).
Measurement of TNF-Induced Cell Death by Flow Cytometry.
L929 cells were kept in suspension by seeding them the day before in uncoated 24-well tissue culture plates (Sarstedt). Cell death was induced by addition of TNF to the cell suspension. Cell death was measured by quantifying PI-positive cells by a fluorescence-activated cell sorter (FACSCalibur, Becton Dickinson) as described (22). The PI dye was excited with an argon-ion laser at 488 nm; PI fluorescence was measured above 590 nm by using a long-pass filter. Three thousand cells were routinely analyzed. Cell death is expressed as the percentage of PI-positive cells in the total cell population.
Identification of the Protein Spot as GLO1 by Matrix-Assisted Laser Desorption Ionization–Mass Spectrometry.
After in-gel digestion of the excised protein with endoproteinase Lys-C (sequencing grade; Boehringer Mannheim), a 10% aliquot of the generated peptide mixture was purified and concentrated on Poros 50 R2 beads and used for matrix-assisted laser desorption ionization–mass spectrometry peptide mass fingerprint analysis (23, 24). The peptide mass map obtained did not lead to an unambiguous protein identification because of contamination with human keratin peptides. The remainder of the peptide mixture was therefore separated by RP-HPLC to obtain 20 eluting peptide fractions; these were then analyzed by matrix-assisted laser desorption ionization–mass spectrometry. Adequate peptide ions were further selected for postsource decay analysis (25). A postsource decay spectrum obtained from a peptide ion with a mass of 902.42 Da (measured in linear mode) present in the first RP-HPLC fraction could be unambiguously assigned to the peptide NH2-SLDFYTR-COOH present in human GLO1 (database entry number 417246) by using the SEQUEST algorithm and a nonredundant protein database. After a search in an EST database, the same peptide sequence was identified in many different mouse EST clones. Additional confirmation of the initial finding was conducted through postsource decay analysis of a peptide present in RP-HPLC fraction 11 with an apparent mass of 1396.53 Da. On the basis of the partially 18O-labeled y-type fragment ions, a peptide sequence tag (391.24)YAI/LF(885.67) could easily be obtained. Furthermore, a SEQUEST search in a nonredundant protein database led to the identification of the peptide NH2-FSLYFLAYEDK-COOH also belonging to human GLO1. Again, the same peptide sequence was found in different mouse EST clones by using the postsource decay data and a SEQUEST search in an EST database. On the basis of the amino acid sequence of human GLO1, masses of peptide ions observed in the different RP-HPLC fractions could be assigned to the identified protein. In this way, a total of 38% of the amino acid sequence of the protein was covered, again confirming the identification of GLO1.
Assay of GLO1 Activity.
GLO1 activity was determined by using the spectrophotometric method, which monitors the initial rate of change in absorbance at 240 nm caused by the formation of S-D-lactoylglutathione (26). The standard assay mixture contained 2-mM MG and 2-mM GSH in a sodium phosphate buffer (50 mM, pH 6.6, 20°C). The reaction mixture was allowed to stand for 10 min before the addition of the cytosolic protein fraction (15–30 μg) to ensure the equilibration of hemithioacetal formation.
d-Lactate Measurements.
D-Lactate measurements were performed by using the fluorometric endpoint assay with D-lactate dehydrogenase (27).
Methylglyoxal Assay.
Intracellular MG was measured by using a modification of the HPLC-based method described in ref. 28. L929 cell samples were harvested, resuspended in ice-cold PBS buffer, acidified with 100 mM acetic acid, and immediately frozen at −80°C until shipment on dry ice between collaborating groups. Samples were frozen at −20°C until assayed. Sample preparation was in 100 mM acetic acid as described (17). HPLC was performed with a mobile phase consisting of 35% acetonitrile/0.1% trifluoroacetic acid, pH 2.4 and 65% 10 mM phosphate/0.1% trifluoroacetic acetic acid in HPLC-grade water, pH 2.4. Under these modified conditions, the MG derivative 2-methylquinoxaline eluted after 7.5 min, and 5-methylquinoxaline (the internal standard) eluted after 11.2 min.
Detection of MG-Derived AGEs.
L929 cells were seeded 48 h before the experiment. TNF incubations (1,000 units/ml) were performed in the presence of CHX to synchronize cell death. After TNF incubations (1.5 or 2.5 h), the cells were rinsed three times with ice-cold PBS buffer, and cell lysates were prepared in a 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate-containing cytosol extraction buffer (29). MG-derived AGEs were detected by Western blotting by using the mAb6B (19). To use the antibody sparingly, SDS-polyacrylamide gels were only run across a distance of 5 cm.
Results
TNF Induces Increased Phosphorylation of Glyoxalase I.
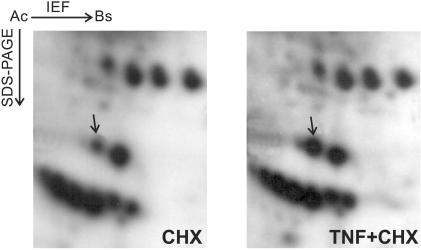
Fig. 1 shows the autoradiogram of 2D gels obtained from TNF-treated and control samples derived from L929 cells labeled with [32P]orthophosphate. The arrow indicates the protein spot corresponding to phosphorylated GLO1. GLO1 was identified by mass spectrometric analysis of a peptide mixture obtained through an in-gel digestion of the excised protein spot. Increased GLO1 phosphorylation was already observed after 15 min of TNF treatment (data not shown), but was much more pronounced after 1.5 h (Fig. 1), which indicates that the TNF-induced phosphorylation of GLO1 is an early but lasting event. Densitometric analysis of the 32P-labeled spot from several experiments revealed a 3-fold or higher increase in phosphorylation in TNF-treated cells (1.5 h) compared with control cells. No previous reports of mammalian GLO1 phosphorylation exist, although the sequence contains several potential phosphorylation sites (12).
Figure 1.
TNF induces increased phosphorylation of GLO1. Autoradiogram of 2-D gels (pH 3–10) of an organelle fraction from TNF-treated (TNF + CHX; 1.5 h) and control (CHX) cells. CHX was used to synchronize cell death. The protein spot representing phosphorylated GLO1 was identified by mass spectrometry and is indicated by an arrow.
TNF-Induced Phosphorylation of Glyoxalase I Is Required for Cell Death.
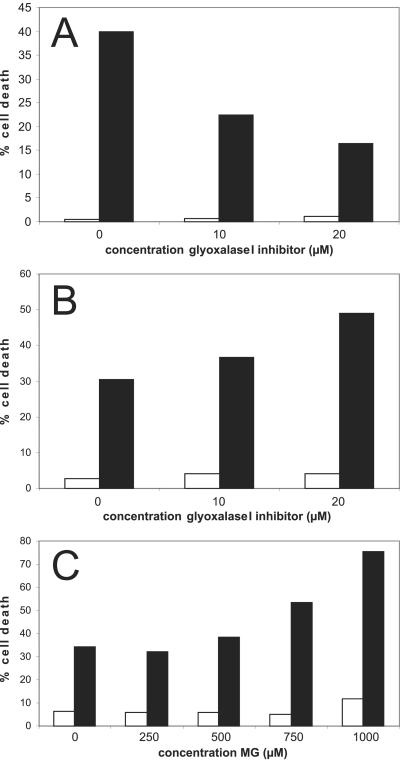
To investigate the role of GLO1 in TNF-induced cell death, cell death was measured in the presence or absence of a well characterized cell-membrane-permeable competitive inhibitor of GLO1, BBGD (30). Preincubating L929 cells for 1 to 1.5 h with BBGD strongly inhibited TNF-induced cell death in a concentration-dependent manner (Fig. 2A). TNF-induced cell death was measured as a function of time by flow cytometry (TNF, 1,000 units/ml); the maximum inhibition (60%) occurred at 20 μM BBGD. Inhibition of cell death was more pronounced at early time points during the TNF treatment. In contrast to the protective effects of preincubation with BBGD, coincubation of L929 cells with TNF and BBGD synergistically increased TNF-induced cell death, particularly at lower doses of TNF (100 units/ml) and at earlier time points. Fig. 2B shows a 50% increase in cell death at 20 μM BBGD. Previous studies show that incubation with BBGD results in intracellular MG accumulation (30). Furthermore, Fig. 2C demonstrates that exogenously added MG has a strong synergistic effect on TNF-induced cell death in a concentration-dependent manner, whereas MG alone and used at the same concentrations is not cytotoxic for L929 cells, suggesting that the increased sensitivity of the L929 cells to TNF on coincubation with BBGD resulted from MG accumulating as BBGD-inhibited GLO1 activity.
Figure 2.
The GLO1 inhibitor BBGD interferes with TNF-induced cell death. (A) Preincubation of L929 cells with the BBGD inhibits TNF-induced cell death. L929 cells were preincubated with BBGD for 1 h and 10 min. The percentage of cell death after 4 h of TNF treatment (1,000 units/ml) is shown. (B) Coincubation of BBGD with TNF has a synergistic effect on cell death. The percentage of cell death after 10 h of TNF treatment (100 units/ml) is shown. (C) Exogeneously added MG is synergistic with TNF-induced cell death. The percentage of cell death after 6 h of TNF treatment (100 units/ml) is shown. White bars, control cells; black bars, TNF-treated cells.
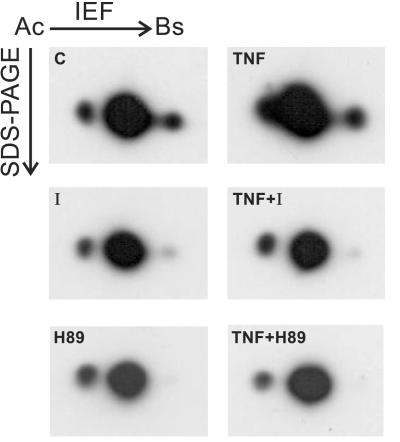
The contrasting effects of BBGD on TNF-induced cell death prompted an investigation of whether BBGD inhibited the TNF-induced phosphorylation of GLO1. Fig. 3 shows immunoblots (anti-human GLO1 polyclonal antibody) of 2D gels (pH 3–10) from total cell lysates derived from TNF-treated and nontreated cultures preincubated with and without BBGD for 1.5 h. In the upper panels, it is evident that TNF induces a more acidic phosphoisoform of GLO1 in the absence of BBGD that is not well separated from nonphosphorylated GLO1. The middle panels demonstrate that TNF does not induce the more acidic phosphoisoform of GLO1 in the presence of BBGD (the 2D patterns are identical with the control).
Figure 3.
TNF-induced phosphorylation of GLO1 is inhibited by preincubation with the GLO1 inhibitor BBGD and the PKA inhibitor H89. Western blots of 2-D gels (pH 3–10) developed with an anti-human GLO1 antibody. The immunocomplexes were visualized with use of an ECL kit (Amersham Pharmacia). The immunocomplexes shown here are the only ones that were detected on the blot. Equal amounts of total cellular lysate as measured by total protein were loaded on each gel. TNF treatments were performed for 1.5 h (1,000 units/ml) in the presence of CHX to synchronize cell death. Preincubation with the GLO1 inhibitor BBGD (20 μM) and PKA inhibitor H89 (5 μM) was performed for 1.5 h. C, control cells; TNF, TNF-treated cells; I, control GLO1 inhibitor; TNF + I, TNF-treated cells that were pretreated with the GLO1 inhibitor; H89, control PKA inhibitor; TNF + H89, TNF-treated cells that were pretreated with the PKA inhibitor.
Taken together, the results indicate that when L929 cells are pretreated with BBGD, BBGD binding to GLO1 hinders TNF-induced phosphorylation, leading to inhibition of TNF-induced cell death, which indicates that phosphorylated GLO1 is essential for TNF-mediated cell death. However, when BBGD is administered to the cells at the same time as TNF, TNF-induced phosphorylation of GLO1 occurs first (by a TNF-receptor-coupled mechanism) and is not affected by BBGD (data not shown), which implies that BBGD can bind and inhibit only the nonphosphorylated form of GLO1. Thus, coincubation of BBGD with TNF leads only to accumulation of MG, which synergizes with cell death. In cells that are pretreated with BBGD, BBGD binding to nonphosphorylated GLO1 has two effects that are counteracting, namely inhibition of the phosphorylation and MG accumulation. This counteraction may explain why inhibition of TNF-induced cell death in cells pretreated with BBGD is more pronounced at early time points of TNF treatment, because accumulation of MG is slower than inhibition of the phosphorylation. Furthermore, the fact that the competitive inhibitor BBGD can inhibit the TNF-induced phosphorylation of GLO1 suggests that the phosphorylation may modulate the substrate recognition site and/or the active site of GLO1.
TNF-Induced Phosphorylation of Glyoxalase I Is Mediated by Protein Kinase A.
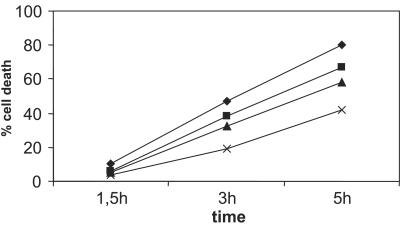
To identify the kinase that is responsible for the TNF-induced phosphorylation of GLO1, the effect of the protein kinase A (PKA) inhibitor H89 on TNF-induced cell death was examined because PKA is, among other things, an important regulator of cellular metabolism and is activated by TNF (31). As shown in Fig. 4, pretreatment (1.5 h) of L929 cells with the PKA inhibitor reduces TNF-induced cell death in a concentration-dependent fashion. This effect was already evident at relatively low concentrations of the PKA inhibitor (1 μM), whereas at a higher concentration (5 μM), a 50% inhibition of TNF-induced cell death was obtained at early time points of TNF treatment. Thus, PKA plays a role in TNF-induced cell death. To determine whether PKA was directly responsible for the TNF-induced phosphorylation of endogeneous GLO1, L929 cells were pretreated with the PKA inhibitor before TNF treatment. As shown in Fig. 3 (Lower), this pretreatment almost completely eliminated the induction of the more acidic phosphoisoform of GLO1 by TNF, suggesting that the inhibitory effect of the PKA inhibitor on TNF-induced cell death could be largely due to the direct inhibition of GLO1 phosphorylation.
Figure 4.
TNF-induced cell death is inhibited by the PKA inhibitor H89. The cells were pretreated with different concentrations of H89 for 1.5 h. TNF-induced cell death (1,000 units/ml) was measured as a function of time. No cell death was observed in the presence of the PKA inhibitor alone. ⧫, TNF; ■, TNF + 1 μM H89; ▴, TNF + 2 μM H89; ×, TNF + 5 μM H89.
Accumulation of MG in TNF-Treated Cells Is Not a Result of Decreased MG Detoxification Through the Glyoxalase System.
For many years, MG has been known to be carcinostatic, but its direct use as an anticancer drug has been prevented by rapid detoxification in vivo by the glyoxalase system. Indeed, this characteristic provided the rationale for the development of GLO1 inhibitors as potential anticancer agents (30, 32). Therefore, the hypothesis that TNF-induced phosphorylation of GLO1 results in MG accumulation and subsequent cell death seemed reasonable. Moreover, measurements of intracellular MG levels in TNF-treated L929 cells confirmed that MG accumulation occurred in the presence of TNF. Two independent experiments were performed, and each sample was measured in triplicate; MG levels increased 32% (from 0.91 μM in control cells to 1.20 μM in TNF-treated cells) and 94% (from 1.24 μM in control cells to 2.39 μM in TNF-treated cells), respectively, after 1.5 h of TNF treatment (that is, just before cells start to die). However, preincubation of L929 cells with BBGD inhibited rather than exacerbated TNF-induced cell death, and measurements of GLO1 activity in lysates derived from TNF-treated and control cells showed a limited increase in GLO1 activity in TNF-treated cells. Activity measurements were repeated several times and gave consistent results, with an average increase of 8% after treatment with TNF for 1 h and 12% after treatment with TNF for1.5 h (from 0.086 ± 0.003 to 0.106 ± 0.001 units/8.5 μg of total protein). Furthermore, measurement of the carbon flux to D-lactate, the end product of the glyoxalase system, showed an increase of 60% in D-lactate production after 1.5 h of TNF treatment compared with control cells (data not shown), thus confirming that TNF treatment did not inhibit the catabolic activity of GLO1. Increased flux of MG through the glyoxalase system in TNF-treated cells might just be a reflection of the increased levels of MG. Taken together, these results indicate that MG accumulates in TNF-treated cells, but not as the result of a decreased flux through the glyoxalase system. Furthermore, if TNF-induced phosphorylation of GLO1 would result in inhibition of its MG-detoxifying activity, then artificial inhibition of this activity by preincubation with BBGD should be synergistic with TNF-induced cell death, which is not the case, suggesting that the TNF-induced phosphorylation of GLO1 does not lead to inhibition of its MG-detoxifying activity, which implies that the phosphorylated form of GLO1 is not involved in MG catabolism, although that is yet to be proven.
Formation of a Specific MG-Derived AGE During TNF-Induced Cell Death.
Given the demonstrated role of MG in AGE formation and the accumulation of MG noted in response to TNF treatment, we next sought to determine whether irreversible protein modification by MG is a critical step in TNF-induced cell death. Immunoblots of L929 protein extracts were performed with a monoclonal antibody raised against in vitro MG-modified keyhole limpet hemocyanin. This antibody (mAb6B) recognizes epitopes in arterial walls of diabetic kidneys and in tissue injured by ischemia/reperfusion (19). The immunoblots showed a distinct differential protein band specifically present in TNF-treated (2.5 h) cells along with several protein bands that were present in both the control and TNF-treated cells (Fig. 5A). The band was already present, although very weakly, after 1.5 h of TNF treatment (data not shown). These data indicate that protein modification by MG is not a random process during TNF-induced cell death, but rather involves specific target molecules for MG.
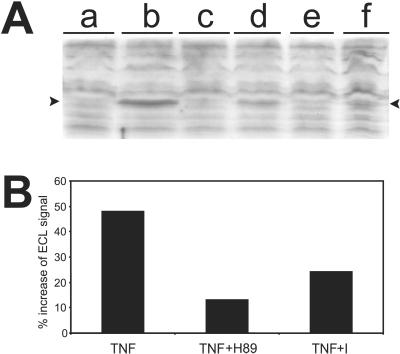
Figure 5.
Formation of a specific MG-derived AGE during TNF-induced cell death and the inhibition by several agents. (A) Western blot with the anti-AGE antibody mAb6B that was developed against MG-modified keyhole limpet hemocyanin (recognizes also MG-modified BSA) (19). To use the antibody sparingly, the SDS-polyacrylamide gels were run over a distance of only 5 cm (separation gel). Immunocomplexes were visualized by ECL and evaluated by scanning densitometry. To analyze AGE formation in TNF-induced cell death, all TNF treatments (1,000 units/ml, 2.5 h) were performed in the presence of CHX to synchronize cell death. Equal amounts of total cytosolic protein from cells incubated under different conditions were loaded in each lane. Lanes: a, control cells; b, TNF-treated cells; c, control BHA (100 μM); d, TNF-treated cells in the presence of BHA (the antioxidant agent BHA was administered 0.5 h after TNF administration to allow initiation of TNF signaling) (6); e, control 2-deoxyglucose; f, TNF-treated cells in the presence of 2-deoxyglucose (2:1 ratio to glucose) (2-deoxyglucose was administered at the same time as TNF). Note the appearance of the specific MG-derived AGE exclusively in TNF-treated cells (indicated by arrowheads), whereas no significant changes in the other AGEs can be observed. The formation of this MG-derived AGE is strongly inhibited (65%) in the presence of BHA and 2-deoxyglucose. (B) Formation of the specific MG-derived AGE is inhibited by the inhibitors that also inhibit the phosphorylation of GLO1. This figure represents the percentage relative increase of the ECL signal (as evaluated by scanning densitometry) of this specific MG-derived AGE in TNF-treated cells (1,000 units/ml in the presence of CHX, 1.5 h) over the background in control cells.
To demonstrate that the TNF-induced MG-derived AGE identified here by the antibody was formed as a consequence of oxidative stress, as in diabetic hyperglycemia (33), and only under cytotoxic conditions, L929 cells were treated with the antioxidant BHA. BHA arrests TNF-induced ROS production and cell death (6). The formation of this specific MG-derived AGE in TNF-treated cells, as measured by densitometric analysis of the ECL signal from immunoblots, was reduced by 65% in the presence of BHA (Fig. 5A). The antiglucose metabolite 2-deoxyglucose was then used to determine whether the TNF-induced increase in MG concentration was derived from glycolytic intermediates, which are usually considered to be the main intracellular source of MG. In the presence of 2-deoxyglucose (2:1 ratio to glucose), TNF-induced cell death was inhibited by 65% (data not shown), as was the formation of the specific MG-derived AGE noted in Fig. 5A. The possibility exists that inhibition of AGE formation in the presence of 2-deoxyglucose is an indirect result of the inhibition of mitochondrial ROS, which would mean the latter are derived from increased glycolysis. Indeed, it has been reported that TNF highly increases glycolysis and glucose uptake in L929 cells (35).¶
Taken together, our results clearly indicate that glycolysis plays an important role in TNF-induced necrosis and that TNF-induced mitochondrial ROS, as in diabetic hyperglycemia, can lead to accumulation of MG and subsequent formation of a specific MG-derived AGE. How TNF might activate and modulate glycolysis in tumor cells, and whether accumulation of MG occurs through inhibition of glyceraldehyde-3-phosphate dehydrogenase by ROS (36) or through activation of aldose reductase, both of which can lead to increased levels of MG, remains to be determined.
The TNF-Induced Phosphorylation of GLO1 Is Involved in Formation of the Specific MG-Derived AGE.
Until now, AGE formation has been described as nonenzymatic, irreversible modifications of Lys and Arg residues slowly formed through long-term exposure to high concentrations of sugars and reactive compounds such as MG. Yet in TNF-induced cell death, MG modification of proteins occurs very rapidly (within 1.5–2.5 h of initiating TNF treatment), which suggests that MG modification of specific target molecules could be enzymatically catalyzed by phosphorylated GLO1. To test this hypothesis, L929 cells were pretreated with BBGD and the PKA inhibitor H89, respectively, to determine whether these inhibitors of GLO1 phosphorylation interfere with the formation of MG-derived protein modifications during TNF-induced cell death. In cell cultures pretreated with BBGD and the PKA inhibitor, the formation of the specific MG-derived AGE, which is recognized by mAb6B, as measured by densitometric analysis of the ECL signal from immunoblots, was reduced by 50% and 70%, respectively, after 1.5 h of TNF treatment (Fig. 5B). Inhibition was less pronounced (10% and 20%, respectively) after 2.5 h of TNF treatment, which could be because, at later time points of TNF treatment, MG accumulation is dominant over the lower levels of the phosphorylated form of GLO1 (basal levels of phosphorylated GLO1 are always present in L929 cells; K.V., unpublished observations). With the PKA inhibitor, we cannot exclude the possibility that the inhibition of MG-derived AGE formation is caused by inhibition of the pathway leading to increased MG accumulation. If that were the case, however, we would expect that the strong inhibition of the specific MG-derived AGE formation would last with longer TNF incubations (2.5 h), similar to what we observe with BHA and 2-deoxyglucose. The inhibitory effects with pretreatment of BBGD on TNF-mediated AGE formation and cell death are less pronounced during longer TNF treatments, possibly as a consequence of MG accumulation as described above. In any event, these data indicate that formation of the specific MG-derived AGE during cell death requires the TNF-induced phosphorylation of GLO1.
Discussion
In this article, we report that phosphorylation of glyoxalase I by PKA plays a key role in TNF-induced cell death in L929 cells, that is caspase-independent and characterized by increased production of mitochondrial ROS (reviewed in ref. 3). TNF treatment of L929 cells also leads to a substantial increase in MG concentrations and the consequent formation of a specific MG-derived AGE. This MG-derived AGE is only formed under cytotoxic conditions and as a consequence of oxidative stress. Furthermore, we provide evidence that the TNF-induced phosphorylation of GLO1 is not involved in the detoxification pathway of MG, but rather that phosphorylated GLO1 is involved in a pathway that leads to the formation of this specific MG-derived AGE. Although the real biological function of phosphorylated GLO1 remains to be determined, a possible mechanism could be that it acts as a modifier enzyme for specific target molecules at elevated MG concentrations and thus mediates the cytotoxic effect of MG.
Protein thiols play an important role in cell death (37) and, in particular, in TNF-induced cell death in L929 cells. For example, the divalent thiol-reactive agent diamide (which mimics disulfide bridge formation) is synergistic with TNF-induced cell death in L929 cells, whereas a monovalent thiol-blocking agent (which impedes disulfide bridge formation caused by thiol oxidation) desensitizes the cells for TNF-induced cell death (7). On the basis of these observations, and the fact that the substrate for the nonphosphorylated GLO1 is the hemithioacetal formed between MG and GSH, we propose that phosphorylated GLO1 could convert the normally reversible hemithioacetals that form between MG and protein thiols in specific target molecules to irreversible adducts. This process may include formation of a protein disulfide bridge and a resulting altered activity of the target molecule. The major epitope that is recognized by the anti-AGE monoclonal antibody mAb6B that was used in this study, and that recognizes the specific MG-derived AGE in TNF-treated L929 cells, is argpyrimidine. Argpyrimidine forms when the guanidine moiety of Arg reacts with two MG molecules or, alternatively, with an MG dimer generating the formation of a condensed-ring product (19). The possibility cannot be excluded that the specific MG-derived AGE formed during TNF-induced cell death is of the argpyrimidine type. However, the epitope recognized by mAb6B in our study may also be simply an MG-derived ring structure whose formation between two protein thiol groups is possibly catalyzed by phosphorylated GLO1. If this epitope were catalyzed by phosphorylated GLO1, the spatial localization of these protein thiol groups would be critical and might determine the specificity of the target molecule for modification by MG and the specificity of MG as a signaling molecule. Whatever the case may be, the identity and function of the MG-modified protein that are formed during TNF-induced cell death are still very much open to question and need to be investigated.
The apparent function of phosphorylated GLO1 and MG as regulators of cell death as described in this study may be related to the theories of the Nobel Laureate Albert Szent-Györgyi. He hypothesized almost 40 years ago that the glyoxalase system and MG were major regulators of cell division based on their almost universal occurrence in Nature and the high reactivity of MG with cellular thiols that are involved in cell division (for review, see ref. 10). Whether the MG-derived modifications noted here are irreversible or transient is still unknown. However, it is possible to conceive of a system for the regulation of cellular growth in which some MG-derived protein modifications are transient, leading to temporary activation or inactivation of target proteins, whereas permanent MG modifications of the same or different target molecules may result in cell death. Phosphorylation of yeast (Saccharomyces cerevisiae) GLO1 has been observed during the arrest of cell division at the G1 phase (34).
In conclusion, we show that phosphorylation of GLO1, mediated through the action of PKA, and the subsequent MG-derived modification of a specific target molecule in the cell, are important steps in the signaling processes associated with necrotic cell death. We provide a direct mechanistic connection between MG, the glyoxalase system, and the regulation of cell death. Furthermore, our results suggest an underlying mechanism for several pathophysiological conditions in vivo, namely, that mitochondrial ROS lead to accumulation of MG, and that subsequent MG modification of specific target molecules, either directly or indirectly by phosphorylated GLO1, then result in cell death and tissue damage that are a consequence of these conditions.
Acknowledgments
We thank Prof. P. Thornalley for the generous gift of the glyoxalase I inhibitor BBGD and the anti-glyoxalase I antibody. We thank Prof. K. Uchida for the monoclonal antibody to MG-derived AGEs. This work was supported by the Fonds voor Wetenschappelijk Onderzoek-Vlaanderen, The Interuniversitaire Attractiepolen and the Geconcerteerde Onderzoeks Actie, and the Whitaker Foundation (to F.W.R.C.). F.V.H. is a fellow with the Vlaams Instituut voor de Bevordering van het Wetenschappelijk-technologisch Onderzoek in de Industrie. K.G. and K.V. are postdoctoral researchers with the Fonds voor Wetenschappelijk Onderzoek-Vlaanderen.
Abbreviations
- AGE
advanced glycation end product
- BBGD
S-p-bromobenzylglutathione cyclopentyl diester
- BHA
butylated hydroxyanisole
- CHX
cycloheximide
- ECL
enhanced chemiluminescence
- GLO1
glyoxalase I
- GSH
glutathione
- MG
methylglyoxal
- PI
propidium iodide
- PKA
protein kinase A
- ROS
reactive oxygen species
- TNF
tumor necrosis factor
- 2D
two-dimensional
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Kim, Y. H. & Kim, S. S. (2001) Cancer Detect. Prev. 24, Suppl. 1 (abstr.).
References
- 1.Locksley R M, Killeen N, Lenardo M J. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 2.Tracey K J, Cerami A. Annu Rev Med. 1994;45:491–503. doi: 10.1146/annurev.med.45.1.491. [DOI] [PubMed] [Google Scholar]
- 3.Fiers W, Beyaert R, Declercq W, Vandenabeele P. Oncogene. 1999;18:7719–7730. doi: 10.1038/sj.onc.1203249. [DOI] [PubMed] [Google Scholar]
- 4.Wallach D, Varfolomeev E E, Malinin N L, Goltsev Y V, Kovalenko A V, Boldin M P. Annu Rev Immunol. 1999;17:331–367. doi: 10.1146/annurev.immunol.17.1.331. [DOI] [PubMed] [Google Scholar]
- 5.Schulze-Osthoff K, Bakker A C, Vanhaesebroeck B, Beyaert R, Jacob W A, Fiers W. J Biol Chem. 1992;267:5317–5323. [PubMed] [Google Scholar]
- 6.Goossens V, Grooten J, De Vos K, Fiers W. Proc Natl Acad Sci USA. 1995;92:8115–8119. doi: 10.1073/pnas.92.18.8115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goossens V, De Vos K, Vercammen D, Steemans M, Vancompernolle K, Fiers W, Vandenabeele P, Grooten J. Biofactors. 1999;10:145–156. doi: 10.1002/biof.5520100210. [DOI] [PubMed] [Google Scholar]
- 8.Vancompernolle K, Boonefaes T, Mann M, Fiers W, Grooten J. J Biol Chem. 2000;275:33876–33882. doi: 10.1074/jbc.M004785200. [DOI] [PubMed] [Google Scholar]
- 9.Thornalley P J. Biochem J. 1990;269:1–11. doi: 10.1042/bj2690001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalapos M P. Biochim Biophys Acta. 1999;1426:1–16. doi: 10.1016/s0304-4165(98)00141-x. [DOI] [PubMed] [Google Scholar]
- 11.Thornalley P J. Gen Pharmacol. 1996;27:565–573. doi: 10.1016/0306-3623(95)02054-3. [DOI] [PubMed] [Google Scholar]
- 12.Ranganathan S, Walsh E S, Godwin A K, Tew K D. J Biol Chem. 1993;268:5661–5667. [PubMed] [Google Scholar]
- 13.Rulli A, Carli L, Romani R, Baroni T, Giovannini E, Rosi G, Talesa V. Breast Cancer Res Treat. 2001;66:67–72. doi: 10.1023/a:1010632919129. [DOI] [PubMed] [Google Scholar]
- 14.Davidson S D, Cherry J P, Choudhury M S, Tazaki H, Mallouh C, Konno S. J Urol. 1999;161:690–691. [PubMed] [Google Scholar]
- 15.Sakamoto H, Mashima T, Kizaki A, Dan S, Hashimoto Y, Naito M, Tsuruo T. Blood. 2000;95:3214–3218. [PubMed] [Google Scholar]
- 16.Lo T W, Westwood M E, McLellan A C, Selwood T, Thornalley P J. J Biol Chem. 1994;269:32299–32305. [PubMed] [Google Scholar]
- 17.Chaplen F W, Fahl W E, Cameron D C. Proc Natl Acad Sci USA. 1998;95:5533–5538. doi: 10.1073/pnas.95.10.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Westwood M E, Thornalley P J. J Protein Chem. 1995;14:359–372. doi: 10.1007/BF01886793. [DOI] [PubMed] [Google Scholar]
- 19.Oya T, Hattori N, Mizuno Y, Miyata S, Maeda S, Osawa T, Uchida K. J Biol Chem. 1999;274:18492–18502. doi: 10.1074/jbc.274.26.18492. [DOI] [PubMed] [Google Scholar]
- 20.Corman B, Duriez M, Poitevin P, Heudes D, Bruneval P, Tedgui A, Levy B I. Proc Natl Acad Sci USA. 1998;95:1301–1306. doi: 10.1073/pnas.95.3.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brownlee M. Annu Rev Med. 1995;46:223–234. doi: 10.1146/annurev.med.46.1.223. [DOI] [PubMed] [Google Scholar]
- 22.Grooten J, Goossens V, Vanhaesebroeck B, Fiers W. Cytokine. 1993;5:546–555. doi: 10.1016/s1043-4666(05)80003-1. [DOI] [PubMed] [Google Scholar]
- 23.Gevaert K, De Mol H, Verschelde J L, Van Damme J, De Boeck S, Vandekerckhove J. J Protein Chem. 1997;16:335–342. doi: 10.1023/a:1026320318489. [DOI] [PubMed] [Google Scholar]
- 24.Gevaert K, Demol H, Sklyarova T, Vandekerckhove J, Houthaeve T. Electrophoresis. 1998;19:909–917. doi: 10.1002/elps.1150190606. [DOI] [PubMed] [Google Scholar]
- 25.Spengler B, Kirsch D, Kaufmann R, Jaeger E. Rapid Commun Mass Spectrom. 1992;6:105–108. doi: 10.1002/rcm.1290060207. [DOI] [PubMed] [Google Scholar]
- 26.Oray B, Norton S J. Methods Enzymol. 1982;90:542–546. doi: 10.1016/s0076-6879(82)90182-3. [DOI] [PubMed] [Google Scholar]
- 27.McLellan A C, Phillips S A, Thornalley P J. Anal Biochem. 1992;206:12–16. doi: 10.1016/s0003-2697(05)80004-1. [DOI] [PubMed] [Google Scholar]
- 28.Chaplen F W, Fahl W E, Cameron D C. Anal Biochem. 1996;238:171–178. doi: 10.1006/abio.1996.0271. [DOI] [PubMed] [Google Scholar]
- 29.Guy G R, Cao X, Chua S P, Tan Y H. J Biol Chem. 1992;267:1846–1852. [PubMed] [Google Scholar]
- 30.Thornalley P J, Edwards L G, Kang Y, Wyatt C, Davies N, Ladan M J, Double J. Biochem Pharmacol. 1996;51:1365–1372. doi: 10.1016/0006-2952(96)00059-7. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y H, Lin J X, Yip Y K, Vilcek J. Proc Natl Acad Sci USA. 1988;85:6802–6805. doi: 10.1073/pnas.85.18.6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vince R, Wadd W B. Biochem Biophys Res Commun. 1969;34:593–598. doi: 10.1016/0006-291x(69)90445-8. [DOI] [PubMed] [Google Scholar]
- 33.Nishikawa T, Edelstein D, Du X L, Yamagishi S, Matsumura T, Kaneda Y, Yorek M A, Beebe D, Oates P J, Hammes H P, et al. Nature (London) 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 34.Inoue Y, Choi B Y, Murata K, Kimura A. J Biochem (Tokyo) 1990;108:4–6. doi: 10.1093/oxfordjournals.jbchem.a123159. [DOI] [PubMed] [Google Scholar]
- 35.Matthews N. Br J Cancer. 1983;48:405–410. doi: 10.1038/bjc.1983.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knight R J, Kofoed K F, Schelbert H R, Buxton D B. Cardiovasc Res. 1996;32:1016–1023. doi: 10.1016/s0008-6363(96)00137-x. [DOI] [PubMed] [Google Scholar]
- 37.Marchetti P, Decaudin D, Macho A, Zamzami N, Hirsch T, Susin S A, Kroemer G. Eur J Immunol. 1997;27:289–296. doi: 10.1002/eji.1830270142. [DOI] [PubMed] [Google Scholar]