Abstract
PURPOSE:
Report primary analysis results from the mantle cell lymphoma (MCL) cohort of the phase I seamless design TRANSCEND NHL 001 (NCT02631044) study.
PATIENTS AND METHODS:
Patients with relapsed/refractory MCL after ≥2 lines of prior therapy, including Bruton tyrosine kinase inhibitor (BTKi), alkylating agent, and CD20-targeted agent, received lisocabtagene maraleucel (liso-cel) at a target dose level of 50×106 (DL1) or 100×106 (DL2) CAR+ T cells. Primary endpoints were adverse events, dose-limiting toxicities, and objective response rate (ORR) by independent review committee per Lugano criteria.
RESULTS:
Of 104 leukapheresed patients, liso-cel was infused into 88. Median (range) number of prior lines of therapy was 3 (1–11) with 30% receiving ≥5 prior lines of therapy, 73% of patients were aged ≥65 years, 69% had refractory disease, 53% had BTKi refractory disease, 23% had TP53 mutation, and 8% had secondary central nervous system lymphoma. Median (range) on-study follow-up was 16.1 months (0.4–60.5). In the efficacy set (n=83; DL1+DL2), ORR was 83.1% (95% CI, 73.3%–90.5%) and complete response (CR) rate was 72.3% (95% CI, 61.4%–81.6%). Median duration of response was 15.7 months (95% CI, 6.2–24.0) and progression-free survival was 15.3 months (95% CI, 6.6–24.9). Most common grade ≥3 treatment-emergent adverse events were neutropenia (56%), anemia (37.5%) and thrombocytopenia (25%). Cytokine release syndrome (CRS) was reported in 61% of patients (grade 3/4, 1%; grade 5, 0), neurological events (NE) in 31% (grade 3/4, 9%; grade 5, 0), grade ≥3 infections in 15%, and prolonged cytopenia in 40%.
CONCLUSION:
Liso-cel demonstrated high CR rate and deep, durable responses with low incidence of grade ≥3 CRS, NE, and infections in patients with heavily pretreated relapsed/refractory MCL, including those with high-risk, aggressive disease.
INTRODUCTION
Patients with mantle cell lymphoma (MCL) who experience disease progression after treatment with covalent Bruton tyrosine kinase inhibitors (BTKi) have historically poor outcomes with subsequent therapy, including conventional chemotherapy (objective response rates [ORR], ~30%; median overall survival [OS], 6–10 months). 1–3 Recent studies have demonstrated improved outcomes after treatment with the chimeric antigen receptor (CAR) T-cell therapy brexucabtagene autoleucel; however, treatment-related toxicity is high. 4 As outcomes decline with successive lines of therapy, 5 a continued unmet need exists for treatments that achieve deep (ie, high complete response [CR] rates) and durable responses with a favorable safety profile in patients with relapsed/refractory (R/R) MCL, including high-risk, aggressive disease.
Lisocabtagene maraleucel (liso-cel) is an autologous, CD19-directed, 4-1BB CAR T-cell product composed of CD8+ and CD4+ CAR+ T cells that has demonstrated rapid and durable efficacy with low rates of severe cytokine release syndrome (CRS) and neurological events (NE) across multiple R/R B-cell malignancies. 6–11
Here, we report primary analysis results from the MCL cohort of TRANSCEND NHL 001 (TRANSCEND; NCT02631044).
METHODS
Study Design and Participants
TRANSCEND is a phase I, open-label, multicenter, multicohort, seamless design study evaluating the safety, antitumor activity, and cellular kinetics of liso-cel in adult patients with R/R B-cell non-Hodgkin lymphoma. 6 The MCL cohort of TRANSCEND (TRANSCEND-MCL) enrolled adults (age ≥18 years) with positron emission tomography (PET)-positive MCL per Lugano 2014 critera, 12 with diagnosis confirmed with cyclin D1 expression or evidence of t(11;14) by cytogenetics, fluorescence in situ hybridization, or polymerase chain reaction. Eligible patients had R/R disease after ≥2 prior lines of therapy, including a BTKi, alkylating agent, and CD20-targeted agent. Patients who had moderate renal and cardiac dysfunction, secondary central nervous system (CNS) lymphoma, or received prior autologous or allogeneic hematopoietic stem cell transplantation (HSCT) were eligible. Full eligibility criteria are available in the Data Supplement.
Procedures
Patients underwent leukapheresis for manufacture of liso-cel. Bridging chemotherapy was allowed during liso-cel manufacturing for disease control at the investigator’s discretion; reconfirmation of PET-positive disease by investigator assessment was required before receiving lymphodepleting chemotherapy (fludarabine 30 mg/m2 and cyclophosphamide 300 mg/m2 intravenously daily for 3 days). Liso-cel was administered 2–7 days later as two sequential intravenous infusions of CD8+ and CD4+ CAR+ T cells at a total target dose of 50×106 CAR+ T cells (dose level [DL] 1) or 100×106 CAR+ T cells (DL2). TRANSCEND-MCL followed the seamless design principle, consisting of dose-finding, dose-expansion, and dose-confirmation phases. 13,14 For the purpose of dose-finding decisions, DLs were assessed for safety based on probability of dose-limiting toxicities (DLT) and activity based on probability of CR per investigator assessment in patients in dose-finding and dose-expansion phases. The recommended dose for TRANSCEND-MCL (DL2) was administered during dose-confirmation. Additional details are available in the Data Supplement. Patients who achieved a CR after liso-cel infusion and subsequently had progressive disease could receive retreatment with liso-cel. Outpatient administration of liso-cel was allowed at the investigator’s discretion.
Endpoints and Assessments
Primary endpoints included frequency and severity of adverse events (AE), probability of DLTs, and ORR, defined as the proportion of patients who achieved a best overall response of CR or partial response (PR). The key secondary endpoint was CR rate; additional secondary endpoints included duration of response (DOR), progression-free survival (PFS), OS, cellular kinetic parameters, health-related quality of life (QOL), and hospitalizations. Response was evaluated by PET/computed tomography per Lugano 2014 criteria12 based on independent review committee (IRC) assessment. Analyses in prespecified patient subgroups were performed for the primary and secondary efficacy endpoints.
Disease status and survival were assessed at visits approximately 29, 60, 90, 180, 270, 365, 545, and 730 (end of study) days after liso-cel infusion. Survival was assessed after day 730 in a long-term follow-up study (NCT03435796; Data Supplement). To assess the impact of the COVID-19 pandemic, post hoc sensitivity analyses were conducted for time-to-event endpoints censoring for patients who died because of COVID-19 (Data Supplement). 15–17
AEs, including NEs, were graded per the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03, except for CRS, which was graded using the Lee 2014 criteria. 18 The treatment-emergent AE (TEAE) period was defined as the time from initiation of liso-cel administration through and including 90 days after liso-cel administration. NEs were defined as investigator-identified neurological AEs related to liso-cel.
Additional information on endpoints and assessments for safety, patient-reported outcomes (PRO), cellular kinetics, and pharmacodynamics are summarized in the Data Supplement.
Statistical Analysis
The prespecified analysis sets are described in Supplementary Table A1. All analysis sets included patients across dose-finding, dose-expansion, and dose-confirmation phases except the DLT-evaluable set, which included patients across dose-finding and dose-expansion phases only. Efficacy outcomes are reported for the leukapheresed (intent to treat) set, the efficacy set (patients with PET-positive disease per IRC who received liso-cel at DL1+DL2), and the primary analysis set (PAS; patients in the efficacy set who received liso-cel at DL2 and received ≥2 prior lines of systemic therapy, including an alkylating agent, BTKi, and CD20-targeted agent (Supplementary Table A1). AEs are reported for the liso-cel–treated set. DLTs are reported in the DLT-evaluable set.
Primary (ORR) and key secondary (CR rate) endpoint hypothesis testing was based on hierarchical procedure (Data Supplement p14) and conducted on the PAS. Time-to-event endpoints were summarized with medians and 95% confidence intervals (CI) using the Kaplan-Meier method.
RESULTS
Patients
Between March 28, 2016, and February 10, 2022, 104 patients were enrolled in TRANSCEND-MCL and underwent leukapheresis. Of those, 92 received CAR T cells (9 patients died before infusion and 3 no longer met eligibility criteria). Eighty-eight patients received liso-cel (liso-cel–treated set) at 13 study sites in the United States (Data Supplement) and 4 received nonconforming product (Fig 1). Median time from leukapheresis to liso-cel availability and liso-cel infusion was 24.5 days (range, 17–80) and 39 days (range, 28–489), respectively. Six patients received DL1 and 82 received DL2 at a median dose of 49.9 (range, 46–54) and 99.6 (range, 62–103) ×106 CAR+ T cells, respectively. At data cutoff (January 19, 2023), median on-study follow-up was 16.1 months (range, 0.4–60.5).
Fig 1.
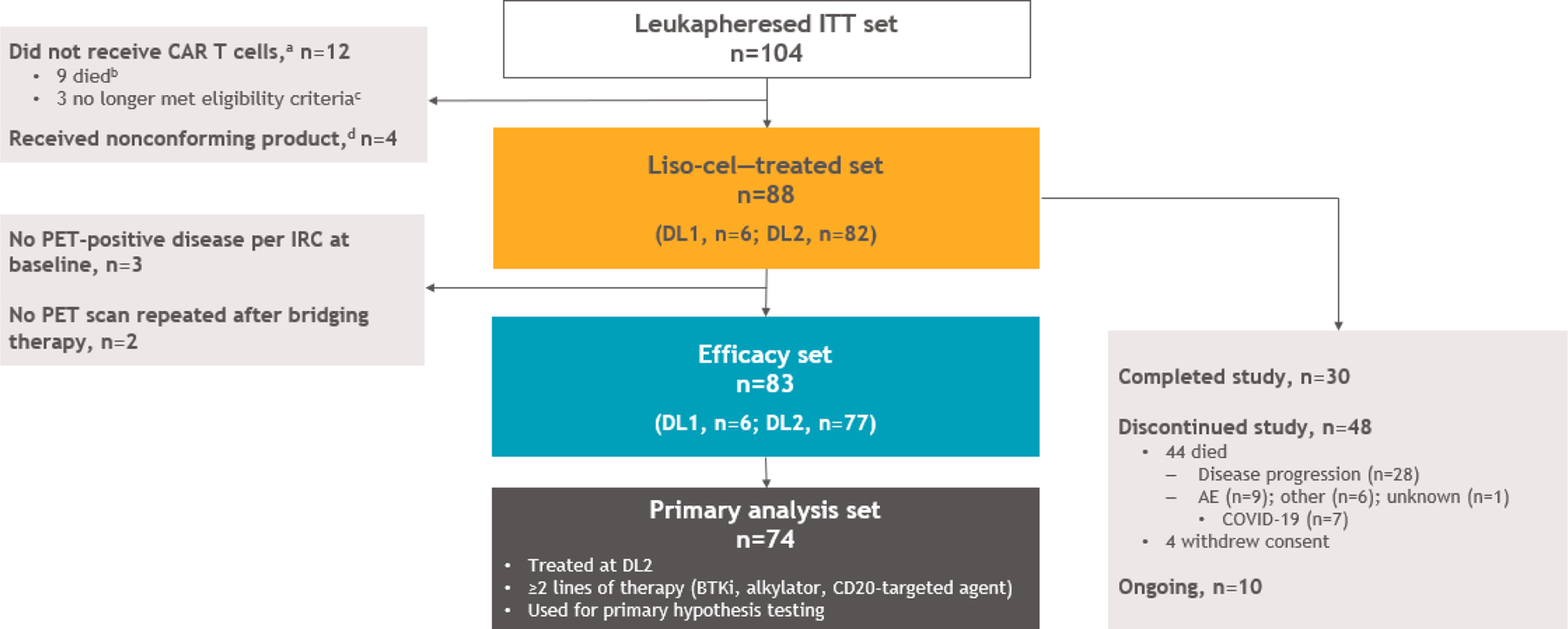
Patient disposition and analysis sets (CONSORT). aNonconforming product was manufactured for 2 patients who did not receive CAR T cells due to death (n=1) and no longer meeting eligibility criteria (n=1). bSeven patients died because of disease progression, 1 because of an AE, and 1 because of other reasons (sepsis and pneumonia). cOne patient was ineligible due to second primary malignancy before LDC and 2 patients remained in ongoing CR after receipt of bridging therapy. dDefined as any product wherein one of the CD8 or CD4 cell components did not meet one of the requirements to be considered liso-cel but was considered appropriate for infusion. AE, adverse event; BTKi, Bruton tyrosine kinase inhibitor; CAR, chimeric antigen receptor; CR, complete response; DL, dose level; IRC, independent review committee; ITT, intent to treat; LDC, lymphodepleting chemotherapy; liso-cel, lisocabtagene maraleucel; PET, positron emission tomography.
Median age was 68.5 years (range, 36–86; age ≥75 years, n=18 [20%]; 66 (75%) patients had Ki-67 proliferation index ≥30%; 27 (31%) had blastoid morphology; 20 (23%) had TP53 mutation; and 7 (8%) had secondary CNS lymphoma at baseline (Table 1; Supplementary Table A2). Median number of prior lines of systemic therapy was 3 (range, 1–11) with 26 (30%) patients having received ≥5 and 29 (33%) previously receiving HSCT. Sixty-one (69%) patients had refractory disease; 47 (53%) had disease refractory to prior BTKi therapy and 36 (41%) had disease that progressed during or after receiving BTKi therapy. Fifty-eight (66%) patients received bridging therapy during liso-cel manufacturing.
Table 1.
Demographics and Baseline Characteristics (liso-cel–Treated Set)
| Liso-cel–Treated Set (N=88) | |
|---|---|
| Age, years | |
| Median (range) | 68.5 (36–86) |
| ≥65, No. (%) | 64 (73) |
| ≥75, No. (%) | 18 (20) |
|
| |
| Male, No. (%) | 67 (76) |
|
| |
| Race, No. (%) | |
| White | 77 (87.5) |
| Other | 8 (9) |
| Unknown | 3 (3) |
|
| |
| Ethnicity, No. (%) | |
| Hispanic or Latino | 4 (5) |
| Not Hispanic or Latino | 81 (92) |
| Unknown | 3 (3) |
|
| |
| ECOG PS at screening, No. (%) | |
| 0 | 48 (55) |
| 1 | 40 (45) |
|
| |
| sMIPI score, No. (%)a | |
| Low risk (0–3) | 36 (41) |
| Intermediate risk (4–5) | 44 (50) |
| High risk (≥6) | 8 (9) |
|
| |
| LDH before LDC, U/L | |
| Median (range) | 233.5 (78–4651) |
| ≥ULN U/L, No. (%) | 39 (44) |
|
| |
| SPD per IRC before LDC, cm2 | |
| Median (range) | 13.9 (0.7–93.8) |
| ≥Median cm2, No. (%) | 38 (43) |
|
| |
| CrCl before LDC, mL/min | |
| Median (range) | 79.7 (39.9–195.7) |
| ≥60, No. (%)b | 68 (78) |
|
| |
| LVEF at screening, % | |
| Median (range) | 60 (45–88) |
| ≥40% to <50%, No. (%) | 5 (6) |
|
| |
| Ki-67 proliferation fraction, % | |
| Median (range) | 60 (5–95) |
| ≥30, No. (%) | 66 (75) |
|
| |
| TP53 mutation, No. (%) | |
| Yes | 20 (23) |
| No | 34 (39) |
| Indeterminate | 4 (5) |
| Not done | 30 (34) |
|
| |
| Blastoid morphology, No. (%) | |
| Yes | 27 (31) |
| No | 48 (55) |
| Not done | 13 (15) |
|
| |
| Complex karyotype, No. (%) | |
| Yes | 26 (30) |
| No | 35 (40) |
| Indeterminate | 4 (5) |
| Not done | 23 (26) |
|
| |
| Median (range) prior lines of systemic therapyc | 3 (1–11) |
| 1 prior line of systemic therapy, No. (%)d | 3 (3) |
| 2 prior lines of systemic therapy, No. (%) | 28 (32) |
| 3 prior lines of systemic therapy, No. (%) | 19 (22) |
| 4 prior lines of systemic therapy, No. (%) | 12 (14) |
| ≥5 prior lines of systemic therapy, No. (%) | 26 (30) |
|
| |
| Prior HSCT, No. (%) | 29 (33) |
| Allogeneic | 6 (7) |
| Autologous | 26 (30) |
|
| |
| Prior BTKi, No. (%)d | 83 (94) |
| Prior ibrutinib | 65 (74) |
| Prior acalabrutinib | 29 (33) |
| Prior zanubrutinib | 2 (2) |
| Prior pirtobrutinib/loxo-305 | 6 (7) |
|
| |
| Prior venetoclax, No. (%) | 23 (26) |
|
| |
| Prior alkylating agent, No. (%) | 88 (100) |
|
| |
| Prior bendamustine, No. (%) | 55 (62.5) |
|
| |
| Refractory or relapsed disease, No. (%)e | |
| Refractory | 61 (69) |
| Relapsed | 27 (31) |
|
| |
| Disease refractory to BTKi, No. (%)f | 47 (53) |
|
| |
| Secondary CNS lymphoma at liso-cel infusion, No. (%) | 7 (8) |
|
| |
| Received bridging therapy, No. (%) | 58 (66) |
| Systemic treatment only | 41 (71) |
| Radiotherapy only | 3 (5) |
| Both | 14 (24) |
NOTE. All percentages are rounded to whole numbers except those with “.5%.”
Abbreviations: BTKi, Bruton tyrosine kinase inhibitor; CNS, central nervous system; CR, complete response; CrCl, creatinine clearance; ECOG PS, Eastern Cooperative Oncology Group performance status; HSCT, hematopoietic stem cell transplantation; IRC, independent review committee; LDC, lymphodepleting chemotherapy; LDH, lactate dehydrogenase; liso-cel, lisocabtagene maraleucel; LVEF, left ventricular ejection fraction; MCL, mantle cell lymphoma; PD, progressive disease; PR, partial response; SD, stable disease; sMIPI, simplified mantle cell lymphoma International Prognostic Index; SPD, sum of the product of perpendicular diameters; TP53, tumor protein 53; ULN, upper limit of normal.
Score used in patients with MCL to assess risk on the basis of age, ECOG PS, LDH, and white blood cells.
Percentages are based on the number of patients with non-missing results.
Bridging anticancer therapy for disease control was not counted as a prior systemic regimen unless the outcome was CR.
Three patients received 1 prior line of therapy and 5 did not receive prior treatment with a BTKi. The original study protocol enrolled patients with ≥1 prior lines of systemic treatment and the protocol was later amended to require ≥2 previous lines of systemic treatment, including a BTKi, an alkylating agent, and anti-CD20 agent.
Relapsed versus refractory disease was defined as a best response of CR versus a best response of PR, SD, or PD to the last systemic treatment or HSCT with curative intent.
Any response to BTKi less than PR.
Efficacy
The primary and key secondary endpoints of ORR and CR rate in the PAS (n=74) were met at 86.5% (n=64; 95% CI, 76.5–93.3; P<.0001) and 74.3% (n=55; 95% CI, 62.8–83.8; P<.0001), respectively. Response rates in the efficacy set (n=83) were consistent with an ORR of 83.1% (n=69; 95% CI, 73.3–90.5) and CR rate of 72.3% (n=60; 95% CI, 61.4–81.6). Median time to first CR or PR was 0.95 months (range, 0.7–3.0). In the efficacy set, ORR and CR rates were consistent across prespecified patient subgroups, including those with high-risk disease such as TP53 mutation, secondary CNS lymphoma, and blastoid morphology (Fig 2A–2B). Median DOR was 15.7 months (95% CI, 6.2–24.0) after a median follow-up of 22.8 months (95% CI, 16.7–23.0) (Fig 3A; Supplementary Fig A1A). DOR in patients with Ki-67 ≥30%, TP53 mutation, and blastoid morphology are shown in Supplementary Fig A2. Median PFS was 15.3 months (95% CI, 6.6–24.9) after a median follow-up of 23.5 months (95% CI, 17.7–23.8) (Fig 3B; Supplementary Fig A1B), and median OS was 18.2 months (95% CI, 12.9–36.3) after a median follow-up of 24.0 months (95% CI, 23.7–24.2) (Fig 3C; Supplementary Fig A1C). In patients who achieved CR, median OS was 36.3 months (95% CI, 15.7–NR). In the leukapheresed set (n=104), ORR was 70.2% (95% CI, 60.4–78.8) and CR rate was 61.5% (95% CI, 51.5–70.9). Efficacy outcomes in all efficacy-assessed sets are shown in Supplementary Tables A3–A4.
Fig 2.
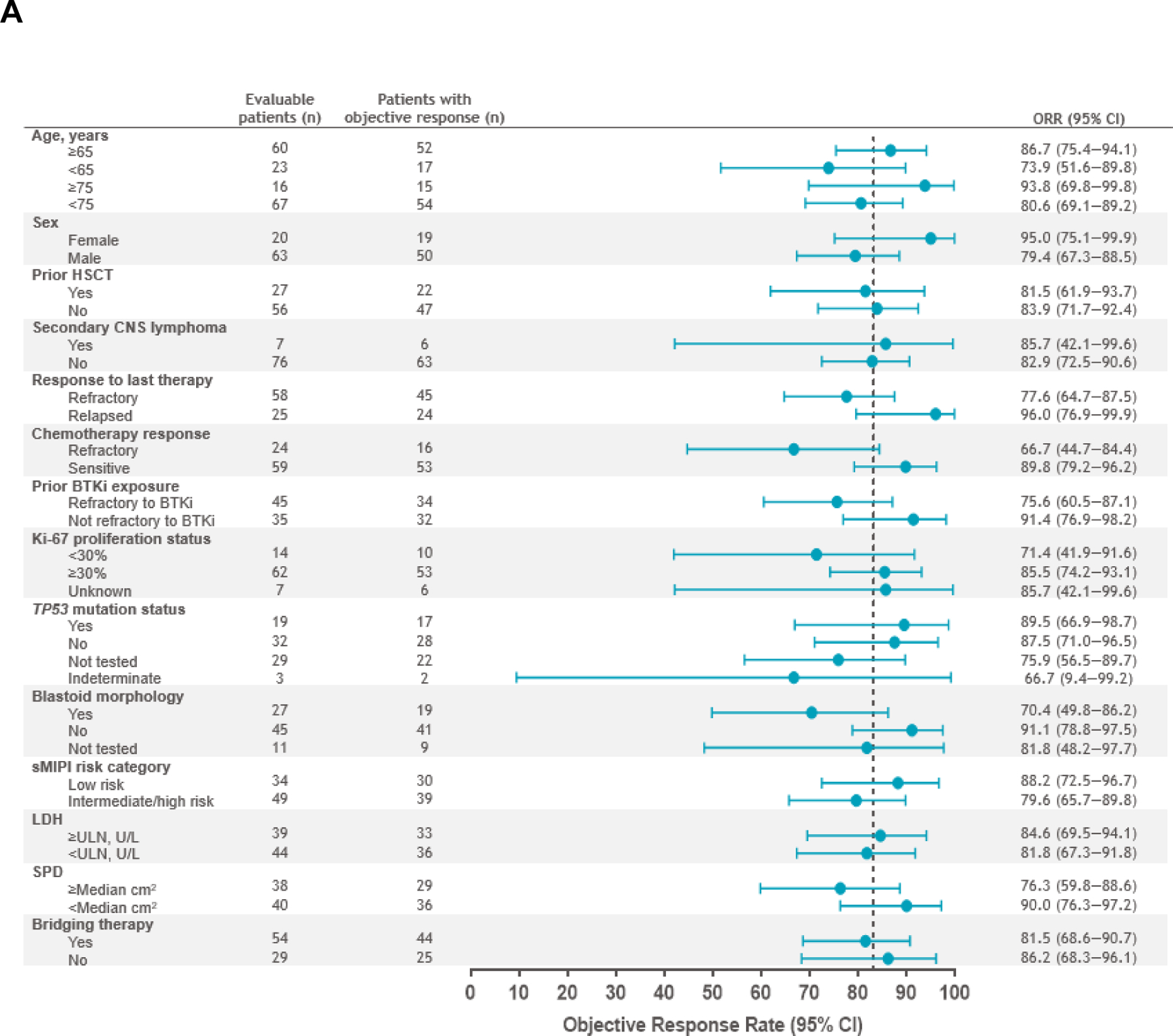
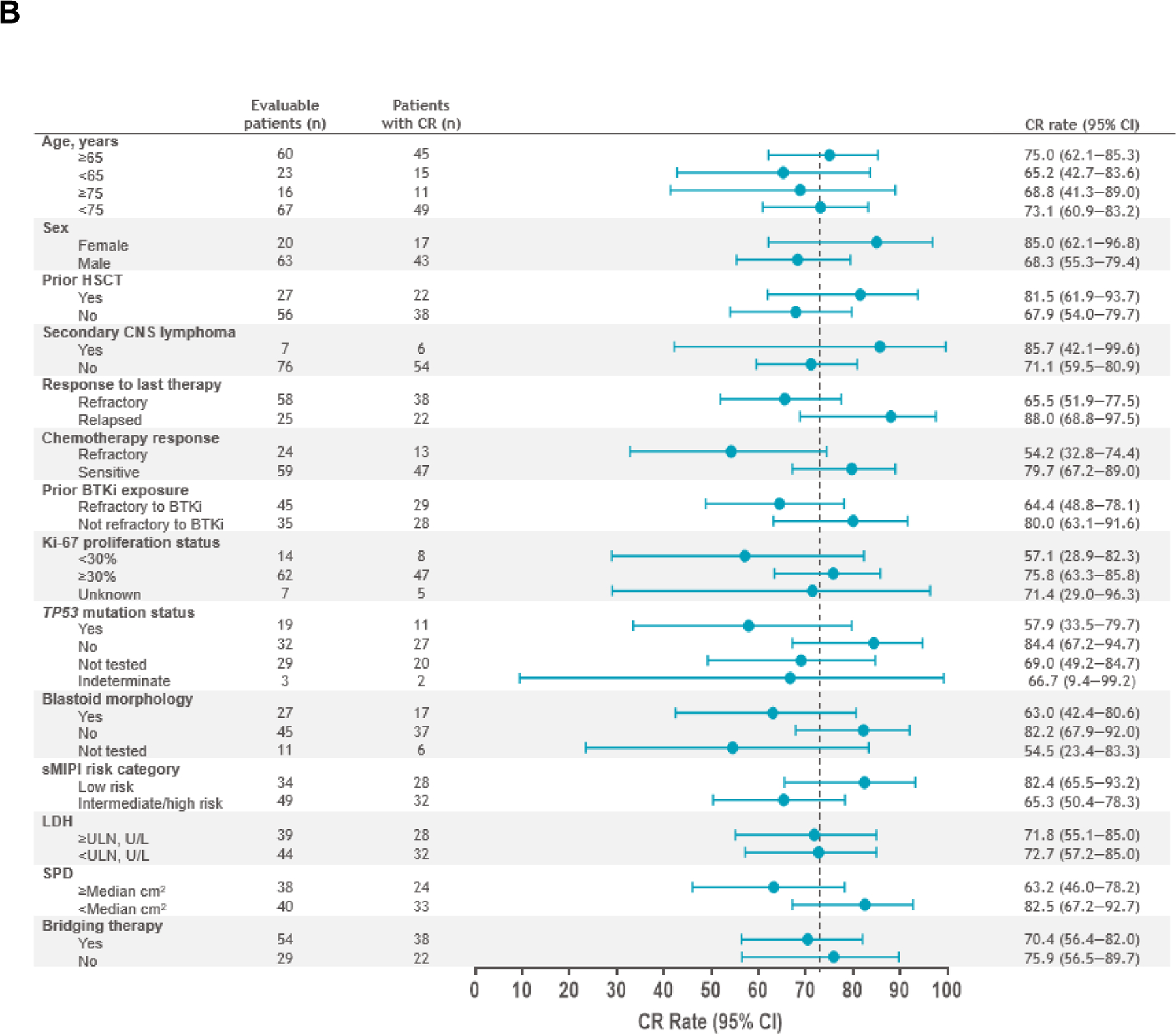
Forest plots of ORR (A) and CR rate (B) per IRC by prespecified subgroups (efficacy set). Response was evaluated by PET/computed tomography according to the Lugano 2014 criteria12 based on IRC assessment. ORR was defined as the proportion of patients who achieved a best response of CR or PR from the time of liso-cel infusion until disease progression, end of study, the start of another anticancer therapy, or HSCT. ORR and two-sided 95% exact Clopper-Pearson CI are shown in panel A. CR rate was defined as the proportion of patients who achieved a best response of CR from the time of liso-cel infusion until disease progression, end of study, the start of another anticancer therapy, or HSCT. CR rate and two-sided 95% exact Clopper-Pearson CI are shown in panel B. BTKi, Bruton tyrosine kinase inhibitor; CI, confidence interval; CNS, central nervous system; CR, complete response; HSCT, hematopoietic stem cell transplantation; IRC, independent review committee; LDH, lactate dehydrogenase; liso-cel, lisocabtagene maraleucel; ORR, objective response rate; PET, positron emission tomography; PR, partial response; sMIPI, simplified mantle cell lymphoma International Prognostic Index; SPD, sum of the product of perpendicular diameters; TP53, tumor protein 53.
Fig 3.
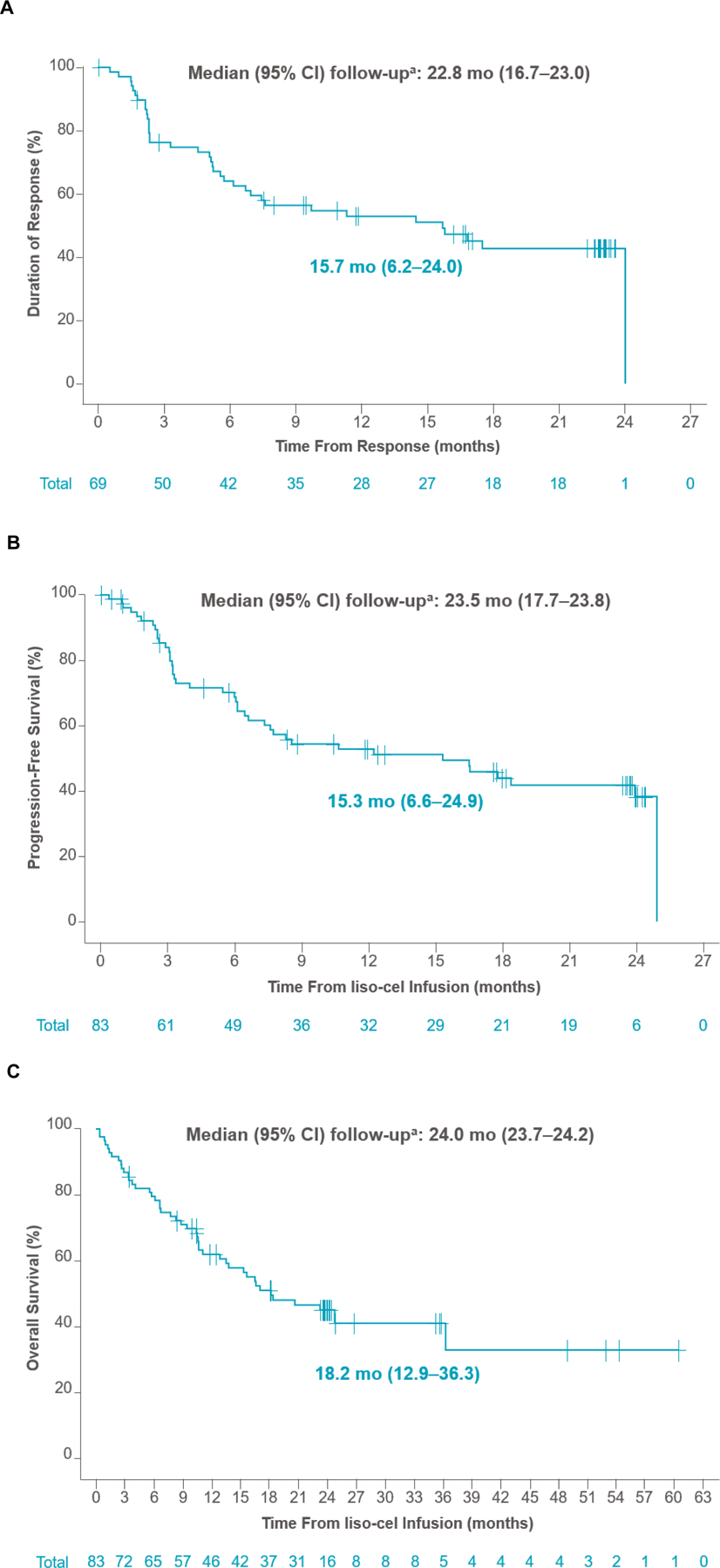
Kaplan-Meier curves of DOR (A), PFS (B), and OS (C) (efficacy set). aReverse Kaplan-Meier method was used to obtain median follow-up and its 95% CI. DOR was defined as the time from first response to progressive disease or death; PFS was defined as the time from liso-cel infusion to progressive disease or death; OS was defined as the time from liso-cel infusion to death. CI, confidence interval; DOR, duration of response; liso-cel, lisocabtagene maraleucel; OS, overall survival; PFS, progression-free survival.
Most patients (75%) were treated during the COVID-19 pandemic; 7 patients died because of COVID-19, including 6 who were in ongoing CR. In the COVID-19 sensitivity analyses (efficacy set, n=83), median DOR was 17.5 months (95% CI, 7.6–24.0), median PFS was 17.8 months (95% CI, 7.6–24.9), and median OS was 24.8 months (95% CI, 15.7–not reached) (Table 2; Supplementary Table A5).
Table 2.
Sensitivity Analyses of Time-to-Event Endpoints Censored for Patients Who Died Because of COVID-19 While in Ongoing CR (Efficacy Set)
| Efficacy Set (n=83) | COVID-19 Sensitivity Analysis in the Efficacy Seta (n=83) | |
|---|---|---|
| DOR | ||
| Median (95% CI),b months | 15.7 (6.2–24.0) | 17.5 (7.6–24.0) |
| Continued response at 12 months, % (95% CI) | 52.9 (40.1–64.2) | 60.4 (47.1–71.4) |
| Continued response at 18 months, % (95% CI) | 42.7 (29.9–54.9) | 48.8 (34.8–61.4) |
| DOR follow-up, median (95% CI),c months | 22.8 (16.7–23.0) | 22.6 (16.2–22.8) |
|
| ||
| PFS | ||
| Median (95% CI),b months | 15.3 (6.6–24.9) | 17.8 (7.6–24.9) |
| PFS rate at 12 months, % (95% CI) | 52.8 (40.6–63.6) | 60.0 (47.5–70.5) |
| PFS rate at 18 months, % (95% CI) | 43.9 (31.8–55.4) | 49.9 (36.9–61.7) |
| PFS follow-up, median (95% CI),c months | 23.5 (17.7–23.8) | 18.2 (12.4–23.7) |
|
| ||
| OS | ||
| Median (95% CI),b months | 18.2 (12.9–36.3) | 24.8 (15.7–NR) |
| OS rate at 12 months, % (95% CI) | 61.8 (50.2–71.4) | 68.2 (56.5–77.3) |
| OS rate at 18 months, % (95% CI) | 50.8 (39.2–61.2) | 56.0 (43.9–66.6) |
| OS follow-up, median (95% CI),c months | 24.0 (23.7–24.2) | 23.8 (23.6–24.2) |
Abbreviations: CI, confidence interval; CR, complete response; DOR, duration of response; NR, not reached; OS, overall survival; PFS, progression-free survival.
Patients in ongoing response who died because of COVID-19 were censored in these analyses.
Kaplan-Meier method was used to obtain two-sided 95% CIs.
Reverse Kaplan-Meier method was used to obtain the median follow-up and its 95% CI.
Safety
Seventy-six (86%) patients experienced grade ≥3 TEAEs (Table 3; Supplentary Tables A6–A7), most commonly cytopenias. Forty-seven (53%) patients had serious TEAEs (Supplementary Table A8); the only serious TEAE to occur in >5 patients was CRS (n=21 [24%]). Thirty-four (39%) patients had serious TEAEs considered related to liso-cel.
Table 3.
Treatment-Emergent Adverse Events (liso-cel–Treated Set)
| Liso-cel–Treated Set (N=88) |
||
|---|---|---|
| Any Grade | Grade ≥3 | |
|
| ||
| Any TEAE,a No. (%) | 88 (100) | 76 (86) |
| Most common TEAEs (≥15%), No. (%) | ||
| CRS | 54 (61) | 1 (1) |
| Neutropenia | 52 (59) | 49 (56) |
| Anemia | 39 (44) | 33 (37.5) |
| Fatigue | 31 (35) | 2 (2) |
| Thrombocytopenia | 26 (30) | 22 (25) |
| Hypokalemia | 21 (24) | 7 (8) |
| Headache | 20 (23) | 0 |
| Decreased appetite | 18 (20) | 4 (5) |
| Nausea | 16 (18) | 2 (2) |
| Diarrhea | 15 (17) | 0 |
| Hypophosphatemia | 15 (17) | 8 (9) |
| Peripheral edema | 15 (17) | 1 (1) |
| Pyrexia | 15 (17) | 0 |
| Confusional state | 14 (16) | 2 (2) |
NOTE. All percentages are rounded to whole numbers except those with “.5%.”
Abbreviations: CRS, cytokine release syndrome; liso-cel, lisocabtagene maraleucel; TEAE, treatment-emergent adverse event.
TEAE period was defined as the time from initiation of liso-cel administration through study day 90. Adverse events occurring after the initiation of a subsequent therapy or liso-cel retreatment were not considered TEAEs.
TEAEs of special interest are summarized in Table 4 and Supplementary Table A9. Any-grade CRS was reported in 54 (61%) patients; there was 1 (1%) grade 4 event and no grade 3 or 5 CRS events (Supplementary Table A10). Median time to onset and resolution of CRS was 4.0 days (range, 1–10) and 4.0 days (range, 1–14), respectively. Any-grade NEs were reported in 27 (31%) patients (Supplementary Table A11). Seven (8%) patients had grade 3 NEs and 1 (1%) had grade 4 NE. No grade 5 NEs occurred. Median time to onset and resolution of NEs was 8.0 days (range, 1–25) and 5.0 days (range, 1–45), respectively. For CRS and/or NE management, 11 (12.5%) patients received tocilizumab only, 6 (7%) received corticosteroids only, and 12 (14%) received both tocilizumab and corticosteroids. No macrophage activation syndrome was reported. Grade ≥3 infections occurred in 13 (15%) patients and prolonged cytopenias (grade ≥3 at day 29) in 35 (40%) patients. Of patients with prolonged cytopenia and laboratory results after day 29, 4/4 (100%) with anemia, 18/21 (86%) with neutropenia, and 22/27 (81%) with thrombocytopenia had recovered to grade ≤2 within 90 days after liso-cel infusion. Tumor lysis syndrome (TLS) was reported in 2 (2%) patients and hypogammaglobulinemia in 6 (7%) patients. Three (3%) patients had second primary malignancies of pancreatic cancer, basal cell carcinoma of the skin, and squamous cell carcinoma of the skin (n=1 each).
Table 4.
Treatment-Emergent Adverse Events of Special Interest (liso-cel–Treated Set)
| Liso-cel–Treated Set (N=88) | |
|---|---|
| CRSa | |
| Any grade, No. (%) | 54 (61) |
| Grade 1/2 | 53 (60) |
| Grade 3 | 0 |
| Grade 4 | 1 (1) |
| Grade 5 | 0 |
| Median (range) time to onset, days | 4.0 (1–10) |
| Median (range) time to resolution, days | 4.0 (1–14) |
|
| |
| NEsb | |
| Any grade, No. (%) | 27 (31) |
| Grade 1/2 | 19 (22) |
| Grade 3 | 7 (8) |
| Grade 4 | 1 (1) |
| Grade 5 | 0 |
| Median (range) time to onset, days | 8.0 (1–25) |
| Median (range) time to resolution, days | 5.0 (1–45) |
|
| |
| Tocilizumab and/or corticosteroid use for CRS and/or NEs,c No. (%) | |
| Tocilizumab and/or corticosteroids | 29 (33) |
| Tocilizumab only | 11 (12.5) |
| Corticosteroids only | 6 (7) |
| Both tocilizumab and corticosteroids | 12 (14) |
|
| |
| Tocilizumab and/or corticosteroid use for CRS,d No. (%) | |
| Tocilizumab and/or corticosteroids | 24 (27) |
| Tocilizumab only | 15 (17) |
| Corticosteroids only | 1 (1) |
| Both tocilizumab and corticosteroids | 8 (9) |
|
| |
| Tocilizumab and/or corticosteroid use for NEs,e No. (%) | |
| Tocilizumab and/or corticosteroids | 15 (17) |
| Tocilizumab only | 1 (1) |
| Corticosteroids only | 14 (16) |
| Both tocilizumab and corticosteroids | 0 |
|
| |
| Other AESIs, No. (%) | |
| Grade ≥3 infections | 13 (15) |
| Hypogammaglobulinemia | 6 (7) |
| Tumor lysis syndrome | 2 (2) |
| Second primary malignancyf | 3 (3) |
| Macrophage activation syndrome | 0 |
| Infusion-related reaction | 2 (2) |
| Autoimmune disorders | 0 |
| Prolonged cytopeniag | 35 (40) |
| Grade ≥3 decreased hemoglobin at the day 29 study visit | 4 (5) |
| Recovered to grade ≤2 by the day 90 study visith | 4 (5) |
| Grade ≥3 decreased platelets at the day 29 study visit | 28 (32) |
| Recovered to grade ≤2 by the day 90 study visith | 22 (25) |
| Grade ≥3 decreased neutrophils at the day 29 study visit | 21 (24) |
| Recovered to grade ≤2 by the day 90 study visith | 18 (20) |
NOTE. All percentages are rounded to whole numbers except those with “.5%.”
Abbreviations: AESI, adverse event of special interest; CRS, cytokine release syndrome; liso-cel, lisocabtagene maraleucel; NE, neurological event.
CRS was graded based on Lee 2014 grading criteria. 18
NEs were defined as investigator-identified neurological adverse events related to liso-cel.
Two (2%) patients were treated with another immunosuppressive agent (siltuximab, anakinra, or etanercept) and 3 (3%) patients received vasopressors for management of CRS and/or NEs.
One (1%) patient was treated with another immunosuppressive agent (siltuximab, anakinra, or etanercept) and 2 (2%) patients received vasopressors for management of CRS.
Two (2%) patients were treated with another immunosuppressive agent (siltuximab, anakinra, or etanercept) and 1 (2%) patient received vasopressors for management of NEs.
Included events of pancreatic cancer, basal cell carcinoma of the skin, and squamous cell carcinoma of the skin (n=1 each).
Prolonged cytopenias were defined as grade ≥3 laboratory result of anemia, neutropenia, or thrombocytopenia not resolved at the day 29 study visit.
Recovery data are presented for patients who had laboratory results after day 29 (decreased hemoglobin, n=4; decreased platelets, n=27; decreased neutrophils, n=21). Percentages are calculated out of the liso-cel–treated set (n=88).
Among 31 patients evaluable for DLTs in the dose-finding (n=17) and dose-expansion (n=14) phases, 2 (6%) patients had 3 DLTs at DL2 (1 patient with high tumor burden experienced grade 5 TLS and 1 patient experienced grade 3 neutropenia and grade 4 thrombocytopenia [Supplementary Tables A12–A15]). No maximum tolerated dose was identified.
A total of 46 deaths occurred in the liso-cel–treated set. Most patients (n=29) died because of disease progression and 7 died because of COVID-19 (only 1 was considered treatment emergent) (Supplementary Table A16). Four (5%) patients had grade 5 TEAEs; 3 were considered related to liso-cel (cryptococcal meningoencephalitis, lung infection [COVID-19 pneumonia], TLS [DLT noted above]) and 1 was considered unrelated to liso-cel (cardiopulmonary arrest).
Thirteen (15%) patients were treated in the outpatient setting; of those, 12 were hospitalized for AEs after receiving liso-cel. Median time from liso-cel infusion to initial hospitalization was 4 days (range, 2–10) and median duration of initial hospitalization was 6.5 days (range, 2–43). One patient was admitted to the intensive care unit (ICU). For the 75 patients treated in the inpatient setting, median duration of hospitalization from liso-cel infusion was 11 days (range, 2–31). Five patients were admitted to the ICU.
Cellular Kinetics and Pharmacodynamics
Seventy-nine of 88 (90%) patients in the liso-cel–treated set had available data for cellular kinetic parameters. The median time to maximum liso-cel transgene levels was 10 days after infusion (Supplementary Table A17). Median maximum transgene level (Cmax) was 29,335 copies/μg and area under the curve for transgene levels from 0 to 28 days after infusion (AUC(0–28d)) was 288,557 days×copies/μg. Higher expansion (Cmax and AUC(0–28d)) was associated with higher CR rate; longer DOR; longer PFS; and higher incidence of any-grade CRS, any-grade NEs, and grade ≥3 NEs (Supplementary Table A18). Persistence of the liso-cel transgene was detected at month 12 in 23/33 (70%) patients and at month 24 in 6/17 (35%) patients (Supplementary Table A19). Most patients (51/88 [58%]) had CD19+ B-cell aplasia at baseline, and after liso-cel infusion, the frequency of B-cell aplasia increased to 98% (60/61 patients) at month 2 then decreased to 73% (24/33 patients) at month 12 (Supplementary Table A20). Immunoglobulin G serum levels <500 mg/dL were 40% (35/87 patients) at baseline, increased to 68% (38/56 patients) at month 6 after infusion, and were 64% at month 12 (27/42 patients) and 24 (14/22 patients).
Elevated levels of serum amyloid A, granulocyte macrophage colony-stimulating factor, interferon gamma, interleukin (IL)-2, IL-4, IL-5, IL-6, and C-reactive protein were associated with any-grade CRS; only elevated IL-2 was associated with any-grade NE (Supplementary Table A21). Median time from liso-cel infusion to peak cytokine levels ranged from 4 to 8 days for all cytokines except for IL-4 and decreased by day 29 (Supplementary Figs A3–A4). None were associated with grade ≥3 NE. As it occurred in only 1 patient, grade ≥3 CRS could not be assessed.
PROs
Completion rates are shown in Supplementary Fig A5. Clinically meaningful improvement (Supplementary Table A22) was observed in the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 items fatigue, global health status/QOL, and physical functioning domains by month 2 and remained consistent thereafter; pain was stable through month 12. The EQ-5D-5L visual analog scale demonstrated sustained clinically meaningful improvement by month 2 through 18. Results of all PRO domains are included in Supplementary Figs A6–A7.
DISCUSSION
TRANSCEND-MCL enrolled patients with R/R MCL post-BTKi, and the primary and key secondary endpoints were met in the PAS (ORR, 86.5%; CR rate, 74.3%), with consistent results in the efficacy set. Responses were rapid (median time to CR or PR, 0.95 months) and durable (median DOR, 15.7 months; median PFS, 15.3 months; median OS, 18.2 months). Importantly, liso-cel treatment was associated with low incidences of grade ≥3 CRS (1%), NEs (9%), and infections (15%).
Patients with MCL whose disease is relapsed or refractory to BTKi therapy have limited treatment options. Currently, two new therapies are available. 19,20 Pirtobrutinib, a noncovalent, reversible BTKi, was approved in the United States for patients with R/R MCL after ≥2 lines of systemic therapy, including a BTKi. 20,21 In clinical practice, pirtobrutinib monotherapy is often used in patients with indolent, relapsed disease and low tumor burden, as single-agent response rate is adequate but not very high (ORR, 58%; CR rate, 20%).20,22 To date, the only CAR T-cell therapy approved in MCL is brexucabtagene autoleucel, which targets CD19 with CD28 co-stimulation. ZUMA-2 assessed the efficacy and safety of brexucabtagene autoleucel in patients with R/R MCL after prior BTKi, demonstrating an ORR of 93% and a CR rate of 67% in the first 60 patients with ≥7 months of follow-up. 4In the ~3 year follow-up analysis, the 30-month OS rate was 60.3%.23 Although brexucabtagene autoleucel has shown high efficacy, substantial toxicity has been reported; in the ZUMA-2 study, 15% of patients had grade ≥3 CRS, 31% had grade ≥3 NEs, and 32% had grade ≥3 infections.
There is an urgent need for CAR T-cell therapy options with low incidence of CRS, NEs, and infections. With a favorable benefit/risk profile and consistent responses in high-risk patient populations (eg, high Ki-67 proliferation index, TP53 mutations, blastoid morphology, secondary CNS lymphoma), liso-cel may help to address this unmet clinical need. The results from TRANSCEND-MCL support liso-cel as a potential new treatment option for R/R MCL, including populations in which toxicity is a significant concern (eg, patients with older age or comorbidities), as this study included patients that have historically been underrepresented or excluded from clinical trials. Furthermore, the safety profile of liso-cel may provide opportunity for outpatient treatment and combination therapy with other targeted and immunotherapies.
The ZUMA-2 study excluded patients who received >5 prior lines of therapy, whereas TRANSCEND-MCL included patients who received ≥2 prior lines of therapy with no upper limit. The median PFS in TRANSCEND-MCL was 15.3 months and median OS was 18.2 months, indicating that patients who relapsed after treatment with liso-cel had very short survival. For many patients in the study, liso-cel was their last treatment. It is likely that the inclusion of patients with such a high number of prior lines of therapy in TRANSCEND-MCL may have impacted efficacy outcomes, and treatment in earlier lines could improve outcomes in R/R MCL. The data from TRANSCEND-MCL highlight the importance of further studies with longer follow-up to guide real-world experiences.
Another urgent unmet need exists for patients with secondary CNS lymphoma; when disease metastasizes to the CNS in patients with R/R MCL, treatment options are limited and prognosis is poor. 24 TRANSCEND-MCL included 7 patients with secondary CNS lymphoma. Among these patients, response rates (ORR, 85.7%; CR rate, 85.7%) were comparable with the overall population. Despite the small sample size, these data are encouraging and provide the first prospective clinical trial data supporting the use of CAR T-cell therapy for patients with R/R MCL and secondary CNS lymphoma.
CAR T-cell therapies continue to evolve in R/R MCL. Both brexucabtagene autoleucel and liso-cel target CD19; however, alternative targets are being explored such as BAFF-R and ROR1. 25,26 Additionally, bispecific antibodies are emerging as a new therapeutic modality, including glofitamab. 27,28 Studies are still ongoing, and the clinical activity of these therapies has not yet been fully established.
This study was limited by the single-arm design. Additionally, the small sample size precludes definitive conclusions in some subgroups, including patients with secondary CNS lymphoma. The study population also included patients who had high-risk features, including a wide range of prior therapies and those with TP53 mutations, which may have influenced efficacy results.
The current study expands knowledge about CAR T-cell therapy and the clinical landscape of R/R MCL in patients with aggressive disease and high-risk features, including those with older age and moderate comorbidities. These results support liso-cel as a potential new treatment option for R/R MCL, particularly in patients for whom limited therapies are available.
Supplementary Material
CONTEXT SUMMARY.
Key Objective
Continued unmet need exists for therapies that achieve deep and durable responses (ie, high and sustained complete response [CR] rates) with a favorable benefit/risk profile in relapsed/refractory (R/R) mantle cell lymphoma (MCL), especially for patients with high-risk, aggressive disease. Lisocabtagene maraleucel (liso-cel), an autologous, CD19-directed, 4–1BB CAR T-cell product, has demonstrated efficacy and manageable safety profile across R/R B-cell malignancies. We report primary analysis results from the MCL cohort of TRANSCEND NHL 001.
Knowledge Generated
In this primary analysis, liso-cel resulted in rapid and durable CR with consistently high ORR and CR rates in patients with heavily pretreated R/R MCL across prespecified patient subgroups, including those with high-risk disease characteristics (eg, TP53 mutation and secondary central nervous system lymphoma). Liso-cel demonstrated favorable benefit/risk profile with low incidence of grade ≥3 cytokine release syndrome and neurological events.
Relevance
Lisocabtagene maraleucel represents a novel treatment option for patients with mantle cell lymphoma refractory to BTK inhibition, including patients with CNS involvement. Given the favorable toxicity profile, future studies should evaluate this treatment earlier in the disease course.
Relevance statement written by Dr. Friedberg
ACKNOWLEDGMENTS
The TRANSCEND NHL 001 study was funded by Juno Therapeutics, a Bristol-Myers Squibb Company. All authors contributed to and approved the manuscript; writing and editorial assistance were provided by Allison Green, PhD, CMPP, and Nikola Vojtov, PhD, of The Lockwood Group (Stamford, CT, USA), funded by Bristol Myers Squibb. We would like to thank Ashvin Singh for assistance with data inquiries and requests.
Footnotes
Previous presentation: Presented in part at the 17th International Conference on Malignant Lymphoma (ICML), Lugano, Switzerland, June 13–17, 2023 (LBA3)
Clinical trial registration: ClinicalTrials.gov registration number: NCT02631044
Research support: Juno Therapeutics, a Bristol-Myers Squibb Company
DATA SHARING STATEMENT
Bristol Myers Squibb policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
REFERENCES
- 1.Martin P, Maddocks K, Leonard JP, et al. : Postibrutinib outcomes in patients with mantle cell lymphoma. Blood 127:1559–63, 2016 [DOI] [PubMed] [Google Scholar]
- 2.Jain P, Kanagal-Shamanna R, Zhang S, et al. : Long-term outcomes and mutation profiling of patients with mantle cell lymphoma (MCL) who discontinued ibrutinib. Br J Haematol 183:578–587, 2018 [DOI] [PubMed] [Google Scholar]
- 3.Cheah CY, Chihara D, Romaguera JE, et al. : Patients with mantle cell lymphoma failing ibrutinib are unlikely to respond to salvage chemotherapy and have poor outcomes. Ann Oncol 26:1175–1179, 2015 [DOI] [PubMed] [Google Scholar]
- 4.Wang M, Munoz J, Goy A, et al. : KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med 382:1331–1342, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar A, Sha F, Toure A, et al. : Patterns of survival in patients with recurrent mantle cell lymphoma in the modern era: progressive shortening in response duration and survival after each relapse. Blood Cancer J 9:50, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abramson JS, Palomba ML, Gordon LI, et al. : Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet 396:839–852, 2020 [DOI] [PubMed] [Google Scholar]
- 7.Abramson JS, Solomon SR, Arnason J, et al. : Lisocabtagene maraleucel as second-line therapy for large B-cell lymphoma: primary analysis of the phase 3 TRANSFORM study. Blood 141:1675–1684, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sehgal A, Hoda D, Riedell PA, et al. : Lisocabtagene maraleucel as second-line therapy in adults with relapsed or refractory large B-cell lymphoma who were not intended for haematopoietic stem cell transplantation (PILOT): an open-label, phase 2 study. Lancet Oncol 23:1066–1077, 2022 [DOI] [PubMed] [Google Scholar]
- 9.Morschhauser F, Dahiya S, Palomba ML, et al. : TRANSCEND FL: phase 2 study results of lisocabtagene maraleucel (liso-cel) in patients (pts) with relapsed/refractory (R/R) follicular lymphoma (FL). Hematol Oncol 41:877–880, 2023. (S2; LBA4)37392141 [Google Scholar]
- 10.Siddiqi T, Soumerai JD, Dorritie KA, et al. : Phase 1 TRANSCEND CLL 004 study of lisocabtagene maraleucel in patients with relapsed/refractory CLL or SLL. Blood 139:1794–1806, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siddiqi T, Maloney DG, Kenderian SS, et al. : Lisocabtagene maraleucel in chronic lymphocytic leukaemia and small lymphocytic lymphoma (TRANSCEND CLL 004): a multicentre, open-label, single-arm, phase 1–2 study. Lancet 402:641–654, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheson BD, Fisher RI, Barrington SF, et al. : Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 32:3059–68, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hobbs BP, Barata PC, Kanjanapan Y, et al. : Seamless designs: current practice and considerations for early-phase drug development in oncology. J Natl Cancer Inst 111:118–128, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.US Food and Drug Administration: Expansion cohorts: use in first-in-human clinical trials to expedite development of oncology drugs and biologics - guidance for industry, https://www.fda.gov/regulatory-information/search-fda-guidance-documents/expansion-cohorts-use-first-human-clinical-trials-expedite-development-oncology-drugs-and-biologics, 2018 [Google Scholar]
- 15.Meyer RD, Ratitch B, Wolbers M, et al. : Statistical issues and recommendations for clinical trials conducted during the COVID-19 pandemic. Stat Biopharm Res 12:399–411, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Degtyarev E, Rufibach K, Shentu Y, et al. : Assessing the impact of COVID-19 on the clinical trial objective and analysis of oncology clinical trials-application of the estimand framework. Stat Biopharm Res 12:427–437, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hemmings R: Under a black cloud glimpsing a silver lining: comment on statistical issues and recommendations for clinical trials conducted during the COVID-19 pandemic. Stat Biopharm Res 12:414–418, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee DW, Gardner R, Porter DL, et al. : Current concepts in the diagnosis and management of cytokine release syndrome. Blood 124:188–95, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.TECARTUS (brexucabtagene autoleucel) [package insert]. Santa Monica, CA: Kite Pharma, Inc.; October 2021 [Google Scholar]
- 20.Wang ML, Jurczak W, Zinzani PL, et al. : Pirtobrutinib in covalent Bruton tyrosine kinase inhibitor pretreated mantle-cell lymphoma. J Clin Oncol 41:3988–3997, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.JAYPIRCA (pirtobrutinib) [package insert]. Indianapolis, IN: Lilly USA, LLC: January 2023 [Google Scholar]
- 22.Wang ML, Shah NN, Jurczak W, et al. : Efficacy of pirtobrutinib in covalent BTK-inhibitor pre-treated relapsed / refractory mantle cell lymphoma: additional patients and extended follow-up from the phase 1/2 BRUIN study. Blood 140:9368–9372, 2022 [Google Scholar]
- 23.Wang M, Munoz J, Goy A, et al. : Three-year follow-up of KTE-X19 in patients with relapsed/refractory mantle cell lymphoma, including high-risk subgroups, in the ZUMA-2 study. J Clin Oncol 41:555–567, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rusconi C, Cheah CY, Eyre TA, et al. : Ibrutinib improves survival compared with chemotherapy in mantle cell lymphoma with central nervous system relapse. Blood 140:1907–1916, 2022 [DOI] [PubMed] [Google Scholar]
- 25.ClinicalTrials.gov: BAFFR-targeting CAR T Cells for Patients With Relapsed or Refractory B-NHL, 2023. https://classic.clinicaltrials.gov/ct2/show/NCT05370430 [Google Scholar]
- 26.ClinicalTrials.gov: First in Human Study of NVG-111 in Relapsed/Refractory ROR1+ Malignancies, 2023. https://clinicaltrials.gov/study/NCT04763083 [Google Scholar]
- 27.Phillips T, Dickinson M, Morschhauser F, et al. : MCL-467 Glofitamab Monotherapy Induces High Complete Response Rates in Patients With Heavily Pretreated Relapsed or Refractory Mantle Cell Lymphoma. Clinical Lymphoma Myeloma and Leukemia 23:S462, 2023 [Google Scholar]
- 28.Phillips TJ, Dickinson M, Morschhauser F, et al. : Glofitamab monotherapy induces high complete response rates in patients with heavily pretreated relapsed or refractory mantle cell lymphoma. Blood 140:178–180, 2022 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


