Abstract
Treatment with antagonists of luteinizing hormone-releasing hormone (LH-RH) leads to down-regulation of pituitary LH-RH receptors. Thus, the effect of LH-RH antagonists is similar to that of the LH-RH agonists, but the mode of action of antagonists is not completely understood. The aim of this study was to investigate the effects of LH-RH antagonist cetrorelix on the binding characteristics and subcellular localization of receptors for LH-RH in rat pituitaries. Radioligand binding studies, performed after in vitro desaturation, revealed that a single s.c. injection of cetrorelix at a dose of 100 μg per rat significantly decreased the number of pituitary membrane receptors for LH-RH in a time-dependent manner with the nadir occurring at 6 h. In contrast, 2–6 h after cetrorelix treatment, the concentration of binding sites for LH-RH in the nuclei of rat pituitaries was significantly higher (P < 0.01) than in controls. Chronic administration of cetrorelix also decreased the level of membrane receptors for LH-RH by 83% (P < 0.01) after 7 days, and 86% (P < 0.01) after 14 days. The number of LH-RH binding sites in the nuclear pellet was increased 3-fold (P < 0.01) by days 7 and 14 after the initiation of treatment with cetrorelix. A single injection or prolonged treatment with LH-RH antagonist also decreased the mRNA expression of pituitary receptors for LH-RH. Our results demonstrate that the down-regulation of LH-RH receptors on the cell membranes of rat pituitaries after therapy with antagonist cetrorelix is associated with an increase in receptor concentration in the nuclei. These phenomena could be related to the internalization and subcellular translocation of LH-RH receptors.
Keywords: LH-RH receptor‖internalization‖translocation to nuclei
The stimulation of the secretion of gonadotropins by luteinizing hormone-releasing hormone (LH-RH) and its analogs is mediated by specific, high-affinity, G protein-coupled receptors present on plasma membranes of pituitary gonadotrophs (1–4). The binding of LH-RH to its receptors is followed by microaggregation, complex formation, and internalization (1, 4, 5). Although complex mechanisms are involved in the release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), the responsiveness of pituitary cells to LH-RH appears to be correlated with the number of pituitary LH-RH receptors (1, 5, 6). The regulation of these receptors is influenced by several factors, such as gonadal steroids, gonadotropins, and inhibin, as well as by their own ligand, LH-RH (1, 6–10). An acute or intermittent administration of LH-RH agonists stimulates the synthesis of receptors and induces a marked and sustained release of gonadotropins (1–4, 11). However, chronic administration of LH-RH or its agonists results in a desensitization of the pituitary gonadotrophs, down-regulation of receptors for LH-RH, and suppression of serum LH, FSH, and sex steroid levels (1, 6, 12–13). In contrast to the LH-RH agonists, which require a continuous administration to induce a down-regulation of receptors, an inhibitory effect on gonadotropin and sex steroid secretion can be achieved after a single injection of LH-RH antagonist, reducing the time of the onset of therapeutic actions (1, 2, 14–17). To take advantage of these properties, various LH-RH antagonists such as cetrorelix, abarelix, and ganirelix are being tested for diverse clinical applications (1–3, 14–17). Cetrorelix has already been approved for uses in gynecology, especially in controlled ovarian stimulation for in vitro fertilization and is under clinical investigation for the therapy of benign prostatic hyperplasia, prostate cancer, and other oncological applications (1–3, 14–17). There are indications that cetrorelix may also exert a direct antiproliferative action on various tumors, including breast, ovarian, endometrial, pancreatic, and prostate cancers (2, 3, 14, 18).
Although the principal mechanism of action of LH-RH antagonists was thought to be a competitive blockade of LH-RH receptors, recent studies revealed that administration of LH-RH antagonist cetrorelix to rats also produces a down-regulation of pituitary LH-RH receptors, which was previously believed to occur only with LH-RH agonists (1, 2, 14, 19, 20). Molecular biology analyses also show a significant decrease in the levels of mRNA for pituitary LH-RH receptors after chronic administration of cetrorelix (1, 20). Investigation of the pattern of changes in the levels and subcellular localization of LH-RH receptors after treatment with cetrorelix might provide further insight into the mechanisms by which LH-RH antagonists down-regulate the expression of pituitary receptors for LH-RH. Thus, the purpose of this study was to examine the concentration of receptors for LH-RH on cell membranes and in nuclei of rat pituitaries as well as the mRNA expression of these receptors after a single injection or repeated administration of cetrorelix.
Materials and Methods
Peptides and Chemicals.
LH-RH antagonist cetrorelix (SB-75), originally synthesized in our laboratory by solid-phase methods (17), was made by Zentaris (Frankfurt on the Main, Germany) as cetrorelix acetate (D-20761). Sodium [125I]iodide-labeled sodium was purchased from Amersham Pharmacia. All other peptides and chemicals, unless otherwise mentioned, were obtained from Sigma, Bachem (Torrance, CA), R & D Systems, or California Peptide Research (Napa, CA).
Animals.
Young adult male Sprague–Dawley rats (Charles River Laboratories) weighing 250–300 g were used in the experiments. The animals were housed and fed as described (19, 20). All animal studies were conducted in accord with institutional ethical guidelines for the care and use of experimental animals.
In Vivo Experimental Procedure.
In experiment 1, a group of 59 rats received a s.c. injection of cetrorelix at a dose of 100 μg per rat, dissolved in distilled water containing 5% (wt/vol) mannitol. Twenty-three control animals received only injections of the vehicle and were killed by decapitation under anesthesia immediately after administration (time 0). Eighteen, 23, and 18 rats from the cetrorelix group were killed under anesthesia 2, 6, and 48 h, respectively, after administration of the LH-RH antagonist. Immediately after decapitation, pituitaries were removed, cleaned, and frozen on dry ice, then stored at −70°C until analyses of LH-RH receptors. Pituitaries of four control rats and five rats killed 6 h after the injection of cetrorelix were separated, homogenized in TRI reagent (Sigma), and stored at −70°C until used for determination of mRNA for LH-RH receptors.
In experiment 2, the rats were divided into two groups that received the following treatments: group 1 (controls), vehicle injection only (23 animals); group 2, cetrorelix injections at a dose of 100 μg per day per animal s.c. (42 animals). Control rats were killed immediately after vehicle injection (day 0). In group 2, 24 rats were killed 7 days and 18 rats were killed 14 days after the initiation of cetrorelix treatment. All pituitaries were processed as detailed above, but pituitaries of 5 control rats and pituitaries of 6 rats treated with cetrorelix for 7 days were separated for analyses of receptor mRNA as described above.
Preparation of Cell Membranes and Nuclei.
Pituitary membrane fractions for receptor studies were prepared as described (19). Briefly, the pituitaries were thawed and cleaned, then separated into two portions. One half of the pituitaries was homogenized on ice in 50 mM Tris⋅HCl buffer (pH 7.4) supplemented with protease inhibitors by using an Ultra-Turrax tissue homogenizer (IKA Works, Wilmington, NC). The homogenate was centrifuged at 500 × g for 10 min at 4°C to remove nuclear debris and lipid layer. The supernatant containing the crude membrane fraction was ultracentrifuged (Beckman L8–80 M) twice at 70,000 × g for 60 min at 4°C after resuspension in fresh buffer. The final pellet was resuspended in homogenization buffer and stored at −70°C until assayed. Protein concentration was determined by the method of Bradford (21) by using a Bio-Rad protein assay kit. Crude nuclear pellets were prepared from the remaining half of the pituitary samples as described (22, 23) with some modifications. Briefly, the samples were homogenized in 50 mM Tris⋅HCl buffer (pH 7.4) by using a Teflon–glass homogenizer (Glas-Col, Terre Haute, IN). The homogenate was filtered through two layers of nylon gauze and centrifuged at 800 × g for 10 min at 4°C. The pellet was resuspended and purified by centrifugation at 30,000 × g for 30 min in 2.3 M sucrose buffer, then stored at −70°C until assayed. DNA content in each nuclear preparation was determined by the method of Labara and Paigen (24).
Radioligand Binding Studies.
Radioiodinated derivatives of [D-Trp6]LH-RH were prepared by the chloramine-T method and purified by reverse-phase HPLC in our laboratory (19). LH-RH receptor-binding assays were carried out as reported (19) by using in vitro ligand competition assays based on binding of 125I-[D-Trp6]LH-RH as radioligand to membrane and nuclear fractions of rat pituitaries. This radioligand shows high-affinity binding to rat pituitary and human breast, prostate, and other cancers and has been well characterized (1, 14). Because a certain portion of LH-RH receptors may remain occupied by the LH-RH antagonist after an in vivo administration, a desaturation of LH-RH receptors in vitro was performed by using 0.2 M MnCl2 as chaotropic agent, following the preparation of pituitary fractions (19). After an in vitro desaturation, membrane and nuclear fractions were incubated in duplicate or triplicate with 60,000-80,000 cpm of 125I-[D-Trp6]LH-RH and increasing concentrations (10−12 to 10−6 M) of nonradioactive peptides as competitors in a total volume of 150 μl of binding buffer. At the end of the incubations, 125-μl aliquots of suspension were transferred onto the top of 1 ml of ice-cold binding buffer containing 1.5% BSA in silane-treated polypropylene microcentrifuge tubes (Sigma). The tubes were centrifuged at 12,000 × g for 3 min at 4°C (Beckman J2-21M). Supernatants were aspirated, and the bottoms of the tubes containing the pellet were cut off and counted in a γ counter (Micromedic System, Huntsville, AL).
RNA Isolation and Reverse Transcription (RT)-PCR Analysis.
Total RNA from pituitary glands was isolated by using the TRI reagent (Sigma) protocol as described (25). After precipitation, RNA samples were quantified spectrophotometrically at 260 and 280 nm. One microgram of total RNA was reverse transcribed and then amplified by using GeneAmp RNA PCR Core kit (Perkin-Elmer) according to the manufacturer's instructions. The methods of RT and PCR amplification have been reported in detail (25). For amplification of cDNA transcripts, gene-specific primers for rat LH-RH receptors (26) and rat β-actin (27) were used as described in detail (25). The number of cycles was determined in preliminary experiments to be within the exponential range of PCR amplification. PCR products were subjected to electrophoresis on 1.5% agarose gels, then stained with ethidium bromide and visualized under UV light. Bands of PCR products were then scanned and analyzed semiquantitatively by using an imaging densitometer (model GS-700, Bio-Rad). The levels of mRNA for rat LH-RH receptors were normalized versus values of mRNA of rat β-actin and expressed as percentage of the vehicle-treated controls.
Analysis of Experimental Data.
Specific ligand-binding capacities and affinities were calculated by the Ligand-PC computerized curve-fitting program of Munson and Rodbard (28). To determine the types of receptor binding, equilibrium dissociation constants (Kd values), and the maximal binding capacity of receptors (Bmax), LH-RH binding data were also analyzed by the Scatchard method (29). Statistical analyses were performed by using a computer software (sigmastat, Jandel, San Rafael, CA). P < 0.01 was accepted as a statistically significant difference. sigmaplot graphing program (Jandel) was used to visualize experimental data and to prepare figures.
Results
Characteristics of Pituitary Receptors for LH-RH After a Single Injection of Cetrorelix.
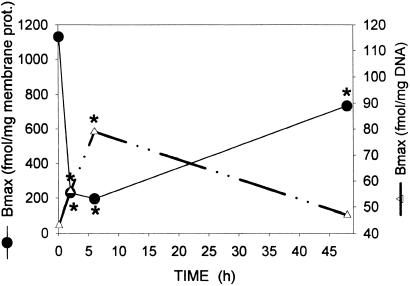
The characteristics of binding of 125I-[D-Trp6]LH-RH to the membrane and nuclear receptors on rat anterior pituitaries following an in vitro desaturation were determined by ligand competition assays. In experiment 1, the computerized nonlinear curve fitting and the Scatchard plot analyses of the binding data demonstrated the presence in pituitaries from control rats of a single class of high-affinity (Kd = 4.82 ± 0.89 nM) binding sites with a mean maximal binding capacity (Bmax) of 1,131.2 ± 66.4 fmol/mg of membrane protein (Table 1). A single injection of cetrorelix at a dose of 100 μg per rat produced a radical decrease in the number of LH-RH binding sites in a time-dependent manner, as compared with that in the control group (time 0) (Table 1, Fig. 1). The concentration of receptors for LH-RH was significantly lower (P < 0.01) 2 h after the administration of cetrorelix, reaching the lowest level 6 h after the initiation of treatment (Table 1, Fig. 1). Although the number of receptors increased markedly at 48 h, this number was still significantly lower than that in controls (Table 1, Fig. 1).
Table 1.
Effects of a single injection of cetrorelix on the binding characteristics of LH-RH receptors
| Time, h | Membrane receptors
|
Nuclear receptors
|
||
|---|---|---|---|---|
| Kd, nM | Bmax, fmol/mg protein | Kd, mM | Bmax, fmol/mg DNA | |
| 0 | 4.82 ± 0.89 | 1,131.2 ± 66.4 | 5.74 ± 0.51 | 42.8 ± 1.91 |
| 2 | 5.67 ± 1.11 | 230.8 ± 26.7* | 6.09 ± 0.64 | 55.8 ± 2.95* |
| 6 | 4.87 ± 0.56 | 196.4 ± 3.60* | 5.19 ± 0.85 | 79.1 ± 4.37* |
| 48 | 4.40 ± 0.83 | 735.7 ± 34.9* | 5.69 ± 0.91 | 47.1 ± 4.31 |
Membrane and nuclear fractions were prepared from rat pituitaries evaluated before (time 0) and at several times after administration of 100 μg of cetrorelix. Results are mean ± SEM of triplicate determinations.
, P < 0.01.
Figure 1.
Concentration of receptors for LH-RH in the membrane (●) and nuclear (▵) fractions of rat pituitaries after a single s.c. injection of LH-RH antagonist cetrorelix at a dose of 100 μg per rat. Each point represents the mean of three determinations. Significant differences from the control (time 0) are marked by asterisks (P < 0.01).
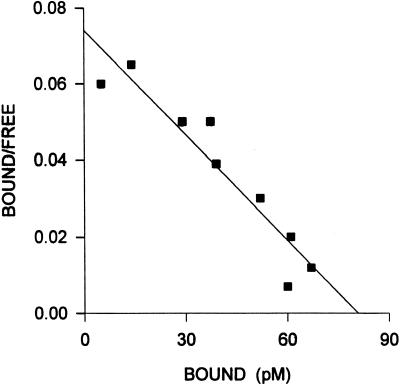
Specific, high-affinity binding sites for LH-RH were also found in the crude nuclear pellet of rat pituitaries (Fig. 2). Before the initiation of treatment (time 0), pituitary nuclear receptors exhibited a mean dissociation constant (Kd) of 5.74 ± 0.51 nM and a Bmax of 42.8 ± 1.91 fmol/mg of DNA (Table 1), as analyzed by complete displacement. After a single injection of cetrorelix, the concentration of nuclear LH-RH receptors increased progressively (Fig. 1). The highest level of binding sites was found at 6 h (P < 0.01), indicating a marked shift in the subcellular distribution of receptors (Table 1, Fig. 1). Forty-eight hours after cetrorelix administration, the concentration of LH-RH receptors in the nuclear fraction returned to the control value (Table 1, Fig. 1).
Figure 2.
Representative Scatchard plot derived from specific 125I-[d-Trp6]LH-RH binding to the nuclear fraction isolated from rat pituitaries. Specific binding was determined as described in the text. Each point represents the mean of three experiments, each done in triplicate.
The affinity of LH-RH receptors in the membrane or nuclei of rat pituitaries was not significantly affected by a single injection of cetrorelix (Table 1). The binding of 125I-[D-Trp6]LH-RH was found to be specific, reversible, and time- and temperature-dependent in both cell membranes and nuclear fractions prepared from rat pituitary glands (data not shown).
Characteristics of Pituitary Receptors for LH-RH After Chronic Treatment with Cetrorelix.
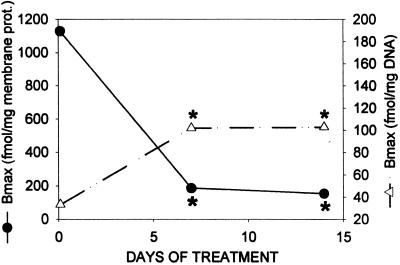
The presence of specific, high-affinity receptors for LH-RH in the membrane fraction of rat pituitaries was also established in experiment 2. In untreated control rats killed on day 0, the radioligand was bound to one class of binding sites with a Kd of 6.76 ± 0.28 nM and a Bmax of 1130.7 ± 69.0 fmol/mg of membrane protein, both values being similar to those in experiment 1 (Table 2). Daily injections of cetrorelix produced a significant (P < 0.01) decrease in the number of LH-RH receptors as measured 7 days after the initiation of treatment (Table 2, Fig. 3). The levels of membrane receptors were also in the same range on day 14, demonstrating an ≈84–86% (P < 0.01) reduction as compared with receptor concentration on day 0 (Fig. 3).
Table 2.
Effects of daily injections of cetrorelix on the binding characteristics of LH-RH receptors
| Days of treatment | Membrane receptors
|
Nuclear receptors
|
||
|---|---|---|---|---|
| Kd, nM | Bmax, fmol/mg protein | Kd, nM | Bmax, fmol/mg DNA | |
| Control (Day 0) | 6.76 ± 0.28 | 1,130.7 ± 69.0 | 5.93 ± 0.85 | 33.3 ± 4.65 |
| Treated | ||||
| Day 7 | 5.39 ± 0.38 | 185.9 ± 17.8* | 5.02 ± 0.87 | 101.0 ± 4.48* |
| Day 14 | 5.08 ± 0.91 | 158.4 ± 22.5* | 5.75 ± 0.80 | 103.4 ± 7.86* |
Membrane and nuclear fractions were prepared from rat pituitaries evaluated before (day 0) and 7 and 14 days after the initiation of treatment (100 μg of cetrorelix per animal per day; s.c.). Results are mean ± SEM of triplicate determinations.
, P < 0.01.
Figure 3.
Concentration of the receptors for LH-RH in the membrane (●) and nuclear (▵) fractions of rat pituitaries after daily s.c. administration of cetrorelix at a dose of 100 μg/day per rat for 7–14 days. Each point represents the mean of three determinations. Significant differences from the control (day 0) are marked by asterisks (P < 0.01).
In accord with experiment 1, there were high-affinity binding sites for LH-RH (Kd = 5.93 ± 0.85 nM) in the nuclear fractions prepared from rat pituitary glands, as determined in control rats on day 0. The maximal binding capacity (Bmax) of the nuclear receptors was 33.3 ± 4.65 fmol/mg DNA (Table 2). A daily administration of cetrorelix produced a major increase in the number of LH-RH binding sites in the nuclear pellet of the pituitaries (Table 2, Fig. 3). The concentration of nuclear LH-RH receptors was significantly higher (P < 0.01) 7 days after treatment with antagonist cetrorelix as compared with the controls, and remained in that range for the period of the study (Fig. 3). This elevated level of nuclear binding sites for LH-RH was more than 3 times higher than the concentration on day 0 (Table 2), demonstrating a major change in subcellular distribution.
The binding affinity of receptors for LH-RH in the membrane or nuclear fractions prepared from rat pituitaries was not altered by daily treatment with cetrorelix for 7–14 days (Table 2).
Effects of Cetrorelix Treatment on the Levels of mRNA for Pituitary LH-RH Receptors.
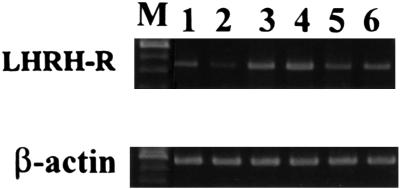
RT-PCR analyses revealed the presence of 441-bp and 542-bp products corresponding to the mRNA for rat LH-RH receptors and rat β-actin, respectively (Fig. 4). Semiquantitative analysis showed that a single injection of 100 μg of LH-RH antagonist cetrorelix caused a significant 26.8% reduction in the mRNA level of receptors for LH-RH 6 h after the treatment (P < 0.05 vs. control) (Fig. 4, lane 2). Daily injections of cetrorelix also significantly (P < 0.01) decreased the level of LH-RH receptor mRNA by 34.5%, 7 days after the initiation of treatment, compared with control values at day 0 (Fig. 4, lanes 5 and 6).
Figure 4.
RT-PCR analysis of mRNA of receptors for LH-RH in rat pituitaries. PCR products were subjected to electrophoresis on 1.5% agarose gel and stained with ethidium bromide. The PCR products were of the expected size of 441 bp (LH-RH receptors) and 542 bp (β-actin). Lanes: M, 100-bp DNA molecular weight marker; lane 1, pituitary of a control rat (time 0); lane 2, pituitary of a rat, 6 h after single injection of cetrorelix; lanes 3 and 4, pituitaries of control rats (day 0); lanes 5 and 6, pituitaries of rats, 7 days after cetrorelix treatment.
Discussion
Numerous studies demonstrated that LH-RH agonists exert their main therapeutic effect through the inhibition of release of LH, follicle-stimulating hormone, and sex steroids resulting from the desensitization of gonadotrophs, down-regulation of LH-RH receptors, and reduction in the mRNA for LH-RH receptors in the pituitary (1–4). Experimental findings indicate that this decrease in the number of receptors could be regulated at the pretranslational level by the expression of the LH-RH receptor gene, but the findings on the posttranslational regulation by recycling, modification, or degradation, and change in the stability of the mRNA have also been reported (1–4, 12, 30). In contrast to LH-RH agonists that require repeated administration to achieve a down-regulation of receptors, LH-RH antagonists produce a competitive blockade of LH-RH receptors, preventing a stimulation by endogenous LH-RH, and cause an immediate inhibition of the release of gonadotropins and sex steroids (1–3, 14). Because antagonistic analogs of LH-RH can suppress LH and sex steroid secretion promptly after the administration, they greatly reduce the time of the onset of therapeutic effects. The use of antagonists can also prevent a clinical flare-up of disease, occasionally seen with the agonists and caused by a transient increase in LH and sex steroid secretion (1–3, 14).
The cellular events that follow the binding of LH-RH antagonists such as cetrorelix to pituitary gonadotrophs are still not completely understood and are a subject of investigations. The principal mechanism of action of LH-RH antagonists was thought to be based only on a competitive occupancy of LH-RH receptors, but recently we demonstrated that acute or chronic administration of LH-RH antagonist cetrorelix to rats also produces a clear down-regulation of membrane receptors for LH-RH in the pituitaries of rats and not merely an occupancy of LH-RH binding sites (19, 20). Receptor assays, carried out after an in vitro desaturation of LH-RH binding sites, demonstrated that pituitary LH-RH receptors were significantly down-regulated for at least 72 h after a single injection of 100 μg of cetrorelix (19). This fall in the number of receptors for LH-RH on rat pituitary membranes was accompanied by suppression of serum LH and testosterone (19). In another study, daily s.c. administration of cetrorelix acetate at dose of 100 μg/day for 4 weeks or a single intramuscular injection of 4.5 mg of a depot formulation of cetrorelix pamoate produced a desensitization of gonadotrophs and a marked down-regulation of pituitary LH-RH receptors in rats (20). A major decrease in the expression of mRNA for pituitary LH-RH receptors was also found after chronic treatment with cetrorelix (20). Our most recent work showed that the degree of suppression of the gene expression of pituitary LH-RH receptors by cetrorelix is correlated with the level of pituitary LH-RH, and that LH-RH antagonists exert their inhibitory effects on the gene expression of pituitary LH-RH receptors by counteracting the stimulatory effect of endogenous LH-RH (25). The exposure of pituitary cells to cetrorelix in the superfusion system in vitro, which lacks endogenous LH-RH, did not influence the mRNA expression of LH-RH receptors (25). However, we observed that cetrorelix causes a significantly higher reduction in LH-RH receptor gene expression in ovariectomized rats, which have a high level of LH-RH in the pituitary portal vessels, than in normal rats (25). It has also been shown that the exposure to the antagonist prevented the up-regulation of the receptor mRNA expression induced by exogenous LH-RH or the LH-RH agonist triptorelin (31). These findings indicate that LH-RH antagonists do not directly influence the gene expression of receptors for LH-RH in the pituitary, but exert their suppressive effect by counteracting the up-regulation caused by LH-RH.
The present study confirms and extends our previous findings (19), which demonstrated that a single injection of cetrorelix significantly decreases the number of pituitary membrane receptors for LH-RH in a time-dependent manner. The lowest receptor level was found 6 h after the injection of cetrorelix, but a marked recovery in receptor number was observed at 48 h. Chronic administration of cetrorelix also decreased the level of membrane receptors for LH-RH by 83% after 7 days, and the number of receptors on day 14 was even lower, presenting an 86% reduction. Semiquantitative analysis of the mRNA expression of pituitary LH-RH receptors showed that the down-regulation of LH-RH binding sites induced by cetrorelix was accompanied by the reduction in gene expression of these receptors.
The most important feature of our present investigation pertains to changes in the subcellular localization of LH-RH binding sites after cetrorelix treatment. We used radioligand binding studies to demonstrate the presence of specific, high-affinity binding sites for LH-RH in the nuclei and not only on membranes of rat pituitaries. Millar et al.§ were the first to report the presence of LH-RH receptors in isolated nuclei from rat anterior pituitaries. These results were consistent with the subsequent findings of Marian and Conn (22), indicating subcellular localization of LH-RH receptors in rat anterior pituitary and ovarian tissue. Nevertheless, our present study demonstrates that the down-regulation of receptors for LH-RH in pituitary membranes after treatment with LH-RH antagonist cetrorelix is accompanied by a significant increase in the concentration of LH-RH binding sites in the nuclear fraction of rat pituitaries. A greater decrease in concentration of receptors in the membrane by prolonged treatment with cetrorelix was associated with a larger increase in the level of nuclear binding sites.
The presence of high-affinity receptors for LH-RH in the nuclear pellet of mammary cancer cells and nitrosamine-induced pancreatic cancer in hamsters, detected by electron microscopic immunohistochemistry and radioligand binding assays, has been reported (32, 33). These observations are in accord with our recent findings demonstrating the presence of specific binding sites for LH-RH in both cell membrane and nuclear fraction of OV-1063 human epithelial ovarian cancers xenografted into nude mice (34). We also demonstrated that the down-regulation of LH-RH receptors on the cell membranes of OV-1063 cells after therapy with the antagonist cetrorelix was associated with an increase in receptor concentration in the nuclei (34). The present study shows that the same phenomenon, that is, an increase in the nuclear fraction of LH-RH receptor, likewise occurs in rat pituitaries after treatment with cetrorelix.
Collectively, these observations suggest that the effects of powerful LH-RH antagonists such as cetrorelix are not limited to the occupation of binding sites for LH-RH and an induction of a competitive inhibition of the binding of the natural ligand. Thus, LH-RH antagonists also appear to initiate major intracellular changes in the distribution of LH-RH receptors. It is likely that the down-regulation of LH-RH receptors on pituitary membranes and the parallel increase in the concentration of LH-RH binding sites in the nuclear fraction of rat pituitaries after therapy with the LH-RH antagonist cetrorelix are linked to the internalization and translocation of the receptors. These phenomena have clinical implications as to the design of therapeutic regimens of cetrorelix.
Acknowledgments
We thank Drs. J. Manuel Arencibia and N. Lamharzi for their experimental help and Dr. Attila Nagy for his valuable advice in the preparation of the manuscript. The work described in this paper was supported by the Medical Research Service of the Department of Veterans Affairs and a grant from Zentaris to Tulane University (all to A.V.S.). Tulane University holds patents on LH-RH antagonist cetrorelix cited in this paper, and A.V.S. is coinventor on that patent.
Abbreviations
- LH-RH
luteinizing hormone-releasing hormone
- RT
reverse transcription
Footnotes
Millar, R., Rosen. H., Pasqualini, C. & Kerdelhue, B. (1982) Program of the 64th Annual Meeting of The Endocrine Society (San Francisco), p. 91 (abstr.).
References
- 1.Schally A V, Halmos G, Rekasi Z, Arencibia J M. In: Infertility and Reproductive Medicine Clinics of North America. Devroey P, editor. Vol. 12. Philadelphia: Saunders; 2001. pp. 17–44. [Google Scholar]
- 2.Schally A V, Comaru-Schally A M, Nagy A, Kovacs M, Szepeshazi K, Plonowski A, Varga J, Halmos G. Front Neuroendocrinol. 2001;22:1–44. doi: 10.1006/frne.2001.0217. [DOI] [PubMed] [Google Scholar]
- 3.Schally A V. Peptides. 1999;20:1247–1262. doi: 10.1016/s0196-9781(99)00130-8. [DOI] [PubMed] [Google Scholar]
- 4.Conn P M. Endocr Rev. 1986;7:3–10. doi: 10.1210/edrv-7-1-3. [DOI] [PubMed] [Google Scholar]
- 5.Gordon K, Hodgen G D. Trends Endocrinol Metab. 1992;7:259–263. doi: 10.1016/1043-2760(92)90128-n. [DOI] [PubMed] [Google Scholar]
- 6.Conn P M, Crawley W F. N Engl J Med. 1991;324:93–103. doi: 10.1056/NEJM199101103240205. [DOI] [PubMed] [Google Scholar]
- 7.Savoy-Moore R T, Schwartz N B, Duncan J A, Marshall J C. Science. 1980;209:942–944. doi: 10.1126/science.6250218. [DOI] [PubMed] [Google Scholar]
- 8.Marian J, Cooper R L, Conn P M. Mol Pharmacol. 1981;19:399–405. [PubMed] [Google Scholar]
- 9.Clayton R N, Catt K J. Endocr Rev. 1981;2:186–209. doi: 10.1210/edrv-2-2-186. [DOI] [PubMed] [Google Scholar]
- 10.Kaiser U B, Jakubowiak A, Steinberger A, Chin W W. Endocrinology. 1993;133:931–934. doi: 10.1210/endo.133.2.8393779. [DOI] [PubMed] [Google Scholar]
- 11.Loumaye E, Catt K J. Science. 1982;215:983–985. doi: 10.1126/science.6296998. [DOI] [PubMed] [Google Scholar]
- 12.Mason D R, Arora K K, Mertz L M, Catt K J. Endocrinology. 1994;135:1165–1170. doi: 10.1210/endo.135.3.8070359. [DOI] [PubMed] [Google Scholar]
- 13.Katt J A, Duncan J A, Herbon L, Barkan A, Marshall J C. Endocrinology. 1985;116:2113–2115. doi: 10.1210/endo-116-5-2113. [DOI] [PubMed] [Google Scholar]
- 14.Schally A V, Comaru-Schally A M. In: Cancer Medicine. 5th Ed. Holland J F, Frei E, Bast R C, Kufe D E, Morton D L, Weichselbaum R R, editors. Hamilton, ON, Canada: Dekker; 2000. pp. 715–729. [Google Scholar]
- 15.Reissmann T, Engel J, Kutscher K, Bernd M, Hilgard P, Peukert M, Szelenyi I, Reichert S, Gonzales-Barcena D, Nieschiag E, Comaru-Schally A M, Schally A V. Drugs of the Future. 1994;19:228–237. [Google Scholar]
- 16.Reissmann T, Schally A V, Bouchard P, Riethmuller H, Engel J. Hum Reprod Update. 2000;6:322–331. doi: 10.1093/humupd/6.4.322. [DOI] [PubMed] [Google Scholar]
- 17.Bajusz S, Csernus V J, Janaky T, Bokser L, Fekete M, Schally A V. Int J Peptide Protein Res. 1988;32:425–435. doi: 10.1111/j.1399-3011.1988.tb01373.x. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez-Barcena D, Banuelos-Alvarez R, Perez-Ochoa E, Cardenas-Cornejo I, Comaru-Schally A M, Schally A V, Engel J, Reissmann T, Riethmuller-Winzen H. Hum Reprod. 1997;12:2028–2035. doi: 10.1093/humrep/12.9.2028. [DOI] [PubMed] [Google Scholar]
- 19.Halmos G, Schally A V, Pinski J, Vadillo-Buenfil M, Groot K. Proc Natl Acad Sci USA. 1996;93:2398–2402. doi: 10.1073/pnas.93.6.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pinski J, Lamharzi N, Halmos G, Groot K, Jungwirth A, Vadillo-Buenfil M, Kakar S S, Schally A V. Endocrinology. 1996;137:3430–3436. doi: 10.1210/endo.137.8.8754771. [DOI] [PubMed] [Google Scholar]
- 21.Bradford M M. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 22.Marian J, Conn M P. Endocrinology. 1983;112:104–112. doi: 10.1210/endo-112-1-104. [DOI] [PubMed] [Google Scholar]
- 23.Graham J M, Rickwood D. Subcellular Fractionation: A Practical Approach. Oxford: IRL Press; 1997. [Google Scholar]
- 24.Labara C, Paigen K. Anal Biochem. 1979;102:344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- 25.Kovacs M, Schally A V, Csernus B, Rekasi Z. Proc Natl Sci USA. 2001;98:1829–1834. doi: 10.1073/pnas.031582398. . (First Published January 30, 2001; 10.1073/pnas.031582398) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaiser U B, Zhao D, Cardona G R, Chin W W. Biochem Biophys Res Commun. 1992;189:1645–1652. doi: 10.1016/0006-291x(92)90266-n. [DOI] [PubMed] [Google Scholar]
- 27.Murata T, Takizawa T, Funaba M, Fujimura H, Murata E, Torii K. Anal Biochem. 1997;244:172–174. doi: 10.1006/abio.1996.9890. [DOI] [PubMed] [Google Scholar]
- 28.Munson P J, Rodbard D. Anal Biochem. 1980;107:220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- 29.Scatchard G. Ann NY Acad Sci. 1949;51:660–662. [Google Scholar]
- 30.Lerrant Y, Kottler M L, Bergametti F, Moumni M, Blumberg-Tick J, Counis R. Endocrinology. 1995;136:2803–2808. doi: 10.1210/endo.136.7.7789305. [DOI] [PubMed] [Google Scholar]
- 31.Kovacs M, Schally A V. Proc Natl Sci USA. 2001;98:12197–12202. doi: 10.1073/pnas.211442598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szende B, Srkalovic G, Timar J, Mulchahey J J, Neill J D, Lapis K, Csikos A, Szepeshazi K, Schally A V. Proc Natl Sci USA. 1991;88:4153–4156. doi: 10.1073/pnas.88.10.4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Szende B, Csikos A, Szepeshazi K, Neill J D, Mulchahey J J, Halmos G, Lapis K, Schally A V. Receptor. 1994;4:201–207. [PubMed] [Google Scholar]
- 34.Halmos G, Schally A V, Kahan Z. Int J Oncol. 2000;17:367–373. doi: 10.3892/ijo.17.2.367. [DOI] [PubMed] [Google Scholar]